We demonstrate an increase in 1-year bleeding rates among patients with AF and thrombocytopenia on oral anticoagulation.
Our findings suggest that baseline platelet counts are an important biomarker for hemorrhagic outcomes in AF.
Visual Abstract
Whether thrombocytopenia substantively increases the risk of hemorrhage associated with anticoagulation in patients with atrial fibrillation (AF) is not established. The purpose of this study was to compare rates of bleeding in patients with AF and thrombocytopenia (platelet count < 100 000/μL) to patients with AF and normal platelet counts (>150 000/μL). We performed a propensity score–matched, retrospective cohort study of adults (n = 1070) with a new diagnosis of AF who received a prescription for an oral anticoagulant between 2015 and 2020. The thrombocytopenia cohort was defined as having at least 2 platelet counts <100 000/μL on separate days in the period spanning the 12 weeks preceding the initiation of anticoagulation to 6 weeks after the initiation of anticoagulation. The primary end point was the 1-year cumulative incidence of major bleeding; secondary end points included clinically relevant bleeding, arterial and venous thrombotic events, and all-cause mortality. Patients with AF and thrombocytopenia experienced a higher 1-year cumulative incidence of major bleeding (13.3% vs 5.7%; P < .0001) and clinically relevant bleeding (24.5% vs 16.7%; P = .005) than the controls. Thrombocytopenia was identified as an independent risk factor for major bleeding (hazard ratio, 2.20; confidence interval, 1.36-3.58; P = .001), with increasing risk based on the severity of thrombocytopenia. The cumulative incidence of arterial thrombosis at 1 year was 3.6% in the group with thrombocytopenia and 1.5% in controls (Gray test, P = .08). These findings suggest that baseline platelet counts are an important biomarker for hemorrhagic outcomes in AF and that the degree of thrombocytopenia is an important factor in determining the level of risk.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with an estimated overall prevalence of 4% to 5% among patients 65 years of age or older.1-3 Anticoagulation reduces the risk of thromboembolic events, including ischemic strokes, in patients with AF but increases the risk of bleeding.4-6 The use of anticoagulation therapy for patients with AF requires the identification of patients with a net clinical benefit. Thrombocytopenia, a common comorbidity affecting 6% to 24% of patients with AF,7,8 may increase the risk of bleeding with anticoagulation, possibly altering the net clinical benefit of anticoagulation therapy. Concurrent thrombocytopenia increases the risk of hemorrhage associated with anticoagulation for acute venous thromboembolism (VTE),9 but whether it substantively alters the risk of bleeding in patients with AF is uncertain.
Despite the frequency of coexisting AF and thrombocytopenia, data to guide clinicians on management in this population are limited. Moderate and severe thrombocytopenia were exclusion criteria in pivotal clinical trials examining the effectiveness and safety of direct oral anticoagulants for stroke prophylaxis in patients with AF.10-13 Older studies that evaluated the safety and efficacy of warfarin for patients with AF included few patients with thrombocytopenia.14,15 Risk calculators developed to predict the risk of major bleeding, such as the HAS-BLED score, often fail to incorporate thrombocytopenia.16,17 The HEMORR2HAGES risk score does incorporate platelet count but is based on a pooled analysis of patients with thrombocytopenia, reduced platelet function, and antiplatelet agents, such that the contribution of thrombocytopenia as a standalone risk factor is undefined.18,19
The paucity of data describing the safety of oral anticoagulants for patients with AF and thrombocytopenia represents a major challenge in clinical practice. Clinical guidelines do not include specific guidance for anticoagulation in patients with coexisting AF and thrombocytopenia, other than suggesting that anticoagulation may be contraindicated in severe thrombocytopenia (platelet count < 50 × 103/μL).5,20 Thus, the purpose of this study was to characterize rates of bleeding in patients with AF and moderate-to-severe thrombocytopenia (ie, platelet count < 100 × 103/μL) in a matched cohort of patients with AF and normal platelet counts (platelet count > 150 × 103/μL).
