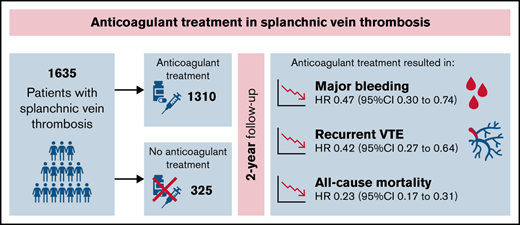
Key Points
Robust evidence on the optimal management of splanchnic vein thrombosis is lacking.
In this meta-analysis of individual patient data, anticoagulant therapy reduced the risks of thrombosis, major bleeding, and mortality.
Abstract
Robust evidence on the optimal management of splanchnic vein thrombosis (SVT) is lacking. We conducted an individual-patient meta-analysis to evaluate the effectiveness and safety of anticoagulation for SVT. Medline, Embase, and clincaltrials.gov were searched up to June 2021 for prospective cohorts or randomized clinical trials including patients with SVT. Data from individual datasets were merged, and any discrepancy with published data was resolved by contacting study authors. Three studies of a total of 1635 patients were included. Eighty-five percent of patients received anticoagulation for a median duration of 316 days (range, 1-730 days). Overall, incidence rates for recurrent venous thromboembolism (VTE), major bleeding, and mortality were 5.3 per 100 patient-years (p-y; 95% confidence interval [CI], 5.1-5.5), 4.4 per 100 p-y (95% CI, 4.2-4.6), and 13.0 per 100 p-y (95% CI, 12.4-13.6), respectively. The incidence rates of all outcomes were lower during anticoagulation and higher after treatment discontinuation or when anticoagulation was not administered. In multivariable analysis, anticoagulant treatment appeared to be associated with a lower risk of recurrent VTE (hazard ratio [HR], 0.42; 95% CI, 0.27-0.64), major bleeding (HR, 0.47; 95% CI, 0.30-0.74), and mortality (HR, 0.23; 95% CI, 0.17-0.31). Results were consistent in patients with cirrhosis, solid cancers, myeloproliferative neoplasms, unprovoked SVT, and SVT associated with transient or persistent nonmalignant risk factors. In patients with SVT, the risk of recurrent VTE and major bleeding is substantial. Anticoagulant treatment is associated with reduced risk of both outcomes.
Introduction
Splanchnic vein thrombosis (SVT) includes portal, mesenteric, or splenic vein thrombosis and Budd-Chiari syndrome.1 SVT is frequently associated with liver cirrhosis, solid cancers, and myeloproliferative neoplasms and less often with oral contraceptive agents, pregnancy, abdominal infections, pancreatitis, or surgery.2-4
Despite several observational studies and a few relatively small randomized controlled trials (RCTs) evaluating the efficacy and safety of anticoagulant treatment for SVT, robust evidence on SVT management is lacking. Recommendations from international societies or expert panels remain highly variable, and, in routine clinical practice, physicians have to decide empirically about the type, dose, and duration of anticoagulant therapy.1,5 ,,, -11 A recent study-level meta-analysis including 7969 patients with SVT suggested a potential benefit of anticoagulant treatment in terms of improved vein recanalization and reduced thrombosis progression, recurrent venous thromboembolism (VTE), major bleeding, and all-cause mortality compared with no anticoagulation.5 Conclusions were limited by the inherent heterogeneity of included populations, the potential for immortal bias, and residual confounding.
To fill these knowledge gaps, we undertook a meta-analysis of individual patient data to evaluate the effectiveness and safety of anticoagulant therapy in patients with SVT and obtain a better understanding of the incidence of clinically relevant outcomes in subgroups at higher risk of thrombotic and bleeding complications.
Methods
This meta-analysis of individual patient data was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guideline.12
Database search and study selection
Medline and Embase were searched from inception up to June 2021 for prospective studies and RCTs including patients with SVT (supplemental Table 1). Furthermore, we searched clincaltrials.gov for ongoing or completed studies and screened the reference lists of relevant studies to retrieve additional records. No language restrictions were applied.
Two authors independently reviewed titles and abstracts identified from the search to select studies that met the following inclusion criteria: (1) prospective study or RCT including ≥50 patients with SVT, (2) reporting of the outcomes of interest (ie, recurrent VTE, major bleeding, and all-cause mortality), and (3) anticoagulant treatment with low molecular weight heparin (LMWH), unfractionated heparin, fondaparinux, vitamin K antagonists (VKAs), direct oral anticoagulants (DOACs), or no anticoagulant therapy. We excluded retrospective cohorts, case series, and case reports.
