Key Points
IDCH differs from LCH in its clinical, molecular, and immunohistochemical features.
IDCH is often associated with other hematopoietic neoplasms, and such cases are associated with an inferior prognosis.
Visual Abstract
Indeterminate dendritic cell histiocytosis (IDCH) is a rare and poorly understood entity characterized by accumulation of CD1a+/S100+ histiocytes (as Langerhans cell histiocytosis [LCH]) but with reduced-absent expression of Langerin/CD207. We assembled 43 cases of IDCH (defined by CD1a+/CD207<20% immunophenotypic profile) examining the clinical, pathologic, and molecular landscape. Median age at presentation was 70 years (interquartile range, 44-80) with cutaneous (31/43; 72%) and nodal (11/43; 26%) involvement predominating. Eighteen (42%) individuals had an associated nonhistiocytic hematopoietic neoplasm (“secondary” IDCH) whereas 7 of 43 (16%) had a concurrent non-IDCH histiocytosis (“mixed” histiocytosis). Most cases exhibited morphology indistinguishable from LCH but with a CD1c+/CSF1R(CD115)− phenotype, mirroring the signature of normal indeterminate cells and conventional DC type 2. Mutational analysis revealed frequent KRAS (13/32; 41%) and BRAF p.V600E (11/36, 31%) mutations that were nearly mutually exclusive. RNA-sequencing analysis uncovered ETV3::NCOA2 fusion in 6 other patients presenting as a sole genetic alteration without any other concurrent histiocytic or hematopoietic neoplasm. BRAF and MAP2K1 alterations were significantly associated with partial/retained (1%-20%) Langerin expression (P = .005) and mixed histiocytosis (P = .002). Remarkably, myeloid alterations (DNMT3A, TET2, and SRSF2) co-occurred in IDCH tissues of several individuals. Paired sequencing of IDCH and concurrent non-IDCH hematopoietic neoplasm in 4 individuals revealed shared mutations. Age at diagnosis and any nodal involvement at diagnosis predicted inferior overall survival, but BRAF/RAS pathway alterations did not affect outcome. These data have implications for the diagnostic evaluation, classification, and therapeutic management of IDCH.
Introduction
The term “histiocyte” embraces dendritic cells (DCs) with antigen-presenting functions and macrophages that excel at antigen processing.1 Histiocytic neoplasms have their origin in cells of the macrophage, dendritic, or monocytic lineages, and are collectively referred to as the histiocytoses. Historically, histiocytoses have been classified as Langerhans cell (LC) histiocytosis (LCH) and non-LCH.2 The underlying reason for this distinction was that initially LCH was thought to be the sole DC disorder, but subsequently other diseases of DC origin were described.1 Current classification systems subclassify the histiocytoses according to the presumed cell of origin based on the immunophenotype when possible, distinctive clinicopathologic features when the immunophenotype is nonspecific, and degree of cytological atypia.3,4
From a historical perspective, LCs are characterized by strong immunoreactivity for S100 protein and CD1a. Furthermore, they contain Birbeck granules, a defining ultrastructural feature of LCs, and currently detected by immunohistochemistry for Langerin/CD207. In 1979, Rowden et al defined the indeterminate cell as an epidermal DC expressing CD1a similar to LCs but without Birbeck granules.5 In 1981, Poppema and Bhan described DCs with similar features in the paracortex of human lymph nodes.6 Because of the immunophenotypic similarities, various hypotheses regarding the relationship between LCs and indeterminate cells have been proposed: (1) indeterminate cells are immature precursors of LCs; (2) LCs are precursors of indeterminate cells; or (3) both are independent types of DCs. It was further suggested that they may represent a precursor pool of LCs.5,7
In recent years, significant progress has been made in our understanding of the developmental pathways of macrophages, monocytes, and DCs. It is now known that although resident epidermal LCs have specialized DC functions, they share ontogeny with tissue resident macrophages.8,9 Dendritic cells (conventional and plasmacytoid) as well as monocytes derive from bone marrow hematopoietic stem cells by way of distinct precursor pathways. In humans, 2 subsets of conventional DCs (cDCs) comprising cDC1 and cDC2 lineages have been defined by expression of BDCA3/CD141 or BDCA1/CD1c, respectively.10 Indeterminate cells appear to have a phenotype compatible with cDC2 lineage, which were first characterized as migratory CD1a+ CD1c+ DCs.11,12 However, it should be noted that neither CD1a nor CD1c are completely restricted to cDC2 and may be expressed by cDC1 and monocyte-derived DCs.10 Moreover, subsequent studies suggested that indeterminate cells seen in human skin and lymph node lack colony stimulating factor 1 receptor expression (CSF1R/CD115), similar to cDCs.13,14
Indeterminate DC histiocytosis (IDCH) was first defined by Wood et al in 1985 as an unusual cutaneous disease characterized by an atypical histiocytic infiltrate with morphological features of LCH but lacking Birbeck granules by electron microscopy.15 They suggested the “indeterminate” cell as the cell of origin. Subsequently, IDCH has been documented in sporadic case reports as a histiocytic neoplasm with variable prognosis.16,17 A few cases were reported with a ETV3::NCOA2 fusion, a finding which the authors proposed as a key feature of this disease.18 Despite these studies, the full clinicopathologic and molecular profile of IDCH remains largely undefined. Moreover, it is unclear whether IDCH is truly distinct from LCH or instead represents a variant form of LCH.