Methods
Study design
The protocol was approved by the institutional review board at Beth Israel Deaconess Medical Center (BIDMC), a quaternary care hospital t Boston, MA. It was conducted according to the Declaration of Helsinki. Data were extracted from the BIDMC electronic medical record from 2015 to 2020. We included all adults aged 18 years or older with a new diagnosis of AF who received a new prescription for an oral anticoagulant. A 12-month look-back period was used to exclude patients with a prior diagnosis of AF or prior anticoagulation use for any indication. AF was identified using codes from the International Classification of Diseases (ICD; ICD-9 and ICD-10). Anticoagulation cases were identified based on prescription codes for warfarin, dabigatran, apixaban, or rivaroxaban. Patients were excluded if there was no documentation of at least 1 platelet count within 14 days of oral anticoagulation onset or if the anticoagulant start date could not be discerned.
Baseline demographics were recorded at the time of anticoagulation initiation. Covariates of interest included the presence or absence of diabetes, hypertension, heart failure, ischemic heart disease, cancer, chronic kidney disease (stage IIIa and higher), and liver disease, along with any history of prior stroke/transient ischemic attack or prior hemorrhage. Covariates were collected at anticoagulation initiation from individual patient problem lists using corresponding codes from the ICD-9 and ICD-10.
Exposure
The thrombocytopenia cohort was defined as having at least 2 platelet counts < 100 × 103/μL on separate days in the period spanning the 12 weeks preceding the initiation of anticoagulation to 6 weeks after the initiation of anticoagulation. The control group was defined as having only platelet counts > 150 × 103/μL in the same window. Patients with only 1 platelet count meeting criteria for their respective cohort were excluded. Individuals from the thrombocytopenia cohort were matched with patients (1:3 ratio) from the control cohort using propensity score matching based on age, sex, CHA2DS2-VASc score, and anticoagulant agent used. Standardized mean differences before and after matching were compared for each of the variables included in the algorithm to examine the balance of the covariate distribution after matching. On subgroup analysis adjusting for severity of thrombocytopenia, the cohort with more severe thrombocytopenia was defined as having at least 2 platelet counts < 75 × 103/μL on separate days in the window previously described.
Sample size and power estimate
A priori analysis demonstrated that there were ∼3500 patients in the BIDMC electronic medical record that met our inclusion criteria; ∼10% of these patients met criteria for the thrombocytopenic cohort. The rate of major bleeding in prior observational studies of patients with AF receiving oral anticoagulation ranged from 1 to 6 per 100 patient years.21,22 Assuming a 1:3 ratio of assignment, a rate of major bleeding of 5% in patients with AF without thrombocytopenia, and a doubling of the rate in those with thrombocytopenia, the study sample was calculated to have 83% power to detect a difference between our cohorts (300 patients with thrombocytopenia and 1:3 matched controls [2-sided α = 0.05]).
Outcomes
Bleeding outcomes were determined by blinded manual chart review and verified by a second reviewer. All bleeding events were classified per the International Society on Thrombosis and Haemostasis criteria.23 The primary end point of the study was the incidence of major bleeding within 1 year of the initiation of anticoagulation. Secondary end points included clinically relevant bleeding, a composite of major bleeding and clinically relevant nonmajor bleeding (CRNMB), fatal bleeding, arterial and venous thrombosis events, and death from all causes.
Analysis
Outcomes were analyzed using cumulative incidence at 1 year, accounting for death as a competing risk with comparison between the 2 groups performed by the Gray test.24 Two-sided P values < .05 were considered statistically significant. The Kaplan-Meier method was used to estimate overall survival, and the log-rank test was used to compare the survival curves between groups. Estimates of 1-year survival rates were also calculated to match the time window used for the primary outcome. Overall survival was defined as the time from the initiation of anticoagulation to the date of death from any cause; individuals alive at the time of data acquisition were censored at that time. Overall survival was further stratified as per the cancer diagnosis to address residual confounding from this covariate. To assess the impact of degree of the thrombocytopenia on outcomes, secondary subgroup analyses divided patients with thrombocytopenia into 2 groups based on baseline platelet counts (<75 × 103/μL and 75-100 × 103/μL) and compared patients in each group with their matched controls. Univariable and multivariable Fine and Gray competing risk regression modeling was used to identify risk factors for major bleeding, CRNMB, venous thrombosis, and arterial thrombosis, with results summarized as hazard ratios (HRs) and 95% confidence intervals (CIs).
