A risk-adapted clinical trial for IDH2-mutant AML demonstrates feasibility of achieving responses with upfront ENA monotherapy.
Mechanisms of therapeutic resistance include clonal evolution and inform future directions of investigation with ENA.
Visual Abstract
Enasidenib (ENA) is an inhibitor of isocitrate dehydrogenase 2 (IDH2) approved for the treatment of patients with IDH2-mutant relapsed/refractory acute myeloid leukemia (AML). In this phase 2/1b Beat AML substudy, we applied a risk-adapted approach to assess the efficacy of ENA monotherapy for patients aged ≥60 years with newly diagnosed IDH2-mutant AML in whom genomic profiling demonstrated that mutant IDH2 was in the dominant leukemic clone. Patients for whom ENA monotherapy did not induce a complete remission (CR) or CR with incomplete blood count recovery (CRi) enrolled in a phase 1b cohort with the addition of azacitidine. The phase 2 portion assessing the overall response to ENA alone demonstrated efficacy, with a composite complete response (cCR) rate (CR/CRi) of 46% in 60 evaluable patients. Seventeen patients subsequently transitioned to phase 1b combination therapy, with a cCR rate of 41% and 1 dose-limiting toxicity. Correlative studies highlight mechanisms of clonal elimination with differentiation therapy as well as therapeutic resistance. This study demonstrates both efficacy of ENA monotherapy in the upfront setting and feasibility and applicability of a risk-adapted approach to the upfront treatment of IDH2-mutant AML. This trial is registered at www.clinicaltrials.gov as #NCT03013998.
Introduction
Acute myeloid leukemia (AML) is a lethal blood cancer characterized by aberrant proliferation of myeloid stem and progenitor cells harboring a block in differentiation.1 The transforming events conferring these malignant properties to hematopoietic cells can be initiated by a combination of leukemogenic mutations and structural alterations that drive aberrant self-renewal and block terminal differentiation.2 Although the mainstay of treatment for younger patients with newly diagnosed AML remains intensive induction chemotherapy,3 advances in understanding the biology of AML has led to the development of targeted therapies, including for patients with mutations in FLT3 and isocitrate dehydrogenase 1/2 (IDH1/2). Both the first-generation FLT3 inhibitor midostaurin4 and second-generation FLT3 inhibitor quizartinib5 improve overall survival when combined with 7 days of cytarabine plus 3 days of an anthracycline (7+3) compared with 7+3 alone. The Food and Drug Administration’s (FDA) approval of midostaurin plus induction chemotherapy for FLT3-mutant AML ushered in a new era of precision oncology for the management of newly diagnosed AML, obligating clinicians to achieve rapid genomic profiling to determine FLT3 mutation status before initiating induction therapy.4 The Beat AML Master Trial further demonstrated the feasibility of integrating prospective genomic profiling into the workflow of newly diagnosed AML therapy.6 For older patients with AML, the use of azacitidine (AZA) with venetoclax leads to high rates of response and improvement in overall survival compared with AZA monotherapy but induces significant myelosuppression and transfusional requirements.7 For newly diagnosed patients with IDH1-mutant AML unfit to receive induction chemotherapy, the FDA recently approved ivosidenib in combination with AZA based on phase 3 randomized data from the AGILE trial comparing AZA alone or together with ivosidenib 500 mg, showing improvement in both overall survival and event-free survival with combination therapy.8 In contrast, patients aged >60 years with IDH2-mutant AML who received intensive induction chemotherapy had poor outcomes, with a 3-year overall survival rate of 15%.9 Therapy that avoids myelosuppression has the potential to improve tolerability, quality of life, and survival in patients with AML.