In hopes of clarifying the clinicopathologic and molecular features of IDCH, we assembled a large cohort of centrally reviewed IDCH cases from 2 institutions, each with a specific research focus on histiocytoses.
Methods
Patient selection
The use of human patient material was performed in accordance with the World Medical Association Declaration of Helsinki and with the approval of the participating institutional review boards (ClinicalTrials.gov identifier: NCT04437381 for cases from France, and protocol 10CN074 for cases from the National Cancer Institute). We identified 51 cases of possible IDCH from the authors’ consultation files and pathology archives that had at least a hematoxylin and eosin stained section, CD1a, and Langerin/CD207 stained slides available for review. Inclusion criteria in this study were as follows: (1) tissue biopsy demonstrating pathological signs of a neoplastic histiocytosis, (2) lack of anaplasia fulfilling diagnosis of histiocytic sarcoma on the cytological grounds, (3) CD1a expression in the great majority of lesional cells, and (4) Langerin/CD207 expression limited to only small subsets of the lesional cells (<20%) or total absence. All cases were centrally reviewed in online group meetings by N.O., S.M.B., E.S.J., and J.-F.E. for confirmation of diagnosis. Of 51 cases evaluated, 43 were eligible for inclusion in this study. Seven cases were excluded, because a reactive histiocytic/DC hyperplasia could not be completely ruled out. One case was excluded because of detected KMT2A::MLLT1 fusion, which raised concerns regarding myeloid sarcoma, despite fulfilling aforementioned histopathologic criteria. Although this patient was excluded from the main cohort, separately discussed below.
Clinicopathologic assessment
For each patient, clinical parameters (age at diagnosis, sex, and sites of involvement), history of any other hematologic neoplasms, and outcome were obtained from medical records. Of note, 18 patients had a concurrent or past history of a nonhistiocytic hematopoietic neoplasm (termed “secondary IDCH”) including myeloid and/or lymphoid neoplasms. These cases were included when a distinct clonal histiocytic infiltrate fulfilling aforementioned criteria was present in the absence of a CD34 or CD117+ blastic infiltrate admixed with the histiocytic lesion. Cases lacking an associated nonhistiocytic hematopoietic neoplasm were labeled as “primary IDCH.” In addition, we assessed the clonal relationship between the IDCH and the associated hematopoietic neoplasm when possible, as detailed below.
Additional immunohistochemical and/or targeted DNA/RNA-sequencing analyses were performed when biopsy material was available. Immunohistochemistry was performed on either a Ventana Benchmark automated immunostainer using Ultra View detection or a Leica (BOND III) system. Langerin expression was stratified into 2 groups based on numbers of lesional cells expressing Langerin: absent (0%) vs partial (1%-20%).
Statistical analysis
Basic descriptive statistics were examined using Mann-Whitney or Fisher exact tests. We looked at all-cause mortality at 6 years (72-month survival) after IDCH diagnosis censoring patients alive at the end of follow-up. For analysis purposes, patients without follow-up after diagnosis were censored at 2 weeks after diagnosis. We used log-rank tests or Cox proportional hazards regression (after verifying proportional hazards assumption) for tested covariates adjusting for age in some analyses. All analysis was conducted with Stata 18 (College Station, TX). Hazard ratios (HR) are depicted with 95% confidence intervals throughout the manuscript.
Molecular genetic analysis
For cases sequenced at the Laboratory of Pathology, NCI (National Cancer Institute, Bethesda, MD), next-generation sequencing (NGS) was performed using a commercial panel TruSight Oncology 500 (TSO500; Illumina, San Diego, CA) as previously described.19,20 The TSO500 panel contents can be found on the manufacturer’s website: https://www.illumina.com/products/by-type/clinical-research-products/trusight-oncology-500.html).
The French cases underwent sequential analyses starting with a targeted BRAFV600E testing, followed by extended NGS panel and RNA sequencing if targeted testing was negative as described.20,21 Ambroise Paré’s custom panel for RNA sequencing comprises 76 genes including the following genes: ALK, BRAF, CSF1, KMT2A, KRAS, MAP2K1, MAP3K8, NCOA2, NRAS, NTRK1, NTRK2, NTRK3, and RET.