A priori covariates of interest were identified for bleeding outcomes (age, gender, choice of anticoagulant, use of antiplatelet agents, presence of thrombocytopenia, and a history of cancer, chronic kidney disease, hypertension, or prior hemorrhage)19,25 separately from venous thrombotic outcomes (age, gender, choice of anticoagulant, use of antiplatelet agents, presence of thrombocytopenia, and a history of cancer)26 and arterial thrombotic outcomes (age, gender, choice of anticoagulant, use of antiplatelet agents, presence of thrombocytopenia, and a history of cancer, ischemic heart disease, hypertension, heart failure, or diabetes).26-28 Notably, the cohort included too few patients with liver disease to include in the bleeding and thrombosis models.
Results
Patient characteristics
A total of 1070 patients with AF were included in the study after matching: 274 patients with thrombocytopenia at baseline and 796 patients in the control cohort (Table 1). The standardized mean differences of all variables used in the matching algorithm were <0.05 after matching (supplemental Figure 2). The median age was 72 years, and 61.8% (n = 661) of patients were male. The most commonly prescribed anticoagulant therapies were warfarin (48.2% [n = 516]) and apixaban (39.5% [n = 423]).
Baseline characteristics
| Characteristic . | Thrombocytopenia, n = 274 . | Control, n = 796 . |
|---|---|---|
| Sex, male, n (%) | 170 (62.0) | 491 (61.7) |
| Age at diagnosis (median, range), y | 72 (37-95) | 72 (26-96) |
| Concurrent antiplatelet treatment, n (%) | 208 (75.9) | 572 (71.9) |
| Comorbidities, n (%) | ||
| Diabetes | 71 (25.9) | 46 (5.8) |
| Hypertension | 79 (28.8) | 62 (7.8) |
| Heart failure | 99 (36.1) | 67 (8.4) |
| Ischemic heart disease | 140 (51.1) | 80 (10.1) |
| Cancer | 61 (22.3) | 85 (10.7) |
| Chronic kidney disease | 13 (4.7) | 17 (2.1) |
| Cirrhosis | 35 (12.8) | 60 (7.5) |
| Prior stroke | 13 (4.7) | 6 (0.8) |
| Prior bleed | 10 (3.6) | 18 (2.3) |
| Anticoagulant agent, n (%) | ||
| Warfarin | 131 (47.8) | 385 (48.4) |
| DOAC∗ | 143 (52.2) | 411 (51.6) |
| Apixaban | 108 (39.4) | 315 (39.6) |
| Dabigatran | 1 (0.4) | 3 (0.4) |
| Rivaroxaban | 34 (12.4) | 93 (11.7) |
| CHA2DS2-VASc score, n (%) | ||
| 0-2 | 112 (40.9) | 313 (39.3) |
| 3-5 | 149 (54.4) | 442 (55.5) |
| 6-9 | 13 (4.7) | 41 (5.2) |
| Characteristic . | Thrombocytopenia, n = 274 . | Control, n = 796 . |
|---|---|---|
| Sex, male, n (%) | 170 (62.0) | 491 (61.7) |
| Age at diagnosis (median, range), y | 72 (37-95) | 72 (26-96) |
| Concurrent antiplatelet treatment, n (%) | 208 (75.9) | 572 (71.9) |
| Comorbidities, n (%) | ||
| Diabetes | 71 (25.9) | 46 (5.8) |
| Hypertension | 79 (28.8) | 62 (7.8) |
| Heart failure | 99 (36.1) | 67 (8.4) |
| Ischemic heart disease | 140 (51.1) | 80 (10.1) |
| Cancer | 61 (22.3) | 85 (10.7) |
| Chronic kidney disease | 13 (4.7) | 17 (2.1) |
| Cirrhosis | 35 (12.8) | 60 (7.5) |
| Prior stroke | 13 (4.7) | 6 (0.8) |
| Prior bleed | 10 (3.6) | 18 (2.3) |
| Anticoagulant agent, n (%) | ||
| Warfarin | 131 (47.8) | 385 (48.4) |
| DOAC∗ | 143 (52.2) | 411 (51.6) |
| Apixaban | 108 (39.4) | 315 (39.6) |
| Dabigatran | 1 (0.4) | 3 (0.4) |
| Rivaroxaban | 34 (12.4) | 93 (11.7) |
| CHA2DS2-VASc score, n (%) | ||
| 0-2 | 112 (40.9) | 313 (39.3) |
| 3-5 | 149 (54.4) | 442 (55.5) |
| 6-9 | 13 (4.7) | 41 (5.2) |
Baseline characteristics are shown after matching. supplemental Figure 2 shows absolute standardized mean differences for individual variables before and after matching.
