TO THE EDITOR:
The recombination-activating genes (RAG1 and RAG2) encode lymphoid-specific proteins responsible for initiation of the V(D)J recombination process, which is critical for the early development of T and B cells.1 The clinical and immunological phenotypes of patients with RAG deficiency are remarkably diverse, varying from severe combined immunodeficiency (SCID) to delayed onset combined immunodeficiency with granulomatous disease and/or autoimmunity (CID-G/AI). The latter phenotype is typically associated with hypomorphic mutations, resulting in residual RAG enzymatic activity and frequent occurrences of autoimmune as well as hyperinflammatory manifestations.2-4 Among them, autoimmune hemolytic anemia (AIHA) is most commonly described, and its pathogenesis has been linked to the breakdown of T- and B-cell tolerance, sometimes triggered by environmental factors (eg, viral infections).5-7 Its therapeutic response to standard treatment (eg, corticosteroids and intravenous immunoglobulins) is often unsatisfactory,3 and patients may benefit from early allogeneic hematopoietic stem cell transplant as the definitive management.8 However, our understanding of this condition remains limited primarily to case reports and small series. Herein, we systematically present clinical, immunological features, treatments, and outcomes of the largest case series, to our knowledge, of AIHA in patients with RAG deficiency.
To assemble a highly annotated and curated clinical database, we retrospectively identified patients with genetically confirmed RAG deficiency who had experienced AIHA during the course of their disease (before allogeneic stem cell transplant if applicable) through an international collaboration. A structured data sheet was created to collect detailed clinical information from the treating physicians. All patients included in this study remained deidentified and were previously consented at their local institutes. In addition, we reviewed all published cases with RAG deficiency accompanied by AIHA in PubMed from 1996 to 2022. We chose 1996 as the starting year of our search because it was when RAG1/2 mutations were first identified as the genetic cause of immunodeficiency in humans.9 Cases with available patient-level data were included. Patients were assigned to relevant clinical and immunological phenotypes (ie, SCID, Omenn syndrome, and atypical SCID [AS]) based on the Primary Immunodeficiency Treatment Consortium criteria.10 CID-G/AI is defined based on the proposed criteria by Delmonte et al.2 Relative V(D)J recombination activity of mutated human RAG1/2 proteins was recorded as described previously11,12 (supplemental Material).
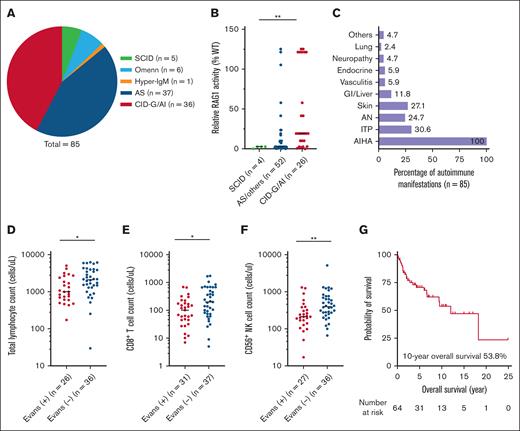
We identified a total of 85 patients with RAG deficiency who developed AIHA during the course of their disease, including 22 from our own case series and 63 extracted from the literature review (supplemental Material). The characteristics of the cohort are shown in Table 1. The median age at clinical and molecular diagnosis of RAG deficiency was 1.3 and 3.0 years, respectively. There was a slight predominance of female patients (55.3%). RAG1 mutations were found in 83.5% of cases, with a median relative RAG1 activity of 7.2%. In terms of clinical phenotypes, patients were classified as SCID (5.9%), CID-G/AI (42.3%), and AS or others (ie, Omenn syndrome and hyper-IgM syndrome; 51.8%) (Figure 1A). As expected, the relative activity of RAG1 mutants correlated with the clinical phenotypes (median, 2.7% in SCID, 2.7% in AS or others, and 19.3% in CID-G/AI, P = .003) (Figure 1B). AIHA was commonly preceded by viral infections (45.2%), and the majority of cases (87.7%) had a documented positive Coombs test. Thirty-seven patients (43.5%) had multiple autoimmune cytopenias (ie, AIHA plus immune thrombocytopenia and/or autoimmune neutropenia), meeting the definition of Evans syndrome.13 Frequencies of other concurrent autoimmune manifestations are shown in Figure 1C.
