Key Points
IFN-1 drives HSCs toward more differentiated progenitors in response to hematopoietic stressors.
NFAT5 has an HSC-intrinsic role, limiting long-term IFN-1 responses to preserve reconstitution potential.
Visual Abstract
Hematopoietic stem cells (HSCs) readily recover from acute stress, but persistent stress can reduce their viability and long-term potential. Here, we show that the nuclear factor of activated T cells 5 (NFAT5), a transcription modulator of inflammatory responses, protects the HSC pool under stress. NFAT5 restrains HSC differentiation to multipotent progenitors after bone marrow transplantation and bone marrow ablation with ionizing radiation or chemotherapy. Correspondingly, NFAT5-deficient HSCs fail to support long-term reconstitution of hematopoietic progenitors and mature blood cells after serial transplant. Evidence from competitive transplant assays shows that these defects are HSC intrinsic. NFAT5-deficient HSCs exhibit enhanced expression of type 1 interferon (IFN-1) response genes after transplant, and suppressing IFN-1 receptor prevents their exacerbated differentiation and cell death after reconstitution and improves long-term regeneration potential. Blockade of IFN-1 receptor also prevented the overdifferentiation of NFAT5-deficient HSCs after bone marrow ablation. These findings show that long-term IFN-1 responses to different hematopoietic stressors drive HSCs toward more differentiated progenitors, and that NFAT5 has an HSC-intrinsic role, limiting IFN-1 responses to preserve reconstitution potential. Our identification of cell-intrinsic mechanisms that strengthen the resistance of HSCs to stress could help to devise approaches to protect long-term stemness during the treatment of hematopoietic malignancies.
Introduction
Adult hematopoietic stem cells (HSCs) are quiescent precursors that can divide to self-renew or differentiate to restock all blood lineages.1 Acute episodes of HSCs exiting from quiescence are clearly beneficial for accelerating hematopoiesis and replenishing depleted immune cells and blood populations.2,3 However, chronic or extensive hematopoietic stress, due to, for instance, aging or persistent inflammation, drives HSCs into prolonged expansion and differentiation, which can cause HSC exhaustion and aplastic anemia.3-5 Likewise, regimes such as γ irradiation or 5-fluorouracil (5-FU) treatment, used for instance for preconditioning or treating patients with leukemia before HSC transplant, cause persistent bone marrow damage.6-8 Indeed, although host bone marrow ablation supports donor HSC engraftment,6 it could also elicit damage responses in host tissues that would affect the transplanted progenitors. Therefore, identifying mechanisms that allow HSCs to withstand hematopoietic stress could be useful for enhancing long-term hematopoietic fitness in different scenarios.
The effect of persistent stress on hematopoietic precursors has been well documented for type 1 interferon (IFN-1) responses in chronic inflammation models,3,5 whereby prolonged inhibition of HSC quiescence by IFN-1 leads to their exhaustion.9 This has also been observed in mouse models deficient in apoptotic mechanisms or in DNA damage and repair responses, which present enhanced IFN-1 production.10-13 Enhanced IFN-1 signals upon DNA damage can also target the mitochondria and increase production of reactive oxygen species (ROS) in hematopoietic progenitors.11
We have shown recently that the transcription factor (TF) nuclear factor of activated T cells 5 (NFAT5) represses acute IFN-1 production in innate immune responses, and that this effect can protect HSC quiescence.14 NFAT5 is a Rel-like TF,15 originally discovered as an osmostress-protective TF.15 NFAT5 activates proinflammatory functions in diverse immune cells by enhancing Toll-like receptor (TLR)-induced transcription of genes such as Nos2, Tnf, Il6, and Il1, but also attenuates IFN-1 expression induced by TLR3 and viruses.14,16-20 NFAT5-deficient mouse models are highly vulnerable to Leishmania major infection because of their defective induction of Nos2 and other TLR-responsive genes,16,21 yet have better control of acute lymphocytic choriomeningitis virus infection as a result of enhanced IFN-1 production.14
The role of NFAT5 in adult hematopoietic precursors has not been explored, but two independent studies pointed to the Nfat5 gene as a target of retroviral insertions that can give rise to mouse myeloma or B-cell lymphoma.22,23 Because leukemia resulting from retroviral insertional mutagenesis can originate from altered hematopoietic precursors, we decided to explore the role of NFAT5 in HSCs. We now show that NFAT5 restrains IFN-1 responses to stress that exacerbate HSC differentiation, and that this preserves the long-term regenerative potential of HSCs. The identification of NFAT5 as a relevant factor in countering the deleterious long-term effects of IFN-1 on HSCs is potentially relevant for improving treatment of hematopoietic malignancies and also may reveal a mechanism that safeguards stem cells in different tissues.
Materials and methods
Mouse models
NFAT5-deficient (Nfat5–/–) mice, hematopoietic cell–specific NFAT5-deficient mice (Nfat5fl/fl Vav-Cre), myeloid cell–specific NFAT5-deficient mice (Nfat5fl/fl LysM-Cre), Ifnar1–/– mice, CD45.1 (Ly5.1) or CD45.2 (Ly5.2) congenic mice, and CD45.1.2 (hybrid F1 with both alleles) mice are described in supplemental Methods.
This study was done under the approval of the animal experimentation and ethics committee (CEEEA) of Barcelona Biomedical Research Park, Universitat Pompeu Fabra. Our animal facility (Animal Facility of the Barcelona Biomedical Research Park) is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). We have different CEEEA-approved protocols that supervise all the procedures and experiments with mice described in this manuscript.
Flow cytometry, blood, and hematopoietic progenitor and stem cell (HSPC) analyses
Flow cytometry was done with an LSRII machine (BD biosciences) equipped with 355-, 405-, 488-, and 633-nm lasers; an LSR Fortessa (BD biosciences) equipped with 405-, 488-, 561-, and 633-nm lasers; a Cytek Aurora Spectral Cytometer 4 Laser: 16V-14B-10YG-8R; and the Cytek Aurora Spectral Cytometer 5 Laser: 16UV-16V-14B-10YG-8R. Cell sorting was done with a BD FACSAria II (BD biosciences) cell sorter equipped with 355-, 405-, 488-, 561-, and 633-nm lasers. Gating strategies and antibodies for flow cytometry analysis are detailed in supplemental Methods and supplemental Table 1.
Bone marrow and HSC transplant
Procedures used in bone marrow and HSC transplants, including serial and competitive transplants are detailed in supplemental Methods.
Challenge with TBI
Total body irradiation (TBI) of Nfat5+/+ Vav-Cre and Nfat5fl/fl Vav-Cre mice was done in a 137Cs-γ IBL 437C H irradiator (Shering CIS Bio International) at 2.56 Gy/min for a single dose of 5 Gy. Irradiated mice were monitored daily and given antibiotic-containing drinking water (Enrovet; 0.6 g/L) for at least 30 days. For IFN-1 receptor (IFNAR) blockade, anti-mouse IFNAR-1 antibody (I-401, Leinco Technologies, Inc) or its immunoglobulin G1 control (I-536, Leinco Technologies, Inc), both at 500 μg per mouse, were injected intraperitoneally in 200 μL of phosphate-buffered saline, 16 hours before and 30 hours after irradiation.
Challenge with 5-FU
5-FU was administered intraperitoneally at 150 mg/kg body weight using 1 single dose or a serial challenge with 3 doses injected 10 or 14 days apart.24 Mice were monitored daily and analyzed 6 to 14 days after 5-FU injection as indicated in the figures. IFNAR blockade was done as above, 16 hours before and 48 hours after each 5-FU injection.
RNA microarray analysis of steady state and posttransplant HSCs and multipotent progenitors (MPPs)
Cell isolation, RNA processing, microarray methodology, and data analysis are described in supplemental Methods. Gene Expression Omnibus accession number: GSE228577.
RNA sequencing analysis of posttransplant HSCs and MPPs
Cell isolation, RNA processing, sequencing methodology, and data analysis are described in supplemental Methods. Gene Expression Omnibus accession number: GSE228634.
Statistical analysis
Data were analyzed with GraphPad Prism 6 and represented as mean ± standard error of the mean. Normality (Gaussian distribution) of samples was determined by a D’Agostino-Pearson normality test before calculating statistical significance with an unpaired t test. Statistical significance for survival curves was determined with a Mantel-Cox test. Statistics analyses used, number of samples, and number of independent experiments done are indicated in each figure legend.