DOAC, direct oral anticoagulant.
The propensity score–matched cohorts were well matched for age, sex, use of antiplatelets, and choice of anticoagulant. Patients with baseline thrombocytopenia had a higher prevalence of cancer (22.3% [n = 61] vs 10.7% [n = 85]; P < .0001), liver disease (4.7% [n = 13] vs 2.1% [n = 17]; P = .03), and kidney disease (12.8% [n = 35] vs 7.5% [n = 60]; P = .01). Concurrent treatment with an antiplatelet agent was common: most patients in the thrombocytopenia (n = 208; 75.9%) and control groups (n = 572; 71.9%) were receiving an antiplatelet agent at the time of anticoagulation initiation. The thrombocytopenia group included 141 patients (51.5%) with moderately suppressed platelet counts (75-100 × 103/μL) at the time of anticoagulation initiation, whereas 133 patients (48.5%) had more severely suppressed counts (<75 × 103/μL).
Bleeding outcomes
Overall, there were a total of 198 bleeding events recorded, including 82 (41.4%) major bleeding events and 116 (58.6%) CRNMB events. The most common types of bleeding events were gastrointestinal (33.3%) and mucocutaneous (38.9%; Table 2). There was 1 fatal bleeding event during follow-up among the patients with baseline thrombocytopenia and 3 fatal bleeding events among the controls.
Bleeding and thrombosis events
| . | Thrombocytopenia n = 274 . | Control n = 796 . |
|---|---|---|
| Total bleeding events, n | 65 | 132 |
| Median platelet count, × 103/μL | 130 | 234 |
| Bleeding type, n (%) | ||
| Major | 36 (55.4) | 45 (34.1) |
| Fatal | 1 (1.5) | 3 (2.3) |
| Bleeding into critical area∗ | 6 (9.2) | 8 (6.1) |
| Overt bleeding† | 28 (43.1) | 31 (23.5) |
| CRNMB | 29 (44.6) | 87 (65.9) |
| Location of bleed, n (%) | ||
| Mucocutaneous | 17 (26.2) | 60 (45.1) |
| Gastrointestinal | 21 (32.3) | 45 (33.8) |
| Intracranial | 3 (4.6) | 7 (5.3) |
| Pulmonary | 3 (4.6) | 7 (5.3) |
| Intramuscular | 5 (7.7) | 4 (3.0) |
| Retroperitoneal | 2 (3.1) | 2 (1.5) |
| Pericardial | 2 (3.1) | 1 (0.8) |
| Other | 12 (18.5) | 6 (4.5) |
| Total thrombosis events, n | 19 | 20 |
| Venous, n (%) | 10 (52.6) | 7 (35.0) |
| Deep vein thrombosis | 4 (21.1) | 5 (25.0) |
| Pulmonary embolism | 2 (10.5) | 0 (0.0) |
| Other | 4 (21.1) | 2 (10.0) |
| Arterial, n (%) | 9 (47.4) | 13 (65.0) |
| Ischemic stroke | 4 (21.1) | 6 (30.0) |
| Acute coronary syndrome | 3 (15.8) | 2 (10.0) |
| Other | 2 (10.5) | 5 (25.0) |
| . | Thrombocytopenia n = 274 . | Control n = 796 . |
|---|---|---|
| Total bleeding events, n | 65 | 132 |
| Median platelet count, × 103/μL | 130 | 234 |
| Bleeding type, n (%) | ||
| Major | 36 (55.4) | 45 (34.1) |
| Fatal | 1 (1.5) | 3 (2.3) |
| Bleeding into critical area∗ | 6 (9.2) | 8 (6.1) |
| Overt bleeding† | 28 (43.1) | 31 (23.5) |
| CRNMB | 29 (44.6) | 87 (65.9) |
| Location of bleed, n (%) | ||
| Mucocutaneous | 17 (26.2) | 60 (45.1) |
| Gastrointestinal | 21 (32.3) | 45 (33.8) |
| Intracranial | 3 (4.6) | 7 (5.3) |
| Pulmonary | 3 (4.6) | 7 (5.3) |
| Intramuscular | 5 (7.7) | 4 (3.0) |
| Retroperitoneal | 2 (3.1) | 2 (1.5) |
| Pericardial | 2 (3.1) | 1 (0.8) |
| Other | 12 (18.5) | 6 (4.5) |
| Total thrombosis events, n | 19 | 20 |
| Venous, n (%) | 10 (52.6) | 7 (35.0) |
| Deep vein thrombosis | 4 (21.1) | 5 (25.0) |
| Pulmonary embolism | 2 (10.5) | 0 (0.0) |
| Other | 4 (21.1) | 2 (10.0) |
| Arterial, n (%) | 9 (47.4) | 13 (65.0) |
| Ischemic stroke | 4 (21.1) | 6 (30.0) |
| Acute coronary syndrome | 3 (15.8) | 2 (10.0) |
| Other | 2 (10.5) | 5 (25.0) |
Defined by International Society on Thrombosis and Haemostasis (ISTH) as the following spaces: intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome.