The characteristics of RAG deficient patients with AIHA
| Variables (median, IQR; n, %) . | All (n = 85) . | Evans (+) (n = 37) . | Evans (–) (n = 48) . | P value∗ . |
|---|---|---|---|---|
| Age (y) | ||||
| Clinical diagnosis | 1.3 (0.7-2.5) (n = 77) | 1.3 (0.9-3.0) (n = 33) | 1.1 (0.4-2.4) (n = 44) | .117 |
| Molecular diagnosis | 3.0 (1.3-8.0) (n = 77) | 4.0 (1.6-10.5) (n = 32) | 2.8 (1.2-7.3) (n = 45) | .345 |
| Male | 34 (44.7) (n = 76) | 12 (34.3) (n = 35) | 22 (53.7) (n = 41) | .109 |
| Clinical phenotype: CID-G/AI | 36 (42.3) | 18 (48.6) | 18 (37.5) | .302 |
| RAG1 genotype | 71 (83.5) | 33 (89.2) | 38 (79.2) | .253 |
| Relative RAG1 activity (per allele) | 7.2 (2.4-23.6) (n = 85) | 10.0 (2.7-49.1) (n = 41) | 2.7 (1.9-17.0) (n = 44) | .045 |
| Relative RAG2 activity (per allele) | 22.1 (9.0-30.8) (n = 26) | 22.1 (22.1-31.2) (n = 8) | 22.1 (2.4-30.8) (n = 18) | .549 |
| History of preceding viral infections | 28 (45.2) (n = 62) | 14 (45.2) (n = 31) | 14 (45.2) (n = 31) | 1.000 |
| Coombs positivity | 57 (87.7) (n = 65) | 31 (96.9) (n = 32) | 26 (78.8) (n = 33) | .054 |
| Other concurrent autoimmunity | 42 (49.4) | 17 (45.9) | 25 (52.1) | .575 |
| Granuloma | 10 (11.8) | 6 (16.2) | 4 (8.3) | .320 |
| Lymphocyte (cells per μL) | 1650 (813-3058) (n = 62) | 1009 (611-2490) (n = 26) | 2120 (1303-3975) (n = 36) | .011 |
| CD3+ (cells per μL) | 454 (197-1161) (n = 77) | 401 (187-697) (n = 32) | 691 (197-1306) (n = 45) | .064 |
| CD4+ (cells per μL) | 172 (87-311) (n = 71) | 150 (82-300) (n = 31) | 202 (101-315) (n = 40) | .480 |
| CD8+ (cells per μL) | 136 (59-360) (n = 68) | 102 (45-180) (n = 31) | 201 (74-673) (n = 37) | .016 |
| CD56+ (cells per μL) | 305 (195-598) (n = 63) | 213 (141-383) (n = 27) | 400 (256-745) (n = 36) | .005 |
| CD19+ (cells per μL) | 87 (29-233) (n = 76) | 113 (44-233) (n = 32) | 81 (21-251) (n = 44) | .296 |
| IgG (mg/dL) | 940 (367-1610) (n = 65) | 973 (400-1705) (n = 29) | 899 (320-1409) (n = 36) | .721 |
| IgA (mg/dL) | 43 (14-105) (n = 54) | 53 (18-123) (n = 25) | 40 (13-94) (n = 29) | .676 |
| IgM (mg/dL) | 128 (38-228) (n = 61) | 109 (38-192) (n = 28) | 128 (36-252) (n = 33) | .728 |
| Allogeneic stem cell transplant | 42 (53.2) (n = 79) | 25 (71.4) (n = 35) | 17 (38.6) (n = 44) | .004 |
| Mortality | 37 (46.8) (n = 79) | 14 (40.0) (n = 35) | 23 (52.3) (n = 44) | .278 |
| Variables (median, IQR; n, %) . | All (n = 85) . | Evans (+) (n = 37) . | Evans (–) (n = 48) . | P value∗ . |
|---|---|---|---|---|
| Age (y) | ||||
| Clinical diagnosis | 1.3 (0.7-2.5) (n = 77) | 1.3 (0.9-3.0) (n = 33) | 1.1 (0.4-2.4) (n = 44) | .117 |