Results
NFAT5 restores HSPC homeostasis after hematopoietic stress
We generated hematopoietic cell–specific NFAT5-deficient (Nfat5fl/fl Vav-Cre) mice in which NFAT5 was deleted from early hematopoietic precursors (supplemental Figure 1A; gating strategies in supplemental Figure 1C-E). NFAT5 expression was high in HSCs but decreased throughout differentiation to MPP subsets or more committed progenitors (supplemental Figure 1A-B). This was consistent with NFAT5 expression data in mouse and human databases (supplemental Figure 1B).25-27
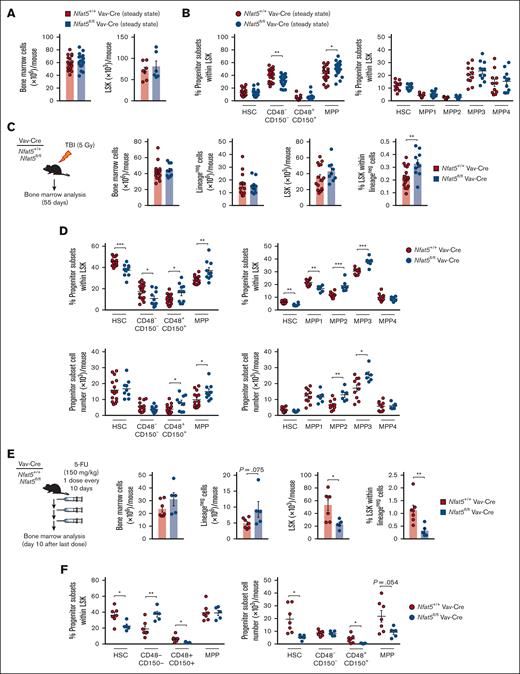
Nfat5fl/fl Vav-Cre mice had normal representation of HSPC subsets, with a modest bias toward more differentiated MPPs (Figure 1A-B; supplemental Figure 2A-B). We challenged them with nonlethal, TBI (5 Gy), and analyzed the hematopoietic compartment after they recovered main hematological parameters (supplemental Figure 2C). Nfat5fl/fl Vav-Cre mice had a higher proportion of lineage-negative (lineageneg) Sca-1+ cKit+ (LSK) cells, reduced frequency of HSCs, and increased representation of the more differentiated MPP2 and the myeloid-biased MPP327 subsets (Figure 1C-D). 5-FU, a chemotherapy drug that depletes proliferating hematopoietic progenitors and forces mobilization of dormant HSCs,28 also caused a substantial decrease in the number of HSCs in Nfat5fl/fl Vav-Cre mice compared with control littermates (Figure 1E-F; supplemental Figure 2D-E). These results pointed to NFAT5 as a relevant factor in protecting HSPCs from radiation and chemotherapeutic stress.
NFAT5 supports the reconstitution of hematopoietic progenitors after γ irradiation or treatment with 5-FU. (A) Number of bone marrow (n = 18 mice) and LSK cells (n = 7 mice) per mouse from WT (Nfat5+/+ Vav-Cre) or hematopoietic cell–specific NFAT5-deficient (Nfat5fl/fl Vav-Cre) mice. (B) Percentage of the indicated hematopoietic stem and progenitor populations in the bone marrow of Nfat5+/+ Vav-Cre or Nfat5fl/fl Vav-Cre mice. n = 20 to 21 mice of each genotype in the left panel, and 11 on the right. (C-D) Analysis of bone marrow from Nfat5+/+ Vav-Cre or Nfat5fl/fl Vav-Cre mice at 55 days after TBI (5 Gy). A schematic diagram of the experiment is shown in panel C (left). Bone marrow cellularity, number of lineageneg, number and percentage of LSK cells (C, right), and percentage and number of hematopoietic progenitor populations (D) are shown. n = 10-17 mice of each genotype from 2 independent experiments in panel C and the left panels in panel D; n = 6-10 mice from 1 experiment in the right panels of panel D. (E-F) Analysis of bone marrow from Nfat5+/+ Vav-Cre or Nfat5fl/fl Vav-Cre mice treated with a regime of 3 consecutive doses of 5-FU (150 mg/kg) administered every 10 days. A schematic diagram of the experiment is shown in panel E (left). Bone marrow cellularity, number of lineageneg, number and percentage of LSK cells (E), and percentage and number of hematopoietic progenitor populations (F) are shown. n = 5-7 mice in panels E-F. Results are shown as mean ± standard error of the mean (SEM). Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated.
NFAT5 supports the reconstitution of hematopoietic progenitors after γ irradiation or treatment with 5-FU. (A) Number of bone marrow (n = 18 mice) and LSK cells (n = 7 mice) per mouse from WT (Nfat5+/+ Vav-Cre) or hematopoietic cell–specific NFAT5-deficient (Nfat5fl/fl Vav-Cre) mice. (B) Percentage of the indicated hematopoietic stem and progenitor populations in the bone marrow of Nfat5+/+ Vav-Cre or Nfat5fl/fl Vav-Cre mice. n = 20 to 21 mice of each genotype in the left panel, and 11 on the right. (C-D) Analysis of bone marrow from Nfat5+/+ Vav-Cre or Nfat5fl/fl Vav-Cre mice at 55 days after TBI (5 Gy). A schematic diagram of the experiment is shown in panel C (left). Bone marrow cellularity, number of lineageneg, number and percentage of LSK cells (C, right), and percentage and number of hematopoietic progenitor populations (D) are shown. n = 10-17 mice of each genotype from 2 independent experiments in panel C and the left panels in panel D; n = 6-10 mice from 1 experiment in the right panels of panel D. (E-F) Analysis of bone marrow from Nfat5+/+ Vav-Cre or Nfat5fl/fl Vav-Cre mice treated with a regime of 3 consecutive doses of 5-FU (150 mg/kg) administered every 10 days. A schematic diagram of the experiment is shown in panel E (left). Bone marrow cellularity, number of lineageneg, number and percentage of LSK cells (E), and percentage and number of hematopoietic progenitor populations (F) are shown. n = 5-7 mice in panels E-F. Results are shown as mean ± standard error of the mean (SEM). Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated.
Whereas Nfat5fl/fl Vav-Cre mice do not exhibit pathological alterations outside immune and hematological parameters, mice with a whole-body deficiency in NFAT5 (NFAT5-deficient mice, Nfat5–/–)29,30 suffer from systemic osmotic stress that might affect hematopoiesis. Nfat5–/– mice were smaller and had bone marrow cytopenia but normal numbers of LSK progenitors (supplemental Figure 2F-G). Nonetheless, their HSPCs presented a marked imbalance toward more differentiated MPP and CD48+ CD150+ cells, and reduced percentage of HSC and CD48− CD150− cells (supplemental Figure 2H). This bias was also detected in the spleen, a site of extramedullary hematopoiesis (supplemental Figure 2I). Altogether, analysis of NFAT5-deficient mice and hematopoietic system–restricted mouse models of NFAT5 deficiency indicate that NFAT5 is required for the appropriate reconstitution of hematopoietic progenitor subsets upon stress.
NFAT5-deficient HSCs have impaired hematopoietic reconstitution capacity
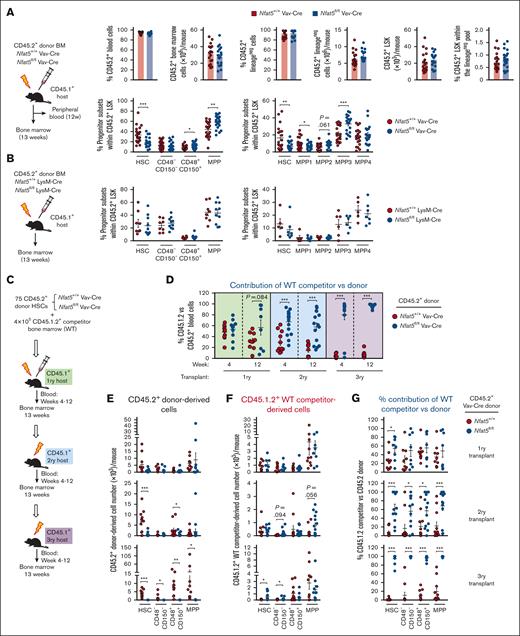
Bone marrow transplant of a lethally irradiated host is commonly used to assess HSC functionality.6Nfat5fl/fl Vav-Cre bone marrow cells reconstituted CD45.2 lineageneg and LSK cells (Figure 2A), but these showed a marked bias toward more differentiated HSPCs, with reduced percentage of HSCs and overrepresentation of MPP3-biased MPPs (Figure 2A; supplemental Figure 3A). Reconstituted mice also had normal representation of the more committed progenitors (common myeloid progenitors [CMPs], granulocyte-monocyte progenitors [GMPs], megakaryocyte-erythroid progenitors [MEPs]) but increased common lymphoid progenitors (CLP) (supplemental Figure 3B). These defects were not attributable to NFAT5 functions in mature myeloid cells,14,16 because reconstitution with bone marrow of myeloid-specific NFAT5-deficient (Nfat5fl/fl LysM-Cre) resulted in normal representation of HSC and MPP subsets (Figure 2B; supplemental Figure 3C-D).