The risk of bias was assessed by 2 independent authors using the Risk Of Bias In Non-randomized Studies of Interventions-I tool.13 Any disagreement was resolved through discussion or involving a third author.
Individual patient data extraction
Authors of included studies were asked to provide individual patient data from their published study, and, in case of ongoing cohorts or registries, data also were requested for patients included after the publication of the main study identified by the search.
The following individual patient data were collected: demographic factors (eg, age and sex), clinical presentation (ie, symptomatic or incidentally detected SVT), site and extension of thrombosis, risk factors for SVT (eg, solid and hematological cancer, liver cirrhosis, myeloproliferative neoplasm, pregnancy, oral contraceptive agents, hormonal therapy, recent surgery, pancreatitis, abdominal infections, and inflammatory bowel disease), laboratory parameters, type and duration of anticoagulant therapy, use of antiplatelet agents or thrombolysis, and outcomes of interest. Data from individual databases were verified and merged into a single electronic database. Any discrepancy with published data were resolved by contacting the corresponding or first author.
Patients were excluded from the analysis if they were aged <18 years, had follow-up <7 days, or were missing relevant information (ie, start or end date of anticoagulation, type of anticoagulant agent, end of follow-up, and date of study outcome).
Study outcomes
The effectiveness outcomes were recurrent VTE (ie, recurrent SVT, deep vein thrombosis [DVT] of the lower or upper extremities, and pulmonary embolism) and all-cause mortality. The safety outcome was major bleeding as defined by the International Society on Thrombosis and Haemostasis.14 All outcomes were evaluated to the longest follow-up available and to a maximum of 2 years. Recurrent VTE or major bleeding were considered fatal events if the patient died within 24 hours of the event.
Statistical analysis
Differences between groups were tested with the Mann-Whitney U test or χ2 test as appropriate. In the primary analysis, follow-up duration was calculated from SVT diagnosis to the occurrence of one of the outcomes, last follow-up available, or end of the 2-year follow-up, whichever came first. Cumulative incidence curves of recurrent VTE and major bleeding were built considering death as competing risk. Subgroup analysis was performed for patients with liver cirrhosis, solid cancers, myeloproliferative neoplasms, unprovoked SVT, and SVT secondary to transient or persistent nonmalignant risk factors. The latter included any of the following: pregnancy, contraceptive agent use, hormonal therapy, recent surgery, inflammatory bowel disease, pancreatitis, or abdominal infections.15 SVT was considered unprovoked in patients without apparent risk factors. Incidence rates for the main outcomes were expressed as the number of events per 100 patient-years (p-y) with relative 95% confidence intervals (CIs) and calculated for the overall cohort during anticoagulation and after treatment withdrawal and in patients who received no anticoagulant treatment. Case fatality rates for major bleeding and recurrent VTE were calculated as the number of patients with fatal bleeding or VTE divided by the total number of patients who experienced that outcome.
Hazard ratios (HRs) were estimated with Cox’s proportional models considering anticoagulant treatment as a time-varying variable.16 The association between each outcome of interest and anticoagulant treatment, age, sex, liver cirrhosis, solid cancer, myeloproliferative neoplasm, and transient or persistent nonmalignant risk factors was first evaluated in univariable analysis. All variables with a significant association were subsequently entered in multivariable models. Proportional hazard assumptions were checked through visual inspection of residual graphs and Schoenfeld test. In sensitivity analysis using univariable Cox’s models, we evaluated the association between study outcomes and anticoagulant treatment as time-varying covariate, age, and sex within the subgroups of patients indicated above. A 2-sided P value <0.05 was considered to indicate statistical significance. All analyses were performed using R software (version 4.0.4 and Rstudio version 1.1.423, 2009-2018; R Foundation for Statistical Computing, Vienna, Austria).17
Results
A total of 5037 records were identified from the search, along with 3 from clinicaltrials.gov (supplemental Figure 1). After removing 708 duplicates and excluding 4320 records by title and abstract screening, 12 studies (in 2525 patients) met the inclusion criteria.3,4,18 ,,,,,, -27 After direct contact with the authors, individual patient data from 5 studies (1871 patients) were made available, merged in a pooled database, checked for completeness, and evaluated for inclusion in the analysis.
