Key Points
Knockout of intestinal DMT1 in βTI mice caused a pathophysiological shift from iron overload to iron depletion with exacerbated anemia.
Oral gavage with nanoparticle-siRNA complexes daily for 16 days repressed intestinal DMT1 and mitigated iron loading in Hbbth3/+ mice.
Visual Abstract
β-thalassemia is an iron-loading anemia caused by homozygous mutation of the hemoglobin subunit β (HBB) gene. In β-thalassemia intermedia (βTI), a non–transfusion-dependent form of the disease, iron overload is caused by excessive absorption of dietary iron due to inappropriately low production of the iron-regulatory hormone hepcidin. Low hepcidin stabilizes the iron exporter ferroportin (FPN) on the basolateral membrane of enterocytes. High FPN activity may deplete intracellular iron and enhance expression of the predominant iron importer divalent metal-ion transporter 1 (DMT1). In mice, DMT1 mediates normal iron absorption under physiological conditions and excessive iron absorption in pathological iron overload (eg, hereditary hemochromatosis). Here, we hypothesized that DMT1 drives elevated iron absorption in βTI. Accordingly, we crossed Hbbth3/+ mice, a preclinical model of βTI, with intestine-specific DMT1-knockout mice. Ablation of intestinal DMT1 in Hbbth3/+ mice caused a pathophysiological shift from iron overload to an iron-deficiency phenotype with exacerbated anemia. DMT1 is thus required for iron absorption and iron loading in Hbbth3/+ mice. Based upon these outcomes, we further logically postulated that in vivo knockdown of intestinal DMT1 would mitigate iron loading in Hbbth3/+ mice. Ginger-derived, lipid nanoparticles carrying DMT1-specific (or control) small interfering RNAs (siRNAs) were administered by oral, intragastric gavage to 4-week-old Hbbth3/+ mice daily for 16 days. siRNA treatment reduced DMT1 expression by >80% and blunted iron loading, as indicated by significant reductions in liver iron and serum ferritin (which reflect body iron stores). These notable experimental outcomes establish intestinal DMT1 as a plausible therapeutic target to mitigate iron overload in βTI.
Introduction
Iron overload is a prominent complication of β-thalassemia major, arising from frequent blood transfusions and inappropriately elevated absorption of dietary iron.1 High intestinal iron transport is driven by erythron expansion and hepcidin suppression.2 In β-thalassemia intermedia (βTI), a less severe, non–transfusion-dependent form of the disease, iron overload is linked primarily to increased iron absorption. Excess tissue iron increases oxidative stress and elevates the risk for liver damage, cardiomyopathy, arthropathies, and endocrine dysfunction.3 Iron chelation remains the standard-of-care treatment to decrease body iron burden in patients with β-thalassemia4,5; however, adverse side effects and poor compliance have spurred the quest for new approaches to mitigate iron loading.6
The Hbbth3/+ (Th3/+) mouse, modeling βTI in humans, has been instrumental in deciphering the pathophysiology of this disorder.7,8 Th3/+ mice have heterozygous ablation of the major and minor β-globin genes and display parenchymal tissue iron loading and severe anemia.8 Liver Hamp (encoding hepcidin) expression is inappropriately low in adolescent Th3/+ mice given their iron status; low hepcidin would be predicted to increase iron absorption and precipitate iron loading. Hepcidin production (and presumably iron absorption), however, then normalizes to wild-type (WT) levels in adult Th3/+ mice.7,9,10
Intestinal divalent metal-ion transporter 1 (DMT1) is required for iron absorption under physiological conditions,11 and for iron loading in hepcidin (Hamp)-knockout (KO) mice (modeling hemochromatosis).12 Also, peroral administration of ginger-derived lipid nanoparticles (NPs) carrying functional DMT1 small interfering RNAs (siRNAs) effectively reduced iron absorption and lessened iron loading in Hamp KO mice.12,13 Here, we tested the hypothesis that DMT1 also mediates the excessive iron absorption that typifies the Th3/+ mouse model, and, if so, we logically predicted that blunting intestinal DMT1 expression will mitigate iron loading. Two complementary experimental approaches were used: in experiment 1, iron homeostasis was examined in Th3/+ mice lacking intestinal DMT1; and in experiment 2, iron loading was assessed in adolescent Th3/+ mice treated with NPs-DMT1 siRNA complexes by gavage for 16 consecutive days. Outcomes revealed a key role for DMT1 in iron loading in Th3/+ mice, implicating intestinal DMT1 as a potential adjunctive, therapeutic target in murine, and, by extension, possibly human βTI.
Materials and methods
Animals and experimental design
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee (UF IACUC). Mice were fed a semipurified, AIN-93–based adequate-iron diet with 50 ppm iron throughout all experiments (TD.130018; Envigo). WT (+/+) females were bred with Hbbth3/+ (Th3/+) males (C57BL/6) to generate control and experimental mice of both sexes (and genotypes). To produce thalassemic mice lacking intestinal DMT1, Hbbth3/+ mice were crossed with Dmt1int/+/villinCre+ (C57BL/6) mice.11,14 Littermate male and female mice of 4 genotypes were studied:
Hbb+/+/Dmt1+/+ (phenotypically normal)
Hbbth3/+/Dmt1+/+ (thalassemic with normal intestinal DMT1 function)
Hbbth3/+/Dmt1int/+ (thalassemic with normal intestinal DMT1 function)
Hbbth3/+/Dmt1int/int (thalassemic lacking intestinal DMT1 function)
Production of FA-GDLVs
Ginger-derived NPs were isolated and purified as described previously.15 Ginger NP lipids were then extracted16 and processed to produce folic acid–coupled ginger NP-derived lipid vectors (FA-GDLVs).13,15 DMT1 (3.75 nmol; catalog no. 4457310, Ambion) or negative-control (catalog no. 4390844, Ambion) siRNAs were dissolved in 300 μL of a sterile 5% glucose solution and mixed gently. Turbofect reagent (Life Technology) was then added (6 μL), and the sample was mixed and incubated for 15 minutes at room temperature. siRNAs were incorporated into the FA-GDLVs by sonication for 5 minutes, and the mixture was then used for in vivo gavage studies within 48 hours of production.