Defective hematopoietic reconstitution in mice that received transplantation with NFAT5-deficient bone marrow or HSCs. (A) Schematic diagram of the experiment (upper left), and donor-derived (CD45.2+) reconstitution of blood, different bone marrow populations, and hematopoietic stem and progenitor subsets in mice that received transplantation with bone marrow (0.5 × 106 cells) from hematopoietic cell–specific NFAT5-deficient mice (Nfat5fl/fl Vav-Cre) or littermate WT mice. Results are from 3 independent experiments, each with 1 bone marrow donor of the respective genotype and 6 to 9 recipients. (B) Donor-derived reconstitution of bone marrow hematopoietic stem and progenitor subsets in mice that received transplantation with bone marrow from myeloid-specific NFAT5-deficient mice (Nfat5fl/fl LysM-Cre) or littermate WT mice (Nfat5+/+ LysM-Cre). Results are from 2 independent experiments (left panel) or 1 experiment (right panel), each with 1 bone marrow donor of each genotype and 3 to 7 recipient mice. (C) Schematic diagram of transplants with Nfat5fl/fl Vav-Cre or WT HSCs together with competitor WT bone marrow cells. For primary transplants, fluorescence-activated cell sorter (FACS)–sorted CD45.2+Nfat5fl/fl Vav-Cre or WT HSCs were mixed with competitor WT CD45.1.2+ bone marrow cells and transplanted into irradiated CD45.1+ recipient mice. For secondary and tertiary transplants, donor cells were from whole bone marrow. (D) Competitor (CD45.1.2+) vs donor (CD45.2+) chimerism in peripheral blood cells isolated from mice serially reconstituted from NFAT5-deficient and control HSCs in competition with WT bone marrow. Blood samples were obtained 4 and 12 weeks after transplant. See supplemental Figure 5A-B for the number and percentage of donor- and competitor-derived blood cells. (E) Numbers of CD45.2+ donor- and (F) CD45.1.2+ competitor- derived reconstitution of bone marrow hematopoietic stem and progenitor subsets through serial transplants derived from Nfat5fl/fl Vav-Cre or WT HSCs. Analysis was done at week 13 after each transplant. (G) Competitor (CD45.1.2+) vs donor (CD45.2+) chimerism in hematopoietic stem and progenitor subsets isolated from mice serially reconstituted from NFAT5-deficient and control HSCs in competition with WT bone marrow. Results in panels D-G are from 2 independent transplant experiments, each with 1 HSC donor of each genotype and 3 to 6 recipients for the primary transplants. Secondary and tertiary transplants were done with pools of reconstituted bone marrow (4-6 WT and 3-5 NFAT5-deficient donors were pooled for the secondary transplant; and 5-7 WT and 3-8 NFAT5-deficient donors were pooled for the tertiary transplant). Results are shown as mean ± SEM. Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated.
Defective hematopoietic reconstitution in mice that received transplantation with NFAT5-deficient bone marrow or HSCs. (A) Schematic diagram of the experiment (upper left), and donor-derived (CD45.2+) reconstitution of blood, different bone marrow populations, and hematopoietic stem and progenitor subsets in mice that received transplantation with bone marrow (0.5 × 106 cells) from hematopoietic cell–specific NFAT5-deficient mice (Nfat5fl/fl Vav-Cre) or littermate WT mice. Results are from 3 independent experiments, each with 1 bone marrow donor of the respective genotype and 6 to 9 recipients. (B) Donor-derived reconstitution of bone marrow hematopoietic stem and progenitor subsets in mice that received transplantation with bone marrow from myeloid-specific NFAT5-deficient mice (Nfat5fl/fl LysM-Cre) or littermate WT mice (Nfat5+/+ LysM-Cre). Results are from 2 independent experiments (left panel) or 1 experiment (right panel), each with 1 bone marrow donor of each genotype and 3 to 7 recipient mice. (C) Schematic diagram of transplants with Nfat5fl/fl Vav-Cre or WT HSCs together with competitor WT bone marrow cells. For primary transplants, fluorescence-activated cell sorter (FACS)–sorted CD45.2+Nfat5fl/fl Vav-Cre or WT HSCs were mixed with competitor WT CD45.1.2+ bone marrow cells and transplanted into irradiated CD45.1+ recipient mice. For secondary and tertiary transplants, donor cells were from whole bone marrow. (D) Competitor (CD45.1.2+) vs donor (CD45.2+) chimerism in peripheral blood cells isolated from mice serially reconstituted from NFAT5-deficient and control HSCs in competition with WT bone marrow. Blood samples were obtained 4 and 12 weeks after transplant. See supplemental Figure 5A-B for the number and percentage of donor- and competitor-derived blood cells. (E) Numbers of CD45.2+ donor- and (F) CD45.1.2+ competitor- derived reconstitution of bone marrow hematopoietic stem and progenitor subsets through serial transplants derived from Nfat5fl/fl Vav-Cre or WT HSCs. Analysis was done at week 13 after each transplant. (G) Competitor (CD45.1.2+) vs donor (CD45.2+) chimerism in hematopoietic stem and progenitor subsets isolated from mice serially reconstituted from NFAT5-deficient and control HSCs in competition with WT bone marrow. Results in panels D-G are from 2 independent transplant experiments, each with 1 HSC donor of each genotype and 3 to 6 recipients for the primary transplants. Secondary and tertiary transplants were done with pools of reconstituted bone marrow (4-6 WT and 3-5 NFAT5-deficient donors were pooled for the secondary transplant; and 5-7 WT and 3-8 NFAT5-deficient donors were pooled for the tertiary transplant). Results are shown as mean ± SEM. Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated.
Serial transplantation assays revealed an extensive defect in the hematopoietic reconstitution potential of Nfat5fl/fl Vav-Cre bone marrow, manifested in tertiary recipient mice as a profound blood cytopenia (supplemental Figure 3E-G) and depletion of all types of LSK progenitors (supplemental Figure 3E). This was already noticeable in secondarily Nfat5fl/fl Vav-Cre–reconstituted mice, which showed a pronounced reduction in HSCs and an increase in MPPs (supplemental Figure 3E). Similar reconstitution defects in serial transplants were seen with purified NFAT5-deficient HSCs (supplemental Figure 4A-G), indicating that NFAT5 maintains the pool of undifferentiated HSCs under stress, thereby preventing their exhaustion and protecting their long-term repopulation capacity.
We then used competitive transplants to interrogate an intrinsic role of NFAT5 in HSCs (Figure 2C). Serial transplants showed that NFAT5-deficient HSCs gave rise to impaired blood reconstitution (supplemental Figure 5A; Figure 2D) and defective regeneration of HSPCs in the LSK compartment (Figure 2E; supplemental Figure 5C). By contrast to NFAT5-deficient cells, competitor bone marrow produced normal numbers and proportions of blood cells and HSPCs (Figure 2D,F; supplemental Figure 5B,D), and outcompeted NFAT5-deficient HSCs in contributing to LSKs, HSPCs, and mature blood cells throughout serial transplants (Figure 2D,G; supplemental Figure 5E). Although wild-type (WT) competitor cells could partially compensate for the hematopoietic defects in hosts reconstituted with NFAT5-deficient HSCs, we observed higher mortality in tertiary hosts reconstituted with NFAT5-deficient cells (supplemental Figure 5F). This likely occurred because competitor WT bone marrow cells were transplanted in low numbers, not sufficient to rescue the NFAT5-deficient hematopoietic system of tertiary hosts (Figure 2C; supplemental Figure 5C-D). Primary competitive transplants also showed that NFAT5-deficient bone marrow cells, and not WT competitor ones in the same host, presented imbalanced reconstitution of the HSPC compartment (supplemental Figure 5G). Altogether, this set of results reveals an HSC-intrinsic function for NFAT5 in its ability to sustain their long-term repopulation capacity.
NFAT5-deficient HSCs exhibit an altered transcriptome with enhanced MPP signatures
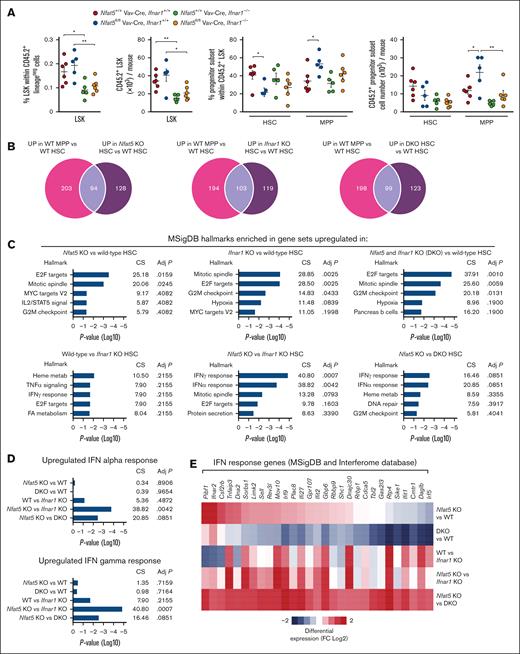
To understand how NFAT5 could limit HSC differentiation, we analyzed the transcriptome of HSCs and MPPs reconstituted from bone marrow of Nfat5fl/fl Vav-Cre or littermate WT mice (see supplemental Figure 6A for the sorting strategy). Reconstituted NFAT5-deficient HSCs had upregulated molecular signature database (MSigDB) hallmarks characteristic of the more differentiated MPPs,31-34 including Myc and early 2 factor (E2F) targets and, with lesser significance, genes involved in G2M cell cycle checkpoint and mTORC1 signaling (Figure 3A-C). Overall, 34% (91/271) of the genes upregulated in NFAT5-deficient vs WT HSCs coincided with genes that in WT progenitors were more expressed in MPPs than HSCs (Figure 3C). Similarly, 31% (39/125) of the genes downregulated in NFAT5-deficient HSCs were genes downmodulated in WT MPPs with respect to HSCs (Figure 3C). NFAT5-deficient HSCs also upregulated gene sets included in the MSigDB curated gene sets, chemical and genetic perturbations (CGP) category, which characterize the more differentiated “short-term hematopoietic stem cell” or “early progenitor” that are characteristic of MPPs35 (Figure 3D). Nonetheless, main markers defining HSCs and MPPs were expressed normally in the respective NFAT5-deficient populations (supplemental Figure 6B), and these showed normal expression of osmoresponsive NFAT5-target genes36 (supplemental Figure 6C), indicating that the bone marrow niche is a nonhypertonic environment. Analysis of HSCs and MPPs from mice in steady state also revealed an overlap, albeit less pronounced, between genes modulated by NFAT5 in HSCs and genes that discriminate MPPs from HSCs (Figure 3E). These results reveal an extensive gene expression program coordinated by NFAT5 to maintain HSC identity.