Results
Clinical features
Characteristics of the 43 patients are summarized in Table 1 and supplemental Table 1 and comprised 30 males and 13 females and included 36 adults and 7 children. The age at presentation ranged from 6 weeks to 89 years (median 70 years): 1 infant had blueberry muffin type rash only 6 weeks after birth. The most common sites of involvement were skin (n = 31) and lymph node (n = 11). Other sites included colon (n = 2), lung (n = 1), and tonsil (n = 1). In 18 patients (42% of all cases), IDCH was associated with an additional nonhistiocytic hematopoietic neoplasm and were designed above as secondary IDCH. These neoplasms were: 14 myeloid and 5 lymphoid neoplasms as detailed in supplemental Table 1. The 25 other patients were designated as primary IDCH.
Characteristics of patients with IDCH
| . | Entire cohort (N = 43) . | Primary IDCH (n = 25, 58%) . | Secondary IDCH (n = 18, 42%) . | |
|---|---|---|---|---|
| Median age (y) | 70 | 64 | 77 | |
| Pediatric patients (n) | 7 | 6 | 1 | |
| Sex | ||||
| Female | 13 | 9 | 4 | |
| Male | 30 | 16 | 14 | |
| Location (n) | ||||
| Skin only | 28 | 20 | 8 | |
| Lymph node only | 8 | 2 | 6 | |
| Skin and lymph node | 3 | 1 | 2 | |
| Colonic polyp | 2 | 1 | 1 | |
| Tonsil | 1 | 0 | 1 | |
| Lung | 1 | 1 | 0 | |
| Immunohistochemistry (n) | ||||
| CD1a expression | 43/43 | 25/25 | 18/18 | |
| S100 protein expression | 31/40 | 16/22 | 15/18 | |
| CSF1R/CD115 expression | 7/29 | 4/16 | 3/13 | |
| CD1c expression | 16/17 | 9/9 | 7/8 | |
| Langerin expression: | ||||
| Langerin negative | 28 | 4/28 pediatric | 16 | 12 |
| 1/28 mixed histiocytosis | ||||
| with BRAF∗/MAP2K1: 5/28 | ||||
| Langerin positive (<20%) | 15 | 3/15 pediatric | 9 (36%) | 6 (33%) |
| 6/15 mixed histiocytosis | ||||
| with BRAF∗/MAP2K1: 9/15 | ||||
| Molecular alterations (n) | ||||
| KRAS | 13 | 4 | 9 | |
| BRAF∗ alterations | 13 | 8 | 5 | |
| ETV3::NCOA2 | 6 | 6 | 0 | |
| Mixed histiocytosis (n) | 7 | 4 | 3 | |
| with BRAF∗/MAP2K1: 7/7 | ||||
| Disease-related mortality | 12/34 (35%) | 2/20 (10%) | 10/14 (71%) | |
| . | Entire cohort (N = 43) . | Primary IDCH (n = 25, 58%) . | Secondary IDCH (n = 18, 42%) . | |
|---|---|---|---|---|
| Median age (y) | 70 | 64 | 77 | |
| Pediatric patients (n) | 7 | 6 | 1 | |
| Sex | ||||
| Female | 13 | 9 | 4 | |
| Male | 30 | 16 | 14 | |
| Location (n) | ||||
| Skin only | 28 | 20 | 8 | |
| Lymph node only | 8 | 2 | 6 | |
| Skin and lymph node | 3 | 1 | 2 | |
| Colonic polyp | 2 | 1 | 1 | |
| Tonsil | 1 | 0 | 1 | |
| Lung | 1 | 1 | 0 | |
| Immunohistochemistry (n) | ||||
| CD1a expression | 43/43 | 25/25 | 18/18 | |
| S100 protein expression | 31/40 | 16/22 | 15/18 | |
| CSF1R/CD115 expression | 7/29 | 4/16 | 3/13 | |
| CD1c expression | 16/17 | 9/9 | 7/8 | |
| Langerin expression: | ||||
| Langerin negative | 28 | 4/28 pediatric | 16 | 12 |
| 1/28 mixed histiocytosis | ||||
| with BRAF∗/MAP2K1: 5/28 | ||||
| Langerin positive (<20%) | 15 | 3/15 pediatric | 9 (36%) | 6 (33%) |
| 6/15 mixed histiocytosis | ||||
| with BRAF∗/MAP2K1: 9/15 | ||||
| Molecular alterations (n) | ||||
| KRAS | 13 | 4 | 9 | |
| BRAF∗ alterations | 13 | 8 | 5 | |
| ETV3::NCOA2 | 6 | 6 | 0 | |
| Mixed histiocytosis (n) | 7 | 4 | 3 | |
| with BRAF∗/MAP2K1: 7/7 | ||||
| Disease-related mortality | 12/34 (35%) | 2/20 (10%) | 10/14 (71%) | |
BRAF V600E mutation or BRAF fusions.
Seven patients were diagnosed with a nonhistiocytic hematopoietic neoplasm before the diagnosis of IDCH (5 months to 6.5 years preceding IDCH diagnosis), 10 were diagnosed simultaneously, and none were diagnosed with a hematologic neoplasm after a diagnosis of IDCH.