Characteristics of included studies
None of the included studies was an RCT. Four studies were international prospective multicenter registries,3,4,26,27 and 1 study included patients from a single center.21 Sample sizes ranged between 57 and 975 patients. After data cleaning, 236 patients, including the whole populations of 2 eligible studies,21,26 were excluded because of patient age <18 years (n = 10), follow-up duration <7 days (n = 33), or missing relevant information (n = 193). A total of 1635 patients from 3 studies (64.8% of the potentially eligible patients) were included in the final analysis.3,4,27 Risk of bias across domains was moderate in 2 studies and serious in 1 (supplemental Table 2).
Characteristics of the included population
Baseline patient characteristics are reported in Table 1. The mean patient age was 56 ± 16 years, and 60.3% of patients were male. SVT was incidentally detected in 41.9% of patients. The most common risk factors for SVT were solid cancers (32.0%) and liver cirrhosis (17.6%); SVT was unprovoked in 31.7% of patients. Laboratory tests for thrombophilia were available in 28.6% of patients and showed the presence of ≥1 thrombophilia in 17.2% (supplemental Table 3). The median follow-up duration was 442 days (range, 7-730 days).
Baseline patient characteristics
| Variable . | Overall (N = 1635) . |
|---|---|
| Mean age ± SD | 56.17 ± 16.16 |
| Male sex | 986 (60.3) |
| Symptomatic patients | 950 (58.1) |
| Involved vein | |
| Portal vein | 562 (34.4) |
| Mesenteric vein | 217 (13.3) |
| Budd-Chiari syndrome | 201 (12.3) |
| Splenic vein | 107 (6.5) |
| Multiple veins | 548 (33.5) |
| Risk factors | |
| Unprovoked | 462 (28.3) |
| Solid cancer | 523 (32.0) |
| Liver cirrhosis | 287 (17.6) |
| Myeloproliferative neoplasm | 118 (7.2) |
| Recent surgery | 155 (9.5) |
| Hormonal therapy | 57 (3.5) |
| Inflammatory bowel disease | 24 (1.5) |
| Pregnancy or puerperium | 15 (0.9) |
| Pancreatitis/abdominal infection | 155 (7.0) |
| Leukemia, lymphoma, myeloma | 20 (1.2) |
| Thrombophilia | |
| Tested negative | 186 (11.4) |
| Tested positive | 281 (17.2) |
| Laboratory test results | |
| Hemoglobin ≤10 g/dL* | 284 (17.4) |
| Platelets* | |
| ≤100 × 103/mm3 | 255 (15.6) |
| ≤50 × 103/mm3 | 60 (3.7) |
| Anticoagulant therapy | |
| None | 325 (19.8) |
| LMWH alone | 521 (31.9) |
| VKAs alone | 416 (25.4) |
| DOACs alone | 27 (1.7) |
| UFH alone | 8 (0.5) |
| Fondaparinux alone | 17 (1.0) |
| Multiple agents | 321 (19.6) |
| Variable . | Overall (N = 1635) . |
|---|---|
| Mean age ± SD | 56.17 ± 16.16 |
| Male sex | 986 (60.3) |
| Symptomatic patients | 950 (58.1) |
| Involved vein | |
| Portal vein | 562 (34.4) |
| Mesenteric vein | 217 (13.3) |
| Budd-Chiari syndrome | 201 (12.3) |
| Splenic vein | 107 (6.5) |
| Multiple veins | 548 (33.5) |
| Risk factors | |
| Unprovoked | 462 (28.3) |
| Solid cancer | 523 (32.0) |
| Liver cirrhosis | 287 (17.6) |
| Myeloproliferative neoplasm | 118 (7.2) |
| Recent surgery | 155 (9.5) |
| Hormonal therapy | 57 (3.5) |
| Inflammatory bowel disease | 24 (1.5) |
| Pregnancy or puerperium | 15 (0.9) |
| Pancreatitis/abdominal infection | 155 (7.0) |
| Leukemia, lymphoma, myeloma | 20 (1.2) |
| Thrombophilia | |
| Tested negative | 186 (11.4) |
| Tested positive | 281 (17.2) |
| Laboratory test results | |
| Hemoglobin ≤10 g/dL* | 284 (17.4) |
| Platelets* | |
| ≤100 × 103/mm3 | 255 (15.6) |
| ≤50 × 103/mm3 | 60 (3.7) |
| Anticoagulant therapy | |
| None | 325 (19.8) |
| LMWH alone | 521 (31.9) |
| VKAs alone | 416 (25.4) |
| DOACs alone | 27 (1.7) |
| UFH alone | 8 (0.5) |
| Fondaparinux alone | 17 (1.0) |
| Multiple agents | 321 (19.6) |
Values in parentheses are percentages.