NFAT5-deficient HSCs exhibit an altered transcriptome with enhanced MPP signatures. (A) MSigDB hallmarks enriched in the set of genes differentially upregulated (left panel) or downregulated (right panel) in HSCs sorted via FACS 13 weeks after bone marrow transplant (BMT) from Nfat5fl/fl Vav-Cre or littermate WT mice (n = 3). See supplemental Figure 6A for the sorting strategy. (B) MSigDB hallmarks upregulated or downregulated in WT MPPs vs WT HSCs 13 weeks after BMT. MSigDB hallmarks analysis was done with Enrichr. (C) Venn diagrams of the number of differentially expressed genes upregulated (left) and downregulated (right) in NFAT5-deficient vs WT HSCs that were also upregulated or downregulated in WT MPPs vs HSCs after BMT. The bottom panels show the Enrichr analysis of MSigDB hallmarks enriched in the set of MPP-like genes upregulated (left panel) or downregulated (right panel) in NFAT5-deficient vs WT HSCs. (D) Enrichment in short-term HSC (ST-HSC) and early progenitor signatures (from MSigDB C2-CGP MM676 and MM1012 data sets respectively) in the set of genes upregulated after BMT in NFAT5-deficient (knockout [KO]) vs WT HSCs, and in WT or NFAT5-deficient (KO) MPP vs HSC. MSigDB were analyzed with gene set enrichment analysis. (E) Venn diagrams of the number of differentially expressed genes upregulated (left) and downregulated (right) in NFAT5-deficient (Nfat5fl/fl Vav-Cre mice) vs WT HSCs that were also upregulated or downregulated, respectively, in WT MPPs vs HSCs in steady-state conditions. The fold-change (FC) values for the pairwise comparisons shown in the Venn diagrams in panels C,E were ≥2, with P values < .05. adj P, adjusted P value; CS, combined score; FDR, false discovery rate; NES, normalized enrichment score.
NFAT5-deficient HSCs exhibit an altered transcriptome with enhanced MPP signatures. (A) MSigDB hallmarks enriched in the set of genes differentially upregulated (left panel) or downregulated (right panel) in HSCs sorted via FACS 13 weeks after bone marrow transplant (BMT) from Nfat5fl/fl Vav-Cre or littermate WT mice (n = 3). See supplemental Figure 6A for the sorting strategy. (B) MSigDB hallmarks upregulated or downregulated in WT MPPs vs WT HSCs 13 weeks after BMT. MSigDB hallmarks analysis was done with Enrichr. (C) Venn diagrams of the number of differentially expressed genes upregulated (left) and downregulated (right) in NFAT5-deficient vs WT HSCs that were also upregulated or downregulated in WT MPPs vs HSCs after BMT. The bottom panels show the Enrichr analysis of MSigDB hallmarks enriched in the set of MPP-like genes upregulated (left panel) or downregulated (right panel) in NFAT5-deficient vs WT HSCs. (D) Enrichment in short-term HSC (ST-HSC) and early progenitor signatures (from MSigDB C2-CGP MM676 and MM1012 data sets respectively) in the set of genes upregulated after BMT in NFAT5-deficient (knockout [KO]) vs WT HSCs, and in WT or NFAT5-deficient (KO) MPP vs HSC. MSigDB were analyzed with gene set enrichment analysis. (E) Venn diagrams of the number of differentially expressed genes upregulated (left) and downregulated (right) in NFAT5-deficient (Nfat5fl/fl Vav-Cre mice) vs WT HSCs that were also upregulated or downregulated, respectively, in WT MPPs vs HSCs in steady-state conditions. The fold-change (FC) values for the pairwise comparisons shown in the Venn diagrams in panels C,E were ≥2, with P values < .05. adj P, adjusted P value; CS, combined score; FDR, false discovery rate; NES, normalized enrichment score.
NFAT5 protects HSPCs from apoptosis after bone marrow transplantation or sublethal irradiation
Because our results suggest that NFAT5 help maintain HSC identity under hematopoietic stress by limiting MPP gene programs, we assessed NFAT5-deficient HSPCs for basic indicators of fitness and activity, such as the cell cycle status, viability, and ROS production. No differences were observed in cell cycle between WT and NFAT5-deficient HSPC populations in steady state or 3 and 13 weeks after transplantation (supplemental Figure 7A). HSPCs of Nfat5fl/fl Vav-Cre mice had normal proportions of apoptotic cells in steady state, showed enhanced apoptosis after primary transplant, and again normal levels of apoptosis after secondary transplant (supplemental Figure 7B). HSPCs of Nfat5fl/fl Vav-Cre mice had normal ROS levels in steady state and upon bone marrow or HSC transplant (supplemental Figure 7C-D), but serial transplants revealed that NFAT5-deficient lineageneg cells of tertiary hosts showed increased ROS accumulation (supplemental Figure 7C-D, right panels). These findings indicate that ROS accumulation is not connected with apoptosis but rather might reflect a dysfunctional or compensatory response. Hematopoietic progenitor subsets of whole-body NFAT5-deficient mice presented normal cell cycle profile but enhanced apoptosis and ROS levels (supplemental Figure 7E). Increased apoptosis in different LSK subsets was also observed in Nfat5fl/fl Vav-Cre mice in the recovery from sublethal TBI but not after a single 5-FU dose (supplemental Figure 8A-B). These findings identify features of compromised function of NFAT5-deficient hematopoietic progenitors under certain stressors.
Suppressing IFNAR signaling rescues NFAT5-deficient HSCs
Persistent inflammation, chemotherapy, and γ irradiation can damage the hematopoietic niche and even cause tissue injury, which triggers the IFN-1 pathway and can cause chronic activation of HSCs.9,37,38 We have previously shown that NFAT5 represses IFN-1 expression and that myeloid-specific NFAT5-deficient mice respond to acute inflammation by overproducing IFN-1, which transiently mobilizes HSCs.14 This raised the question of whether the intrinsic role of NFAT5 in HSCs could involve IFN-1 responses. Because the MSigDB analysis did not reveal interferon response signatures in NFAT5-regulated HSC genes, we used the Interferome database (Interferome version 2.01),39 which provides a more comprehensive coverage. We now found that 373 of 22 142 genes (1.69%) in HSCs reconstituted 13 weeks after transplant were annotated in Interferome as IFN-1 response (supplemental Figure 8C, left panel). A similar proportion (1.99%) was found in genes differentially upregulated in WT HSCs upon bone marrow transplant with respect to basal conditions. However, this proportion was higher (2.75%) in reconstituted NFAT5-deficient HSCs (supplemental Figure 8C, left panel). Analysis of genes differentially expressed between NFAT5-deficient or WT-reconstituted HSCs also showed a greater proportion of IFN-1 response genes among those upregulated in NFAT5-deficient HSCs (supplemental Figure 8C, right panel). These results suggest that reconstituted NFAT5-deficient HSCs might have enhanced expression of IFN-1 response genes.
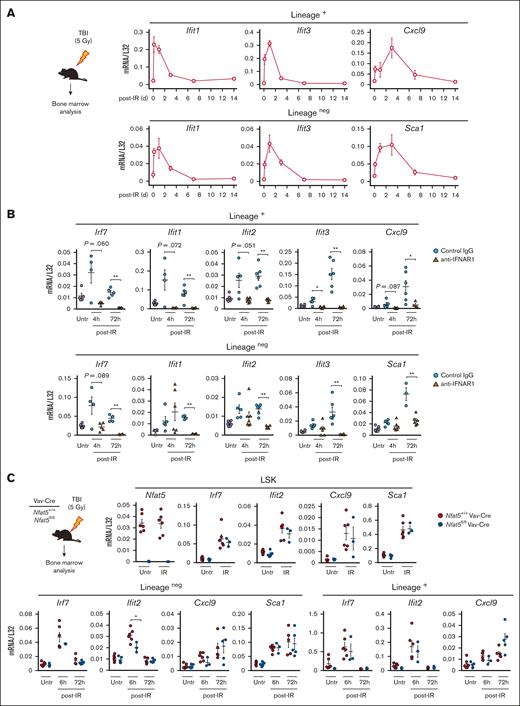
We then analyzed whether sublethal irradiation could induce IFN-1 responses in the hematopoietic niche and observed that it triggered an immediate wave of IFN-1 response genes in lineage-positive and lineageneg cells that vanished one week later (Figure 4A). Antibody blockade of IFNAR prevented expression of these genes (Figure 4B), confirming that they were IFN-1 induced. Early expression of IFN-1–response genes in LSKs, lineage-positive, and lineageneg cells was NFAT5-independent (Figure 4C), indicating that Nfat5fl/fl Vav-Cre mice mount normal IFN-1 responses soon after irradiation-induced tissue damage.
