Key Points
Major bleeding and recurrent/progressive thrombosis were frequent complications in cancer-associated splanchnic vein thrombosis.
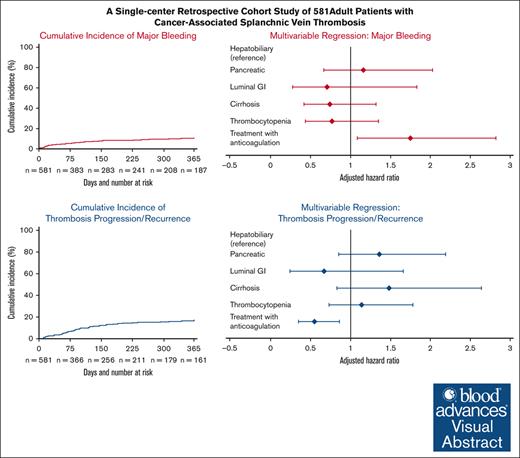
Treatment with anticoagulation was associated with increased major bleeding and decreased thrombotic progression or recurrence.
Visual Abstract
Malignancy is a risk factor for splanchnic vein thrombosis (SpVT). Data on the natural history of cancer-associated SpVT are limited. This was a single-center, retrospective cohort study of 581 adult patients with cancer and SpVT. We aimed to characterize the impact of thrombocytopenia on major bleeding and progression or recurrence of SpVT within 1 year of an initial cancer-associated SpVT diagnosis. Baseline thrombocytopenia (platelet <100 × 103/μL within 15 days of SpVT diagnosis) was present in 39.5% of patients. A total of 39.2% of patients received therapeutic anticoagulation within 2 weeks of an SpVT diagnosis. The cumulative 1-year incidence of major bleeding was 10.7% (95% confidence interval [CI], 8.2-13.2) and 16.2% (95% CI, 13.2-19.2) for SpVT recurrence/progression. In the multivariable regression analysis, therapeutic anticoagulation was associated with increased major bleeding (adjusted risk ratio [aRR], 1.74; 95% CI, 1.08-2.81) and decreased progression/recurrence of SpVT (aRR, 0.55; 95% CI, 0.35-0.86). Baseline thrombocytopenia was not independently associated with either major bleeding (aRR, 0.76; 95% CI, 0.43-1.34) or progression/recurrence of SpVT (aRR, 1.14; 95% CI, 0.73-1.78). A secondary analysis using inverse probability of treatment weighting with propensity scores for baseline thrombocytopenia corroborated that patients with thrombocytopenia did not have an increased bleeding risk (adjusted hazard ratio [aHR], 0.81; 95% CI, 0.48-1.39). The multivariable analysis in which platelets were treated as a time varying covariate also did not reveal an association with major bleeding (aHR, 0.89; 95% CI, 0.55-1.45). Bleeding and thrombosis progression were frequent in patients with cancer-associated SpVT. Anticoagulation was associated with increased major bleeding and decreased thrombotic progression; thrombocytopenia did not impact the outcomes.
Introduction
A venous thromboembolism (VTE) is a frequent complication among patients with cancer and is associated with increased morbidity and mortality.1,2 Splanchnic vein thrombosis (SpVT) refers to thrombosis of the hepatic vein, portal vein, splenic vein, or mesenteric veins.3 Cancers, especially gastrointestinal (GI) malignancies, are associated with an increased risk for SpVT. This risk is especially pronounced in patients with concomitant liver disease or those who have undergone intra-abdominal surgery.4,5 Although SpVT may be detected incidentally during surveillance imaging, it can present with complications of portal hypertension, GI bleeding, hepatic ischemia, and bowel infarction.6,7 Moreover, SpVT has been associated with increased mortality in the general population8 and specifically in patients with localized or metastatic malignancy.9
Patients with cancer-associated thrombosis can be particularly challenging to manage because of the high risk for VTE recurrence10 and bleeding complications associated with anticoagulation therapy when compared with patients without cancer.11 The optimal management of cancer-associated, usual-site VTE (deep vein thrombosis or pulmonary embolism) has now been assessed in several large randomized control trials.12 However, the existing evidence regarding the outcomes in cancer-associated SpVT and the impact of anticoagulation therapy are based primarily on limited observational data.13 The current society guidelines reflect this uncertainty surrounding treatment with anticoagulants in patients with cancer-associated SpVT. The American Society of Hematology guidelines suggest either short-term anticoagulation treatment or observation in patients with cancer-associated SpVT,14 and the American Society of Clinical Oncology recommends treatment of incidental cancer-associated SpVT on a case-by-case basis.15 The International Society on Thrombosis and Hemostasis (ISTH) proposes treatment of symptomatic SpVT in both the general population and in those with cancer based on weak quality of evidence.16
Concurrent thrombocytopenia is frequent in cancer-associated thrombosis, which can be attributed to the malignancy itself, comorbidities, or cancer-directed therapies,17 and can further complicate decisions surrounding anticoagulation therapy.18 This is particularly relevant to patients with cancer-associated SpVT because portal hypertension is associated with both lower platelet counts and an increased risk for GI bleeding.19 However, data on how thrombocytopenia modulates thrombotic and hemorrhagic risk in this high risk subpopulation are lacking. This study aimed to describe the clinical characteristics, anticoagulation strategies used, and bleeding and thrombotic outcomes in patients with cancer-associated SpVT. We further aimed to determine the impact of thrombocytopenia at the time of SpVT diagnosis on these outcomes.
Methods
Study design
This was a single-center, retrospective cohort study. The inclusion criteria were age ≥18 years, a radiographic SpVT diagnosis between 2010 and 2021, and a cancer diagnosis made before or up to 1 month after the SpVT diagnosis. Diagnoses of SpVT were confirmed with radiology reports. The exclusion criteria included squamous and basal cell carcinomas of the skin, benign neuroendocrine tumors, in situ neoplasms, neoplasms of uncertain behavior, and myeloproliferative neoplasms.
Patients with cancer-associated SpVT were initially identified using billing codes. We subsequently conducted manual chart review for all patients to confirm their eligibility and to collect data on the patient characteristics, treatment strategies, and outcomes. Cancer status was classified as active if the diagnosis had been made within 6 months before the SpVT diagnosis, if the cancer was metastatic, or if the patient was undergoing cancer-directed systemic therapy at the time of SpVT diagnosis. We defined baseline thrombocytopenia as at least 1 platelet count of <100 × 103/μL within 15 days before or after the diagnosis of SpVT. The cutoff for thrombocytopenia based on the Common Terminology Criteria for Adverse Events (v.5.0),20 which classifies grade 1 thrombocytopenia as a platelet count less than the lower limit of normal to 75 × 103/μL. In keeping with previous work in our group on thrombocytopenia in cancer-associated thrombosis, we defined the lower limit of normal as <100 × 103/μL platelets per μL to discount temporary or clinically inconsequential decreases in the platelet count in the range of 100 × 103/μL to 150 × 103/μL.17
Outcomes
All outcomes were defined and measured up to 12 months after the initial diagnosis of SpVT. The primary outcome was major bleeding. Bleeding outcomes were classified according to the ISTH criteria.21 Secondary outcomes included clinically relevant nonmajor bleeding (CRNMB) according to the ISTH criteria22; clinically relevant bleeding, which was defined as a composite of major bleeding and CRNMB; progression or recurrence of SpVT with progression defined as extension of the thrombus contiguously into a new vein and recurrence defined as a new interval thrombus, which was noncontiguous with the initial thrombus; and usual-site VTE, which included deep vein thrombosis and pulmonary embolism.