SD, standard deviation; UFH, unfractionated heparin.
Measurements in 104 patients were missing.
Eighty-five percent of patients received anticoagulant therapy, including parenteral treatment alone (mostly LMWH) in 33.4%, VKAs alone in 25.4%, DOACs alone in 1.7%, and ≥1 anticoagulant agent in 19.6% (Table 1; supplemental Table 4). Overall, 75 patients (5.7%) received a DOAC as single drug (n = 27) or after switching to or from another anticoagulant drug (n = 48). DOACs prescribed were rivaroxaban in 56 patients (4.2%), apixaban in 13 (1.0%), and edoxaban and dabigatran in 3 (0.2%) each. Information on the dose of DOACs was not available.
The overall median duration of anticoagulant treatment was 316 days (range, 1-730 days), with 263 patients (16.1%) treated for <3 months, 338 (20.7%) for 3 to 6 months, and 706 (43.2%) for >6 months. Solid cancer, liver cirrhosis, male sex, and symptomatic presentation of SVT were less common in patients who received anticoagulation therapy compared with untreated patients (supplemental Table 5). Concomitant antiplatelet therapy was used in 49 of 1310 patients (3.0%) and was the only treatment for SVT in 8 patients (0.5%). Thrombolysis was used in 27 of 1635 patients (1.7%).
Recurrent VTE
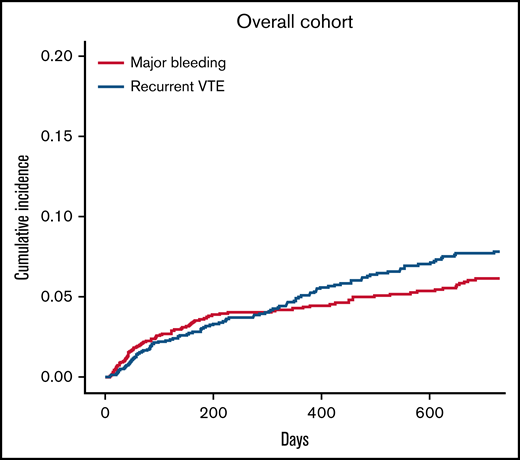
A total of 104 patients had recurrent VTE during follow-up, for an incidence rate of 5.3 per 100 p-y (95% CI, 5.1-5.5; Table 2). Cumulative incidences of recurrent VTE in the overall population and patient subgroups are shown in Figures 1 and 2, respectively. Recurrent VTE included recurrent SVT in 40 patients (38.5%), usual-site VTE (DVT of the lower extremities or pulmonary embolism with or without DVT) in 46 patients (44.2%), and thrombosis in other sites in 18 patients (17.3%). Fifty percent of recurrent SVTs occurred in patients with cirrhosis, and 54.3% of usual-site recurrent VTE and 66.7% of other-site DVT developed in patients with solid cancers. None of the recurrent VTE events were fatal. Median time from index SVT to recurrent VTE was 253 days (range, 9-719).
Incidence rates for recurrent VTE, major bleeding, and all-cause mortality
| Event . | Overall cohort . | Never treated . | Treated, on treatment . | Treated, off treatment . |
|---|---|---|---|---|
| Total p-y | 1975 | 376 | 1132 | 467* |
| Recurrent VTE | ||||
| Events per 100 p-y | 104 | 35 | 38 | 31 |
| Relative 95% CI | 5.3 (5.1-5.5) | 9.3 (8.4-10.3) | 3.4 (3.2-3.6) | 6.6 (6.0-7.2) |
| Major bleeding | ||||
| Events per 100 p-y | 86 | 24 | 35 | 27 |
| Relative 95% CI | 4.4 (4.2-4.6) | 6.4 (5.8-7.1) | 3.1 (2.9-3.3) | 5.8 (5.3-6.4) |
| All-cause mortality | ||||
| Events per 100 p-y | 136 | 82 | 65 | 109 |
| Relative 95% CI | 13.0 (12.4-13.6) | 21.8 (19.7-24.1) | 5.7 (5.4-6.0) | 23.3 (21.3-25.3) |
| Event . | Overall cohort . | Never treated . | Treated, on treatment . | Treated, off treatment . |
|---|---|---|---|---|
| Total p-y | 1975 | 376 | 1132 | 467* |
| Recurrent VTE | ||||
| Events per 100 p-y | 104 | 35 | 38 | 31 |
| Relative 95% CI | 5.3 (5.1-5.5) | 9.3 (8.4-10.3) | 3.4 (3.2-3.6) | 6.6 (6.0-7.2) |
| Major bleeding | ||||
| Events per 100 p-y | 86 | 24 | 35 | 27 |
| Relative 95% CI | 4.4 (4.2-4.6) | 6.4 (5.8-7.1) | 3.1 (2.9-3.3) | 5.8 (5.3-6.4) |
| All-cause mortality | ||||
| Events per 100 p-y | 136 | 82 | 65 | 109 |
| Relative 95% CI | 13.0 (12.4-13.6) | 21.8 (19.7-24.1) | 5.7 (5.4-6.0) | 23.3 (21.3-25.3) |
Values are expressed as number of events per 100 p-y with relative 95% CIs.