Also, 7 patients (17%) had a concurrent or prior histiocytosis with non-IDCH histology including 3 patients with LCH, 2 patients with Erdheim-Chester disease (ECD), 1 patient with ECD/LCH, and 1 patient with Rosai-Dorfman disease, consistent with mixed histiocytosis.22,23 Of note, mixed histiocytosis cases were associated with secondary IDCH in 3 cases.
Histopathologic findings
Morphologically, the nuclear features of the lesional cells in IDCH resembled those of LCH in most samples. However, unlike LCH, admixed eosinophils were rare to absent in most biopsies (Figure 1A-C). Less frequently “blastoid” morphology with small to medium-sized lesional cells, scant cytoplasm, and round to irregular nuclei with finely dispersed chromatin was noted (Figure 1D-E). However, blastoid morphology was neither exclusive to secondary IDCH cases nor present in all of them. Necrosis was present in 5 cases, all of which were secondary IDCH.
Cytologic and immunohistochemical features seen in IDCH. (A) A skin biopsy with characteristic ETV3::NCOA2 fusion showing cytologic features of LCH. However, unlike LCH, admixed eosinophils are absent. (B-C) Skin and lymph node biopsies from the same patient with primary IDCH, which harbors TET2 and KRAS mutations. Variation in cytologic features even in the same patient is noted in different sites. (D) Skin biopsy from a patients with secondary IDCH, who has underlying chronic myelomonocytic leukemia (CMML), showing mostly similar features seen in panels A,C with a relatively higher nucleus-to-cytoplasm ratio. (E) Lymph node biopsy from 1 of the excluded patients who was detected to have a KMT2A::MLLT1 fusion showing somewhat similar morphologic features seen in panel D. (F) Skin biopsy of a patient with primary IDCH showing epidermotropism, which was occasionally present in IDCH. (G-J) Representative case with ETV3::NCO2 fusion: lesional cells are diffusely positive for (G) CD1a and (H) BDCA1/CD1c; whereas negative for (I) Langerin/CD207 and (J) CSF1R/CD115.
Cytologic and immunohistochemical features seen in IDCH. (A) A skin biopsy with characteristic ETV3::NCOA2 fusion showing cytologic features of LCH. However, unlike LCH, admixed eosinophils are absent. (B-C) Skin and lymph node biopsies from the same patient with primary IDCH, which harbors TET2 and KRAS mutations. Variation in cytologic features even in the same patient is noted in different sites. (D) Skin biopsy from a patients with secondary IDCH, who has underlying chronic myelomonocytic leukemia (CMML), showing mostly similar features seen in panels A,C with a relatively higher nucleus-to-cytoplasm ratio. (E) Lymph node biopsy from 1 of the excluded patients who was detected to have a KMT2A::MLLT1 fusion showing somewhat similar morphologic features seen in panel D. (F) Skin biopsy of a patient with primary IDCH showing epidermotropism, which was occasionally present in IDCH. (G-J) Representative case with ETV3::NCO2 fusion: lesional cells are diffusely positive for (G) CD1a and (H) BDCA1/CD1c; whereas negative for (I) Langerin/CD207 and (J) CSF1R/CD115.
In most of the skin biopsies, the neoplastic cells demonstrated diffuse infiltration within the superficial and deep dermis. The overlying epidermis was mostly unremarkable without epidermotropism. However, a few biopsies exhibited unusual features including a perivascular/periadnexal infiltration pattern, and focal epidermal ulceration or epidermotropism (Figure 1F).
Lymph node and tonsil biopsies were characterized by either an extensive diffuse infiltrate or multifocal small aggregates of lesional cells embedded in a lymphoid background. In 4 patients, concurrent lymphoma was present in the background of IDCH within the same specimen. Two patients presented with a colonic polyp, which showed a submucosal IDCH nodule with destructive infiltration of colonic crypt. One patient presented with solitary pulmonary nodule showed a solid appearing nodule with indistinct edges and clusters of lesional cells within the nodule.
Immunohistochemical findings are shown in Table 1, supplemental Table 1, and Figure 1G-J. All cases were positive for CD1a in 43 of 43 tested cases (inclusion criterion for study). CD4 was positive in 26 of 27 and BDCA1/CD1c in 16 of 17, whereas CD68 and CD163 were positive in only 12 of 20 and 5 of 26 cases, respectively. S100 protein was at least focally positive in 31 of 40 tested cases. Langerin/CD207 expression in lesional cells varied from 0% to up to 20%. The majority of the cases (n = 28) were essentially negative for Langerin/CD207. Fifteen cases showed restricted staining for Langerin/CD207 in a minor subset of lesional cells: 5 cases, ≤1% to <5%; 6 cases ≤5% to <10%, and 4 cases ≤10% to <20% staining. Interestingly 5 cases with clear epidermotropism on hematoxylin and eosin sections demonstrated Langerin/CD207 expression restricted to the intraepidermal component whereas Langerin/CD207 was largely negative in rest of the infiltrate in the dermis. Of 28 cases (including both primary and secondary IDCH) 20 were variably positive for CD56. In cases with extensive CD56 expression, CD123 and/or TCF4 stains were either negative or showed positive staining in small subset, excluding blastic plasmacytoid DC neoplasm. Of 29 tested cases, 22 (76%) were negative for CSF1R/CD115 expression.