γ irradiation triggers a wave of IFN-1 response genes in the bone marrow. (A) Expression of IFN-1 response genes in lineage-positive (lineage+) and lineageneg cells in the bone marrow of mice exposed to sublethal, TBI (5 Gy). Samples were analyzed 4 hours, and 1, 3, 7, and 14 days after irradiation. Results are shown as mean ± SEM (n = 4 mice). (B) Effect of neutralizing IFNAR1 on the expression of IFN-1 response genes in lineage+ and lineageneg bone marrow cells after TBI (5 Gy). Samples were analyzed 4 and 72 hours after irradiation in mice treated with control immunoglobulin G1 (IgG1) or neutralizing anti-IFNAR1 antibody as described in “Materials and methods.” Nonirradiated cells (Untr) are shown. Results are shown as mean ± SEM (n = 3-6 mice per condition). (C) Expression of IFN-1 response genes in LSK cells, lineage+, and lineageneg bone marrow cells of Nfat5fl/fl Vav-Cre or littermate WT mice after TBI (5 Gy). LSK cells were analyzed 6 hours after irradiation, and lineage+ and lineageneg cells at 6 and 72 hours. Nonirradiated cells (Untr) are shown. Results are shown as mean ± SEM (n = 3-6 mice of each genotype). Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01. P values < .1 are also indicated.
γ irradiation triggers a wave of IFN-1 response genes in the bone marrow. (A) Expression of IFN-1 response genes in lineage-positive (lineage+) and lineageneg cells in the bone marrow of mice exposed to sublethal, TBI (5 Gy). Samples were analyzed 4 hours, and 1, 3, 7, and 14 days after irradiation. Results are shown as mean ± SEM (n = 4 mice). (B) Effect of neutralizing IFNAR1 on the expression of IFN-1 response genes in lineage+ and lineageneg bone marrow cells after TBI (5 Gy). Samples were analyzed 4 and 72 hours after irradiation in mice treated with control immunoglobulin G1 (IgG1) or neutralizing anti-IFNAR1 antibody as described in “Materials and methods.” Nonirradiated cells (Untr) are shown. Results are shown as mean ± SEM (n = 3-6 mice per condition). (C) Expression of IFN-1 response genes in LSK cells, lineage+, and lineageneg bone marrow cells of Nfat5fl/fl Vav-Cre or littermate WT mice after TBI (5 Gy). LSK cells were analyzed 6 hours after irradiation, and lineage+ and lineageneg cells at 6 and 72 hours. Nonirradiated cells (Untr) are shown. Results are shown as mean ± SEM (n = 3-6 mice of each genotype). Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01. P values < .1 are also indicated.
Next, we analyzed whether suppressing IFNAR could compensate for the long-term defects of NFAT5-deficient HSCs. Double-knockout (DKO) mice (knock out of NFAT5 and IFNAR) compensated the MPP bias of Nfat5fl/fl Vav-Cre mice in steady state (supplemental Figure 9A), and their HSC to MPP3 overdifferentiation upon sublethal irradiation (Figure 5A; supplemental Figure 9B-D) or serial 5-FU treatment (Figure 5B; supplemental Figure 9E). In line with this, antibody blockade of IFNAR corrected the HSC and MPP imbalance caused by 5-FU (Figure 5C; supplemental Figure 9F-H). These experiments also showed that irradiation and 5-FU differed in their ability to induce apoptosis in HSPCs: NFAT5 protected HSPCs from irradiation, and this protective effect was partially enhanced by IFNAR1 (Figure 5D), whereas neither NFAT5 nor IFNAR1 affected apoptosis in 5-FU–treated mice (supplemental Figure 9I-J). Regarding the serial 5-FU experiments, we changed our initial protocol from 10 days between doses (Figure 1E-F) to 14 days (Figure 5B-C), to allow a better recovery of blood cells after each dose.24 This 14-day spacing rendered NFAT5-dependent effects more similar to those of sublethal irradiation (Figures 1C-D and 5A). Finally, overdifferentiation of NFAT5-deficient HSCs to MPP3 upon bone marrow ablation was not paralleled by enhanced committed myeloid progenitors or mature myeloid cell output in the blood (supplemental Figure 9D-E; and data not shown), and, conversely, attenuating the MPP3 bias in the serial 5-FU regime by IFNAR blockade was not paralleled by reduced production of committed myeloid progenitors (supplemental Figure 9H). This recalls our findings after transplantation (Figure 2A; supplemental Figure 3A-B) and points to an enhanced myeloid potential in NFAT5-deficient HSPCs in their adaptation to stress.
Neutralization of IFNAR attenuates the reconstitution defects of NFAT5-deficient hematopoietic progenitors upon γ irradiation or treatment with 5-FU. (A) Percentage of the indicated hematopoietic progenitor populations in the bone marrow of mice of the different genotypes 55 days after TBI (5 Gy). WT (Nfat5+/+ Vav-Cre, Ifnar1+/+), hematopoietic cell–specific NFAT5-deficient (Nfat5fl/fl Vav-Cre, Ifnar1+/+), IFNAR1-deficient (Nfat5+/+ Vav-Cre, Ifnar1–/–), and double deficient (Nfat5fl/fl Vav-Cre, Ifnar1–/–) mice. Results are shown as mean ± SEM (n = 6-12 mice of each genotype). (B) Percentage of hematopoietic progenitor populations in the bone marrow of mice of the indicated genotypes treated with 3 consecutive doses of 5-FU (150 mg/kg) administered every 14 days, and analyzed 14 days after the last dose. Results are shown as mean ± SEM (n = 4-7 mice of each genotype). (C) Effect of neutralizing IFNAR1 during a 3-dose 5-FU regime on the percentage of hematopoietic progenitor populations. Mice were treated with control IgG or neutralizing anti-IFNAR1 antibody as described in “Materials and methods,” and analyzed 14 days after the last dose of 5-FU. Results are shown as mean ± SEM (n = 5 mice per condition). (D) Percentage of apoptotic cells (annexin V–positive [annexin V+] DAPI− [4′,6-diamidino-2-phenylindole–negative]) in hematopoietic progenitor populations in the bone marrow of mice of the indicated genotypes 55 days after TBI (5 Gy). Results are shown as mean ± SEM (n = 5-9 mice of each genotype). Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated.
Neutralization of IFNAR attenuates the reconstitution defects of NFAT5-deficient hematopoietic progenitors upon γ irradiation or treatment with 5-FU. (A) Percentage of the indicated hematopoietic progenitor populations in the bone marrow of mice of the different genotypes 55 days after TBI (5 Gy). WT (Nfat5+/+ Vav-Cre, Ifnar1+/+), hematopoietic cell–specific NFAT5-deficient (Nfat5fl/fl Vav-Cre, Ifnar1+/+), IFNAR1-deficient (Nfat5+/+ Vav-Cre, Ifnar1–/–), and double deficient (Nfat5fl/fl Vav-Cre, Ifnar1–/–) mice. Results are shown as mean ± SEM (n = 6-12 mice of each genotype). (B) Percentage of hematopoietic progenitor populations in the bone marrow of mice of the indicated genotypes treated with 3 consecutive doses of 5-FU (150 mg/kg) administered every 14 days, and analyzed 14 days after the last dose. Results are shown as mean ± SEM (n = 4-7 mice of each genotype). (C) Effect of neutralizing IFNAR1 during a 3-dose 5-FU regime on the percentage of hematopoietic progenitor populations. Mice were treated with control IgG or neutralizing anti-IFNAR1 antibody as described in “Materials and methods,” and analyzed 14 days after the last dose of 5-FU. Results are shown as mean ± SEM (n = 5 mice per condition). (D) Percentage of apoptotic cells (annexin V–positive [annexin V+] DAPI− [4′,6-diamidino-2-phenylindole–negative]) in hematopoietic progenitor populations in the bone marrow of mice of the indicated genotypes 55 days after TBI (5 Gy). Results are shown as mean ± SEM (n = 5-9 mice of each genotype). Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated.
Mice reconstituted upon DKO bone marrow transplant also compensated the imbalance of NFAT5-deficient HSPCs (Figure 6A-B; supplemental Figure 10A). This was more noticeable in HSC and MPP3 subsets (supplemental Figure 10A). Reconstituted DKO HSCs now had improved viability than NFAT5-deficient HSCs (Figure 6C). DKO hematopoietic progenitors also performed better than NFAT5-deficient hematopoietic progenitors in serial transplants, with secondary recipients improving peripheral blood reconstitution, including platelet size and number (supplemental Figure 10B; Figure 6D). IFN-1 can limit platelet production in response to inflammation40,41; our results suggest that this effect is inhibited by NFAT5. Secondary recipients of DKO bone marrow cells also had fairly normal proportions of HSCs and MPP subsets (Figure 6E; supplemental Figure 10C). Consistent with these findings, IFNAR1 deletion also improved the survival of mice reconstituted from NFAT5-deficient bone marrow in tertiary serial transplants (Figure 6F).