Recurrent mutations in isocitrate dehydrogenase (IDH) are present in ∼20% of patients with AML.10 These enzymes function as homodimers involved in critical cellular processes including chromatin modification and metabolism.11 Wild-type isoforms of IDH1 and IDH2 catalyze a decarboxylation reaction converting isocitrate to α-ketoglutarate. However, oncogenic IDH1/2 mutations confer neomorphic activity that produces 2-hydroxyglutarate (2-HG), an onco-metabolite that poisons α-ketoglutarate–dependent cellular processes and perturbs epigenetic states that contribute to the differentiation blockade seen in IDH-mutant hematopoietic stem and progenitor cells.12,13
Enasidenib (ENA) is a first-in-class, orally available small-molecule inhibitor of IDH2 that potently suppresses the neomorphic production of 2-HG mediated by mutant IDH2.14 ENA monotherapy exhibits less myelosuppression compared with cytotoxic chemotherapy and has clinical activity in the relapsed setting, which led to its FDA approval based on complete remission/complete remission with partial hematologic recovery (CR/CRh) rates of 23%, a median duration of CR/CRh of 8.2 months, and independence from red blood cell and platelet transfusions.15,16
AZA is a hypomethylating agent (HMA) that inhibits and degrades DNA methyltransferase 1 and exhibits modest clinical activity as monotherapy17 in AML. IDH inhibitors have been used in combination with AZA with promising results.8,18,19 AG221-AML-005 was a phase 1b/2, multicenter, open-label trial that enrolled patients with newly diagnosed IDH2-mutant AML.18 The phase 1b dose-finding portion of the study established ENA 100 mg as the selected dose to be studied in phase 2 in combination with AZA. When compared with the AZA-only arm, ENA plus AZA resulted in a doubling of both overall response (74% for ENA/AZA and 36% for AZA alone) and CR rates (54% for ENA/AZA and 12% for AZA alone), but no difference in event-free survival or overall survival was observed between the 2 treatment arms.18 Notably, grade 3 or 4 thrombocytopenia, neutropenia, and anemia were reported as adverse events with a frequency that was comparable in both treatment groups, suggesting that these adverse effects were largely driven by the known myelosuppressive effects of AZA.
In the era of HMA/venetoclax combination therapy for AML, induction-ineligible patients with IDH1- or IDH2-mutant AML exhibit high response rates with durable remissions after upfront treatment with AZA/venetoclax. However, in a pooled analysis that included patients enrolled in the phase 3 VIALE-A study, Pollyea et al reported high rates of hematologic treatment-emergent adverse events within the IDH-mutant population of patients with AML treated with AZA/venetoclax (82.7%, grade 3 or higher).20 Similar to AG221-AML-005, AZA seemed to be a major contributor to these toxicities because 67.9% of patients with IDH-mutant AML treated with AZA alone experienced hematologic treatment-emergent adverse events of grade 3 or higher.
Within this context, we hypothesized that a stepwise approach to the treatment of IDH2-mutant AML, beginning with ENA monotherapy, followed by the subsequent addition of HMA therapy in patients who do not achieve an adequate response, would be a safe and effective targeted approach to upfront AML-directed therapy for patients aged ≥60 years, particularly those patients with specific comorbidities who would be unable to tolerate hematologic toxicities conferred by AZA or other HMA combinations. We conducted this phase 2/1b clinical trial as part of the Beat AML umbrella study master protocol. The combined phase 2 portion of this study was designed to assess the overall response to ENA monotherapy, and the phase 1b portion of the trial was designed to assess the safety of adding AZA to ENA in patients who do not achieve a response to monotherapy within 5 cycles.
Methods
Study population
Patients aged ≥60 years with suspected AML were eligible for genetic screening under the Beat AML Master Trial (www.clinicaltrials.gov: #NCT03013998).6 Patients with a confirmed new diagnosis of AML harboring R140/R172 mutations in IDH2 and Eastern Cooperative Oncology Group performance status (PS) scores of 0 to 2 were assigned to and enrolled in this substudy, which accrued patients across 16 sites throughout the United States. Full inclusion and exclusion criteria are described in supplemental Table 1. All patients provided written informed consent before enrollment. The study protocol and all amendments were approved by review boards and ethics committees at all accruing sites.