Defined by ISTH as bleeding causing a drop in hemoglobin ≥2g/dL or leading to a transfusion of at least 2 units of red blood cells.
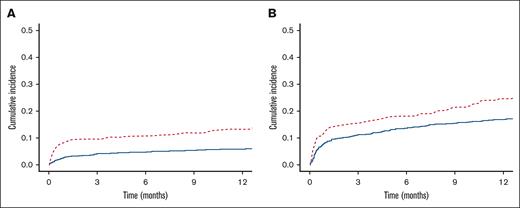
The cumulative incidence of major bleeding at 1 year was significantly higher among patients with baseline thrombocytopenia than among controls (13.3% vs 5.7%; P < .0001; Figure 1A). The cumulative incidence of clinically relevant bleeding at 1 year was also significantly higher in the thrombocytopenia cohort (24.5% vs 16.7%; P = .005; Figure 1B).
Cumulative incidence of bleeding. One-year cumulative incidence of bleeding in patients with baseline thrombocytopenia vs in those with normal platelet counts. (A) Major bleeding. (B) Clinically relevant bleeding. Those with thrombocytopenia in red hashed lines and controls in solid blue.
Cumulative incidence of bleeding. One-year cumulative incidence of bleeding in patients with baseline thrombocytopenia vs in those with normal platelet counts. (A) Major bleeding. (B) Clinically relevant bleeding. Those with thrombocytopenia in red hashed lines and controls in solid blue.
The median platelet count at the time of bleeding events was 130 × 103/μL in the thrombocytopenia cohort compared with 234 × 103/μL in patients with normal platelet counts at baseline (P < .0001).
Variables associated with bleeding
Univariable Fine and Gray competing risk regression was performed to identify variables associated with major bleeding (supplemental Table 2). Among the variables analyzed, age (HR, 1.96; 95% CI, 1.10-3.49; P = .02), hypertension (HR, 2.18; 95% CI, 1.38-3.42; P = .0008), and thrombocytopenia (HR, 2.48; 95% CI, 1.60-3.84; P < .0001) were significantly associated with an increased risk of major bleeding.
The multivariable competing risk regression reinforced the effect of thrombocytopenia on major bleeding. In the primary analysis, thrombocytopenia (<100 × 103/μL) and older age were the variables independently associated with major bleeding, with HRs of 2.20 (95% CI, 1.36-3.58; P = .001) and 1.96 (95% CI, 1.08-3.58; P = .03), respectively (Table 3). In a secondary analysis, the degree of thrombocytopenia at baseline also appeared to affect the risk of hemorrhage. For patients with baseline platelet counts below <75 × 103/μL, the associated HR for major bleeding was 2.92 (95% CI, 1.45-5.88; P = .003), compared with a HR of 1.88 (95% CI, 0.96-3.67; P = .07) for patients with less severe thrombocytopenia (75-100 × 103/μL). In the cohort with more severe thrombocytopenia, age was also associated with major bleeding (HR, 3.68; 95% CI, 1.29-10.54; P = .01).