| Molecular diagnosis | 3.0 (1.3-8.0) (n = 77) | 4.0 (1.6-10.5) (n = 32) | 2.8 (1.2-7.3) (n = 45) | .345 |
| Male | 34 (44.7) (n = 76) | 12 (34.3) (n = 35) | 22 (53.7) (n = 41) | .109 |
| Clinical phenotype: CID-G/AI | 36 (42.3) | 18 (48.6) | 18 (37.5) | .302 |
| RAG1 genotype | 71 (83.5) | 33 (89.2) | 38 (79.2) | .253 |
| Relative RAG1 activity (per allele) | 7.2 (2.4-23.6) (n = 85) | 10.0 (2.7-49.1) (n = 41) | 2.7 (1.9-17.0) (n = 44) | .045 |
| Relative RAG2 activity (per allele) | 22.1 (9.0-30.8) (n = 26) | 22.1 (22.1-31.2) (n = 8) | 22.1 (2.4-30.8) (n = 18) | .549 |
| History of preceding viral infections | 28 (45.2) (n = 62) | 14 (45.2) (n = 31) | 14 (45.2) (n = 31) | 1.000 |
| Coombs positivity | 57 (87.7) (n = 65) | 31 (96.9) (n = 32) | 26 (78.8) (n = 33) | .054 |
| Other concurrent autoimmunity | 42 (49.4) | 17 (45.9) | 25 (52.1) | .575 |
| Granuloma | 10 (11.8) | 6 (16.2) | 4 (8.3) | .320 |
| Lymphocyte (cells per μL) | 1650 (813-3058) (n = 62) | 1009 (611-2490) (n = 26) | 2120 (1303-3975) (n = 36) | .011 |
| CD3+ (cells per μL) | 454 (197-1161) (n = 77) | 401 (187-697) (n = 32) | 691 (197-1306) (n = 45) | .064 |
| CD4+ (cells per μL) | 172 (87-311) (n = 71) | 150 (82-300) (n = 31) | 202 (101-315) (n = 40) | .480 |
| CD8+ (cells per μL) | 136 (59-360) (n = 68) | 102 (45-180) (n = 31) | 201 (74-673) (n = 37) | .016 |
| CD56+ (cells per μL) | 305 (195-598) (n = 63) | 213 (141-383) (n = 27) | 400 (256-745) (n = 36) | .005 |
| CD19+ (cells per μL) | 87 (29-233) (n = 76) | 113 (44-233) (n = 32) | 81 (21-251) (n = 44) | .296 |
| IgG (mg/dL) | 940 (367-1610) (n = 65) | 973 (400-1705) (n = 29) | 899 (320-1409) (n = 36) | .721 |
| IgA (mg/dL) | 43 (14-105) (n = 54) | 53 (18-123) (n = 25) | 40 (13-94) (n = 29) | .676 |
| IgM (mg/dL) | 128 (38-228) (n = 61) | 109 (38-192) (n = 28) | 128 (36-252) (n = 33) | .728 |
| Allogeneic stem cell transplant | 42 (53.2) (n = 79) | 25 (71.4) (n = 35) | 17 (38.6) (n = 44) | .004 |
| Mortality | 37 (46.8) (n = 79) | 14 (40.0) (n = 35) | 23 (52.3) (n = 44) | .278 |
IQR, interquartile range.
Comparison between patients with and without Evans syndrome, with statistical significance indicated in bold.
AIHA in RAG deficiency. (A) Distribution of clinical phenotypes. (B) Relative activities of RAG1 mutants across different clinical phenotypes (n = allele number). (C) Frequencies of autoimmune cytopenias and other autoimmune manifestations. (D-F) Comparisons of total lymphocyte, CD8+ T cell and CD56+ NK cell counts between patients with and without Evans syndrome (statistical significance, ∗P < .05, ∗∗P < .01). (G) Kaplan-Meier curve of the overall survival.