Analysis
The cumulative incidences of the primary and secondary outcomes were calculated at 12 months after the incident SpVT, and death was considered a competing risk. We performed a Poisson regression with robust variance estimators to identify factors associated with major bleeding, including age at thrombosis (continuous), sex, the presence of cirrhosis, a previous episode of major hemorrhage, abdominal surgery within the last 3 months, type of SpVT (bland vs tumor thrombus or mixed), thrombocytopenia, therapeutic anticoagulation treatment within 2 weeks of an SpVT diagnosis, baseline creatinine, degree of thrombus occlusion (partially occlusive vs completely occlusive), and vessel involvement (single vessel vs multiple vessels). Although we defined thrombocytopenia as a platelet count of less than 100 × 103/μL as mentioned above, we also tested lower thresholds of thrombocytopenia, namely 75 × 103/μL and 50 × 103/μL (within 15 days before or after the diagnosis of SpVT), as covariates in secondary analyses. In univariable and multivariable regression models, we used age at thrombosis (continuous), sex, previous major bleed, baseline creatinine (continuous), use of antiplatelet therapy, and use of therapeutic anticoagulants within 2 weeks of diagnosis as covariates. The data were reported using risk ratios (RRs), adjusted RRs (aRRs), and 95% confidence intervals (CIs).
In a secondary analysis, we used Cox models with inverse probability of treatment weighting with propensity scores for thrombocytopenia (less than 100 × 103 platelets per μL at baseline) to calculate the adjusted hazard ratios (aHRs) and 95% CIs for the primary and secondary outcomes, including death as a competing risk. Covariates included in the Cox models were the same as those in the outcome-specific Poisson models. The independent variables that were used to create the propensity scores included the type of cancer (hematologic, solid tumor) and all other variables included in the adjusted regression models. Propensity scores were truncated at the 1st and 99th percentiles. Patients were censored from the analysis at the time of an event, separately by outcome; at their last clinical encounter; or at 1 year after their SpVT diagnosis, whichever occurred first.
Given the dynamic nature of platelet counts that can fluctuate over time, we performed Cox proportional hazards models using the same covariates listed but using thrombocytopenia (<100 × 103 platelets per μL) as a time-varying exposure to assess the impact of thrombocytopenia on major bleeding.
A robust Poisson regression was also performed to assess these same risk factors for the association with the secondary outcome of recurrence or progression of SpVT. The model included the same covariates as those included in the model for major bleeding detailed previously, except that previous VTE was substituted for a previous major bleed.
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and GraphPad Prism (GraphPad Software for Windows, La Jolla, CA).
Results
Patient characteristics
A total of 581 patients met the inclusion criteria. (Table 1). Overall, 63.6% (n = 368) were male, 44.2% (n = 257) of patients had a previous diagnosis of cirrhosis, 5.2% (n = 30) had a history of VTE, and 6.0% (n = 35) had abdominal surgery in the last 3 months. The most common cancer types were hepatobiliary (55.2%; n = 321), pancreatic (22.0%; n = 128), and colorectal (5.3%; n = 31). Hematologic malignancies accounted for 6.2% (n = 36) of cancers. Most (92.8%; n = 525) cancers were classified as active at the time of index SpVT. The most common presenting symptom of SpVT was abdominal pain (42.3%; n = 246), whereas 32.7% (n = 190) were diagnosed incidentally. The most commonly involved site in the splanchnic bed was the portal vein (89.3%; n = 519). Based on radiographic characteristics, 70.6% (n = 408) of patients had bland SpVT, whereas 19.9% (n = 115) had tumor SpVT and 5.5% (n = 32) were mixed type. Of cases for which data were available, the majority of SpVT (68.2%, n = 264) were radiologically classified as partially occlusive.
Patient characteristics
| Characteristic . | N = 581 n (%) . |
|---|---|
| Age at thrombosis (y), mean ± SD | 64.2 ± 11.4 |
| Sex | |
| Female | 211 (36.4) |
| Male | 368 (63.6) |
| Comorbidities | |
| Cirrhosis | 257 (44.2) |
| Previous major hemorrhage | 67 (11.5) |
| Abdominal surgery in past 3 mo | 35 (6.0) |
| Previous VTE | 30 (5.2) |
| Cancer diagnosis∗ | |
| Hepatobiliary | 321 (55.2) |
| Pancreatic | 128 (22.0) |
| Colorectal | 31 (5.3) |
| Lymphoma | 23 (4.0) |
| Stomach | 17 (2.9) |
| Breast | 15 (2.6) |
| Leukemia | 13 (2.2) |
| Kidney | 11 (1.9) |
| Prostate | 8 (1.4) |
| Esophageal | 5 (0.9) |
| Lung | 5 (0.9) |
| Small intestine | 5 (0.9) |
| Endometrial | 4 (0.7) |
| Melanoma | 4 (0.7) |
| Ovarian | 4 (0.7) |
| Sarcoma | 4 (0.7) |
| Bladder | 3 (0.5) |
| Head and neck | 3 (0.5) |
| Myeloma | 2 (0.3) |
| Nonmelanoma skin | 1 (0.2) |
| Thyroid | 1 (0.2) |
| Other | 23 (4.0) |
| Cancer stage (n = 560) | |
| Local | 133 (23.8) |
| Locally advanced | 199 (35.5) |
| Metastatic | 228 (40.7) |
| Cancer status | |
| Active (diagnosed or treated within last 6 mo) | 525 (92.8) |
| In remission | 41 (7.2) |
| Medications at time of SpVT | |
| Antiplatelet | 113 (19.4) |
| Anticoagulant | 32 (5.5) |
| Systemic cancer therapy | 116 (20.0) |
| Thrombosis location∗ | |
| Portal vein | 519 (89.3) |
| Hepatic vein | 35 (6.0) |
| Splenic vein | 66 (11.4) |
| Superior mesenteric vein | 119 (20.5) |
| Inferior mesenteric vein | 6 (1.0) |
| Extent of SpVT (n = 387) | |
| Completely occlusive | 123 (31.8) |
| Partially occlusive | 264 (68.2) |
| Type of SpVT | |
| Bland | 408 (70.6) |
| Tumor | 115 (19.9) |
| Mixed | 32 (5.5) |
| Uncertain | 23 (4.0) |
| Presenting signs or symptoms of SpVT∗ | |
| Abdominal pain | 246 (42.3) |
| Nausea or vomiting | 49 (8.4) |