p-y includes time before treatment initiation and time after treatment withdrawal.
Cumulative incidence for recurrent VTE and major bleeding with death as competing risk in the overall study population.
Cumulative incidence for recurrent VTE and major bleeding with death as competing risk in the overall study population.
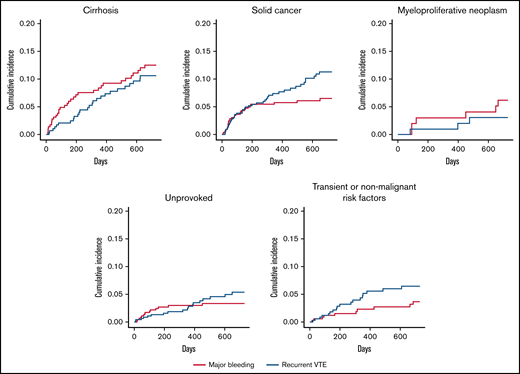
Cumulative incidence of recurrent VTE and major bleeding with death as competing risk in several patient subgroups.
Cumulative incidence of recurrent VTE and major bleeding with death as competing risk in several patient subgroups.
The incidence rate of recurrent VTE was lower during anticoagulant treatment (3.4 per 100 p-y; 95% CI, 3.2-3.6) than after treatment discontinuation (6.6 per 100 p-y; 95% CI, 6.0-7.2) or in patients who received no anticoagulation (9.3 per 100 p-y; 95% CI, 8.4-10.3; Table 2). The incidence rates of recurrent VTE in patient subgroups are reported in supplemental Table 6.
On-treatment recurrent VTE occurred in 21 patients treated with LMWH (7.9 per 100 p-y; 95% CI, 7.0-8.9), 16 patients receiving VKAs (2.0 per 100 p-y; 95% CI, 1.9-2.1), 1 patient receiving unfractionated heparin (25.0 per 100 p-y; 95% CI, 9.4-66.6), and none of those receiving DOACs.
Major bleeding
Major bleeding occurred in 86 patients, for an incidence rate of 4.4 per 100 p-y (95% CI, 4.2-4.6; Table 2). The site of major bleeding was gastrointestinal in 69 patients (4.2%), intracranial in 7 (0.4%), and other in 10 (0.6%). The cumulative incidences of major bleeding in the overall population and patient subgroups are shown in Figures 1 and 2, respectively. In total, there were 5 fatal major bleeding events (overall case fatality rate, 5.8%), of which 4 occurred in patients who received no anticoagulation (case fatality rate in untreated patients, 16.6%) and 1 in a patient after unfractionated heparin withdrawal. Sites of fatal bleeding were gastroesophageal varices in 2 cases, nonvariceal gastrointestinal site in 1 case (postcolectomy hemorrhage), and not reported for the other 2 events. Median time to major bleeding was 197 days (range, 8-685).
The incidence rate of major bleeding was lower during anticoagulant treatment (3.1 per 100 p-y; 95% CI, 2.9-3.3) than after treatment discontinuation (5.8 per 100 p-y; 95% CI, 5.3-6.4) or compared with patients who did not undergo anticoagulation (6.4 per 100 p-y; 95% CI, 5.8-7.1; Table 2). The incidence rates of major bleeding in patient subgroups are reported in supplemental Table 7.
On-treatment major bleeding occurred in 23 patients treated with LMWH (8.6 per 100 p-y; 95% CI, 7.6-9.7), 11 patients receiving VKAs (1.3 per 100 p-y; 95% CI, 1.2-1.4), and 1 patient treated with DOACs (3.0 per 100 p-y; 95% CI, 2.1-4.2).