In vivo knockdown of intestinal DMT1
Four-week-old Th3/+ mice of both sexes were fasted for 2 hours and then gavaged with saline, FA-GDLVs, or FA-GDLVs loaded with DMT1-specific or negative-control siRNAs (3.75 nmol per dose) daily for 16 days. Mice were given food immediately thereafter. Fecal samples were collected every third day and freshly frozen at −80°C. Subsequently, mice were euthanized, and blood was collected and processed for complete blood count analysis and reticulocyte counting. A duodenal mucosal scrape was taken for protein isolation. The liver, spleen, kidney, heart, and pancreas were frozen in liquid nitrogen and stored at −80°C. Bone marrow was flushed from the femur for messenger RNA (mRNA) isolation. Enzyme-linked immunosorbent assays were used to quantify serum ferritin (ab157713, Abcam), erythropoietin (EPO; MEP00B, R&D Systems), hepcidin (HMC-001, Intrinsic Lifesciences), C-reactive protein (MCRP00, R&D Systems), and interleukin-6 (IL-6; M6000B, R&D Systems). Serum and tissue nonheme iron (NHI) concentrations and total iron-binding capacity (TIBC) were determined using a standard colorimetric method.12,17,18 Percent transferrin saturation (TSAT) was calculated as serum iron/TIBC × 100.
Intestinal iron (59Fe) absorption and distribution experiments
Mice were fasted for 2 hours and then gavaged with a transport solution containing 2.5 μCi of 59Fe-HCl (Perkin Elmer) diluted into 0.2 mL of phosphate-buffered saline containing 0.5 M ascorbate, 0.15 M NaCl, and 5 mg of FeSO4.19 Food was provided immediately thereafter, and mice were euthanized 24 hours later. The 24-hour time point was selected to ensure full gastric emptying and allow complete passage of the test dose through the entire intestinal tract (transit time in mice is ∼11 hours),20 and also to capture iron absorbed during the slow, or lag phase that occurs ≥12 hours after iron is consumed.21,22 This approach thus allowed us to quantify the total amount of iron absorbed (as percent of dose). The total amount of 59Fe in the animals after 24 hours should represent total iron absorption because iron excretion is very low in mice, equating to ∼0.5% of a test dose of radioactive iron administered per day.23-25 Significant loss of already absorbed iron was thus not a major concern within the timeframe of this experiment. Blood samples were collected, and the stomach and small and large intestines were removed. 59Fe activity was measured in the blood, small and large intestine, and carcass using a γ counter (2480 Wizard2; Perkin Elmer). Other organs were then removed and 59Fe activity was measured. Intestinal iron absorption was calculated as: ([radioactivity in the carcass minus radioactivity in the gastrointestinal tract divided by total radioactivity in the oral gavage test dose] × 100).
Fecal lipocalin-2 assay
Frozen fecal samples were reconstituted in phosphate-buffered saline containing 0.1% Tween 20, homogenized in a bullet blender for 20 minutes at setting 10, and then centrifuged for 10 minutes at 12 000g at 4°C. Cleared supernatants were collected and stored at −20°C. Fecal lipocalin-2 levels were determined by enzyme-linked immunosorbent assay (DY1857, R&D Systems) and expressed as ng/g feces.
Quantitative reverse transcription polymerase chain reaction and western blot analysis
mRNA isolation and SYBR-Green quantitative reverse transcription polymerase chain reaction was performed as described previously.26,27 Protein was isolated from duodenal scrapes and the liver and spleen using a membrane protein extraction kit (catalog no. 89842, Thermo Fisher Scientific). Proteins (20 μg) were separated in 10% polyacrylamide gels, then transferred to Polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in Odyssey blocking buffer (Licor), and then incubated with the following antibodies: DMT1 (1:2000; kindly provided by François Canonne-Hergaux); ferroportin 1 (FPN1; 1:2000; catalog no. MTP11-A; Alpha Diagnostics), ferritin heavy chain (FTH; 1:2000; catalog no. ab75973; Abcam), and β-actin (1:50 000; catalog no. 66009-1-lg, Proteintech). Blots were then incubated with secondary antibodies (1:10 000) (catalog no. 925-32213 or 926-68072; Licor), and then imaged using a Licor Odyssey CLx instrument (model 9141-02V). Experimental protein band intensities were normalized to β-actin band intensities.
Statistical analyses
Data were analyzed by analysis of variance (GraphPad Prism, version 8.0). Departures from normal distribution were detected using the D’Agostino and Pearson goodness-of-fit test. The Brown-Forsythe test or Levene test was used to check for equal variance. Some data were log transformed before statistical analyses. P <.05 was considered statistically significant.
Results
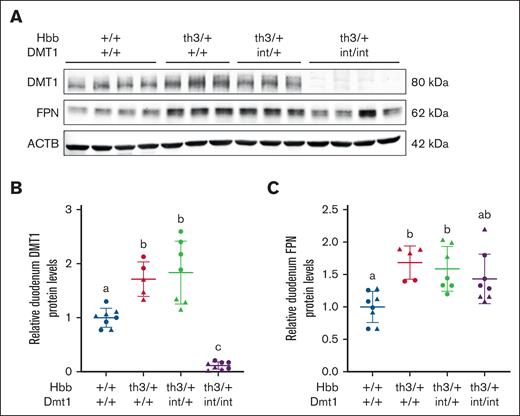
Intestinal DMT1 and FPN protein expression increased in Th3/+ mice
To confirm intestinal DMT1 ablation and determine whether expression of intestinal iron transporters reflects the magnitude of iron absorption, western blot experiments were undertaken using previously validated antibodies.18 Intestinal DMT1 and FPN protein levels increased by ∼60% to 75% in 6-week-old thalassemic mice (Figure 1), which is consistent with elevated iron absorption, and iron loading.7 In Hbbth3/+ / Dmt1int/int mice, DMT1 expression was reduced by >95% but FPN protein levels were unaltered. These data confirm the almost total loss of DMT1 protein and show that lack of intestinal DMT1 activity did not influence FPN protein levels.