Features of cases with partial Langerin/CD207 expression
Fifteen patients had partial Langerin/CD207 expression (≥1% staining in lesional cells; Table 1; supplemental Table 1; Figure 2). In comparison with rest of the cohort, this subgroup contained a slightly higher proportion of pediatric patients (3/15 vs 4/28, P = .6), a significantly higher proportion of concurrent mixed histiocytosis (6/15 vs 1/28; P = .002), and more frequent BRAF (BRAFV600E mutation or BRAF fusion) or MAP2K1 alterations (9/15 vs 5/28; P = .005) compared with the subgroup with absent Langerin expression. These features were present even in cases with minimal Langerin expression (≥1% to <5%) in which 4 of 5 cases had BRAF alterations.
Molecular findings
Results of molecular analysis are summarized in Table 1, supplemental Table 1, Figures 2 and 3. DNA was successfully amplified in 36 cases, of which putative oncogenic mutations were identified in 28 (78%), encompassing mutually exclusive cancer-associated KRAS and BRAF p.V600E mutations in 13 and 11 cases, respectively. However, 1 case, in which the patient had history of chronic lymphocytic leukemia, harbored KRAS mutation co-occurring with BRAF p.N581S mutation. Six cases had activating NRAS mutations, 2 of which co-occurred with activating cancer-associated KRAS mutations (namely p.K117N and p.A146T), 2 with BRAF p.V600E, 1 with activating BRAF p.F595L along with inactivating NF1-splice site 4577+1G>A mutations, and 1 with 2 inactivating NF1 mutations (NF1 p.R1276Q and NF1 p.Y2556∗). One case had 2 activating MAP2K1 mutations (MAP2K1 p.61_65del and MAP2K1 p.58_62del).
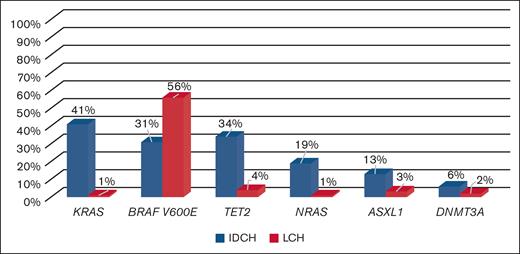
Comparison to LCH,24IDCH differs in its mutational profile. IDCH showed significant enrichment in KRAS and NRAS mutations, which are extremely rare in LCH. Although both diseases have frequent BRAFV600E mutations, IDCH appears to have concurrent mutations in myeloid neoplasm–associated genes such as TET2, DNMT3A, and ASXL1, unlike LCH.
Comparison to LCH,24IDCH differs in its mutational profile. IDCH showed significant enrichment in KRAS and NRAS mutations, which are extremely rare in LCH. Although both diseases have frequent BRAFV600E mutations, IDCH appears to have concurrent mutations in myeloid neoplasm–associated genes such as TET2, DNMT3A, and ASXL1, unlike LCH.
RNA was also successfully amplified for analysis in 34 cases. Ten of these cases (29%) demonstrated gene fusions including ETV3::NCOA2 in 6 cases, LMNA::NTRK1, TPD52::BRAF, and PIP4P1::BRAF in 1 case each as well as IGH::BCL2 in 1 follicular lymphoma-associated secondary IDCH case confirming a clonal relationship between IDCH and follicular lymphoma.
In addition to harboring well-characterized histiocytosis-driving alterations, the lesions exhibited concurrent mutations in genes commonly mutated in myeloid neoplasms including TET2, DNMT3A, and ASXL1 in 20 patients including 8 and 12 primary and secondary IDCH, respectively (supplemental Table 1; Figures 2 and 3). Presence of myeloid neoplasm-associated mutations were independent from the presence of an overt myeloid neoplasm and suggested the presence of underlying clonal hematopoiesis. The single case associated with follicular lymphoma harbored well-defined follicular lymphoma alterations such as TNFRSF14 mutation and BCL2 translocation without mutations in myeloid neoplasm-associated genes, suggesting a different evolutionary mechanism in follicular lymphoma–associated cases (supplemental Table 1; Figure 2). Four patients had paired NGS studies performed both for IDCH and associated nonhistiocytic hematopoietic neoplasm. All were detected to have shared common mutations between IDCH and associated nonhistiocytic hematopoietic neoplasm (patients 14, 16, 34, and 38 with chronic myelomonocytic leukemia, myelodysplastic syndrome/acute myeloid leukemia [AML], chronic myelomonocytic leukemia, and myelodysplastic syndrome, respectively).