Multivariable analysis and major bleeding
| Characteristic . | All patients n = 1 070 . | Platelet count < 75 × 103/μL n = 519 . | Platelet count of 75 to 100 × 103/μL n = 551 . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age > 65 y | 1.96 (1.08-3.58) | .03 | 3.68 (1.29-10.54) | .01 | 1.31 (0.61-2.83) | .48 |
| Sex∗ | 0.94 (0.59-1.49) | .78 | 1.07 (0.55-2.07) | .84 | 0.85 (0.44-1.64) | .63 |
| Hypertension | 1.36 (0.82-2.26) | .24 | 0.91 (0.43-1.93) | 0.80 | 1.92 (0.96-3.84) | .06 |
| Kidney disease | 1.42 (0.75-2.69) | .28 | 1.49 (0.55-3.99) | .43 | 1.39 (0.59-3.29) | .45 |
| Cancer | 0.66 (0.32-1.35) | .26 | 0.48 (0.16-1.41) | .18 | 0.95 (0.37-2.41) | .91 |
| Prior bleed | 0.94 (0.22-3.93) | .93 | 1.07 (0.14-7.96) | .95 | 0.89 (0.11-7.31) | .92 |
| Thrombocytopenia | 2.20 (1.36-3.58) | .001 | 2.92 (1.45-5.88) | .003 | 1.88 (0.96-3.67) | .07 |
| Anticoagulant† | 1.24 (0.79-1.94) | .35 | 1.23 (0.64-2.36) | .54 | 1.31 (0.7-2.44) | .39 |
| Antiplatelet | 1.28 (0.74-2.24) | .38 | 1.98 (0.81-4.86) | .13 | 0.91 (0.44-1.9) | .80 |
| Characteristic . | All patients n = 1 070 . | Platelet count < 75 × 103/μL n = 519 . | Platelet count of 75 to 100 × 103/μL n = 551 . | |||
|---|---|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Age > 65 y | 1.96 (1.08-3.58) | .03 | 3.68 (1.29-10.54) | .01 | 1.31 (0.61-2.83) | .48 |
| Sex∗ | 0.94 (0.59-1.49) | .78 | 1.07 (0.55-2.07) | .84 | 0.85 (0.44-1.64) | .63 |
| Hypertension | 1.36 (0.82-2.26) | .24 | 0.91 (0.43-1.93) | 0.80 | 1.92 (0.96-3.84) | .06 |
| Kidney disease | 1.42 (0.75-2.69) | .28 | 1.49 (0.55-3.99) | .43 | 1.39 (0.59-3.29) | .45 |
| Cancer | 0.66 (0.32-1.35) | .26 | 0.48 (0.16-1.41) | .18 | 0.95 (0.37-2.41) | .91 |
| Prior bleed | 0.94 (0.22-3.93) | .93 | 1.07 (0.14-7.96) | .95 | 0.89 (0.11-7.31) | .92 |
| Thrombocytopenia | 2.20 (1.36-3.58) | .001 | 2.92 (1.45-5.88) | .003 | 1.88 (0.96-3.67) | .07 |
| Anticoagulant† | 1.24 (0.79-1.94) | .35 | 1.23 (0.64-2.36) | .54 | 1.31 (0.7-2.44) | .39 |
| Antiplatelet | 1.28 (0.74-2.24) | .38 | 1.98 (0.81-4.86) | .13 | 0.91 (0.44-1.9) | .80 |
P < .05 was considered statistically significant.
HRs are for the female group as compared with the male group.
HRs are for the warfarin group as compared with the direct oral anticoagulant group.
Thrombosis outcomes
There were a total of 39 thrombotic events during follow-up, including 19 events among patients with baseline thrombocytopenia and 20 among patients with normal platelet counts. The most common types of thrombotic events were VTE (n = 17; 43.6%) and ischemic strokes (n = 10; 25.6%; Table 2).
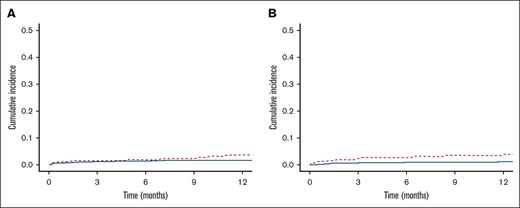
The cumulative incidence of thrombotic events at 1 year was significantly higher in those with thrombocytopenia at the time of starting anticoagulation than in the control cohort (7.5% vs 2.5%; P = .005). This was driven by the cumulative incidence of venous thrombosis, which was significantly higher in the thrombocytopenia cohort (3.9% vs 0.9%; P = .001; Figure 2B). In contrast, the cumulative incidence of arterial thrombosis at 1 year was not significantly different between groups (3.6% vs 1.5%; P = .08; Figure 2A).