AIHA in RAG deficiency. (A) Distribution of clinical phenotypes. (B) Relative activities of RAG1 mutants across different clinical phenotypes (n = allele number). (C) Frequencies of autoimmune cytopenias and other autoimmune manifestations. (D-F) Comparisons of total lymphocyte, CD8+ T cell and CD56+ NK cell counts between patients with and without Evans syndrome (statistical significance, ∗P < .05, ∗∗P < .01). (G) Kaplan-Meier curve of the overall survival.
More detailed information about the AIHA was available from our own case series (n = 22). The median age at onset of the first AIHA episode was 1.5 years, and AIHA was the first autoimmune manifestation in 9 cases (40.9%). Patients typically presented with severe symptomatic anemia (median hemoglobin 6.4 g/dL) that required packed red blood cell transfusion (70%; median 3 units). Most cases were warm AIHA with immunoglobulin G (IgG) autoantibodies (94.4%) on direct Coombs tests. In parallel, anticytokine autoantibodies against interferon-α (85.7%), interferon-ω (57.1%), and interleukin-12 (28.6%) were frequently found in these patients (n = 7). One of them had available serum samples 16 months before AIHA onset, and anti-interferon-α/ω autoantibodies were already detected at that time. In the remaining cases, autoantibodies were only measured either at the onset of AIHA or after its development, depending on the availability of samples. The disease course was usually recurrent in nature (75%). Despite treatment with corticosteroids (86.4%) and high-dose intravenous immunoglobulins (54.5%), half of them required additional immunosuppressive therapy (50%) (supplemental Material), and ultimately, 16 patients (72.7%) underwent allogeneic stem cell transplant. Of note, refractory cytopenia was among the transplant indications in 8 patients (50%). No recurrence of AIHA was observed in transplant survivors.
Patients who were diagnosed with Evans syndrome (n = 37, 43.5%) exhibited a unique baseline immunological profile (Table 1), characterized by a higher relative activity of RAG1 mutants (10.0% vs 2.7%, P = .045), lower total lymphocytes (median 1009 vs 2120/μL, P = .011), CD8+ T (median 102 vs 201/μL, P = .016), and CD56+ natural killer cell counts (median 213 vs 400/μL, P = .005) (Figure 1D-F). Levels of IgG, IgA, or IgM were not significantly different between the 2 groups. More people with Evans syndrome underwent allogeneic stem cell transplant (71.4% vs 38.6%, P = .004). Of the entire cohort, the 10-year overall survival was 53.8% (n = 64 with follow-up data available) (Figure 1G) with infections as the main cause of death (supplement), and allogeneic stem cell transplant was associated with a reduced mortality (33.3% vs 62.2%; P = 0.010).
RAG1/2 mutations affect not only the composition of adaptive immune receptor repertoires but T- and B-cell tolerance.1 Although immune dysregulation, exemplified by the development of a broad range of serum autoantibodies as well as diverse clinical autoimmunity,3,6 has been recognized as a key feature of RAG deficiency, the underlying pathophysiology is not yet fully understood (supplemental Material). Importantly, the defects of B-cell tolerance worsen with age and autoantibodies such as those targeting cytokines appear along the course of disease in RAG deficiency, suspectedly contributed by repeated exposure to environmental antigens, such as viral infections.7 In parallel, the corresponding clinical phenotype could also evolve in response to an environmental trigger with the subsequent development of new clinical autoimmunity, for instance the onset of AIHA within several months after a viral illness.14 In this study, we summarized the clinical and immunological features of AIHA, a common hematological manifestation of immune dysregulation, observed in patients with RAG deficiency. It typically manifests as severe, recurrent, and treatment-refractory anemia despite the efforts of standard transfusion support and various immunosuppressive treatments. Allogeneic stem cell transplant represents a potentially curative approach, although it is not without challenges8 (supplemental Material). Anticytokine autoantibodies, potential biomarkers of widespread B-cell activation in RAG deficiency, were frequently found in these patients, and 1 patient had detectable anti-interferon-α/ω antibodies 16 months before AIHA onset. These findings support the idea that abnormal B-cell activation precedes the emergence of autoimmune manifestations, which may raise the possibility of early intervention to prevent the progression toward symptomatic clinical autoimmunity.7 Notably, the coexistence of multiple autoimmune cytopenias (ie, Evans syndrome) is not uncommon, and these patients demonstrated a unique immunological profile with reduced numbers of cytotoxic cells. However, it remains unclear whether this finding directly contributes to the development of autoimmune cytopenias or if it is a bystander reflecting the severity of underlying immunodeficiency. Further mechanistic investigations are warranted, such as evaluating the cytotoxic functions in these patients.