| Ascites | 57 (9.8) |
| Jaundice | 46 (7.9) |
| Abnormal liver function tests | 66 (11.4) |
| GI bleed | 27 (4.6) |
| Ischemic bowel | 4 (0.7) |
| Incidental finding | 190 (32.7) |
| Other | 147 (25.3) |
| Concurrently diagnosed usual-site VTE | |
| DVT | 15 (2.6) |
| PE | 14 (2.4) |
| Baseline laboratory results, mean ± SD | |
| Hemoglobin (g/dL) | 10.8 ± 2.1 |
| Platelets (K/μL) | 194 ± 137 |
| Creatinine (mg/dL) | 1.1 ± 0.8 |
| Prothrombin time (s) | 18.3 ± 13.2 |
| Partial thromboplastin time (s) | 47.7 ± 32.3 |
| Treatment within 2 wk of diagnosis | |
| Therapeutic anticoagulation | 228 (39.2) |
| Unfractionated heparin | 33 (14.5) |
| Warfarin | 42 (18.4) |
| LMWH | 98 (43.0) |
| DOAC | 55 (24.1) |
| Fondaparinux | 0 (0.0) |
| Mechanical thrombectomy | 23 (4.0) |
| Baseline platelet count | |
| ≥100 × 103/μL | 310 (60.6) |
| 75 × 103/μL - 99 × 103/μL | 65 (12.7) |
| 50 × 103/μL - 74 × 103/μL | 72 (14.1) |
| <50 × 103/μL | 65 (12.7) |
| Characteristic . | N = 581 n (%) . |
|---|---|
| Age at thrombosis (y), mean ± SD | 64.2 ± 11.4 |
| Sex | |
| Female | 211 (36.4) |
| Male | 368 (63.6) |
| Comorbidities | |
| Cirrhosis | 257 (44.2) |
| Previous major hemorrhage | 67 (11.5) |
| Abdominal surgery in past 3 mo | 35 (6.0) |
| Previous VTE | 30 (5.2) |
| Cancer diagnosis∗ | |
| Hepatobiliary | 321 (55.2) |
| Pancreatic | 128 (22.0) |
| Colorectal | 31 (5.3) |
| Lymphoma | 23 (4.0) |
| Stomach | 17 (2.9) |
| Breast | 15 (2.6) |
| Leukemia | 13 (2.2) |
| Kidney | 11 (1.9) |
| Prostate | 8 (1.4) |
| Esophageal | 5 (0.9) |
| Lung | 5 (0.9) |
| Small intestine | 5 (0.9) |
| Endometrial | 4 (0.7) |
| Melanoma | 4 (0.7) |
| Ovarian | 4 (0.7) |
| Sarcoma | 4 (0.7) |
| Bladder | 3 (0.5) |
| Head and neck | 3 (0.5) |
| Myeloma | 2 (0.3) |
| Nonmelanoma skin | 1 (0.2) |
| Thyroid | 1 (0.2) |
| Other | 23 (4.0) |
| Cancer stage (n = 560) | |
| Local | 133 (23.8) |
| Locally advanced | 199 (35.5) |
| Metastatic | 228 (40.7) |
| Cancer status | |
| Active (diagnosed or treated within last 6 mo) | 525 (92.8) |
| In remission | 41 (7.2) |
| Medications at time of SpVT | |
| Antiplatelet | 113 (19.4) |
| Anticoagulant | 32 (5.5) |
| Systemic cancer therapy | 116 (20.0) |
| Thrombosis location∗ | |
| Portal vein | 519 (89.3) |
| Hepatic vein | 35 (6.0) |
| Splenic vein | 66 (11.4) |
| Superior mesenteric vein | 119 (20.5) |
| Inferior mesenteric vein | 6 (1.0) |
| Extent of SpVT (n = 387) | |
| Completely occlusive | 123 (31.8) |
| Partially occlusive | 264 (68.2) |
| Type of SpVT | |
| Bland | 408 (70.6) |
| Tumor | 115 (19.9) |
| Mixed | 32 (5.5) |
| Uncertain | 23 (4.0) |
| Presenting signs or symptoms of SpVT∗ | |
| Abdominal pain | 246 (42.3) |
| Nausea or vomiting | 49 (8.4) |
| Ascites | 57 (9.8) |
| Jaundice | 46 (7.9) |
| Abnormal liver function tests | 66 (11.4) |
| GI bleed | 27 (4.6) |
| Ischemic bowel | 4 (0.7) |
| Incidental finding | 190 (32.7) |
| Other | 147 (25.3) |
| Concurrently diagnosed usual-site VTE | |
| DVT | 15 (2.6) |
| PE | 14 (2.4) |
| Baseline laboratory results, mean ± SD | |
| Hemoglobin (g/dL) | 10.8 ± 2.1 |
| Platelets (K/μL) | 194 ± 137 |
| Creatinine (mg/dL) | 1.1 ± 0.8 |
| Prothrombin time (s) | 18.3 ± 13.2 |
| Partial thromboplastin time (s) | 47.7 ± 32.3 |
| Treatment within 2 wk of diagnosis | |
| Therapeutic anticoagulation | 228 (39.2) |
| Unfractionated heparin | 33 (14.5) |
| Warfarin | 42 (18.4) |
| LMWH | 98 (43.0) |
| DOAC | 55 (24.1) |
| Fondaparinux | 0 (0.0) |
| Mechanical thrombectomy | 23 (4.0) |
| Baseline platelet count | |
| ≥100 × 103/μL | 310 (60.6) |
| 75 × 103/μL - 99 × 103/μL | 65 (12.7) |
| 50 × 103/μL - 74 × 103/μL | 72 (14.1) |
| <50 × 103/μL | 65 (12.7) |
DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; PE, pulmonary embolism; SD, standard deviation.
Patients can have multiple attributes, and thus totals may sum to more than 100%.
Of the 512 patients with available baseline platelet count data, 39.5% (n = 202) of patients had a platelet count of <100 × 103/μL; overall, 12.7% (n = 65) had platelet counts of 75 × 103/μL to 99 × 103/μL, 14.1% (n = 72) had counts of 50 × 103/μL to 74 × 103/μL, and 12.7% (n = 65) had counts of <50 × 103/μL. A total of 5.5% (n = 32) of patients were on anticoagulation therapy at baseline at the time of SpVT diagnosis, and 19.4% (n = 113) were on antiplatelet therapy. Less than half (39.2%; n = 228) of patients received therapeutic anticoagulation within 2 weeks of diagnosis, and 4.0% (n = 23) of patients received a mechanical thrombectomy. Most common anticoagulants used were low molecular weight heparin (43.0%; n = 98), direct oral anticoagulants (24.1%; n = 55), and warfarin (18.4%; n = 42). The median duration of anticoagulation treatment was 3.0 months (interquartile range, 0.5-6.0). Survival at 1 year was 54.4%, and the median follow-up time was 5.3 months (interquartile range, 1.3-12.0).
Bleeding outcomes
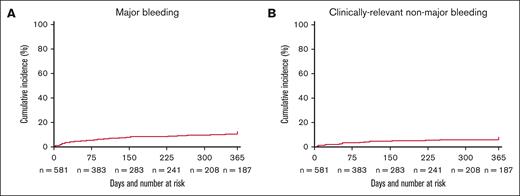
The cumulative incidence of major bleeding events within 1 year of the SpVT diagnosis was 10.7% (95% CI, 8.2-13.2) (Table 2; Figure 1A). Of these major bleeding episodes, 69.4% (n = 43) were upper GI bleeds. The cumulative incidence of CRNMB at 1 year was 6.2% (95% CI, 4.2-8.2) (Figure 1B), and the cumulative incidence of clinically relevant bleeding was 16.9% (95% CI, 13.8-19.9). Among the 62 patients who had major bleeding, 31 (50%) had been started on therapeutic anticoagulation for their SpVT; of the patients started on anticoagulation, 29 (93.5%) remained on anticoagulation up until their bleeding event. Among the 36 patients who had CRNMB, 17 (47.2%) had been started on anticoagulation therapy; of the patients started on anticoagulation, all remained on anticoagulants at the time of the bleeding event. Outcomes were further stratified by tumor type, stage, the presence of symptoms, and treatment with therapeutic anticoagulation (supplemental Table 1).