Study design
This was a phase 2 study assessing the efficacy of single-agent ENA in treating IDH2-mutant AML, with subsequent response-driven addition of AZA. Patients were treated with single-agent 100 mg ENA daily, continuously in 28-day cycles for up to 5 cycles. Patients who achieved a response, defined as CR or incomplete blood count recovery (CRi), by the end of 5 cycles continued with single-agent ENA until patients exhibited disease progression, proceeded to allogeneic stem cell transplantation, or experienced unacceptable toxicity. Patients who did not achieve a response by the end of cycle 5 or who progressed before this time were administered AZA (75 mg/m2, days 1-7) concurrently with ENA in the phase 1b portion of this study to evaluate the safety of this combination. Patients who achieved a response (CR/CRi) after up to 4 more cycles of combination continued therapy, whereas those who did not achieve a CR/CRi discontinued treatment.
Sample preparation for correlative studies
Mononuclear cells were isolated from either the bone marrow aspirate or peripheral blood using a Ficoll gradient and cryopreserved until analysis by flow cytometry and next-generation sequencing. Plasma samples for pharmacokinetics and 2-HG analysis were isolated immediately after drawing blood and frozen until testing.
Flow cytometry
Cryopreserved mononuclear cells from serial bone marrow aspirate and peripheral blood samples were assessed for clonal and immunophenotypic changes by flow cytometry. Samples were thawed, washed, and stained with LIVE/DEAD Fixable Near-IR Dead Cell Stain (Invitrogen, no. L10119), followed by suspension in fluorescence-activated cell sorting buffer containing Brilliant stain buffer (BD, no. 566349). Samples were Fc-blocked using human immunoglobulin G (Athens Research & Technology, no. 16-16-090707) at a concentration of 10 μg/100 μL. Samples were then stained with the following antibodies along with appropriate controls: CD66b (Beckman Coulter, no. IM0531U), CD117 (BD, no. 567132), CD14 (BD, no. 562335), CD11b (BioLegend, no. 301328), CD56 (BioLegend, no. 392412), CD34 (BD, no. 555824), CD16 (BioLegend, no. 302026), CD163 (BD, no. 562643), CD3 (BioLegend, no. 344828), CD19 (BioLegend, no. 302244), CD38 (BioLegend, no. 356620), CD45 (BD, no. 563716), and CD123 (BD, no. 564195). All samples were analyzed on a BD LSRFortessa Cell Analyzer. Data analyses were done in Kaluza (Beckman Coulter) and Cytobank (Beckman Coulter). Figures were generated in Cytobank.21
Next-generation sequencing
DNA and RNA were isolated from cryopreserved mononuclear cells from serial bone marrow aspirate and peripheral blood samples. DNA sequencing libraries were prepared and enriched using the VariantPlex Myeloid Panel (ArcherDx/Integrated DNA Technologies, Carlsbad, IA), and RNA sequencing libraries were prepared and enriched using the FusionPlex Myeloid Panel (ArcherDx/Integrated DNA Technologies). Libraries were sequenced on the NovaSeq platform according to the manufacturer’s suggested protocol (Illumina, San Diego, CA). Variants were predicted using ArcherAnalysis (ArcherDx/Integrated DNA Technologies). Baseline mutations were identified by sequencing described in the Beat AML Master Trial and visualized using OncoPrinter from cBioPortal.6,22,23
Pharmacodynamics (2-HG) and pharmacokinetics
Serial plasma samples were collected as described above and analyzed for AG-221 and its active metabolite AGI-0016903. The plasma pharmacokinetic parameters of AG-221 and AGI-0016903, including, but not limited to, Cmax, Tmax, and area under the curve, were determined using noncompartmental methods in Phoenix WinNonlin Version 6.3 (Certara, Princeton, NJ). Serial pharmacodynamic samples were similarly analyzed for 2-HG and correlated with flow cytometry and next-generation sequencing data.