Intestinal DMT1 and FPN expression is elevated in Th3/+ mice. Duodenal DMT1 and FPN protein expression was assessed in male (triangles) and female (circles) littermates at 6 weeks of age. A representative western blot for DMT1, FPN, and ACTB is shown (A), and quantitative data from all experiments are also shown (B-C). Data are mean ± standard deviation (SD) for n = 5 to 8 mice per group and were analyzed by 1-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Genotype main effects: P < .0001 for panel B; P < .01 for panel C. ACTB, beta actin.
Intestinal DMT1 and FPN expression is elevated in Th3/+ mice. Duodenal DMT1 and FPN protein expression was assessed in male (triangles) and female (circles) littermates at 6 weeks of age. A representative western blot for DMT1, FPN, and ACTB is shown (A), and quantitative data from all experiments are also shown (B-C). Data are mean ± standard deviation (SD) for n = 5 to 8 mice per group and were analyzed by 1-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Genotype main effects: P < .0001 for panel B; P < .01 for panel C. ACTB, beta actin.
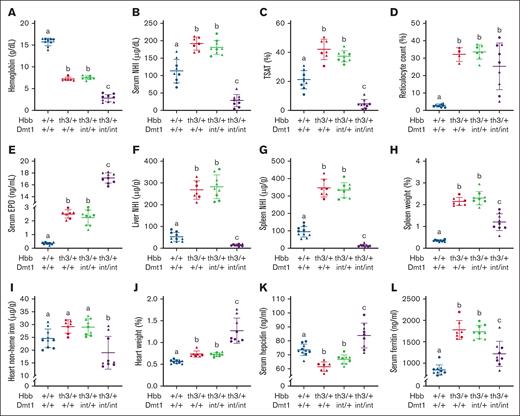
Ablation of intestinal DMT1 in Th3/+ mice caused iron depletion with exacerbated anemia
To assess how intestinal DMT1 contributes to iron loading in βTI, comprehensive studies were undertaken in WT mice and in Th3/+ mice with and without functional DMT1. Six-week-old Th3/+ / Dmt1int/int mice weighed less than all other groups at euthanasia (supplemental Figure 1A). Spleen weights were >12-fold higher in thalassemic mice with normal DMT1 function but only approximately threefold higher in Th3/+ mice lacking intestinal DMT1 (Figure 2H). Heart weight was ∼30% higher in Th3/+ mice, and ∼230% higher in Th3/+ mice lacking DMT1 (Figure 2J). Liver and kidney weights were similar between experimental groups (supplemental Figure 1B-C). Impaired growth, splenomegaly, and cardiac hypertrophy more broadly typify the severe anemia that was noted in thalassemic mice (as detailed below).28-30 Splenomegaly is likely the result of expansion of the red pulp due to activation of stress erythropoiesis.31 How ablation of intestinal DMT1 partially mitigated the splenomegaly in Th3/+ mice despite exacerbating the anemia is unclear, but it could relate to a reversal from splenic iron loading to splenic iron depletion (Figure 2G), which would presumably reduce oxidative stress associated with excess iron. The noted cardiomegaly in thalassemic mice lacking intestinal DMT1 likely resulted from severe anemia/hypoxia,28 but cardiac iron depletion (Figure 2I) could also have contributed. Notably, Dmt1int/int mice with severe iron-deficiency anemia (IDA) also displayed these same physiological derangements,11 raising the possibility that these outcomes related specifically to impaired intestinal iron transport and not thalassemia per se.
Lack of intestinal DMT1 causes iron depletion and exacerbates the anemia in Th3/+ mice. Tissue NHI levels, hematologic biomarkers, and serum hepcidin and serum EPO were assessed in male (triangles) and female (circles) littermates at 6 weeks of age. Shown are Hb (A), serum NHI (B), TSAT (C), retic count (D), serum EPO (E), liver NHI (F), spleen NHI (G), spleen weight (H), heart NHI (I), heart weight (J), serum hepcidin (K), and serum ferritin (L). Data are mean ± SD for n = 7 to 10 mice per group and were analyzed by 1-way ANOVA followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Genotype main effects: P < .0001 for panels A,C,E-J; P < .001 for panels B,L; P < .01 for panels D,K.
Lack of intestinal DMT1 causes iron depletion and exacerbates the anemia in Th3/+ mice. Tissue NHI levels, hematologic biomarkers, and serum hepcidin and serum EPO were assessed in male (triangles) and female (circles) littermates at 6 weeks of age. Shown are Hb (A), serum NHI (B), TSAT (C), retic count (D), serum EPO (E), liver NHI (F), spleen NHI (G), spleen weight (H), heart NHI (I), heart weight (J), serum hepcidin (K), and serum ferritin (L). Data are mean ± SD for n = 7 to 10 mice per group and were analyzed by 1-way ANOVA followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Genotype main effects: P < .0001 for panels A,C,E-J; P < .001 for panels B,L; P < .01 for panels D,K.