Cumulative incidence of primary and secondary outcomes by thrombocytopenia status
| Outcome (within 1 y of SpVT diagnosis) . | Overall cohort N = 581 . | Platelet <100 × 103/μL n = 202 . | Platelet ≥100 × 103/μL n = 310 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | Incidence (95% CI) . | Time to event (d), median (IQR) . | n . | Incidence (95% CI) . | Time to event (d), median (IQR) . | n . | Incidence (95% CI) . | Time to event (d), median (IQR) . | |
| Major bleeding | 62 | 10.7% (8.2-13.2) | 70.0 (17.0-146) | 21 | 10.4% (6.2-14.6) | 23.0 (12.0-146) | 34 | 11.0% (7.5-14.5) | 77.0 (23.0-144) |
| Upper GI | 43 | 7.4% (5.3-9.5) | 75.0 (14.0-160) | 13 | 6.4% (3.1-9.8) | 13.0 (3.0-146) | 24 | 7.7% (4.8-10.7) | 82.0 (22.5-152) |
| Lower GI | 4 | 0.7% (0.02-1.4) | 55.5 (37.0-83.0) | 1 | 0.5% (0.00-1.5) | 46.0 (46.0-46.0) | 3 | 1.0% (0.00-2.1) | 65.0 (28.0-101) |
| Intracranial | 5 | 0.9% (0.01-1.6) | 129 (110-244) | 2 | 1.0% (0.00-2.4) | 187 (129-244) | 2 | 0.7% (0.00-1.5) | 141 (21.0-261) |
| Retroperitoneal | 2 | 0.3% (0.00-0.08) | 23.5 (6.0-41.0) | 0 | 0.0% | -- | 2 | 0.7% (0.00-1.5) | 23.5 (6.0-41.0) |
| Intraarticular/intramuscular | 3 | 0.5% (0.00-1.1) | 23.0 (16.0-54.0) | 3 | 1.5% (0.00-3.2) | 23.0 (16.0-54.0) | 0 | 0.0% | -- |
| Mucocutaneous | 0 | 0.0% | -- | 0 | 0.0% | -- | 0 | 0.0% | -- |
| Pericardial | 0 | 0.0% | -- | 0 | 0.0% | -- | 0 | 0.0% | -- |
| CRNMB | 36 | 6.2% (4.2-8.2) | 56.0 (19.0-117) | 13 | 6.4% (3.1-9.8) | 97.0 (55.0-220) | 18 | 5.8% (3.2-8.4) | 21.5 (5.0-90.0) |
| Clinically relevant bleeding | 98 | 16.9% (13.8-19.9) | 60.5 (18.0-141) | 34 | 16.8% (11.7-22.0) | 55.5 (16.0-154) | 52 | 16.8% (12.6-20.9) | 56.0 (20.5-101) |
| Progression and/or recurrence of SpVT∗ | 94 | 16.2% (13.2-19.2) | 87.0 (51.0-146) | 29 | 14.4% (9.5-19.2) | 82.0 (37.0-161) | 40 | 12.9% (9.2-16.6) | 73.0 (44.5-125) |
| Progression | 79 | 13.6% (10.8-16.4) | 89.0 (56.0-151) | 24 | 11.9% (7.4-16.3) | 81.0 (37.5-159) | 33 | 10.7% (7.2-14.1) | 87.0 (56.0-130) |
| Recurrence | 20 | 3.4% (2.0-4.9) | 110 (24.5-195) | 6 | 3.0% (0.6-5.3) | 174 (17.0-224) | 8 | 2.6% (0.8-4.4) | 53.0 (15.5-95.5) |
| Usual-site VTE (DVT or PE) | 30 | 5.2% (3.4-7.0) | 80.5 (43.0-137) | 5 | 2.5% (0.3-4.6) | 134 (92.0-174) | 21 | 6.8% (4.0-9.6) | 71.0 (43.0-137) |
| DVT | 16 | 2.8% (1.4-4.1) | 81.0 (35.5-185) | 5 | 2.5% (0.3-4.6) | 134 (92.0-174) | 9 | 2.9% (1.0-4.8) | 66.0 (43.0-196) |
| PE | 14 | 2.4% (1.2-4.0) | 80.5 (54.0-100) | 0 | 0.0% | -- | 12 | 3.9% (1.7-6.0) | 77.0 (48.0-119) |
| Outcome (within 1 y of SpVT diagnosis) . | Overall cohort N = 581 . | Platelet <100 × 103/μL n = 202 . | Platelet ≥100 × 103/μL n = 310 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n . | Incidence (95% CI) . | Time to event (d), median (IQR) . | n . | Incidence (95% CI) . | Time to event (d), median (IQR) . | n . | Incidence (95% CI) . | Time to event (d), median (IQR) . | |
| Major bleeding | 62 | 10.7% (8.2-13.2) | 70.0 (17.0-146) | 21 | 10.4% (6.2-14.6) | 23.0 (12.0-146) | 34 | 11.0% (7.5-14.5) | 77.0 (23.0-144) |
| Upper GI | 43 | 7.4% (5.3-9.5) | 75.0 (14.0-160) | 13 | 6.4% (3.1-9.8) | 13.0 (3.0-146) | 24 | 7.7% (4.8-10.7) | 82.0 (22.5-152) |
| Lower GI | 4 | 0.7% (0.02-1.4) | 55.5 (37.0-83.0) | 1 | 0.5% (0.00-1.5) | 46.0 (46.0-46.0) | 3 | 1.0% (0.00-2.1) | 65.0 (28.0-101) |
| Intracranial | 5 | 0.9% (0.01-1.6) | 129 (110-244) | 2 | 1.0% (0.00-2.4) | 187 (129-244) | 2 | 0.7% (0.00-1.5) | 141 (21.0-261) |
| Retroperitoneal | 2 | 0.3% (0.00-0.08) | 23.5 (6.0-41.0) | 0 | 0.0% | -- | 2 | 0.7% (0.00-1.5) | 23.5 (6.0-41.0) |
| Intraarticular/intramuscular | 3 | 0.5% (0.00-1.1) | 23.0 (16.0-54.0) | 3 | 1.5% (0.00-3.2) | 23.0 (16.0-54.0) | 0 | 0.0% | -- |
| Mucocutaneous | 0 | 0.0% | -- | 0 | 0.0% | -- | 0 | 0.0% | -- |
| Pericardial | 0 | 0.0% | -- | 0 | 0.0% | -- | 0 | 0.0% | -- |
| CRNMB | 36 | 6.2% (4.2-8.2) | 56.0 (19.0-117) | 13 | 6.4% (3.1-9.8) | 97.0 (55.0-220) | 18 | 5.8% (3.2-8.4) | 21.5 (5.0-90.0) |
| Clinically relevant bleeding | 98 | 16.9% (13.8-19.9) | 60.5 (18.0-141) | 34 | 16.8% (11.7-22.0) | 55.5 (16.0-154) | 52 | 16.8% (12.6-20.9) | 56.0 (20.5-101) |
| Progression and/or recurrence of SpVT∗ | 94 | 16.2% (13.2-19.2) | 87.0 (51.0-146) | 29 | 14.4% (9.5-19.2) | 82.0 (37.0-161) | 40 | 12.9% (9.2-16.6) | 73.0 (44.5-125) |
| Progression | 79 | 13.6% (10.8-16.4) | 89.0 (56.0-151) | 24 | 11.9% (7.4-16.3) | 81.0 (37.5-159) | 33 | 10.7% (7.2-14.1) | 87.0 (56.0-130) |
| Recurrence | 20 | 3.4% (2.0-4.9) | 110 (24.5-195) | 6 | 3.0% (0.6-5.3) | 174 (17.0-224) | 8 | 2.6% (0.8-4.4) | 53.0 (15.5-95.5) |
| Usual-site VTE (DVT or PE) | 30 | 5.2% (3.4-7.0) | 80.5 (43.0-137) | 5 | 2.5% (0.3-4.6) | 134 (92.0-174) | 21 | 6.8% (4.0-9.6) | 71.0 (43.0-137) |
| DVT | 16 | 2.8% (1.4-4.1) | 81.0 (35.5-185) | 5 | 2.5% (0.3-4.6) | 134 (92.0-174) | 9 | 2.9% (1.0-4.8) | 66.0 (43.0-196) |
| PE | 14 | 2.4% (1.2-4.0) | 80.5 (54.0-100) | 0 | 0.0% | -- | 12 | 3.9% (1.7-6.0) | 77.0 (48.0-119) |
DVT, deep vein thrombosis; IQR, interquartile range; PE, pulmonary embolism.