Statistics
The initial phase 2 portion of the study used a 3-outcome, 2-stage design to test the null hypothesis of a 20% composite complete response (cCR) rate (H0: P ≤ .2) against the alternative hypothesis of 50% (Ha: P ≥ .5). The 3 possible outcomes of this design included the following: (i) reject the alternative hypothesis and declare futility of single-agent ENA, (ii) reject the null hypothesis and declare sufficient efficacy of the single-agent ENA, and (iii) reject neither and proceed to test the agent in combination in a new phase 2 study. A sample size of 24 patients ensured a probability of 83% of correctly declaring futility if the null hypothesis was true and a probability of 73% of correctly declaring efficacy if the alternative hypothesis was true. An interim analysis occurred after the first 9 patients were treated.
Given that 11 of the first 24 patients achieved a cCR, the original phase 2 trial was expanded to enroll 36 additional patients, and the null hypothesis was revised to a 30% cCR rate (H0: P ≤ .3). In the final analysis, if ≥29 responses were observed out of all 60 patients, the revised null hypothesis was rejected. The overall sample size of 60 patients ensured a 1-sided type 1 error rate of 0.025 and provided a 77% chance of correctly declaring efficacy when the alternative hypothesis was true, conditional on the 2 prior interim analyses.
Any patient who did not achieve a response in the first 5 cycles of single-agent ENA was allowed to enroll in the phase 1b portion of the study, in which they received the combination ENA plus AZA. Patients were allowed 5 cycles to achieve a response based on the first-in-human phase 1/2 study of ENA in which 87.3% responding patients with relapse/refractory AML achieved their first response by cycle 5, suggesting that responses occurring after the addition of AZA would likely be due to the combined therapy.16 The phase 1b portion of the study used a 3+3 design evaluating ENA doses of 100 mg/day and 1 possible de-escalated dose of 50 mg/day in combination with AZA (75 mg/m2, days 1-7).
Overall survival was measured from date of initiation of single-agent ENA for phase 2 and date of initiation of combination of ENA and AZA for phase 1b; patients who were alive were censored at time of last follow-up. Overall survival was estimated using the Kaplan-Meier method.
Results
Between 13 April 2017 and 19 November 2019, 64 patients with newly diagnosed, dominant-clone, IDH2-mutant AML consented to the S3 substudy of the Beat AML trial (Figure 1). The mutation is considered to be a part of the dominant clone, per the treatment assignment algorithm defined in the BeatAML Master Trial, if it has a variant allelic frequency (VAF) >30%.6 If all mutations occur at a VAF of <30%, then the mutation with the highest VAF is considered part of the dominant clone and prioritized for assignment. Two patients were ineligible, and 2 patients did not start therapy because of other reasons. The remaining 60 patients who consented were given ENA monotherapy at a dose of 100 mg daily upfront for up to 5 cycles, as part of either the initial phase 2 cohort (n = 24) or the subsequent phase 2 expansion cohort (n = 36). The median age of the patients enrolled on study was 75 years (range, 60-89 years). Although the majority of the patients had a PS of 0 or 1, 20% of the patients had a PS of 2. In addition, 10% of the patients had therapy-related AML. Although prior studies have demonstrated that patients with IDH2-mutant disease most commonly present with a normal karyotype and intermediate-risk disease, 53% of older patients with AML in this study had adverse-risk AML by European LeukemiaNet criteria.3 The remainder of the baseline demographic and disease characteristics are provided in Table 1. The primary end point of the study was overall response, which includes rates of CR or complete response with CRi, as per European LeukemiaNet criteria, to ENA monotherapy therapy. In the initial phase 2 cohort, 11 of 24 patients achieved a CR/CRi after 5 cycles of monotherapy (Table 2; cCR rate of 46%; 95% exact confidence interval [CI], 26-67). Three patients receiving monotherapy discontinued treatment before the completion of 5 cycles owing to death (n = 2) or treatment failure (n = 1). Having met the prespecified primary end point of the initial phase 2 cohort, a phase 2 expansion cohort was established, in which 18 of 36 patients achieved a CR/CRi on 100 mg of ENA monotherapy (CR rate of 50%; 95% exact CI, 33-67). Combining both portions of the phase 2 cohorts, 29 of 60 patients achieved a CR/CRi for a cCR rate of 48% (95% exact CI, 35-62). The median duration of response in the phase 2 cohorts was 11.1 months (95% CI, 5.6-41.4), with 69% of patients without progression at 6 months and 38% of patients without progression at 24 months (Table 3; Figure 2B).