Anemia was evident in βTI mice, as expected, with lower hemoglobin (Hb) in Th3/+ mice (7.4 g/dL) than in normal controls (15.7 g/dL; Figure 2A). Hb levels were even lower in Th3/+ mice lacking DMT1 (2.8 g/dL). A similar pattern, with highest values in normal controls, intermediate values in thalassemic mice, and lower values in Th3/+ mice with ablated DMT1, was also seen for red blood cell counts, hematocrit, mean corpuscular volume, and mean corpuscular Hb (Table 1). Mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW) and hemoglobin distribution width (HDW) values (Table 1), and reticulocyte counts (Figure 2D), were different in controls as compared with the other 3 groups (which did not vary). Serum EPO levels increased approximately sevenfold in Th3/+ mice, and >48-fold in DMT1 KO Th3/+ mice (Figure 2E). Lack of intestinal DMT1 thus exacerbated the anemia in thalassemic mice to levels congruent with those observed in nonthalassemic mice lacking intestinal DMT.11,14 The anemia was worsened most likely because of iron restriction caused by impaired intestinal iron transport, depletion of storage iron (Figure 2F-G), and hypoferremia (Figure 2B).
Hematologic parameters of experimental mice
| Genotype . | RBCs (106/μL) . | Hct (%) . | MCV (fL) . | Mean cell Hb (pg) . | Mean cell Hb concentration (g/dL) . | Hb distribution width (g/dL) . | Red cell distribution width (%) . |
|---|---|---|---|---|---|---|---|
| +/+ Dmt1+/+ | 10 ± 0.4a | 55 ± 2a | 54 ± 0.7a | 16 ± 1a | 29 ± 12a | 2 ± 0.1a | 15 ± 1a |
| Th3/+ Dmt1+/+ | 7 ± 0.4b | 31 ± 1b | 42 ± 2b | 10 ± 1b | 23 ± 0.6b | 5 ± 0.2b | 38 ± 1b |
| Th3/+ Dmt1int/+ | 7 ± 1b | 32 ± 3b | 44 ± 3b | 10 ± 1b | 23 ± 1b | 4 ± 0.5b | 37 ± 1b |
| Th3/+ Dmt1int/int | 4 ± 1c | 13 ± 4c | 34 ± 1c | 7 ± 1c | 21 ± 1b | 5 ± 0.3b | 39 ± 4b |
| P value∗ | <.01 | <.01 | <.01 | <.01 | <.01 | <.01 | <.01 |
| Genotype . | RBCs (106/μL) . | Hct (%) . | MCV (fL) . | Mean cell Hb (pg) . | Mean cell Hb concentration (g/dL) . | Hb distribution width (g/dL) . | Red cell distribution width (%) . |
|---|---|---|---|---|---|---|---|
| +/+ Dmt1+/+ | 10 ± 0.4a | 55 ± 2a | 54 ± 0.7a | 16 ± 1a | 29 ± 12a | 2 ± 0.1a | 15 ± 1a |
| Th3/+ Dmt1+/+ | 7 ± 0.4b | 31 ± 1b | 42 ± 2b | 10 ± 1b | 23 ± 0.6b | 5 ± 0.2b | 38 ± 1b |
| Th3/+ Dmt1int/+ | 7 ± 1b | 32 ± 3b | 44 ± 3b | 10 ± 1b | 23 ± 1b | 4 ± 0.5b | 37 ± 1b |
| Th3/+ Dmt1int/int | 4 ± 1c | 13 ± 4c | 34 ± 1c | 7 ± 1c | 21 ± 1b | 5 ± 0.3b | 39 ± 4b |
| P value∗ | <.01 | <.01 | <.01 | <.01 | <.01 | <.01 | <.01 |
Hct, hematocrit; MCV, mean corpuscular volume; RBC, red blood cell.
Statistical analysis by 1-way analysis of variance. P values indicate significance of the genotype main effect for data in that column.
Serum hepcidin levels were lower in Th3/+ mice but increased in Th3/+ mice lacking intestinal DMT1 (Figure 2K). Because the Hamp gene is transcriptionally regulated,32 we also quantified hepcidin mRNA expression. Liver hepcidin transcript levels reflected circulating hepcidin levels in 3 groups of experimental mice, but unexpectedly, hepatic Hamp was repressed in thalassemic mice lacking intestinal DMT1 (which had the highest serum hepcidin levels; supplemental Figure 1D). Very low mRNA levels but higher circulating protein levels could result from a defect in renal hepcidin clearance. Renal iron depletion (supplemental Figure 1E) with concurrent hypoxia may have impaired kidney function in Th3/+/Dmt1int/int mice, reducing urinary hepcidin excretion and causing a paradoxical increase in circulating hepcidin levels.33 This is consistent with the reduced renal function previously noted in Th3/+ mice.34
Serum (Figure 2B), liver (Figure 2F), spleen (Figure 2G), and kidney (supplemental Figure 1E) NHI levels, and TSAT (Figure 2C), were elevated in Th3/+ mice with normal DMT1 levels but significantly reduced in Th3/+ mice lacking intestinal DMT1. NHI in the heart was lower by ∼25% (Figure 2I), and duodenum NHI iron was reduced by approximately two-thirds (supplemental Figure 1F), whereas TIBC was elevated (by ∼25%) in Th3/+ mice lacking intestinal DMT1 (supplemental Figure 1G). Furthermore, serum ferritin values were higher in all 3 groups of thalassemic mice (compared with controls), but the elevation was significantly lower in thalassemic mice lacking intestinal DMT1 (Figure 2L). And finally, 2 inflammatory biomarkers, C-reactive protein, and IL-6, were found to be low and mainly invariant in the serum of all mouse groups (supplemental Figure 1H-I), demonstrating that observed changes in iron homeostasis did not relate to systemic inflammation.
Collectively, these observations demonstrate that ablation of intestinal Dmt1 in Th3/+ mice shifted the pathophysiological phenotype from iron overload with anemia to iron depletion with exacerbated anemia due to iron restriction. Intestinal DMT1 is thus required for iron absorption and iron loading in βTI mice.