For patients with progression and recurrence, time to event is time to the earlier event.
Cumulative incidence of bleeding outcomes. Cumulative incidence of (A) major bleeding and (B) CRNMB using the Kaplan-Meier method with death as a competing risk within 1 year of diagnosis of cancer-associated SpVT.
Cumulative incidence of bleeding outcomes. Cumulative incidence of (A) major bleeding and (B) CRNMB using the Kaplan-Meier method with death as a competing risk within 1 year of diagnosis of cancer-associated SpVT.
A multivariable Poisson regression analysis identified male sex and therapeutic anticoagulation within 2 weeks of diagnosis as independent predictors of major bleeding (aRR, 2.42; 95% CI, 1.27-4.59; and aRR, 1.74; 95% CI, 1.08-2.81, respectively) (Table 3). Baseline thrombocytopenia (<100 × 103/μL) was not associated with major bleeding (aRR, 0.76; 95% CI, 0.43-1.34). Notably, antiplatelet use at baseline (aRR, 1.46; 95% CI, 0.85-2.52) was not significantly associated with major bleeding at 12 months.
Univariable and multivariable analysis for major bleeding within 1 year of cancer-associated SpVT diagnosis
| Characteristic . | Major bleeding n (%) . | Unadjusted RR (95% CI) . | Adjusted∗ RR (95% CI) . |
|---|---|---|---|
| Age at thrombosis | |||
| ≤65 y | 39 (12.7) | Ref | Ref |
| >65 y | 23 (8.4) | 0.67 (0.41-1.08) | 0.67 (0.30-1.51) |
| Sex | |||
| Female | 11 (5.2) | Ref | Ref |
| Male | 51 (13.9) | 2.66 (1.42-4.99) | 2.42 (1.27-4.59) |
| Cirrhosis | |||
| Absent | 35 (10.8) | Ref | Ref |
| Present | 27 (10.5) | 0.97 (0.61-1.56) | 0.73 (0.41-1.31) |
| Abdominal surgery within past 3 mo | |||
| Absent | 58 (10.6) | Ref | Ref |
| Present | 4 (11.4) | 1.08 (0.41-2.79) | 1.03 (0.37-2.83) |
| Previous major bleed | |||
| Absent | 52 (10.1) | Ref | Ref |
| Present | 10 (14.9) | 1.48 (0.79-2.76) | 1.50 (0.79-2.84) |
| Baseline creatinine | |||
| ≤1.0 | 43 (9.5) | Ref | Ref |
| >1.0 | 19 (14.6) | 1.53 (0.93-2.54) | 1.44 (0.83-2.49) |
| Recent systemic chemotherapy | |||
| Absent | 51 (11.0) | Ref | Ref |
| Present | 11 (9.5) | 0.86 (0.47-1.61) | 1.13 (0.60-2.11) |
| Use of antiplatelet therapy at baseline | |||
| Absent | 45 (9.6) | Ref | Ref |
| Present | 17 (15.0) | 1.56 (0.93-2.63) | 1.46 (0.85-2.52) |
| Type of thrombus | |||
| Bland/mixed | 46 (10.5) | Ref | Ref |
| Tumor | 14 (12.2) | 1.16 (0.66-2.04) | 1.24 (0.71-2.18) |
| Thrombus occlusion | |||
| Partial | 20 (16.3) | Ref | Ref |
| Complete | 27 (10.2) | 0.63 (0.37-1.08) | 0.69 (0.40-1.17) |
| Anticoagulation within 2 wk | |||
| Absent | 31 (8.8) | Ref | Ref |
| Present | 31 (13.6) | 1.55 (0.97-2.48) | 1.74 (1.08-2.81) |
| Thrombocytopenia | |||
| None | 34 (11.0) | Ref | Ref |
| Platelets 75 × 103/μL - 99 × 103/μL | 8 (12.3) | 1.12 (0.55-2.31) | 0.86 (0.39-1.90) |
| Platelets 50 × 103/μL - 74 × 103/μL | 3 (4.2) | 0.38 (0.12-1.20) | 0.33 (0.10-1.03) |
| Platelets <50 × 103/μL | 10 (15.4) | 1.40 (0.73-2.69) | 1.21 (0.60-2.44) |
| Thrombocytopenia at baseline | |||
| None | 34 (11.0) | Ref | Ref |
| Platelets <100 × 103/μL | 21 (10.4) | 0.95 (0.57-1.59) | 0.76 (0.43-1.34) |
| Vessel involvement | |||
| Single | 49 (10.8) | Ref | Ref |
| Multiple | 13 (10.3) | 0.96 (0.54-1.71) | 0.89 (0.48-1.65) |
| Type of cancer | |||
| Hepatobiliary | 37 (11.5) | Ref | Ref |
| Pancreatic | 16 (12.7) | 1.10 (0.64-1.91) | 1.15 (0.66-2.02) |
| Luminal GI | 4 (8.2) | 0.71 (0.26-1.90) | 0.70 (0.27-1.82) |
| Other | 5 (5.9) | 0.51 (0.21-1.26) | 0.57 (0.23-1.46) |
| Characteristic . | Major bleeding n (%) . | Unadjusted RR (95% CI) . | Adjusted∗ RR (95% CI) . |
|---|---|---|---|
| Age at thrombosis | |||
| ≤65 y | 39 (12.7) | Ref | Ref |
| >65 y | 23 (8.4) | 0.67 (0.41-1.08) | 0.67 (0.30-1.51) |
| Sex | |||
| Female | 11 (5.2) | Ref | Ref |
| Male | 51 (13.9) | 2.66 (1.42-4.99) | 2.42 (1.27-4.59) |
| Cirrhosis | |||
| Absent | 35 (10.8) | Ref | Ref |
| Present | 27 (10.5) | 0.97 (0.61-1.56) | 0.73 (0.41-1.31) |
| Abdominal surgery within past 3 mo | |||
| Absent | 58 (10.6) | Ref | Ref |
| Present | 4 (11.4) | 1.08 (0.41-2.79) | 1.03 (0.37-2.83) |
| Previous major bleed | |||
| Absent | 52 (10.1) | Ref | Ref |
| Present | 10 (14.9) | 1.48 (0.79-2.76) | 1.50 (0.79-2.84) |
| Baseline creatinine | |||
| ≤1.0 | 43 (9.5) | Ref | Ref |
| >1.0 | 19 (14.6) | 1.53 (0.93-2.54) | 1.44 (0.83-2.49) |
| Recent systemic chemotherapy | |||
| Absent | 51 (11.0) | Ref | Ref |
| Present | 11 (9.5) | 0.86 (0.47-1.61) | 1.13 (0.60-2.11) |
| Use of antiplatelet therapy at baseline | |||
| Absent | 45 (9.6) | Ref | Ref |
| Present | 17 (15.0) | 1.56 (0.93-2.63) | 1.46 (0.85-2.52) |
| Type of thrombus | |||
| Bland/mixed | 46 (10.5) | Ref | Ref |
| Tumor | 14 (12.2) | 1.16 (0.66-2.04) | 1.24 (0.71-2.18) |
| Thrombus occlusion | |||
| Partial | 20 (16.3) | Ref | Ref |
| Complete | 27 (10.2) | 0.63 (0.37-1.08) | 0.69 (0.40-1.17) |
| Anticoagulation within 2 wk | |||
| Absent | 31 (8.8) | Ref | Ref |
| Present | 31 (13.6) | 1.55 (0.97-2.48) | 1.74 (1.08-2.81) |
| Thrombocytopenia | |||
| None | 34 (11.0) | Ref | Ref |
| Platelets 75 × 103/μL - 99 × 103/μL | 8 (12.3) | 1.12 (0.55-2.31) | 0.86 (0.39-1.90) |
| Platelets 50 × 103/μL - 74 × 103/μL | 3 (4.2) | 0.38 (0.12-1.20) | 0.33 (0.10-1.03) |
| Platelets <50 × 103/μL | 10 (15.4) | 1.40 (0.73-2.69) | 1.21 (0.60-2.44) |
| Thrombocytopenia at baseline | |||
| None | 34 (11.0) | Ref | Ref |
| Platelets <100 × 103/μL | 21 (10.4) | 0.95 (0.57-1.59) | 0.76 (0.43-1.34) |
| Vessel involvement | |||
| Single | 49 (10.8) | Ref | Ref |
| Multiple | 13 (10.3) | 0.96 (0.54-1.71) | 0.89 (0.48-1.65) |
| Type of cancer | |||
| Hepatobiliary | 37 (11.5) | Ref | Ref |
| Pancreatic | 16 (12.7) | 1.10 (0.64-1.91) | 1.15 (0.66-2.02) |
| Luminal GI | 4 (8.2) | 0.71 (0.26-1.90) | 0.70 (0.27-1.82) |
| Other | 5 (5.9) | 0.51 (0.21-1.26) | 0.57 (0.23-1.46) |
Adjusted for age at thrombosis (continuous), sex, previous major bleed, baseline creatinine (continuous), use of antiplatelets, use of therapeutic anticoagulants within 2 weeks of diagnosis, and type of cancer.