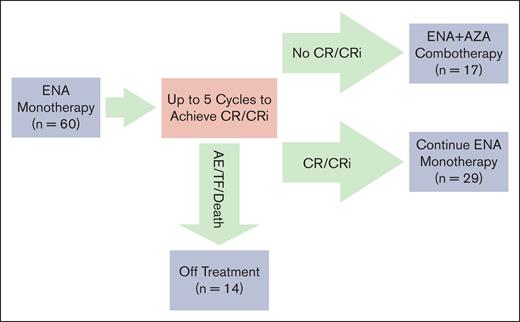
Patients with suspected AML harboring IDH2 R140/R172 mutations were screened under the Beat AML Master Trial and consented for this study (n = 64). Eligible patients started on ENA monotherapy with up to 5 cycles to achieve a CR or CRi (n = 60). Patients who achieved a CR/CRi by 5 cycles continued on ENA monotherapy until relapse (n = 29). Patients who did not achieve a CR/CRi started ENA plus AZA combination therapy (n = 17). Patients who experienced treatment failure, death, withdrew, or an adverse event warranting discontinuation during the first 5 cycles of ENA monotherapy went off study (n = 14). Patients were accrued in 2 stages, n = 24 and n = 36, respectively.
Patients with suspected AML harboring IDH2 R140/R172 mutations were screened under the Beat AML Master Trial and consented for this study (n = 64). Eligible patients started on ENA monotherapy with up to 5 cycles to achieve a CR or CRi (n = 60). Patients who achieved a CR/CRi by 5 cycles continued on ENA monotherapy until relapse (n = 29). Patients who did not achieve a CR/CRi started ENA plus AZA combination therapy (n = 17). Patients who experienced treatment failure, death, withdrew, or an adverse event warranting discontinuation during the first 5 cycles of ENA monotherapy went off study (n = 14). Patients were accrued in 2 stages, n = 24 and n = 36, respectively.
Demographics and baseline characteristics
| Characteristic . | Phase 2 and Exp (n = 60) . | Phase 1b∗ (n = 17) . |
|---|---|---|
| Age, median (range), y | 75 (60-89) | 75 (60-86) |
| Age ≥75 y, n (%) | 31 (52) | 9 (53) |
| Gender, n (%) | ||
| Female | 31 (52) | 7 (41) |
| Male | 29 (48) | 10 (59) |
| Race, n (%) | n = 57 | |
| Caucasian | 47 (82) | 13 (76) |
| African American | 5 (9) | 0 (0) |
| Asian | 4 (7) | 4 (24) |
| Hispanic | 1 (2) | 0 (0) |
| Unknown | 3 | 0 |
| PS, n (%) | ||
| 0 | 16 (27) | 7 (41) |
| 1 | 32 (53) | 6 (35) |
| 2 | 12 (20) | 4 (24) |
| Hemoglobin, median (range), g/dL | 8.3 (6.8-13.1) | 9.1 (6.8-12.4) |
| Platelets, median (range), 109/L | 58 (5-517) | 91 (17-335) |
| WBC, median (range), 109/L | 3.2 (0.4-54.5) | 3 (0.9-54.5) |
| WBC >50, n (%) | 2 (3) | 1 (6) |
| Creatinine, U/L2 | 0.9 (0.5-1.9) | 0.9 (0.6-1.9) |
| LDH, U/L | 276.5 (104-3350) | 184 (131-500) |
| ALT, U/L3 | 20 (6-93) | 18 (8-33) |
| AST, U/L | 22 (7-74) | 19 (11-34) |