Collectively, patients with RAG deficiency often experience autoimmune cytopenias, particularly AIHA during the course of their disease. Multiple lines of AIHA-directed therapies are typically required and allogeneic stem cell transplant, when appropriate, may improve the long-term outcomes in this population.
Acknowledgments: Research laboratory studies were performed on deidentified samples under institutional review board approved protocols at the University of South Florida (USF-Pro00035468 [principal investigator (PI), J.E.W.], USF-Pro00025693 [PI, J.E.W.]) and Johns Hopkins Medical Institute/Johns Hopkins All Children’s Hospital (JHMI-IRB00175372 [PI, J.E.W.]).
C.W. is supported in part by the Division of Clinical Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.R.F. was supported by a faculty development award from the American Academy of Allergy Asthma & Immunology and the National Institute on Minority Health and Health Disparities of the National Institutes of Health under award number R01MD017816. L.D.N. is supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant ZIA AI001222). J.E.W. is supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health 5K08AI103035, sub-R01AI100887-05 and R01AI153830-05, Robert A. Good Endowment at University of South Florida, and Jeffrey Modell Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contribution: C.W. collected the data, performed analyses, and wrote the manuscript; K.W. collected the data; B.U., S.G., and K. Csomos measured anticytokine autoantibodies; B.S., J.R.F., C.B.G., E.W.-C., S.S., E.S., R.S., K. Chen, J.J.J., C.M.D., M.G.K., M.A., P.P., C.B., E.L., B.W.-K., G.D., J.B., D.M., B.N., C.S., R.S.G., L.D.N., M.M., D.K.B., W.W., J.-Y.W., and X.W. contributed data; and J.E.W. supervised the study and revised the manuscript.
Conflict-of-interest: J.R.F. is an ongoing consultant for Pharming and has received investigator-initiated research grants from Pfizer, Bristol Myers Squibb, and Pharming, with no direct relation to the work presented. K. Chen is a consultant at Immune Deficiency Foundation and Horizon Therapeutics, and is a part of the speaker’s bureau for Takeda. J.E.W. reports grant/research/clinical trial support provided by Takeda (Immunology), Janssen (Hematology), Chiesi (Immunology), MustangBio (Immunology), ADMA Biologicals (Immunology), Octapharma (Immunology), X4-Pharmaceuticals (Immunology), Novartis (Allergy), Regeneron (Allergy), and Bristol Myers Squibb (Immunology); consultant/advisory board fees from Takeda (Immunology), X4-Pharmaceuticals (Immunology), CSL-Behring (Immunology), Grifols (Immunology), ADMA Biologicals (Immunology), Enzyvant (Immunology), Regeneron (Immunology), and Pharming (Immunology); speaker’s bureau fees from Takeda (Immunology) and Pharming (Immunology); and is a medical writer for UpToDate. The remaining authors declare no competing financial interests.
The current affiliation for C.W. is National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Correspondence: Jolan E. Walter, Division of Pediatric Allergy/Immunology, University of South Florida, 601 4th St South (CRI 4008), Tampa, FL 33701; email: jolanwalter@usf.edu.
References
Author notes
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 December 2022.
Data that support the findings of this study are available on request from the corresponding author, Jolan E. Walter (jolanwalter@usf.edu).
The full-text version of this article contains a data supplement.