In the secondary Cox models with inverse probability of treatment weighting with propensity scores for thrombocytopenia, baseline thrombocytopenia (<100 × 103 platelets per μL) was not associated with a significant risk for major bleeding within 1 year (aHR, 0.81; 95% CI, 0.48-1.39). Similarly, in the multivariable Cox model with platelet count as a time-varying covariate over the course of the follow-up period, thrombocytopenia was not associated with major bleeding (aHR, 0.89; 95% CI, 0.55-1.45) (supplemental Table 2).
Thrombotic outcomes
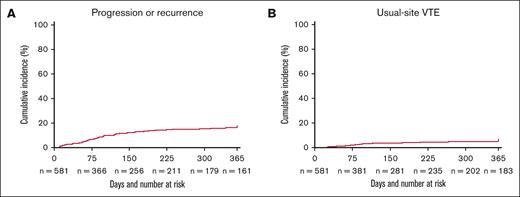
The cumulative incidence of progression or recurrence of SpVT at 1 year was 16.2% (95% CI, 13.2-19.2) (Figure 2A) and usual-site VTE at 1 year was 5.2% (95% CI, 3.4-7.0) (Figure 2B). Of the 99 patients who had progression or recurrence, 27 (27.3%) had been treated with therapeutic anticoagulation at the time of their initial SpVT; of the patients treated, 19 (70.3%) were still on anticoagulation at the time of their thrombotic event. Of the 30 patients who had usual-site VTE, 18 (60.0%) had been treated with therapeutic anticoagulation for their SpVT; of these patients, only 10 (55.6%) were still on anticoagulation therapy at the time of their usual-site VTE. In the multivariable regression model, therapeutic anticoagulation within 2 weeks of diagnosis was associated with a lower incidence of progression or recurrence of SpVT (aRR, 0.55; 95% CI, 0.35-0.86) (Table 4). Of note, baseline thrombocytopenia (aRR, 1.14; 95% CI, 0.73-1.78) was not associated with progression/recurrence of SpVT.
Cumulative incidence of thrombotic outcomes. Cumulative incidence of (A) progression/recurrence of SpVT and (B) usual-site VTE using the Kaplan-Meier method with death as a competing risk within 1 year of diagnosis of cancer-associated SpVT.
Cumulative incidence of thrombotic outcomes. Cumulative incidence of (A) progression/recurrence of SpVT and (B) usual-site VTE using the Kaplan-Meier method with death as a competing risk within 1 year of diagnosis of cancer-associated SpVT.
Univariable and multivariable regression for progression or recurrence of cancer-associated SpVT within 1 year of SpVT diagnosis
| Characteristic . | Progression or recurrence, n (%) . | Unadjusted RR (95% CI) . | Adjusted∗ RR (95% CI) . |
|---|---|---|---|
| Age at thrombosis | |||
| ≤65 y | 50 (16.2) | Ref | Ref |
| >65 y | 44 (16.1) | 0.99 (0.69-1.44) | 0.78 (0.45-1.37) |
| Sex | |||
| Female | 32 (15.2) | Ref | Ref |
| Male | 62 (16.9) | 1.11 (0.75-1.64) | 1.13 (0.74-1.73) |
| Cirrhosis | |||
| Absent | 46 (14.2) | Ref | Ref |
| Present | 48 (18.7) | 1.32 (0.91-1.90) | 1.48 (0.83-2.64) |
| Abdominal surgery within past 3 mo | |||
| Absent | 92 (16.9) | Ref | Ref |
| Present | 2 (5.7) | 0.34 (0.09-1.32) | 0.43 (0.11-1.65) |
| Previous VTE | |||
| Absent | 88 (16.0) | Ref | Ref |
| Present | 6 (20.0) | 1.25 (0.60-2.63) | 1.34 (0.64-2.78) |
| Baseline creatinine | |||
| ≤1.0 | 82 (18.2) | Ref | Ref |
| >1.0 | 12 (9.2) | 0.51 (0.29-0.90) | 0.51 (0.25-1.04) |
| Recent systemic chemotherapy | |||
| Absent | 75 (16.1) | Ref | Ref |
| Present | 19 (16.4) | 1.02 (0.64-1.61) | 1.11 (0.68-1.81) |
| Use of antiplatelets at baseline | |||
| Absent | 77 (16.5) | Ref | Ref |
| Present | 17 (15.0) | 0.91 (0.56-1.48) | 0.93 (0.56-1.52) |
| Type of thrombus | |||
| Bland/mixed | 68 (15.5) | Ref | Ref |
| Tumor | 24 (20.9) | 1.35 (0.89-2.05) | 1.16 (0.76-1.79) |
| Thrombus occlusion | |||
| Partial | 16 (13.0) | Ref | Ref |
| Complete | 42 (15.9) | 1.22 (0.72-2.09) | 1.37 (0.79-2.37) |
| Anticoagulation within 2 wk | |||
| Absent | 69 (19.6) | Ref | Ref |
| Present | 25 (11.0) | 0.56 (0.37-0.86) | 0.55 (0.35-0.86) |
| Thrombocytopenia at baseline | |||
| None | 40 (12.9) | Ref | Ref |
| Platelets 75 × 103/μL - 99 × 103/μL | 8 (12.3) | 0.95 (0.47-1.94) | 0.89 (0.44-1.83) |
| Platelets 50 × 103/μL - 74 × 103/μL | 15 (20.8) | 1.61 (0.95-2.76) | 1.67 (0.97-2.88) |
| Platelets <50 × 103/μL | 6 (9.2) | 0.72 (0.32-1.62) | 0.76 (0.33-1.73) |
| Thrombocytopenia | |||
| None | 40 (12.9) | Ref | Ref |
| Platelets <100 × 103/μL | 29 (14.4) | 1.11 (0.71-1.73) | 1.14 (0.73-1.78) |
| Vessel involvement | |||
| Single | 77 (16.9) | Ref | Ref |
| Multiple | 17 (13.5) | 0.80 (0.49-1.30) | 0.79 (0.47-1.32) |
| Type of cancer | |||
| Hepatobiliary | 55 (17.1) | Ref | Ref |
| Pancreatic | 24 (19.1) | 1.11 (0.72-1.71) | 1.36 (0.85-2.19) |
| Luminal GI | 5 (10.2) | 0.60 (0.25-1.41) | 0.67 (0.27-1.66) |
| Other | 10 (11.8) | 0.69 (0.37-1.29) | 0.84 (0.44-1.61) |
| Characteristic . | Progression or recurrence, n (%) . | Unadjusted RR (95% CI) . | Adjusted∗ RR (95% CI) . |
|---|---|---|---|
| Age at thrombosis | |||
| ≤65 y | 50 (16.2) | Ref | Ref |
| >65 y | 44 (16.1) | 0.99 (0.69-1.44) | 0.78 (0.45-1.37) |
| Sex | |||
| Female | 32 (15.2) | Ref | Ref |
| Male | 62 (16.9) | 1.11 (0.75-1.64) | 1.13 (0.74-1.73) |
| Cirrhosis | |||
| Absent | 46 (14.2) | Ref | Ref |
| Present | 48 (18.7) | 1.32 (0.91-1.90) | 1.48 (0.83-2.64) |
| Abdominal surgery within past 3 mo | |||
| Absent | 92 (16.9) | Ref | Ref |
| Present | 2 (5.7) | 0.34 (0.09-1.32) | 0.43 (0.11-1.65) |
| Previous VTE | |||
| Absent | 88 (16.0) | Ref | Ref |
| Present | 6 (20.0) | 1.25 (0.60-2.63) | 1.34 (0.64-2.78) |
| Baseline creatinine | |||
| ≤1.0 | 82 (18.2) | Ref | Ref |
| >1.0 | 12 (9.2) | 0.51 (0.29-0.90) | 0.51 (0.25-1.04) |
| Recent systemic chemotherapy | |||