Differences between primary and secondary IDCH cases
Secondary IDCH cases were older (median age 77 vs 64 years for primary IDCH). However, 1 pediatric case associated with T-cell acute lymphoblastic leukemia was also present in our cohort. Primary IDCH cases showed cutaneous involvement in 21 cases and lymph node involvement in 3 cases (isolated lymph node disease in 2 of them), and presented as a colonic polyp and lung nodule in 1 case each. Myeloid neoplasm–associated IDCH cases showed cutaneous involvement in 10 cases, lymph node involvement in 5 cases (isolated lymph node disease in 3 of them) and presented as a colonic polyp in 1 case. In contrast, none of the lymphoid neoplasm associated cases showed cutaneous involvement but isolated lymph node involvement was present in 4 cases and an isolated tonsil mass in 1 case. Although KRAS mutations were slightly more enriched in secondary IDCH group (9/18 cases) in comparison to BRAF alterations (7/18 cases), neither was exclusive for primary or secondary IDCH.
Clinical outcome
Clinical outcome data were available for 34 patients (20 primary IDCH and 14 secondary IDCH). The median duration of follow-up was 14.0 months (range, 0.2-280.0) with 14 deaths (4 primary IDCH and 10 secondary IDCH) in 43 patients and a death rate of 12.4 per 1000 patient-years, with a 72-month survival of 46% (95% confidence interval, 24.8-4.9). Of note, none of the pediatric patients with available follow-up data died of disease (supplemental Tables 1 and 2).
Primary IDCH cases who died of disease included 2 patients with mixed histiocytosis (IDCH/LCH and IDCH/LCH/ECD). All other primary patients with IDCH with known follow-up data were either alive or died of unrelated causes.
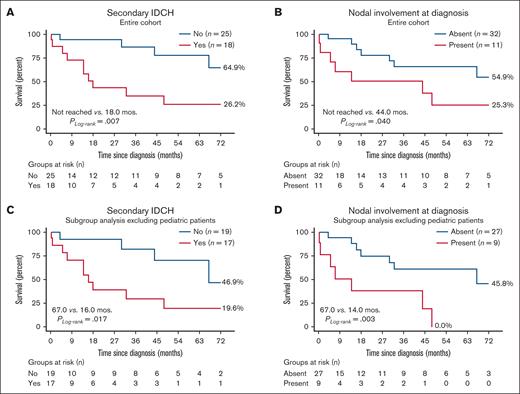
Age at diagnosis (hazard ratio [HR], 1.1 [1.0-1.1]; P = .021), and any nodal involvement at diagnosis (HR, 3.2 [1.1-9.5]; P = .036) predicted inferior overall survival (Figure 4). Although secondary IDCH also adversely affected survival, this was not relevant after adjusting for age at diagnosis (P = .051; Figure 4). Associated mixed histiocytosis (P = .15) and Langerin expression levels (<5% vs ≥5%) did not affect outcomes (HR, 2.0 [0.6-6.9]; P = .26). Although RAS alterations (KRAS or NRAS) were significantly associated with secondary IDCH (76.5% vs 22.7%; P < .001) and presence of concurrent myeloid gene alterations (58.8% vs 22.7%; P = .022), neither RAS nor BRAF alterations individually affected 72-month overall survival.
Impact of secondary IDCH and nodal involvement at diagnosis. Both (A) secondary IDCH and (B) nodal involvement at diagnosis were associated with inferior median and 72-month survival. (C-D) Subgroup analysis excluding the 7 pediatric patients aged <18 years showed similar findings. Separate analysis of pediatric patients was underpowered but there were no deaths in these patients during the analysis time of 72 months after excluding pediatric patients from the analysis.
Impact of secondary IDCH and nodal involvement at diagnosis. Both (A) secondary IDCH and (B) nodal involvement at diagnosis were associated with inferior median and 72-month survival. (C-D) Subgroup analysis excluding the 7 pediatric patients aged <18 years showed similar findings. Separate analysis of pediatric patients was underpowered but there were no deaths in these patients during the analysis time of 72 months after excluding pediatric patients from the analysis.
Discussion
This collaborative international study provides, to our knowledge, for the first time, comprehensive clinicopathologic and genomic analysis of IDCH from a well-characterized cohort of 43 patients. We show that IDCH is a clinically and genetically heterogeneous disease and only a minority of patients harbor an ETV3::NCOA2 fusion, contrary to what was previously suggested.18 IDCH, as defined by a CD1a+/Langerin−/+, differs from LCH in its clinical features and molecular profile. Notably, it is often a secondary event with other hematologic neoplasms of varied lineages: myeloid, lymphoid, and histiocytic. Additionally, the prognostic features of primary and secondary IDCH cases show important differences. These findings have critical implications for the diagnostic evaluation, classification, and therapeutic management of IDCH.