All-cause mortality
A total of 256 patients died during follow-up, for an incidence rate of 13 per 100 p-y (95% CI, 12.4-13.6; Table 2). Causes of death were cancer progression (62.9%), sepsis (8.6%), liver failure (7.4%), cardiovascular disease (3.5%), multi-organ failure (1.6%), respiratory failure (1.6%), bowel obstruction (1.2%), kidney failure (0.8%), suicide (0.4%), and fatal bleeding (2.0%). In the remaining 10.0% of cases, the cause of death was unknown.
The incidence rate of all-cause mortality was lower during anticoagulant treatment (5.7 per 100 p-y; 95% CI, 5.4-6.0) than after treatment discontinuation (23.3 per 100 p-y; 95% CI, 21.3-25.3) or compared with patients who received no anticoagulation (21.8 per 100 p-y; 95% CI, 19.7-24.1; Table 2). The incidence rates of all-cause mortality in patient subgroups are reported in supplemental Table 6.
Time-varying analysis
Table 3 shows the results of univariable and multivariable time-varying analyses. Anticoagulant therapy appeared to be associated with lower risks of recurrent VTE (HR, 0.42; 95% CI, 0.27-0.64), major bleeding (HR, 0.47; 95% CI, 0.30-0.74), and all-cause mortality (HR, 0.23; 95% CI, 0.17-0.31) after adjusting for age, sex, liver cirrhosis, solid cancer, myeloproliferative neoplasms, and transient or persistent nonmalignant risk factors. The risks of recurrent VTE (HR, 2.02; 95% CI, 1.35-3.04) and all-cause mortality (HR, 8.68; 95% CI, 6.24-12.07) appeared to be increased in patients with solid cancer, whereas the risk of major bleeding was higher in patients with liver cirrhosis (HR, 1.92; 95% CI, 1.21-3.05).
Time-varying analysis for recurrent VTE, major bleeding, and all-cause mortality in the overall study population
| Variable . | Recurrent VTE . | Major bleeding . | All-cause mortality . | |||
|---|---|---|---|---|---|---|
| Univariable . | Multivariable . | Univariable . | Multivariable . | Univariable . | Multivariable . | |
| Anticoagulant treatment | 0.35 (0.23-0.52) | 0.42 (0.27-0.64) | 0.38 (0.24-0.58) | 0.47 (0.30-0.74) | 0.17 (0.13-0.23) | 0.23 (0.17-0.31) |
| Age, 1-y increase | 1.03 (1.01-1.04) | 1.02 (1.00-1.03) | 1.03 (1.02-1.05) | 1.03 (1.01-1.04) | 1.04 (1.03-1.05) | 1.02 (1.01-1.03) |
| Sex, male | 1.78 (1.16-2.74) | 1.54 (1.00-2.37) | 1.16 (0.75-1.79) | – | 1.15 (0.89-1.49) | – |
| Cirrhosis | 1.62 (1.04-2.52) | 1.10 (0.70-1.73) | 2.87 (1.85-4.45) | 1.92 (1.21-3.05) | 1.57 (1.18-2.09) | 0.95 (0.71-1.27) |
| Solid cancer | 2.78 (1.88-4.12) | 2.02 (1.35-3.04) | 1.70 (1.09-2.67) | 1.22 (0.77-1.92) | 12.92 (9.46-17.65) | 8.68 (6.24-12.07) |
| Myeloproliferative neoplasm | 0.31 (0.10-1.00) | – | 0.86 (0.38-1.98) | – | 0.19 (0.07-0.51) | 0.96 (0.34-2.69) |
| Transient or persistent nonmalignant risk factor* | 0.70 (0.42-1.16) | – | 0.45 (0.23-0.87) | 0.63 (0.35-1.25) | 0.34 (0.22-0.52) | 0.50 (0.32-0.77) |
| Variable . | Recurrent VTE . | Major bleeding . | All-cause mortality . | |||
|---|---|---|---|---|---|---|
| Univariable . | Multivariable . | Univariable . | Multivariable . | Univariable . | Multivariable . | |
| Anticoagulant treatment | 0.35 (0.23-0.52) | 0.42 (0.27-0.64) | 0.38 (0.24-0.58) | 0.47 (0.30-0.74) | 0.17 (0.13-0.23) | 0.23 (0.17-0.31) |
| Age, 1-y increase | 1.03 (1.01-1.04) | 1.02 (1.00-1.03) | 1.03 (1.02-1.05) | 1.03 (1.01-1.04) | 1.04 (1.03-1.05) | 1.02 (1.01-1.03) |
| Sex, male | 1.78 (1.16-2.74) | 1.54 (1.00-2.37) | 1.16 (0.75-1.79) | – | 1.15 (0.89-1.49) | – |
| Cirrhosis | 1.62 (1.04-2.52) | 1.10 (0.70-1.73) | 2.87 (1.85-4.45) | 1.92 (1.21-3.05) | 1.57 (1.18-2.09) | 0.95 (0.71-1.27) |
| Solid cancer | 2.78 (1.88-4.12) | 2.02 (1.35-3.04) | 1.70 (1.09-2.67) | 1.22 (0.77-1.92) | 12.92 (9.46-17.65) | 8.68 (6.24-12.07) |
| Myeloproliferative neoplasm | 0.31 (0.10-1.00) | – | 0.86 (0.38-1.98) | – | 0.19 (0.07-0.51) | 0.96 (0.34-2.69) |
| Transient or persistent nonmalignant risk factor* | 0.70 (0.42-1.16) | – | 0.45 (0.23-0.87) | 0.63 (0.35-1.25) | 0.34 (0.22-0.52) | 0.50 (0.32-0.77) |
Values are expressed as HR (95% CI). Only covariates that were associated on univariable analysis were tested in multivariable analysis.