Deletion of IFNAR attenuates the reconstitution defects of NFAT5-deficient hematopoietic progenitors in BMT. (A) Donor-derived (CD45.2+) reconstitution of lineageneg bone marrow (upper panel), and percentage and number of LSK cells (middle and bottom panels) 13 weeks after primary BMT with the indicated genotypes. (B) Percentage and number of donor-derived hematopoietic progenitor populations in the bone marrow of mice reconstituted with bone marrow of the indicated genotypes. Results for HSC and MPP are from 2 independent transplants, each with 1 donor bone marrow of the respective genotype and 5 to 7 recipients. LT-HSC and ST-HSC distribution was analyzed in 1 experiment. (C) Percentage of donor-derived apoptotic cells (annexin V+ DAPI−) in hematopoietic progenitor subsets 13 weeks after BMT of the indicated genotypes. (D-E) Donor-derived reconstitution of bone marrow hematopoietic progenitor subsets (E) and blood platelets (D, plateletcrit [PCT], blood platelets per mL, and platelet distribution width [PDW]) after secondary transplant with bone marrow of the indicated genotypes. Secondary transplants used bone marrow from 1 of 2 experiments shown in panel B, using a pool of 6 donors of each genotype to reconstitute 6 to 7 secondary recipients. (F) Survival of mice in tertiary transplants with bone marrow cells derived from the indicated genotypes. Tertiary transplants used donor bone marrow pools of secondary transplanted mice from panel E to reconstitute 7 recipients. Results are shown as mean ± SEM. Statistical significance is shown for comparisons between the indicated genotypes, and was determined with an unpaired t test for panels A-E and a log-rank Mantel-Cox test for panel F. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated. LT, long-term; ST, short-term.
Deletion of IFNAR attenuates the reconstitution defects of NFAT5-deficient hematopoietic progenitors in BMT. (A) Donor-derived (CD45.2+) reconstitution of lineageneg bone marrow (upper panel), and percentage and number of LSK cells (middle and bottom panels) 13 weeks after primary BMT with the indicated genotypes. (B) Percentage and number of donor-derived hematopoietic progenitor populations in the bone marrow of mice reconstituted with bone marrow of the indicated genotypes. Results for HSC and MPP are from 2 independent transplants, each with 1 donor bone marrow of the respective genotype and 5 to 7 recipients. LT-HSC and ST-HSC distribution was analyzed in 1 experiment. (C) Percentage of donor-derived apoptotic cells (annexin V+ DAPI−) in hematopoietic progenitor subsets 13 weeks after BMT of the indicated genotypes. (D-E) Donor-derived reconstitution of bone marrow hematopoietic progenitor subsets (E) and blood platelets (D, plateletcrit [PCT], blood platelets per mL, and platelet distribution width [PDW]) after secondary transplant with bone marrow of the indicated genotypes. Secondary transplants used bone marrow from 1 of 2 experiments shown in panel B, using a pool of 6 donors of each genotype to reconstitute 6 to 7 secondary recipients. (F) Survival of mice in tertiary transplants with bone marrow cells derived from the indicated genotypes. Tertiary transplants used donor bone marrow pools of secondary transplanted mice from panel E to reconstitute 7 recipients. Results are shown as mean ± SEM. Statistical significance is shown for comparisons between the indicated genotypes, and was determined with an unpaired t test for panels A-E and a log-rank Mantel-Cox test for panel F. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated. LT, long-term; ST, short-term.
We then analyzed how IFNAR influenced gene expression in NFAT5-deficient progenitors after HSC transplantation, having first confirmed that DKO HSCs compensated NFAT5-deficient HSPC reconstitution (Figure 7A). RNA-sequencing analysis showed that both NFAT5 and IFNAR1 restricted the expression of MPP genes in HSCs (Figure 7B). MSigDB analysis again showed that NFAT5-deficient HSCs had upregulated MPP-associated hallmarks, such as E2F targets and mitotic spindle (Figure 7C; see also Figure 3A-B). Intriguingly, although DKO cells compensated viability, differentiation, and long-term reconstitution defects of NFAT5-deficient HSCs (see Figure 6; supplemental Figure 10A-C), they still showed upregulated E2F targets and mitotic spindle hallmarks as seen in NFAT5-deficient HSCs (Figure 7C). These comparisons also revealed that NFAT5-deficient HSCs had enhanced IFN responses compared with IFNAR1-deficient cells, which were attenuated in DKO cells (Figure 7C-D). Here, we noticed that of 411 genes upregulated (fold change of ≥2) in NFAT5-deficient HSCs vs DKO HSCs, only 29 were annotated as IFN-1 responsive in MSigDB and Interferome (Figure 7E). Likewise, of 84 genes upregulated by twofold or more in an IFNAR1-dependent manner in NFAT5-deficient HSCs vs WT cells, only 5 were annotated as IFN response in MSigDB and Interferome (supplemental Figure 11). This suggests that the genes we have identified by week 13 after transplantation mostly represent secondary targets of an early IFN-1 response that had already dissipated (as detected in Figure 4A), or previously unrecognized IFN-1 targets. Altogether, our findings showed that NFAT5 supports the function of HSCs under stress by restricting a long-term program of IFN-1–responsive genes.
Transcriptome analysis of hematopoietic progenitors reconstituted upon transplant of IFNAR1- and NFAT5-deficient HSCs. (A) Proportion and number of donor-derived LSK cells, HSCs and MPPs in bone marrow of mice that received transplantation with HSC donor cells (200 HSCs + 3 × 105 CD45.1 Sca1neg helper cells as in supplemental Figure 4A) of the indicated genotypes, and used for the RNA-sequencing analysis below. Results are shown as mean ± SEM. Statistical significance (unpaired t test) is shown for comparisons between the indicated genotypes. ∗P < .05, ∗∗P < .01. Transplants were done with 1 donor of HSCs of each genotype transplanted in 6 recipient mice. Equal numbers of FACS-sorted HSCs and MPPs from 6 recipient mice per genotype were pooled and processed for RNA sequencing. (B) Venn diagrams illustrating the numbers of differentially upregulated (FC ≥2.5) MPP-enriched (WT MPPs vs WT HSCs) genes in NFAT5-deficient (Nfat5 KO) vs WT HSCs; IFNAR1-deficient (Ifnar1 KO) vs WT HSCs; and double-deficient (DKO) vs WT HSCs. (C) Main MSigDB hallmarks enriched in the set of genes differentially upregulated (FC ≥2) in the indicated HSC comparisons. MSigDB hallmarks analysis was done with Enrichr. (D) Relative upregulation of IFN response genes in the MSigDB hallmarks database in pairwise comparisons of HSCs of the indicated genotypes. (E) Heat map showing the differential expression of a subset of 29 IFN-response genes in the comparisons indicated. This subset comprises genes annotated as IFN responsive in MSigDB and/or Interferome database, within the set of genes differentially upregulated (411 genes, FC ≥2) in NFAT5-deficient (Nfat5 KO) vs NFAT5 and IFNAR1 double-deficient HSCs (DKO). adj P, adjusted P value; CS, combined score.
Transcriptome analysis of hematopoietic progenitors reconstituted upon transplant of IFNAR1- and NFAT5-deficient HSCs. (A) Proportion and number of donor-derived LSK cells, HSCs and MPPs in bone marrow of mice that received transplantation with HSC donor cells (200 HSCs + 3 × 105 CD45.1 Sca1neg helper cells as in supplemental Figure 4A) of the indicated genotypes, and used for the RNA-sequencing analysis below. Results are shown as mean ± SEM. Statistical significance (unpaired t test) is shown for comparisons between the indicated genotypes. ∗P < .05, ∗∗P < .01. Transplants were done with 1 donor of HSCs of each genotype transplanted in 6 recipient mice. Equal numbers of FACS-sorted HSCs and MPPs from 6 recipient mice per genotype were pooled and processed for RNA sequencing. (B) Venn diagrams illustrating the numbers of differentially upregulated (FC ≥2.5) MPP-enriched (WT MPPs vs WT HSCs) genes in NFAT5-deficient (Nfat5 KO) vs WT HSCs; IFNAR1-deficient (Ifnar1 KO) vs WT HSCs; and double-deficient (DKO) vs WT HSCs. (C) Main MSigDB hallmarks enriched in the set of genes differentially upregulated (FC ≥2) in the indicated HSC comparisons. MSigDB hallmarks analysis was done with Enrichr. (D) Relative upregulation of IFN response genes in the MSigDB hallmarks database in pairwise comparisons of HSCs of the indicated genotypes. (E) Heat map showing the differential expression of a subset of 29 IFN-response genes in the comparisons indicated. This subset comprises genes annotated as IFN responsive in MSigDB and/or Interferome database, within the set of genes differentially upregulated (411 genes, FC ≥2) in NFAT5-deficient (Nfat5 KO) vs NFAT5 and IFNAR1 double-deficient HSCs (DKO). adj P, adjusted P value; CS, combined score.