IDCH histologically resembles LCH in most cases but has been less well characterized in terms of its clinical features and molecular profile. A major question we chose to address in this study was the relationship between LCH and IDCH. Our study shows major differences from LCH. The molecular profile of IDCH showed significant enrichment in KRAS and NRAS mutations, which are extremely rare in LCH (Figure 3).20,22,24 Although both diseases harbor frequent BRAFV600E mutations, IDCH appears to have concurrent mutations in myeloid neoplasm–associated genes unlike LCH.24 Immunohistochemical studies showed CSF1R/CD115 expression in only a small subset of our patients with IDCH (24% in our cohort). In contrast, recent studies reported universal CSFR1/CD115 expression in LCH, a finding that may have relevance for the cell of origin of IDCH. Finally, the median age at diagnosis of IDCH is significantly higher than that of LCH (70 years for IDCH vs 6.2 years for LCH according to 2826 patients with LCH included in the French histiocytosis registry). However, the present retrospective series may have some bias of inclusion.
An unexpected finding was the striking association of concomitant nonhistiocytic hematopoietic neoplasms in patients with IDCH comprising both myeloid and lymphoid lesions (42% of all cases). This phenomenon has been reported occasionally in LCH25; however, it is much more common in IDCH as documented in our study as well as noted in a recent literature review.26 The phenomenon of “mixed histiocytosis” has been recently emphasized.22,23 Mixed histiocytosis also was found in 7 IDCH cases in our cohort, mainly LCH and ECD, confirming the previous proposal of possible relationship of these entities.27 Notably, in the mixed histiocytosis cases, IDCH was nearly always positive for the BRAFV600E mutation (6 of 7 cases) rather than KRAS or other point mutations and/or fusions, further suggesting a close relationship to LCH for this subset.
Another important question was whether cases with low expression of Langerin/CD207 can be considered as LCH. Current diagnostic criteria have never established the required threshold for expression of Langerin to justify a diagnosis of LCH, although 75% positivity was proposed in 1 study.28 Therefore, we included cases with minor degrees of Langerin/CD207 expression (<20%) in this study. The 15 cases with low levels of Langerin expression more often showed BRAF alterations (9/15), had a history of another histiocytosis (6/15), and were more likely to present in the pediatric age group (3/15). However, cases with partial Langerin expression were seen in both primary (36%) and secondary (33%) IDCH (Table 1). One can argue that cases with partial Langerin/CD207 expression (≥1%) may biologically be closer to mixed histiocytosis or LCH than true IDCH. In such cases, final diagnosis should be made based on clinical and molecular findings rather than relying on only Langerin expression.
Our findings highlighted genetic heterogeneity with common occurrence of MAPK pathway alterations along with myeloid neoplasm–associated mutations in IDCH. We observed substantial mutual exclusivity in kinase alterations across patients; however, a few cases with mutations in multiple kinases are noted but with disparate allele frequencies. Although it is difficult to make a definitive assessment given the paucity of accompanying bone marrow and absence of peripheral blood sequencing data, this is likely due to underlying clonal hematopoiesis or myeloid neoplasms in those cases.
Given the diverse genetic landscape, we also questioned whether any single genetic alteration is sufficient to delineate clinical subgroups. Although it is rare overall, the presence of ETV3::NCOA2 appears to define a specific clinicopathologic subgroup. These cases appear to be limited to the skin-limited form of IDCH without any Langerin/CD207 expression (<1%) and lack epidermotropism. So far, IDCH with ETV3::NCOA2 fusion had been reported exclusively in adults. However, our study identified 2 pediatric patients with ETV3::NCOA2 fusion, which were clinically similar to cases in adults. These cases lacked an association with other hematologic neoplasms and were negative for other genetic alterations.18,29,30 However, it should be noted that rare non-IDCH histiocytosis patients bearing the ETV3::NCOA2 rearrangement have been described,31 suggesting that this fusion is not restricted to IDCH but likely defines a unique subtype. Apart from the ETV3::NCOA2 fusion, no distinct molecular subgroup was identified.
The cell of origin of IDCH still remains to be determined. Recent reports suggested a similar immunophenotype between indeterminate cells and the cDC2 lineage.10 To explore this point, when possible, we examined our cases of IDCH for expression of CD1c and CSF1R/CD115. The vast majority of tested samples were CD1c+ and CSF1R/CD115−, similar to normal indeterminate cells and the cDC2 lineage. CSF1R is a receptor of the tyrosine kinase family and is known to play a major role in the biology of histiocytes.32 It is known that in humans, CSF1R/CD115 is highly expressed on monocytes, tissue-resident macrophages, normal LCs, DC progenitors, and monocyte-derived DCs, whereas cDCs (circulating and tissue) are negative for CSF1R/CD115 expression and are not dependent on CSF1R for their development.10,33 CD1c is also known to be positive in cDC2 lineage but is not completely restricted to cDC2 and may be expressed in monocyte-derived DCs.10 This combination of findings may suggest immunophenotypic similarities between IDCH and the cDC2 lineage.