| Absent | 75 (16.1) | Ref | Ref |
| Present | 19 (16.4) | 1.02 (0.64-1.61) | 1.11 (0.68-1.81) |
| Use of antiplatelets at baseline | |||
| Absent | 77 (16.5) | Ref | Ref |
| Present | 17 (15.0) | 0.91 (0.56-1.48) | 0.93 (0.56-1.52) |
| Type of thrombus | |||
| Bland/mixed | 68 (15.5) | Ref | Ref |
| Tumor | 24 (20.9) | 1.35 (0.89-2.05) | 1.16 (0.76-1.79) |
| Thrombus occlusion | |||
| Partial | 16 (13.0) | Ref | Ref |
| Complete | 42 (15.9) | 1.22 (0.72-2.09) | 1.37 (0.79-2.37) |
| Anticoagulation within 2 wk | |||
| Absent | 69 (19.6) | Ref | Ref |
| Present | 25 (11.0) | 0.56 (0.37-0.86) | 0.55 (0.35-0.86) |
| Thrombocytopenia at baseline | |||
| None | 40 (12.9) | Ref | Ref |
| Platelets 75 × 103/μL - 99 × 103/μL | 8 (12.3) | 0.95 (0.47-1.94) | 0.89 (0.44-1.83) |
| Platelets 50 × 103/μL - 74 × 103/μL | 15 (20.8) | 1.61 (0.95-2.76) | 1.67 (0.97-2.88) |
| Platelets <50 × 103/μL | 6 (9.2) | 0.72 (0.32-1.62) | 0.76 (0.33-1.73) |
| Thrombocytopenia | |||
| None | 40 (12.9) | Ref | Ref |
| Platelets <100 × 103/μL | 29 (14.4) | 1.11 (0.71-1.73) | 1.14 (0.73-1.78) |
| Vessel involvement | |||
| Single | 77 (16.9) | Ref | Ref |
| Multiple | 17 (13.5) | 0.80 (0.49-1.30) | 0.79 (0.47-1.32) |
| Type of cancer | |||
| Hepatobiliary | 55 (17.1) | Ref | Ref |
| Pancreatic | 24 (19.1) | 1.11 (0.72-1.71) | 1.36 (0.85-2.19) |
| Luminal GI | 5 (10.2) | 0.60 (0.25-1.41) | 0.67 (0.27-1.66) |
| Other | 10 (11.8) | 0.69 (0.37-1.29) | 0.84 (0.44-1.61) |
Adjusted for age at thrombosis (continuous), sex, previous VTE, baseline creatinine (continuous), use of antiplatelets, use of therapeutic anticoagulants within 2 weeks of diagnosis, and type of cancer.
In the Cox models with inverse probability of treatment weighting with propensity scores for thrombocytopenia, patients with thrombocytopenia did not have a significantly increased risk for progression or recurrence of SpVT (aHR, 1.14; 95% CI, 0.70-1.84).
Discussion
In our cohort of patients with cancer-associated SpVT, both major bleeding and thrombosis progression/recurrence were frequent complications of SpVT. Treatment with therapeutic anticoagulation within 2 weeks of diagnosis was associated with increased major bleeding and decreased progression or recurrence or SpVT within 1 year. Thrombocytopenia (<100 × 103 platelets per μL) at baseline or analyzed as a time-varying covariate was not an independent risk factor for major bleeding or recurrent/progressive thrombosis at 1 year after the SpVT diagnosis.
Notable characteristics of our cohort include the high prevalence of cirrhosis (44.2%) and nonluminal intraabdominal tumors (77.3%). The proportion of patients with SpVT with cirrhosis at our institution was higher than that in previously published cohorts of SpVT in the general population, namely 17.6% of patients in an individual-patient meta-analysis,23 11.3% in a national Danish registry,8 and 2.9% in a retrospective cohort of 1561 patients.9 It is uncertain whether this reflects a higher prevalence of cirrhosis in patients with cancer-associated SpVT when compared with patients with SpVT in the general population or whether this reflects unique characteristics of the population at our institution, a major liver transplant referral center. Similarly, the proportion of patients with hepatobiliary tumors is higher in our study (55.2%) than in other cohorts of cancer-associated SpVT, namely 18.8% with hepatobiliary tumors in a recent cohort24 and 57.6% with combined hepatobiliary and pancreatic tumors in a separate study.13 This may relate to the high prevalence of cirrhosis in our population. Coincident thrombocytopenia at the time of SpVT diagnosis was also frequent in our study. A retrospective cohort study of patients at our institution noted that VTE was associated with thrombocytopenia (<100 × 103/μL platelets per μL) in 22% of patients with solid tumors.17 The data presented here suggest that thrombocytopenia may be more prevalent in patients with cancer with SpVT than in those with usual-site VTE.
Uncertainty regarding the optimal treatment of SpVT is reflected in the fact that a minority of patients in our cohort were treated with anticoagulants within 2 weeks of diagnosis. The reported rates of treatment of SpVT with anticoagulants vary in the literature. A prospective cohort study of 132 patients with SpVT with or without cancer found that 68.9% were treated with anticoagulation as opposed to 99.2% of patients in a control group with usual-site VTE.13 In an individual patient meta-analysis of prospective trials of patients with SpVT, 85% of patients overall received anticoagulation treatment, and 73.6% of those with solid cancers were treated.23 In contrast, a retrospective cohort study of SpVT in the general population found that only 23.9% of patients aged >65 years received anticoagulation treatment within 1 month.9 Notably, in a recent cohort study of 298 patients with cancer-associated SpVT among which 49% were hepatocellular carcinoma, approximately 15% were treated with anticoagulants.25 We postulate that the reasons for the relatively low rates of anticoagulation treatment in our cohort may reflect institutional practice and the significant proportion of patients with of thrombocytopenia (39.5%), tumor thrombi (19.9%), and incidentally diagnosed thrombi (32.7%), which could be interpreted as more likely to be chronic in the appropriate clinical setting.