Discussion
We report that IFN-1 drives HSCs toward more differentiated progenitors in response to hematopoietic stressors, and that NFAT5 has an HSC-intrinsic role limiting long-term IFN-1 responses to preserve their reconstitution potential. This is evidenced by the failure of NFAT5-deficient HSCs to reconstitute a hematopoietic system in serial transplants, and by the abnormal differentiation and apoptosis observed upon bone marrow transplant from panhematopoietic but not myeloid-restricted NFAT5-deficient mice. The cell-intrinsic role for NFAT5 in safeguarding HSC fitness was revealed by competitive transplant assays.
Acute and chronic stress affect adult HSCs in different manners,4,5,42 and this is also the case for inflammatory responses. For instance, acute IFN-1 responses transiently mobilize HSCs but do not deteriorate long-term regeneration,2,9 whereas prolonged inflammatory insults, such as caused by tissue damage, can activate IFN-1 signals that undermine HSC function.3-5,43 NFAT5 prevented the HSC to MPP bias upon transplantation, sublethal irradiation or serial 5-FU treatment by attenuating their response to IFN-1. Although this protective effect is evident in long-term scenarios, our previous work showed that NFAT5 does not influence acute responses of HSC to IFN-1.14 The detrimental role of persistent IFN-1 signaling in HSCs has been identified in pathological settings, such as chronic infections;3,44,45 and our findings now suggest that restraining the effect of IFN-1 might also improve long-term HSC reconstitution after bone marrow ablation. This adverse effect of IFN-1 contrasts with its beneficial role when locally produced in tumors during radiotherapy, which is fundamental for activating antitumor immunity.37,46 Our results showing improved viability and regenerative potential in NFAT5-deficient HSCs that lack IFN-1 responses also recall reports showing that IFNα administration to patients with leukemia increases toxicity upon TBI.47
Steady state HSCs and other stem cells express the IFNAR but are relatively refractory to the cytokine.48-51 However, extensive cellular and DNA damage trigger persistent IFN-1 signaling in the hematopoietic niche and cause HSC exhaustion.10,11 IFN-1 can also lead to death of hematopoietic progenitors as part of its ability to block the spread of infection.9,52 Our findings advance the understanding of mechanisms that protect HSCs by identifying NFAT5 as a factor that limits the detrimental effects of IFN-1 in HSCs.
Intriguingly, lack of IFNAR in NFAT5-deficient HSCs corrected enhanced apoptosis, differentiation toward MPPs, and reduced long-term potential during transplantation, yet it did not neutralize gene responses characteristic of HSC activation, such as overexpression of E2F targets and mitotic spindle hallmarks. Therefore, our results suggest that NFAT5 protects HSCs by modulating IFN-1–dependent and –independent responses. IFNAR-independent effects of NFAT5 in HSCs might involve conventional inflammatory pathways, such as TLR responses activated upon cellular damage.53 In this regard, NFAT5 enhances expression of TLR-induced inflammatory mediators such as interleukin-1, tumor necrosis factor α, or interleukin-6,16-18,20 which directly or indirectly activate HSCs.4
We propose that the main mechanism by which NFAT5 and IFNAR regulate the response to different stressors is the cell-intrinsic ability of NFAT5 to restrain IFN-1–induced long-term HSC differentiation to other hematopoietic progenitors, and particularly MPP3s, which can undermine the pool of HSCs and therefore compromise their long-term function. In contrast, NFAT5 and IFNAR contribute differently to HSC viability upon different hematopoietic insults. Although apoptosis of NFAT5-deficient HSCs reconstituted after transplant involves IFN-1, it is IFNAR-independent in irradiated mice, and neither NFAT5 nor IFNAR influence HSC viability after 5-FU treatment. These differences could reflect that different stressors might cause a variable amount of damage in the niche of HSCs, that their effect might last for a shorter or longer time, or even that they have a different mode of action in the target cell.54,55 For instance, HSCs transplanted in lethally irradiated hosts are exposed to stronger damage-induced inflammation signals than under sublethal irradiation,56 and 5-FU kills proliferating cells, and so selects for quiescent HSCs.28
The role of NFAT5 limiting HSC to MPP3 differentiation after transplant reconstitution, and after recovering from irradiation or chemoablation, was IFN-1-dependent, suggesting that NFAT5 might regulate overlapping sets of genes in these scenarios. However, our independent transcriptomics assays with transplant-reconstituted HSCs did not identify a common IFN-1 response signature, and moreover uncovered IFNAR and NFAT5-regulated genes not annotated previously as IFN-1 targets.39 This raises the possibility that NFAT5 might repress IFN-1 response genes indirectly, through other transcription regulators or chromatin modifications, as it does in other contexts.14,18
Persistent IFN-1 responses are proposed to have a long-term effect on adult HSCs and influence their repopulation capacity and future response to inflammation.9,44 This is more evident under enforced hematopoietic reconstitution upon transplantation, irradiation, or myeloablation, but can also be detected in steady-state in mouse models lacking inhibitors of the IFN-1 pathway.44,45 These reports are in line with our findings with hematopoietic-restricted NFAT5-deficient mice, which show a modest degree of increased HSC differentiation to MPPs (MPP3) under steady-state that is exacerbated upon transplantation, irradiation, or serial 5-FU treatment. Our identification of NFAT5 as a factor that limits the detrimental effect of IFN-1 in long-term HSC function reveals a mechanism by which the impact of sustained inflammation on stem cell exhaustion is reduced. Understanding how to exploit this mechanism has the potential to improve current treatments of hematopoietic malignancies.
Acknowledgments
The authors thank Alex Bote, Eva Julià, and Erika Ramírez and the Universitat Pompeu Fabra/Centre for Genomic Regulation (UPF/CRG) Flow Cytometry Unit for excellent guidance with flow cytometry. The authors also thank Berta Fusté, Marta Gut, Lidia Agueda, Julie Marie Blanc, and Matthew Ingham of Centro Nacional de Análisis Genómico (CNAG) for library preparation and RNA sequencing; the microarrays facility of Institut Hospital del Mar d'Investigacions Mèdiques (IMIM) for microarray assays, and particularly Lara Nonell, Marta Bódalo, and Eulàlia Puigdecanet. The authors are grateful to Anna Bigas and José Yélamos (IMIM), Alberto Yañez-Boyer (University of Valencia), and Maria Carolina Florian (Bellvitge Biomedical Research Institute) for helpful discussions. The authors thank Laura Higuera, Maria Val, and Marta Riera-Borrull of the laboratory of C.L.-R./J.A. for help with mouse and bone marrow processing and analysis, and for discussions. Veronica A. Raker is acknowledged for manuscript editing.
This work was supported by the Spanish Ministry of Science and Innovation and European Fund for Regional Development (FEDER) (RTI2018-095902-B-I00 and PID2021-128721OB-I00), Fundació la Marató de TV3 (201619-30), and the Worldwide Cancer Research United Kingdom Foundation (grant 20-0144). A.E.-C. was funded by ISCIII/MINECO (PT17/0009/0019) and cofunded by FEDER. Research in the laboratory of C.L.-R. and J.A. was also supported by the Generalitat de Catalunya (2021SGR00683) and the Spanish Ministry of Science Innovation through the “Unidad de Excelencia María de Maeztu” program funded by the Agencia Estatal de Investigación (MDM-2014-0370 and CEX2018-000792-M). L.T. was funded by a predoctoral contract (BES-2015-074170) associated to MDM-2014-0370. V.C.P. and H.H.E. were funded by the program Formación de Profesorado Universitario of Ministerio de Universidades (FPU19-04119 to V.C.P., and FPU13/01798 to H.H.E.). Additional funding to C.L.-R. is from the ICREA Acadèmia 2014 and 2022 awards of Institució Catalana de Recerca i Estudis Avançats (ICREA, Generalitat de Catalunya).
Authorship
Contribution: L.T. and V.C.P. performed and analyzed most of the experimental work and drafted the figures; H.H.E. collaborated in setting up the transplant assays; S.A. analyzed immediate IFN-1 responses to irradiation; A.E.-C. performed the bioinformatics analysis of the RNA sequencing data; O.F. optimized the flow cytometry analysis of hematopoietic precursors; and J.A. and C.L.-R. designed and supervised the work, interpreted the data, prepared the figures, wrote the manuscript, and obtained funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for H.H.E. is Haematopoietic Stem Cell Laboratory, The Francis Crick Institute, London, United Kingdom.
Correspondence: Cristina Lopez-Rodriguez, Department of Medicine and Life Sciences, Universitat Pompeu Fabra, Carrer Doctor Aiguader, 88, 08003 Barcelona, Spain; email: cristina.lopez-rodriguez@upf.edu; and Jose Aramburu, Department of Medicine and Life Sciences, Universitat Pompeu Fabra, Carrer Doctor Aiguader, 88, 08003 Barcelona, Spain; email: jose.aramburu@upf.edu.