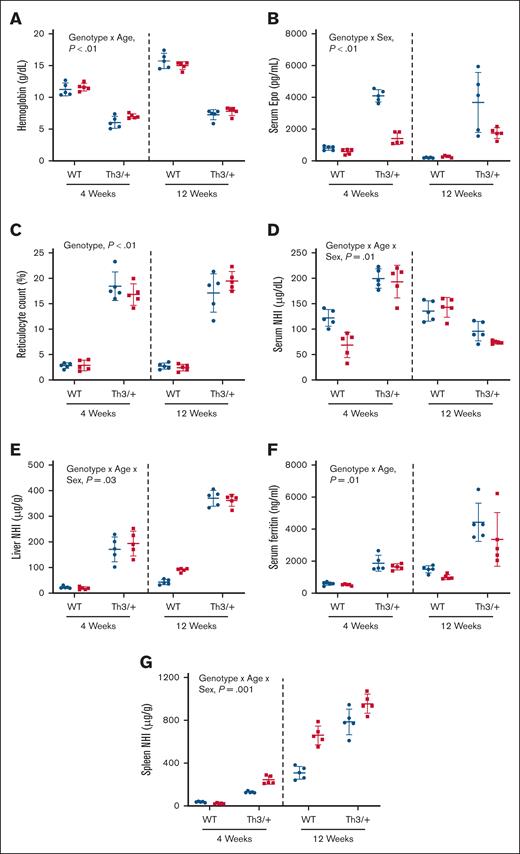
Th3/+ mice were anemic, and iron loaded, by 4 weeks of age
To determine the relative chronology of iron loading in Th3/+ mice, we assessed the iron-related phenotype of weanling (4 weeks) and adult (12 weeks) mice. The spleens and hearts were enlarged (supplemental Table 1), whereas Hb levels were lower in Th3/+ mice at both ages (Figure 3A). Notably, WT mice showed an age-related rise in Hb levels of >4 g/dL (11-15.4 g/dL) from 4 to 12 weeks of age, whereas the increase in Th3/+ mice was ∼1.0 g/dL between age groups. Hematocrit levels were also lower in both sexes of Th3/+ mice at both ages (supplemental Table 1). Consistent with the anemia, serum EPO levels increased in male and female Th3/+ mice at both ages, relative to controls, with higher levels seen in males (Figure 3B). Also, reticulocyte counts were higher in male and female Th3/+ mice at both ages (∼18%), as compared to controls (∼3%; Figure 3C). Both sexes of 4-week-old Th3/+ mice had higher serum NHI levels (∼197 μg/dL), as compared to control males (∼122 μg/dL) and females (∼68 μg/dL; Figure 3D). At 12 weeks, however, serum NHI was lower in thalassemic mice (∼85 μg/dL) as compared to controls (∼140 μg/dL; Figure 3D). Consistent with serum NHI levels, TSAT was elevated in young Th3/+ mice but decreased in older thalassemic mice (supplemental Table 1). Spleen and kidney NHI content was elevated in both sexes of Th3/+ mice at both ages (supplemental Table 1). Moreover, liver NHI levels increased as Th3/+ mice aged, rising from ∼180 μg/g at 4 weeks to ∼370 μg/g at 12 weeks (Figure 3E). Consistent with hepatic iron loading, serum ferritin was elevated in Th3/+ mice at both ages, with values being higher in adult mice, indicating more severe iron overload (Figure 3F). Spleen iron loading was also apparent in Th3/+ mice at both ages (Figure 3G). Collectively, these data demonstrate that the iron-overload, anemic phenotype of Th3/+ mice had emerged by 4 weeks of age.
βTI mice are anemic and iron loaded by 4 weeks of age. Shown are blood Hb (A) levels, serum EPO (B), reticulocyte count (C), serum NHI (D), liver NHI (E), serum ferritin (F), and spleen NHI levels (G) in 4- and 12-week-old male (blue circles) and female (red squares) WT and Th3/+ mice. Data are presented as mean ± SD for n = 5 mice per group and were analyzed by 3-way ANOVA. Genotype, age, and sex interactions and main effect P values are shown in each panel.
βTI mice are anemic and iron loaded by 4 weeks of age. Shown are blood Hb (A) levels, serum EPO (B), reticulocyte count (C), serum NHI (D), liver NHI (E), serum ferritin (F), and spleen NHI levels (G) in 4- and 12-week-old male (blue circles) and female (red squares) WT and Th3/+ mice. Data are presented as mean ± SD for n = 5 mice per group and were analyzed by 3-way ANOVA. Genotype, age, and sex interactions and main effect P values are shown in each panel.
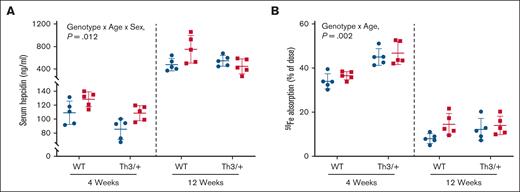
Iron (59Fe) absorption is elevated in weanling Th3/+ mice
Next, we sought to determine whether iron loading in Th3/+ mice related to suppression of hepcidin production and elevated iron absorption. Knowledge of when iron absorption is elevated informed our intervention approach with DMT1 siRNA (as outlined below). Circulating hepcidin levels were lower in 4-week-old mice, as compared with adults (Figure 4A). Serum hepcidin was lower in both sexes of 4-week-old Th3/+ mice, compared with controls, whereas in 12-week-old mice, hepcidin levels were essentially invariant in males but lower in Th3/+ females. Consistent with low hepcidin, intestinal iron absorption was elevated in young thalassemic mice (∼46% of dose) as compared with controls (∼35% of dose; Figure 4B). In adult mice, serum hepcidin was higher and iron absorption was lower (12%-15% of dose), but differences between genotypes were less significant as compared to 4-week-old mice (Figure 4A-B). Moreover, the percentage of absorbed 59Fe distributed to the liver was higher in young Th3/+ mice but lower or invariant in adults, whereas 59Fe distributed to the spleen was markedly higher in thalassemic mice at both ages (supplemental Table 2). 59Fe distributed to the kidney was lower in Th3/+ mice at both ages, but the magnitude of changes was larger in 4-week-old mice (supplemental Table 2). Collectively, these data (and those discussed above) suggest that inappropriately elevated iron absorption precipitates iron loading in 4-week-old Th3/+ mice, and that iron overload persists (or worsens) into adulthood despite a relative normalization of hepcidin expression and iron absorption at this life stage.