Cumulative incidence of thrombosis. One-year cumulative incidence of thrombosis in patients with baseline thrombocytopenia vs normal platelet counts. (A) Arterial thrombosis. (B) Venous thrombosis. Those with thrombocytopenia in red hashed lines and controls in solid blue.
Cumulative incidence of thrombosis. One-year cumulative incidence of thrombosis in patients with baseline thrombocytopenia vs normal platelet counts. (A) Arterial thrombosis. (B) Venous thrombosis. Those with thrombocytopenia in red hashed lines and controls in solid blue.
Variables associated with thrombosis
Univariable Fine and Gray competing risk regression was performed to identify variables associated with thrombosis (supplemental Table 5). Among venous events, only thrombocytopenia was significantly associated with a higher risk of clotting (HR, 4.31; 95% CI, 1.64-11.33; P = .003). Multivariable analysis also confirmed that thrombocytopenia was the only variable independently associated with a higher risk of venous thrombosis (HR, 4.17; 95% CI, 1.60-10.85; P = .003). In a multivariable model of arterial thrombosis, no variables were significantly associated with a higher risk of thrombosis (supplemental Table 6).
All-cause mortality
Overall survival was shorter in patients with baseline thrombocytopenia compared with controls (log-rank P < .001). One-year overall survival was 86.1% (95% CI, 82.0-90.4) in patients with thrombocytopenia vs 93.4% (95% CI, 91.7-95.2) in controls (Figure 3). In a subset analysis based on the presence of cancer, the overall survival was significantly shorter in patients without cancer who had baseline thrombocytopenia (log-rank P < .006) but not in patients with cancer with baseline thrombocytopenia (log-rank P = .09; supplemental Figure 1).
All-cause mortality. All-cause mortality in patients with baseline thrombocytopenia vs normal platelet counts. Those with thrombocytopenia in red and controls in blue.
All-cause mortality. All-cause mortality in patients with baseline thrombocytopenia vs normal platelet counts. Those with thrombocytopenia in red and controls in blue.
Discussion
Anticoagulation management for patients with AF and concurrent thrombocytopenia is challenging. In this population, concerns about hemorrhage must be weighed against the risk of stroke. Our findings demonstrate that moderate-to-severe thrombocytopenia at the time of starting oral anticoagulation for AF is a dominant predictor for both major bleeding and clinically relevant bleeding after the initiation of anticoagulation.
To our knowledge, this is the largest study examining the association between thrombocytopenia and anticoagulation-related outcomes in patients with AF. A subgroup analysis of major bleeding events from the START (Survey on anTicoagulated pAtients RegisTer) database demonstrated that patients with AF and platelet counts < 100 × 103/μL (n = 50) were more likely to experience bleeding (HR, 3.7; 95% CI, 1.17-11.69; P = .026) than patients without thrombocytopenia.29 A single-center analysis from South Korea also found an increased risk of bleeding in patients with AF with moderate-to-severe thrombocytopenia (platelet count < 100 × 103/μL; HR, 2.19; 95% CI, 1.77-2.70; P < .001) relative to those with normal platelet counts; the group, however, did not examine the association with anticoagulation, which was only being used in about half the patients in their cohort.7
Here, factors at the time of starting anticoagulation associated with a significant risk of major bleeding were age and thrombocytopenia. Age is already an established risk factor for hemorrhage in AF.30,31 No other variables were associated with bleeding, including diagnosis of malignancy, presence of renal disease, concurrent use of antiplatelet agents, or type of anticoagulant. Hypertension, although significant in the univariable analysis, was not retained in the multivariable analysis. Other differences in baseline comorbidities (eg, renal disease, liver disease, prior stroke; Table 1) did not influence the risk of bleeding either. Overall, the 1-year rate of major bleeding in our control cohort (5.7%) is in line with prior observational data21 and is at the upper end of that reported in the US direct oral anticoagulant registration trials, which ranged between 2.6% and 5.6%.11,32,33
The degree of thrombocytopenia also influenced the risk of bleeding in our study. In multivariable analyses, more severe thrombocytopenia (<75 × 103/μL) was associated with an increased risk of major bleeding. These findings are consistent with observational data describing the safety and efficacy of anticoagulant dose adjustment for more severe thrombocytopenia in cancer-associated VTE.34-37 This observation led to the clinical recommendation of using reduced-dose anticoagulation for patients with cancer with platelet counts < 50 × 103/μL.38,39 However, in the absence of randomized data, there remains persistent equipoise regarding the optimal treatment strategy in this population, particularly with regard to precise platelet thresholds and the relative safety of various anticoagulants. Future work will need to take into account similar considerations in AF and thrombocytopenia.