References
Author notes
L.T., and V.C.P. contributed equally to this study.
Data sets included in this manuscript are deposited in Gene Expression Omnibus database. Accession numbers are indicated below. SuperSerie: GSE228577: Microarray analysis of mouse bone marrow hematopoietic progenitors from wild-type and NFAT5-deficient (Nfat5 fl/fl Vav-Cre) mice in steady state and after bone marrow transplantation. GSE228634: RNA-sequencing analysis of mouse bone marrow hematopoietic progenitors from wild-type, NFAT5-deficient (Nfat5 fl/fl Vav-Cre), IFN-1 receptor (IFNAR1)-deficient and NFAT5– and IFNAR1–double-deficient mice, after bone marrow transplantation.
For original data, requests should be addressed to the corresponding authors Cristina Lopez-Rodriguez (cristina.lopez-rodriguez@upf.edu) and Jose Aramburu (jose.aramburu@upf.edu).
The full-text version of this article contains a data supplement.

![NFAT5-deficient HSCs exhibit an altered transcriptome with enhanced MPP signatures. (A) MSigDB hallmarks enriched in the set of genes differentially upregulated (left panel) or downregulated (right panel) in HSCs sorted via FACS 13 weeks after bone marrow transplant (BMT) from Nfat5fl/fl Vav-Cre or littermate WT mice (n = 3). See supplemental Figure 6A for the sorting strategy. (B) MSigDB hallmarks upregulated or downregulated in WT MPPs vs WT HSCs 13 weeks after BMT. MSigDB hallmarks analysis was done with Enrichr. (C) Venn diagrams of the number of differentially expressed genes upregulated (left) and downregulated (right) in NFAT5-deficient vs WT HSCs that were also upregulated or downregulated in WT MPPs vs HSCs after BMT. The bottom panels show the Enrichr analysis of MSigDB hallmarks enriched in the set of MPP-like genes upregulated (left panel) or downregulated (right panel) in NFAT5-deficient vs WT HSCs. (D) Enrichment in short-term HSC (ST-HSC) and early progenitor signatures (from MSigDB C2-CGP MM676 and MM1012 data sets respectively) in the set of genes upregulated after BMT in NFAT5-deficient (knockout [KO]) vs WT HSCs, and in WT or NFAT5-deficient (KO) MPP vs HSC. MSigDB were analyzed with gene set enrichment analysis. (E) Venn diagrams of the number of differentially expressed genes upregulated (left) and downregulated (right) in NFAT5-deficient (Nfat5fl/fl Vav-Cre mice) vs WT HSCs that were also upregulated or downregulated, respectively, in WT MPPs vs HSCs in steady-state conditions. The fold-change (FC) values for the pairwise comparisons shown in the Venn diagrams in panels C,E were ≥2, with P values < .05. adj P, adjusted P value; CS, combined score; FDR, false discovery rate; NES, normalized enrichment score.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/21/10.1182_bloodadvances.2023011306/2/m_blooda_adv-2023-011306-gr3.jpeg?Expires=1769095044&Signature=0KMruCovIaR99FcGIH-sLlf~yNPT71omCVyts9OuQ~eNQ0~DcNEkgKs5i8ilf9itUxgXy4ztz69pFxgt9uwplGwJxlfaUEvtyh9yCUaTUWLp-blUgEHkiLtT5RApkizKPKqM2px00QDTJAZIsVYn4bN~FGIbK7UxMzK4bCyNGNZ100q8aQA0xvQEyhsiUkwJFd4Mrm1fiXcURSflyEc90Zu6bCA7rtFydJ-Kh6WCUgrOzrZvuhmocfxXR5AaK956ZXZMa7vnYjtM5uNm2OqlNTQeNUaBDzUqkVHom5o4vEXl0-qVKn5UX7alvO2Fs~TaE9BWnWG~TU7mPLOoL-Mugg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Neutralization of IFNAR attenuates the reconstitution defects of NFAT5-deficient hematopoietic progenitors upon γ irradiation or treatment with 5-FU. (A) Percentage of the indicated hematopoietic progenitor populations in the bone marrow of mice of the different genotypes 55 days after TBI (5 Gy). WT (Nfat5+/+ Vav-Cre, Ifnar1+/+), hematopoietic cell–specific NFAT5-deficient (Nfat5fl/fl Vav-Cre, Ifnar1+/+), IFNAR1-deficient (Nfat5+/+ Vav-Cre, Ifnar1–/–), and double deficient (Nfat5fl/fl Vav-Cre, Ifnar1–/–) mice. Results are shown as mean ± SEM (n = 6-12 mice of each genotype). (B) Percentage of hematopoietic progenitor populations in the bone marrow of mice of the indicated genotypes treated with 3 consecutive doses of 5-FU (150 mg/kg) administered every 14 days, and analyzed 14 days after the last dose. Results are shown as mean ± SEM (n = 4-7 mice of each genotype). (C) Effect of neutralizing IFNAR1 during a 3-dose 5-FU regime on the percentage of hematopoietic progenitor populations. Mice were treated with control IgG or neutralizing anti-IFNAR1 antibody as described in “Materials and methods,” and analyzed 14 days after the last dose of 5-FU. Results are shown as mean ± SEM (n = 5 mice per condition). (D) Percentage of apoptotic cells (annexin V–positive [annexin V+] DAPI− [4′,6-diamidino-2-phenylindole–negative]) in hematopoietic progenitor populations in the bone marrow of mice of the indicated genotypes 55 days after TBI (5 Gy). Results are shown as mean ± SEM (n = 5-9 mice of each genotype). Statistical test: unpaired t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/21/10.1182_bloodadvances.2023011306/2/m_blooda_adv-2023-011306-gr5.jpeg?Expires=1769095044&Signature=f0KhktAUBgBXuu6E3CR8VlFuatLN3Whd0cSmyoADSdANElIUg9giCCEyMM41E-OlEskwYMjJgpQxp5ki-PofPJFvIXQvaxt1eehLyCuH1sWyHaUcK84EssDXgl-pqtgn5dfQuS7x3PpAzL270iamARLmhPEN0GQTPX4jCh0WLWjoGfqzO2Z6g4S5XK3Fi8e9X6FeW7QQIeYZzZO0SerC-JxXiHlq37r2QCJZ-bfG~TolC8b6KHpG0WjFWFADYBRpiXpUsCPQOLXjzlyxk5y~6vZ1tArJvHWSUY1uAwTRKhqki-pC0ICMYOHIpl6bOq4bjFy5ZtvQyyAGY0AqqfhbNg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Deletion of IFNAR attenuates the reconstitution defects of NFAT5-deficient hematopoietic progenitors in BMT. (A) Donor-derived (CD45.2+) reconstitution of lineageneg bone marrow (upper panel), and percentage and number of LSK cells (middle and bottom panels) 13 weeks after primary BMT with the indicated genotypes. (B) Percentage and number of donor-derived hematopoietic progenitor populations in the bone marrow of mice reconstituted with bone marrow of the indicated genotypes. Results for HSC and MPP are from 2 independent transplants, each with 1 donor bone marrow of the respective genotype and 5 to 7 recipients. LT-HSC and ST-HSC distribution was analyzed in 1 experiment. (C) Percentage of donor-derived apoptotic cells (annexin V+ DAPI−) in hematopoietic progenitor subsets 13 weeks after BMT of the indicated genotypes. (D-E) Donor-derived reconstitution of bone marrow hematopoietic progenitor subsets (E) and blood platelets (D, plateletcrit [PCT], blood platelets per mL, and platelet distribution width [PDW]) after secondary transplant with bone marrow of the indicated genotypes. Secondary transplants used bone marrow from 1 of 2 experiments shown in panel B, using a pool of 6 donors of each genotype to reconstitute 6 to 7 secondary recipients. (F) Survival of mice in tertiary transplants with bone marrow cells derived from the indicated genotypes. Tertiary transplants used donor bone marrow pools of secondary transplanted mice from panel E to reconstitute 7 recipients. Results are shown as mean ± SEM. Statistical significance is shown for comparisons between the indicated genotypes, and was determined with an unpaired t test for panels A-E and a log-rank Mantel-Cox test for panel F. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. P values < .1 are also indicated. LT, long-term; ST, short-term.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/21/10.1182_bloodadvances.2023011306/2/m_blooda_adv-2023-011306-gr6.jpeg?Expires=1769095044&Signature=fMeGOkxzOiLijI~rfcTaKTZ1yaquMp1cyJfw-IVZaIKzp~07lO~cJMwRNezOdODJATRHIVqG4P5rkCzSwuSYg1RqKecSyMtdml2m1Tte~U5p-Fdo~SXuOADvsWpSSILeNlA5xrve3a5xrdwNjVEoq2Hd-3YbEJwxGP7P-XTKPPi1ESMfusi0m~WdCSfjWruV2p6im5aKjy0sIKCeSlBx3ZJ3KXtfscITXRnYoWrObhAmFOZAMKM4cYQZV51T21kcz7KQaKil2kuuN6TwVuQtKJVZ~1styVqAvIUo1~CZmwbxUPwVUOo8W8tzpV5pjLrAv4sYbD6kBYEaTJOrr0glBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)