Circulating hepcidin is lower, and intestinal iron absorption is higher in 4-week-old Th3/+ mice. Serum hepcidin (A) and intestinal iron absorption (B) were quantified in 4- and 12-week-old male (blue circles) and female (red squares) WT and Th3/+ mice. Data are mean ± SD for n = 5 mice per group and were analyzed by 3-way ANOVA. Genotype, age, and sex interaction P values are shown in each panel.
Circulating hepcidin is lower, and intestinal iron absorption is higher in 4-week-old Th3/+ mice. Serum hepcidin (A) and intestinal iron absorption (B) were quantified in 4- and 12-week-old male (blue circles) and female (red squares) WT and Th3/+ mice. Data are mean ± SD for n = 5 mice per group and were analyzed by 3-way ANOVA. Genotype, age, and sex interaction P values are shown in each panel.
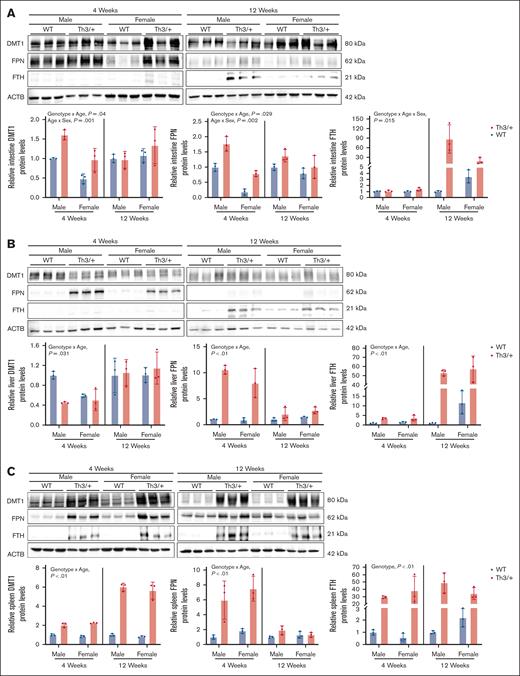
Variable expression of iron transporters underlies altered iron flux in Th3/+ mice
Our next goal was to test the hypothesis that altered expression of iron transporters underlies perturbations in iron absorption and distribution in Th3/+ mice. Accordingly, western blotting experiments were undertaken using protein samples isolated from key tissues involved in iron flux/storage and experimentally validated antibodies. Outcomes showed that expression profiles of DMT1 and FPN mirrored the observed patterns of intestinal iron absorption. For example, at 4 weeks of age, Th3/+ mice expressed higher levels of DMT1 and FPN proteins in the duodenal mucosa (as compared with controls; Figure 5A), which is consistent with elevated iron absorption in 4-week-old thalassemic mice (Figure 4B). In adult mice, the relative increase in intestinal DMT1 and FPN protein expression was lesser than in adolescent mice (Figure 5A), which is also congruent with smaller increases in iron absorption in adult Th3/+ mice (Figure 4B). FTH expression was also elevated in the duodenum of 12-week-old mice (Figure 5A), which coincides with lower iron absorption in adults.
Variations in the expression of iron transporters correlates with perturbations in iron flux in Th3/+ mice. Expression of DMT1, FPN, and FTH was quantified in duodenal epithelial tissues (A) and in the liver (B) and spleen (C) of 4- and 12-week-old male and female WT (blue bars) and Th3/+ (red bars) mice. Data are presented as mean ± SD for n = 3 mice per group and were analyzed by 3-way ANOVA. P values in each panel indicate the significance of genotype, age, and sex interactions and main effects.
Variations in the expression of iron transporters correlates with perturbations in iron flux in Th3/+ mice. Expression of DMT1, FPN, and FTH was quantified in duodenal epithelial tissues (A) and in the liver (B) and spleen (C) of 4- and 12-week-old male and female WT (blue bars) and Th3/+ (red bars) mice. Data are presented as mean ± SD for n = 3 mice per group and were analyzed by 3-way ANOVA. P values in each panel indicate the significance of genotype, age, and sex interactions and main effects.
In the liver, DMT1 protein expression varied little when comparing WT and Th3/+ mice at both ages. FPN protein expression, however, was elevated in Th3/+ mice at both ages, with a particularly notable increase in 4-week-old mice (Figure 5B). High FPN expression is consistent with iron mobilization from hepatocytes and Kupffer cells to support enhanced erythropoiesis due to severe anemia. Moreover, liver FTH expression was higher in 12-week-old Th3/+ mice, as compared with WT controls (Figure 5B), paralleling the elevated hepatic NHI content of these mice (Figure 3D).
In the spleen, DMT1 protein expression was elevated in Th3/+ mice at both ages (Figure 5C). High splenic DMT1 levels may relate to stress erythropoiesis, which occurs during thalassemia.35,36 Developing splenic erythrocytes acquire iron via the transferrin cycle, which requires endosomal DMT1 for iron release into the cytosol and cellular use for heme synthesis.37,38 Splenic FPN expression was higher in 4-week-old Th3/+ mice, but FPN levels were invariant in adult mice of both genotypes (Figure 5C). High FPN expression in 4-week-old mice likely relates to mobilization of iron stores from splenic macrophages, which is required to meet the increased iron demand necessary to support rapid growth. Furthermore, spleen FTH levels were elevated in Th3/+ mice across both age groups (Figure 5C), consistent with spleen iron accumulation.