We identified major different clinical scenarios between primary and secondary IDCH. Primary IDCH is an indolent disease. Indeed, disease-related deaths were seen only in 2 cases with mixed histiocytosis. In contrast, secondary IDCH cases generally had a more adverse prognosis, which was generally linked to the subtype of accompanying hematologic neoplasm. Cytologically most primary and secondary cases were similar. Also, the type of kinase alteration did not definitively predict the presence or nature of the underlying hematopoietic neoplasm. For example, both BRAF and KRAS mutations were found in the full spectrum of clinical subgroups including primary cases, myeloid neoplasm–associated cases, and lymphoid neoplasm–associated cases.
Lack of S100 protein expression has been described in several IDCH case reports34-36; however, the exact frequency is not known. In our series, nearly 23% of IDCH samples were negative. No significant differences in clinicopathologic features emerged between S100 protein–positive and S100 protein–negative cases. Altogether our experience confirms the notion that S100 protein expression does not exclude IDCH; however, the majority of cases show at least focal expression.
One patient in our cohort was excluded because of the presence of KMT2A::MLLT1 fusion, detected only in the lymph node biopsy, despite fulfilling histopathological inclusion criteria. Presence of KMT2A::MLLT1 fusion raised concern about myeloid sarcoma and/or AML. Bone marrow biopsy findings were consistent with involvement by IDCH and did not show convincing evidence of blast increase. This patient had aggressive clinical course with progressive multiorgan failure and died of disease within months. This is a currently relevant issue, because the International Consensus Classification and the classification by the World Health Organization 2022 criteria now consider KMT2A rearrangement to be AML defining for myeloid neoplasms with <20% blasts as opposed to the World Health Organization 2017 criteria. Yet, diagnostic criteria were not clarified for the cases mainly with extramedullary disease and revealing histopathological features of a histiocytic neoplasm as seen in our patient. Supported by this case, assessment of KMT2A rearrangement should be a part of histiocytosis workup and such cases are likely best classified as myeloid sarcoma regardless of histological features. However, further case studies are required to elucidate this finding.
Based on the above considerations, our recommendations for the diagnosis of IDCH can be summarized as follows: (1) histiocytic lesion with CD1a expression in substantial proportion of the lesional cells, (2) <1% Langerin/CD207 expression, (3) focal expression and even absence of S100 protein expression does not exclude IDCH, (4) presence or absence of CD163 and CD68 can be encountered, (5) demonstration of a corroborating genetic alteration because reactive DC hyperplasia may mimic IDCH, and (6) careful exclusion of myeloid sarcomas along with genetic studies because they may have overlapping histopathological features with histiocytic neoplasms. Lastly, the distinction of primary vs secondary IDCH is critical for clinical management, because the prognosis is generally related to the associated hematologic neoplasm.
Acknowledgment
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, and by grants from PRTK (Programme de Recherche Translationnelle en Cancérologie) 19-143.
Authorship
Contribution: N.O., E.S.J., and J.-F.E. conceptualized the study, curated data, performed the central pathology review, wrote the original draft, and submitted the manuscript for publication; S.M.B. performed central pathology review, and provided data; G.V. curated data and performed statistical analysis; L.J.K., S.F., J.R., S. Pittaluga, M.B., F.L.P., A.M., G.V., J.D., J.H., E.S.J., and J.-F.E. contributed patients; I.L., S. Pack, Z.H.-R., and M.R. performed molecular analyses; and all authors reviewed, edited, and approved the manuscript.
Conflict-of-interest disclosure: Z.H.R. and J.-F.E. received honoraria for speaker or advisory role from Qiagen. The remaining authors declare no competing financial interests. The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
The current affiliation for N.O. is BC Cancer Agency, Vancouver, BC, Canada.
Correspondence: Elaine S. Jaffe, Hematopathology Section, Laboratory of Pathology, National Institute of Health/National Cancer Institute, Bldg 10/Room 3S235, 10 Center Dr MSC-1500, Bethesda, MD 20892-1500; email: ejaffe@mail.nih.gov; and Jean-François Emile, EA4340 Laboratory and Department of Pathology, Ambroise Paré Hospital, 9 Avenue Charles de Gaulle, 92104 Boulogne, France; email: jean-francois.emile@uvsq.fr.
References
Author notes
E.S.J. and J.-F.E. contributed equally to this study.
The authors agree to sharing of genomic data in accordance with recommended standards. For the National Cancer Institute cases, data access requests can be made to the corresponding author, Elaine S. Jaffe (ejaffe@mail.nih.gov) at the Center for Cancer Research. For patients of the French registry, data are available upon reasonable request from the corresponding author, Jean-François Emile (jean-francois.emile@uvsq.fr).
The full-text version of this article contains a data supplement.