Risk factors included any of the following: pregnancy, contraceptive, hormonal therapy, recent surgery, and inflammatory bowel disease.
In sensitivity analysis, the effects of anticoagulant treatment on recurrent VTE, major bleeding, and all-cause mortality appeared to be consistent in all patient subgroups (supplemental Table 6).
Discussion
The present work provides prospective data on the management and clinical outcomes of SVT in a large study population with long-term follow-up. The risk of recurrent VTE, major bleeding, and all-cause mortality was lower during anticoagulant treatment after adjustment for potentially relevant patient characteristics and across high-risk patient subgroups. The incidence rates of all outcomes increased after anticoagulant treatment discontinuation and were high in patients who did not receive any anticoagulation.
In agreement with previous observations, the present data confirm that a nonnegligible proportion of patients with SVT remained untreated (15%), and an additional 16% received treatment for <3 months. Although the risk of recurrent VTE in patients with SVT is relevant, concerns for bleeding complications remain major drivers of the decision whether to use anticoagulation.6 Several studies suggested that anticoagulant therapy may actually lower bleeding risk in these patients, presumably by promoting vein recanalization with decreased intravascular pressure in the splanchnic circulation and gastrointestinal varices.28-31 A recent study-level meta-analysis seemed to support this concept, showing increased rates of vein recanalization, lower rates of thrombosis progression, and reduced risk of bleeding complications in anticoagulated patients with SVT.5 Our results strengthen and extend these earlier observations, confirming the lower risks of recurrent VTE and major bleeding with anticoagulant treatment after adjustment for relevant confounders. We also explored the effectiveness and safety of anticoagulant therapy in high-risk subgroups such as those with liver cirrhosis, solid cancers, or myeloproliferative neoplasms.24,28,32,33 Our findings seem reassuring and suggest a lower risk of recurrent VTE, major bleeding, and all-cause mortality during anticoagulant therapy compared with off-treatment periods. These results support the recommendations of recent guidelines to promptly start anticoagulation for acute SVT irrespective of underlying risk factors in the absence of major contraindications.3,6,32 Sixty-one percent of recurrent VTEs occurred outside the splanchnic venous circulation, most often in patients with persistent risk factors. The latter have a chronic procoagulant state and, as for usual-site VTE, may warrant long-term anticoagulation. Interestingly, even though none of the recurrent VTEs resulted in death, the case-fatality rate of major bleeding was approximately 6%, largely accounted for by the number of fatal bleeding episodes (80% of total) occurring among untreated patients. In this latter group, the case-fatality rate was as high as 17%, which seems to support the physician decision not to provide anticoagulant therapy. Further studies are needed to identify patient subgroups with a very high risk of life-threatening bleeding in whom an individualized approach would be advisable. It is worth noting that we could not evaluate the safety of anticoagulation in patients with prior major bleeding, hematological malignancy, or severe thrombocytopenia as a result of the low number of patients with these risk factors.