Altered expression of iron transporters, and the iron-storage protein ferritin, thus underlies perturbations in iron absorption and distribution that occur in thalassemic mice.
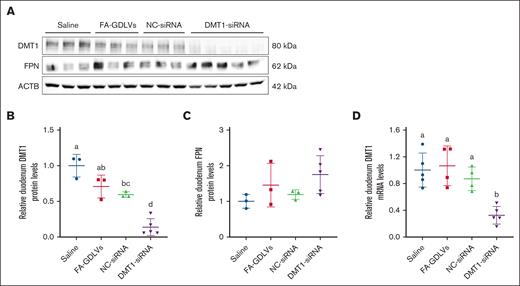
In vivo siRNA treatment decreased intestinal DMT1 expression in Th3/+ mice
Mice were treated with FA-GDLV–siRNA complexes daily for 16 days, and then DMT1 expression was assessed. Intestinal DMT1 mRNA and protein expression was significantly blunted in mice treated with DMT1 siRNA (∼90% reduction), as compared with all control groups (Figure 6A-B). As expected, intestinal FPN expression was unaffected by DMT1 siRNA exposure (Figure 6C). These data validated the experimental approach and provided the opportunity to directly test the hypothesis that knockdown of intestinal DMT1 will mitigate iron loading in Th3/+ mice.
FA-GDLV–mediated oral delivery of DMT1 siRNA decreased intestinal DMT1 protein levels in Th3/+ mice. Expression of intestinal NHI transporters was assessed in 6-week-old male Th3/+ mice that had been treated with saline, empty NPs (FA-GDLVs), or with FA-GDLVs carrying negative-control (NC), or DMT1 siRNA daily for 16 days. Western blots (A) and quantitative data (B-C) are shown. For comparison, duodenal DMT1 mRNA levels are also shown (D). Data are mean ± SD for n = 3 to 5 mice per group and were analyzed by 1-way ANOVA followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Treatment main effect: P < .001 for panels B,D.
FA-GDLV–mediated oral delivery of DMT1 siRNA decreased intestinal DMT1 protein levels in Th3/+ mice. Expression of intestinal NHI transporters was assessed in 6-week-old male Th3/+ mice that had been treated with saline, empty NPs (FA-GDLVs), or with FA-GDLVs carrying negative-control (NC), or DMT1 siRNA daily for 16 days. Western blots (A) and quantitative data (B-C) are shown. For comparison, duodenal DMT1 mRNA levels are also shown (D). Data are mean ± SD for n = 3 to 5 mice per group and were analyzed by 1-way ANOVA followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Treatment main effect: P < .001 for panels B,D.
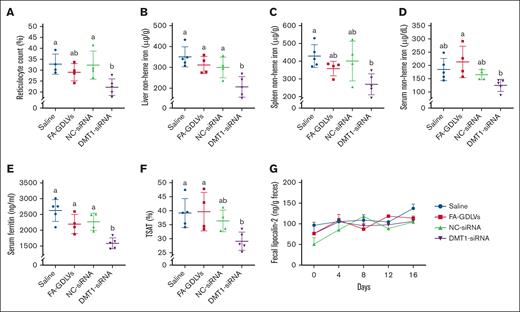
DMT1 siRNA treatment partially mitigated iron loading in Th3/+ mice
Biomarkers of erythropoietic stress and iron status were assessed after the NP-siRNA treatment regimen. Data from the DMT1 siRNA group was lower than the other groups for all tested parameters, but, in most cases, at least 1 other experimental group also had similar outcomes, including reticulocyte counts (Figure 7A), spleen NHI (Figure 7C), serum NHI (Figure 7D), and TSAT (Figure 7F). It was thus not possible to definitively state that in vivo knockdown of intestinal DMT1 reduced these parameters. However, DMT1 knockdown significantly reduced liver NHI levels by ∼30% to 35% and serum ferritin by ∼40%, as compared with all other experimental groups. Fecal lipocalin-2, a biomarker of intestinal inflammation (Figure 7G), serum IL-6 (a biomarker of systemic inflammation; data not shown), and Hb, hematocrit, mean corpuscular volume, serum hepcidin, and spleen and bone marrow erythroferrone mRNA expression (supplemental Figure 2) were invariable among groups at the end of the experimental period. Collectively, these observations demonstrate that blunting iron import via intestinal DMT1 reduces body iron burden in thalassemic mice.
In vivo knockdown of intestinal DMT1 reduced reticulocyte counts and mitigated iron loading in Th3/+ mice. Reticulocyte counts (A), liver NHI (B), spleen NHI (C), serum NHI (D), serum ferritin (E), TSAT (F), and fecal lipocalin-2 levels (G) were quantified in 6-week-old male Th3/+ mice that had been treated with saline, empty NPs (FA-GDLVs), or with FA-GDLVs carrying NC, or DMT1 siRNA daily for 16 days. Data are presented as mean ± SD for n = 4 to 5 mice per group and were analyzed by 1-way ANOVA followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Treatment main effects: P < .001 for panel E; P < .01 for panel B; P < .05 for panels A,C-D,F.
In vivo knockdown of intestinal DMT1 reduced reticulocyte counts and mitigated iron loading in Th3/+ mice. Reticulocyte counts (A), liver NHI (B), spleen NHI (C), serum NHI (D), serum ferritin (E), TSAT (F), and fecal lipocalin-2 levels (G) were quantified in 6-week-old male Th3/+ mice that had been treated with saline, empty NPs (FA-GDLVs), or with FA-GDLVs carrying NC, or DMT1 siRNA daily for 16 days. Data are presented as mean ± SD for n = 4 to 5 mice per group and were analyzed by 1-way ANOVA followed by Tukey multiple comparisons test. Groups labeled with different letters are significantly different from one another. Treatment main effects: P < .001 for panel E; P < .01 for panel B; P < .05 for panels A,C-D,F.