The existing literature on the association between treatment with anticoagulants and clinical outcomes in patients with cancer-associated SpVT is unsettled. In our cohort, treatment with anticoagulants was associated with increased major bleeding and decreased recurrence or progression of thrombosis in the regression models. Our findings regarding the thrombotic outcomes are generally in agreement with prospective data in the general population (including patients without cancer), which also suggest a lower incidence of recurrent thrombosis in patients treated with anticoagulation.23,26 The data regarding anticoagulation and its association with bleeding in patients with SpVT is more mixed. Smaller retrospective studies have demonstrated increased rates of hemorrhage among patients with SpVT on anticoagulation treatment.27 In contrast, some prospective analyses of patients with SpVT in the general population have reported that anticoagulation treatment is correlated with a lower risk for bleeding.6,23 This association has been proposed to be because of an increased recanalization in patients treated with anticoagulants, which in turn leads to lower portal venous pressures and a reduced variceal bleeding risk.23 A retrospective cohort of 298 patients with cancer-associated SpVT found that anticoagulation treatment was not associated with major bleeding but was associated with increased rates of recanalization on re-imaging.25 Taken together, these data highlight the clinical challenge of cancer-associated SpVT and the relative equipoise of anticoagulation strategies that clinicians face in many instances.
The existing data on the relationship between thrombocytopenia and bleeding in cancer-associated SpVT are limited. A 2021 single-center retrospective cohort of 1561 patients with SpVT included 1056 (71.0%) with cancer, although the bleeding and thrombotic outcomes were not reported separately.9 A 2022 individual patient meta-analysis of prospective studies included 1635 patients with SpVT of whom 523 had solid cancer, 118 had myeloproliferative neoplasms, and 20 had other hematologic cancers.23 In this meta-analysis, patients with thrombocytopenia were underrepresented and only 3.7% of all patients had a platelet count of less than 50 × 103/μL. Our study with 581 patients with cancer-associated SpVT, including 39.5% of patients with a platelet count of <100 × 103/μL and 12.7% with a count of <50 × 103/μL, is one of the largest cohorts of patients with cancer with SpVT to our knowledge and aims to fill a gap in the literature by characterizing the outcomes of those with concomitant thrombocytopenia. Our finding that thrombocytopenia was not associated with bleeding risk has several possible explanations. First, the data are retrospective, and the possibility of an untested confounder cannot be excluded. Certain characteristics of our patient population, such as the high incidence of cirrhosis, could affect the relationship between platelet count and bleeding; the relationship between liver dysfunction and bleeding is complex, and thrombocytopenia does not necessarily predict spontaneous major bleeding in patients with cirrhosis.28,29 Moreover, platelet count was measured as a baseline variable, which may not accurately reflect the platelet count at the time of the bleeding episode. Although we attempted to address this by testing thrombocytopenia as a time-dependent covariate in the multivariable regression, platelet count data were not necessarily collected at regular and frequent intervals because of the retrospective nature of the study, and thus the degree of thrombocytopenia immediately preceding a bleeding event for any individual patient may be over- or underestimated based on the data available. In addition, we defined thrombocytopenia as a platelet count of 100 × 103/μL, which is higher than what may be conventionally considered to be the threshold at which the risk for bleeding substantially increases. We specifically chose 100 × 103/μL as our threshold based on recent data suggesting that thrombocytopenia of this degree may confer an increased bleeding risk in patients with cancer-associated VTE who are receiving anticoagulation.30 Similarly, previous data showed an increased bleeding risk at this threshold in patients with hepatitis C–related chronic liver disease31 and in those with atrial fibrillation on anticoagulation.32 Furthermore, we tested a platelet count threshold of 50 × 103/μL in our regression model and did not find an association with major bleeding.
The reason for male sex being independently associated with higher major bleeding incidence in the adjusted multivariable analysis is not clear. Such an effect is not consistent with the existing literature. In a large retrospective study, the rate of bleeding in patients in the general population treated with anticoagulation for VTE was higher in women, but there was no significant association between sex and major bleeding in a multivariable regression model.33 The difference in bleeding rates by sex in our cohort could suggest an unidentified confounder associated with male sex. Given that this finding is incongruous with existing literature and lacks a clear mechanistic explanation, the clinical significance of this finding should be interpreted with caution.
To our knowledge, in this study, we reported on one of the largest cohorts of patients with cancer-associated SpVT to date. However, we acknowledge certain limitations. Because patients were included based on imaging criteria, there may be a selection bias in the sample toward those who received more frequent imaging or those with greater access to health care. Although we attempted to adjust for several covariates, the retrospective nature of the study introduced the possibility of unmeasured confounders. The single-center study design could influence the generalizability of the findings; for example, a relatively high proportion of patients in our sample had a previous diagnosis of cirrhosis. Moreover, the high incidence of cirrhosis indicates that malignancy may not have been the sole etiology of the thrombosis in a significant proportion of patients. Data on the major clinical outcomes and key covariates were collected by manual chart extraction by trained clinicians. However, retrospective data collection may miss relatively minor clinical events that were not documented in the medical record and thus might be undercounted, which may explain why the rates of CRNMB in our cohort were lower than major bleeding rates. An additional limitation is the reliability of baseline clinical characteristics at the time of diagnosis of SpVT; certain variables, in particular laboratory data, may fluctuate significantly over the course of the study period, and baseline data do not reflect this variability. Furthermore, although patients with thrombocytopenia were well-represented in this cohort, our analysis of patients with severe thrombocytopenia (<50 × 103 platelets per μL) was still limited by sample size. Finally, certain characteristics of the SpVT at the time of diagnosis, such as its chronicity and the extent to which it is composed of bland or tumor thrombus, are not always clear based on radiographic characteristics, especially with certain modalities, such as ultrasound. Consequently, features of the thrombus that have the potential to affect outcomes could have been underappreciated in our analysis.
In this retrospective cohort study, we found that in patients with cancer-associated SpVT, both bleeding and thrombotic complications were frequent. Less than half of the patients were treated with therapeutic anticoagulation, highlighting the challenges of management in this high-risk population. Anticoagulation therapy was associated with increased major bleeding and decreased SpVT progression or recurrence. Although thrombocytopenia was a frequent comorbidity, it was not independently associated with a higher risk for bleeding. Prospective studies of patients with cancer-associated SpVT with adequate representation of patients with thrombocytopenia are needed to better characterize the outcomes of these patients and to define optimal treatment strategies.
Acknowledgments
The authors acknowledge George Silva and Karla Pollick at Beth Israel Deaconess Medical Center Integration of Standard Information Gathered using Healthcare Technology Core for their assistance with clinical and laboratory data extraction.
J.I.Z. was partly supported through National Institutes of Health/National Cancer Institute Cancer Center support grant P30 CA008748. R.P. was partly supported through a career development award through the Conquer Cancer Foundation.
Authorship
Contribution: M.A. Jr, J.I.Z., and R.P. designed the study; M.A. Jr and M.J.F.T. collected the data; L.E.D. performed the statistical analyses; M.A. Jr, M.J.F.T., L.E.D, and R.P. drafted the manuscript; and all other authors critically reviewed and approved the final manuscript.
Conflict-of-interest disclosure: J.I.Z. reports personal fees from Calyx, CSL Berhing, and Sanofi, and grants from Incyte and Quercegen outside the submitted work. R.P. reports personal fees from Merck Research outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Rushad Patell, Division of Hematology/Oncology, Department of Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215; email: rpatell@bidmc.harvard.edu.
References
Author notes
Original data are available on request from the corresponding author, Rushad Patell (rpatell@bidmc.harvard.edu).
The full-text version of this article contains a data supplement.