There were limitations inherent to a retrospective study of thrombocytopenia, such as variability in the number and frequency of platelet measurements per patient. Accordingly, we elected to designate cohort assignment based on the platelet count at the time of anticoagulation initiation rather than based on platelet trends after anticoagulation. Confirming the validity of this classification is the nearly 100 × 103/μL difference in platelet counts at the time of bleeding events. Additionally, despite our use of propensity scores to adjust for baseline confounding, there were anticipated imbalances between the 2 cohorts in terms of underlying conditions associated with thrombocytopenia, such as cancer40 and liver disease.41 When accounting for these variables, thrombocytopenia remained an independent and dominant risk factor for hemorrhage.
An unexpected observation was that thrombocytopenia at the time of starting oral anticoagulation for AF was also associated with an increased risk of thrombotic events. This association appeared to be primarily driven by an increased risk of VTE rather than arterial events. On multivariable analysis, the increased risk of VTE was independent of the diagnosis of underlying cancer; we suspect that this may be a product of the small event rate or residual confounding because of unobserved variables. For example, given the retrospective nature of this study, it was not possible to obtain reliable anticoagulant dosing or adherence data in either arm or information regarding the cause of patients’ thrombocytopenia. Patients in the thrombocytopenia cohort might have had suppressed counts because of immune thrombocytopenic purpura or clonal hematopoiesis, both of which have been associated with an increased risk of hemorrhage and thrombosis.42,43 These counterintuitive findings point to the complicated nature of an observational cohort with thrombocytopenia, whereby bleeding and thromboembolic outcomes may be driven by underlying disease states rather than absolute platelet count.
Our findings are based on data from the electronic medical record at a single quaternary care center and may not be generalizable to patients receiving care in other settings. For example, the high rate of concurrent antiplatelet use in our study cohort likely reflects the relatively sick population and the fact that these data are from an era when aspirin was routinely used for primary prevention of ischemic heart disease. Although rates of antiplatelet use were similar across both arms and we accounted for antiplatelet use in our analyses, this study should be repeated in a cohort that reflects more contemporary levels of antiplatelet use.
In summary, our findings show that oral anticoagulation for AF in patients with thrombocytopenia at the time of starting anticoagulation is associated with a significant increase in the risk of major and clinically relevant bleeding. Improved understanding of the net clinical benefit of anticoagulation in this high-risk population and the identification of optimal treatment regimens (eg, platelet cutoffs, dose modification) are urgently needed.
Acknowledgments
The authors acknowledge Karla Pollick and George Silva from the Beth Israel Deaconess Medical Center InSIGHT Core for help with clinical data retrieval. J.I.Z. is supported in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Authorship
Contribution: V.I., R.P., and J.I.Z. conceived the research study; V.I., S.M., A.P., A.B., and P.E. collected data. V.I., R.P., D.S.K., and J.I.Z. interpreted the data and drafted the manuscript; S.R. and D.N. performed statistical analyses; and all other authors critically reviewed and approved the final manuscript.
Conflict-of-interest disclosure: J.I.Z. reports prior research funding from Incyte and Quercegen and consultancy for Sanofi, CSL Behring, and Calyx. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey I. Zwicker, Division of Hematology, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10021; email: zwickerj@mskcc.org.
References
Author notes
∗V.I. and R.P. contributed equally to this study.
Data are available on request from the corresponding author, Jeffrey I. Zwicker (zwickerj@mskcc.org).
The full-text version of this article contains a data supplement.