Discussion
Iron overload exacerbates pathological outcomes in patients suffering from βTI. Iron loading occurs because of repression of hepatic HAMP, possibly mediated by EPO signaling via the erythroblast-derived hormone erythroferrone,10 leading to inappropriately low hepcidin production for any given level of body iron. Low hepcidin leads to excessive absorption of enteral iron and eventual iron loading of parenchymal tissues. The Th3/+ mouse model of βTI has proven to be instrumental in elucidating mechanisms of disease pathogenesis and for testing new therapeutic interventions.39,40 Low hepcidin in young Th3/+ mice may increase iron absorption and precipitate iron loading; however, unlike the situation in humans,41-43 hepcidin levels normalize in Th3/+ mice as they approach adulthood.7,9 Data presented herein confirm these previous observations but also extend them by quantifying intestinal iron absorption. Functionally, low hepcidin increases iron absorption by extending the half-life of the FPN protein on the basolateral membrane of duodenal enterocytes, leading to intracellular iron depletion and a secondary increase in the expression of DMT144 (likely by transcript stabilization via the iron-responsive element/iron-regulatory protein system12,45). Excessive absorption of dietary iron in βTI then is likely the result of the combined elevations of DMT1 and FPN expression/activity, but this concept has yet to be experimentally verified.
One therapeutic approach proposed to improve outcomes in patients with β-thalassemia is to prevent/mitigate iron loading. Several hepcidin-directed interventions decreased iron loading and improved the anemia in Th3/+ mice, including hepcidin agonists,10 transgenic overexpression of hepcidin,40 and silencing of matriptase 2,46,47 which is a negative regulator of hepatic HAMP. All of these strategies, theoretically, increase circulating hepcidin levels, which would be expected to attenuate iron absorption and lead to a gradual loss of body iron. Reducing iron burden, in turn, can have positive hematologic benefits by reducing oxidative stress, decreasing apoptosis, and lessening α-globin chain aggregation.10 Another logical approach to mitigate iron loading in βTI is to directly block or attenuate the absorption of dietary iron.48 Targeting DMT1 has been considered, as evidenced by an array of DMT1 blockers that have been developed over the past several years49-53; however, extensive testing of these blockers in preclinical disease models is lacking. Here, we used a novel in vivo siRNA approach to accomplish a similar goal aimed at reducing DMT1 expression/activity and thus iron import into duodenal enterocytes.
Iron in the diet of omnivorous humans consists of mainly NHI, and heme iron. Laboratory mice poorly absorb and assimilate dietary heme iron,54 so commercially available mouse chows, and the semipurified diets we used in this investigation, contain only NHI. Absorption of dietary NHI is primarily mediated by the iron importer DMT1 and the iron exporter FPN. Under physiological conditions, in mice, DMT1 and FPN are both required for iron absorption.11,14,55 The specific contributions of DMT1 and FPN to the excessive iron absorption that occurs in iron-loading anemias (such as βTI), however, has yet to be defined.
Our intent here was thus to use Th3/+ mice to examine the necessity of intestinal DMT1 for iron absorption, and to determine whether in vivo silencing of intestinal DMT1 would mitigate the iron loading that typifies the murine disorder. We focused on DMT1 for 3 reasons: (1) DMT1 is necessary for the bulk of vectorial iron flux in mice under physiological conditions11; (2) DMT1 is required for efficient iron absorption in humans, with mutations in SLC11A2 (encoding DMT1) leading to severe IDA56; and (3) intestinal DMT1 mediates the excessive iron absorption and iron loading that typifies murine hemochromatosis, and in vivo silencing of intestinal DMT1 mitigated iron loading in Hamp KO mice.12 Our experimental approach here included creating Th3/+ mice with ablation of intestinal DMT1 and in vivo targeting of DMT1 in Th3/+ mice by FA-GDLV–mediated peroral delivery of DMT1-silencing siRNAs. Outcomes demonstrated that intestinal DMT1 is required for iron absorption and iron loading in β-thalassemic mice, and in vivo silencing of intestinal DMT1 is an effective strategy to blunt iron loading and possibly improve the anemia in these mice. This investigation thus reveals a novel strategy for mitigating iron overload in βTI mice. Given that molecular pathways of NHI absorption are highly conserved between mice and humans,57 targeting intestinal DMT1 in the human disorder could be an effective adjunctive therapeutic option.
Acknowledgments
The authors thank Didier Merlin and Chunhua Yang at Georgia State University (Atlanta, Georgia) for expert assistance with the production of folic acid–coupled ginger nanoparticle–derived lipid vectors and intellectual contributions. The divalent metal-ion transporter 1 antibody was a kind gift from François Canonne-Hergaux, French Institute of Health and Medical Research, Bordeaux, France.
This investigation was funded by grants R01DK074867, R01DK109717, and R56DK137863.
Authorship
Contribution: Y.Y. and J.F.C. conceptualized this investigation; Y.Y., R.R.W., J.K.L., P.O.E.-U., J.S.S., S.Z., and Y.H. performed experiments; Y.Y. and J.F.C. designed experiments and interpreted data; Y.Y. drafted the manuscript, performed statistical analyses, and prepared the figures; and all authors participated in writing the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James F. Collins, Food Science & Human Nutrition Department, University of Florida, FSHN Building #441, 572 Newell Dr, Gainesville, FL 32607; email: jfcollins@ufl.edu.
References
Author notes
Data are available on request from the corresponding author, James F. Collins (jfcollins@ufl.edu).
The full-text version of this article contains a data supplement.