There is scant information about the optimal type, dose, and duration of anticoagulant therapy for SVT, and most evidence stems from observational studies in which the decision to use or withhold anticoagulation was at the discretion of the treating physician. Furthermore, most of these studies included patients with liver cirrhosis who received LMWH or VKAs, limiting generalizability to other patient groups or types of anticoagulant agents. Data on the effectiveness and safety of DOACs for the treatment of SVT are limited. In a recently completed prospective study of 100 noncirrhotic patients with acute SVT who were treated with rivaroxaban for 3 months, the incidence of both recurrent VTE and major bleeding was 2.1%.34 Despite only 75 patients having received a DOAC in the present study, our findings seem consistent with earlier observations and suggest that DOACs may represent an alternative to standard anticoagulation with LMWH and VKAs for SVT.
The major strength of our study is the inclusion of a large population that was prospectively followed for as long as 2 years. The use of time-varying and multivariable analyses allowed a more precise estimation of the effects of anticoagulant treatment while accounting for potential confounders.
The present work has also some limitations that warrant discussion. First, some of the risk factors (eg, prior VTE, prior bleeding, or severe thrombocytopenia) were poorly represented, precluding the evaluation of their impact on study outcomes. Second, variables such as stage of thrombosis (ie, acute or chronic), presence of thrombophilia, JAK-2 mutation, and use of concomitant antiplatelet agents were missing in a significant proportion of patients and could not be accounted for in the analysis. Third, the use of a specific anticoagulant regimen as well as the decision to withhold anticoagulation were not randomized or protocol-mandated and could be influenced by patients’ characteristics and prognoses. In this regard, the reduction of all-cause mortality observed in anticoagulated patients may be the result of selection bias, as physicians could be more reluctant to administer anticoagulation to sicker patients with poor prognoses. For these reasons, the comparison between different types of anticoagulant agents remains exploratory. Most of the included patients (81.3%) had a persistent risk factor or unprovoked SVT, potentially limiting the generalizability of our findings to patients with SVT associated with transient risk factors. However, subgroup analysis suggested consistent results in this latter group of patients. Finally, the heterogeneity of anticoagulant treatment did not allow a more granular analysis of effectiveness and safety according to specific agents or doses.
In conclusion, patients with SVT have a substantial risk of recurrent VTE and major bleeding that appears to be reduced during anticoagulant treatment. These benefits are consistent in high-risk subgroups.
Acknowledgments
The authors thank all investigators of the included studies for their contribution on data collection in the original reports. A full list of investigators that participated to the included studies is provided in the supplemental Appendix.
Authorship
Contribution: M.D.N. conceived and designed the study; M.C. and M.D.N. performed data acquisition; M.C. performed statistical analysis; all authors interpreted the data; and M.C., E.V., and M.D.N. drafted the manuscript, which was critically revised for important intellectual content and approved as a final manuscript by all authors.
Conflict-of-interest disclosure: M.D.N. received honoraria for participation at advisory boards from Bayer, Daiichi Sankyo, Pfizer, Leo Pharma, Sanofi, and Viatris, outside the submitted work. W.A. has received a research grant from Bayer to support a clinical study in patients with SVT, received honoraria for participation at advisory boards from Bayer, BMS/Pfizer, Sanofi, Leo Pharma, and Portola, and reports grants and personal fees from Bayer, and personal fees from BMS/Pfizer, Daiichi Sankyo, Sanofi, Aspen, Janssen, and Portola, Werfen, outside the submitted work. M.B.M. is a recipient of a Río Hortega grant from Instituto de Salud Carlos III, Spain. J.-C.G.-P. is a consulting for Cook Medical, Shionogi, W.L. Gore y Asociados, Vifor Pharma, and Boehringer Ingelheim and has received a research frant from Mallinckrodt and Noorik. J.B.W. received honoraria and institutional research support from Bayer AG, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Daiichi Sankyo, DOASENSE, Norgine, Sanofi, and Alexion. S.S. has received research funding from Boehringer Ingelheim and Octapharma and honoraria from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Pfizer, and Sanofi. J.J.L.-N. has received honoraria for lectures from Bayer, Pfizer, and Rovi; educational events from Leo Pharma and Pfizer; support for attending meetings and travel from Bayer, BMS, Pfizer, Leo Pharma, Rovi, and Sanofi; and advisory board participation from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Matteo Candeloro, Department of Innovative Technologies in Medicine and Dentistry, “G. D’Annunzio” University, Via Dei Vestini 31, 66100 Chieti-Pescara, Italy; e-mail: matteo.candeloro@unich.it.
References
Author notes
Data will be made available upon reasonable request: matteo.candeloro@unich.it.
The full-text version of this article contains a data supplement.