| Total bilirubin, mg/dL | 0.6 (0.2-1.6) | 0.6 (0.2-1.0) |
| Blood blasts, % | ||
| Median (range) | 26.5 (0-98) | 13 (0-46.8) |
| Not assessed/unknown | 6 | 3 |
| Bone marrow blasts, % | ||
| Median (range) | 52 (13-95) | 60 (13-95) |
| Not assessed/unknown | 1 | 0 |
| Treatment-related AML, n (%) | ||
| No | 54 (90) | 15 (88) |
| Yes | 6 (10) | 2 (12) |
| IDH2 mutation | ||
| Median VAF (range) | 40.5 (6-58) | 36 (14-51) |
| R140, n (%) | 44 (73) | 15 (88) |
| R172, n (%) | 16 (27) | 2 (12) |
| ELN risk, n (%) | ||
| Adverse | 32 (53) | 8 (47) |
| Intermediate | 17 (28) | 5 (29) |
| Favorable | 11 (18) | 4 (24) |
| Characteristic . | Phase 2 and Exp (n = 60) . | Phase 1b∗ (n = 17) . |
|---|---|---|
| Age, median (range), y | 75 (60-89) | 75 (60-86) |
| Age ≥75 y, n (%) | 31 (52) | 9 (53) |
| Gender, n (%) | ||
| Female | 31 (52) | 7 (41) |
| Male | 29 (48) | 10 (59) |
| Race, n (%) | n = 57 | |
| Caucasian | 47 (82) | 13 (76) |
| African American | 5 (9) | 0 (0) |
| Asian | 4 (7) | 4 (24) |
| Hispanic | 1 (2) | 0 (0) |
| Unknown | 3 | 0 |
| PS, n (%) | ||
| 0 | 16 (27) | 7 (41) |
| 1 | 32 (53) | 6 (35) |
| 2 | 12 (20) | 4 (24) |
| Hemoglobin, median (range), g/dL | 8.3 (6.8-13.1) | 9.1 (6.8-12.4) |
| Platelets, median (range), 109/L | 58 (5-517) | 91 (17-335) |
| WBC, median (range), 109/L | 3.2 (0.4-54.5) | 3 (0.9-54.5) |
| WBC >50, n (%) | 2 (3) | 1 (6) |
| Creatinine, U/L2 | 0.9 (0.5-1.9) | 0.9 (0.6-1.9) |
| LDH, U/L | 276.5 (104-3350) | 184 (131-500) |
| ALT, U/L3 | 20 (6-93) | 18 (8-33) |
| AST, U/L | 22 (7-74) | 19 (11-34) |
| Total bilirubin, mg/dL | 0.6 (0.2-1.6) | 0.6 (0.2-1.0) |
| Blood blasts, % | ||
| Median (range) | 26.5 (0-98) | 13 (0-46.8) |
| Not assessed/unknown | 6 | 3 |
| Bone marrow blasts, % | ||
| Median (range) | 52 (13-95) | 60 (13-95) |
| Not assessed/unknown | 1 | 0 |
| Treatment-related AML, n (%) | ||
| No | 54 (90) | 15 (88) |
| Yes | 6 (10) | 2 (12) |
| IDH2 mutation | ||
| Median VAF (range) | 40.5 (6-58) | 36 (14-51) |
| R140, n (%) | 44 (73) | 15 (88) |
| R172, n (%) | 16 (27) | 2 (12) |
| ELN risk, n (%) | ||
| Adverse | 32 (53) | 8 (47) |
| Intermediate | 17 (28) | 5 (29) |
| Favorable | 11 (18) | 4 (24) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELN, European LeukemiaNet; LDH, lactate dehydrogenase.
The baseline characteristics of phase 1b patients were measured at the start of the study.
Treatment outcomes
| Treatment outcomes . | Phase 2 and Exp (n = 60) . | Phase 1b (n = 17) . |
|---|---|---|
| Best response, n (%) | ||
| CR | 22 (37) | 4 (24) |
| CRh | 5 (8)∗ | 2 (12) |
| CRi | 2 (3) | 1 (6) |
| MLFS | 1 (2) | 1 (6) |
| Partial remission | 2 (3) | 1 (6) |
| Stable disease | 21 (35) | 3 (18) |
| Progressive disease | 2 (3) | 0 (0) |
| Treatment failure | 1 (2) | 0 (0) |
| Not evaluated | 4 (7)† | 5 (29)‡ |
| cCR rate [CR/CRi], n (%, 95% CI) | 29 (48, adjusted: 30.3-60.5)§ | 7 (41, 18-67) |
| Median time to best response [CR/CRi] | (n = 29) | (n = 7) |
| Months (range) | 3 (0.9-11) | 3.7 (0.7-6.8) |
| Overall response rate [CR/CRi/MLFS], n (%, 95% CI) | 30 (50, 37-63) | 8 (47, 23-72) |
| Treatment outcomes . | Phase 2 and Exp (n = 60) . | Phase 1b (n = 17) . |
|---|---|---|
| Best response, n (%) | ||
| CR | 22 (37) | 4 (24) |
| CRh | 5 (8)∗ | 2 (12) |
| CRi | 2 (3) | 1 (6) |
| MLFS | 1 (2) | 1 (6) |
| Partial remission | 2 (3) | 1 (6) |
| Stable disease | 21 (35) | 3 (18) |
| Progressive disease | 2 (3) | 0 (0) |
| Treatment failure | 1 (2) | 0 (0) |
| Not evaluated | 4 (7)† | 5 (29)‡ |
| cCR rate [CR/CRi], n (%, 95% CI) | 29 (48, adjusted: 30.3-60.5)§ | 7 (41, 18-67) |
| Median time to best response [CR/CRi] | (n = 29) | (n = 7) |
| Months (range) | 3 (0.9-11) | 3.7 (0.7-6.8) |
| Overall response rate [CR/CRi/MLFS], n (%, 95% CI) | 30 (50, 37-63) | 8 (47, 23-72) |
MLFS, morphologic leukemia-free state.
All 5 CRh also qualified as CRi.
Early death within the first cycle before assessment could be performed.
Went off treatment before finishing the first cycle of combination therapy.
Given the conditional expansion design, which was conditional on the success of 2 prior interim stages, an adjusted 95% CI is provided for the primary end point. The naïve 95% CI on the CR/CRi rate of 48% that does not consider prior interim analyses is 35% to 62%.
Response duration
| Duration of response∗ . | Phase 2 and Exp (n = 29) . | Phase 1b (n = 7) . |
|---|---|---|
| Response duration | ||
| No. of events | 19 | 4 |
| Median (95% CI) | 11.1 (5.6-41.4) | 14.6 (0.5-NE) |
| % without event at 6 months (95% CI) | 69 (49-82) | 57 (17-84) |
| % without event at 24 months (95% CI) | 38 (21-55) | 43 (10-73) |
| Median follow-up (range) | 34.2 (19.6-47.8) | 45.7 (43.1-52.3) |
| Duration of response∗ . | Phase 2 and Exp (n = 29) . | Phase 1b (n = 7) . |
|---|---|---|
| Response duration | ||
| No. of events | 19 | 4 |
| Median (95% CI) | 11.1 (5.6-41.4) | 14.6 (0.5-NE) |
| % without event at 6 months (95% CI) | 69 (49-82) | 57 (17-84) |
| % without event at 24 months (95% CI) | 38 (21-55) | 43 (10-73) |
| Median follow-up (range) | 34.2 (19.6-47.8) | 45.7 (43.1-52.3) |
Duration of response was calculated from the time of first response (CR/CRi) to progression or death, whichever occurred first; patients without event were censored at the time of last follow-up.
Durability of responses for patients who achieved a remission. Duration of response estimated by the Kaplan-Meier method for phase 1b (A) and phase 2 and Exp (B).
Durability of responses for patients who achieved a remission. Duration of response estimated by the Kaplan-Meier method for phase 1b (A) and phase 2 and Exp (B).
Consistent with data from the initial first-in-human ENA phase 1/2 study in relapsed or refractory AML,16 ENA monotherapy in the phase 2 cohort was well tolerated. The most common treatment-related adverse event in the phase 2 cohort was grade 1 or 2 hyperbilirubinemia.16 Treatment-related grade 3 or higher adverse events seen in >1 patient (Table 4) were differentiation syndrome (20%), neutropenia (6.7%), thrombocytopenia (5%), and anemia (3.3%). There were no grade 5 adverse events related to differentiation syndrome.
Treatment-related adverse events captured in greater than 10% of patients per cohort
| Adverse event, n (%)∗ . | Phase 2 and Exp (n = 60) . | Phase 1b (n = 17) . | ||||
|---|---|---|---|---|---|---|
| Grade . | 1/2 . | 3+ . | All . | 1/2 . | 3+ . | All . |
| Neutropenia | 0 (0) | 4 (6.7) | 4 (6.7) | 0 (0) | 7 (41.2) | 7 (41.2) |
| Anemia | 1 (1.7) | 2 (3.3) | 3 (5) | 2 (11.8) | 1 (5.9) | 3 (17.6) |
| Thrombocytopenia | 0 (0) | 3 (5) | 3 (5) | 2 (11.8) | 3 (17.6) | 5 (29.4) |
| Leukopenia | 1 (1.7) | 1 (1.7) | 2 (3.3) | 1 (5.9) | 5 (29.4) | 6 (35.3) |
| Lymphopenia | 0 (0) | 1 (1.7) | 1 (1.7) | 1 (5.9) | 3 (17.6) | 4 (23.5) |
| Nausea | 21 (35) | 0 (0) | 21 (35) | 3 (17.6) | 2 (11.8) | 5 (29.4) |
| Diarrhea | 13 (21.7) | 0 (0) | 13 (21.7) | 2 (11.8) | 1 (5.9) | 3 (17.6) |
| Vomiting | 6 (10) | 0 (0) | 6 (10) | 3 (17.6) | 1 (5.9) | 4 (23.5) |
| Blood bilirubin increased | 23 (38.3) | 1 (1.7) | 24 (40) | 7 (41.2) | 2 (11.8) | 9 (52.9) |
| Decreased appetite | 11 (18.3) | 1 (1.7) | 12 (20) | 2 (11.8) | 0 (0) | 2 (11.8) |
| Differentiation syndrome | 1 (1.7) | 12 (20) | 13 (21.7) | 0 (0) | 2 (11.8) | 2 (11.8) |
| Adverse event, n (%)∗ . | Phase 2 and Exp (n = 60) . | Phase 1b (n = 17) . | ||||
|---|---|---|---|---|---|---|
| Grade . | 1/2 . | 3+ . | All . | 1/2 . | 3+ . | All . |
| Neutropenia | 0 (0) | 4 (6.7) | 4 (6.7) | 0 (0) | 7 (41.2) | 7 (41.2) |
| Anemia | 1 (1.7) | 2 (3.3) | 3 (5) | 2 (11.8) | 1 (5.9) | 3 (17.6) |
| Thrombocytopenia | 0 (0) | 3 (5) | 3 (5) | 2 (11.8) | 3 (17.6) | 5 (29.4) |
| Leukopenia | 1 (1.7) | 1 (1.7) | 2 (3.3) | 1 (5.9) | 5 (29.4) | 6 (35.3) |
| Lymphopenia | 0 (0) | 1 (1.7) | 1 (1.7) | 1 (5.9) | 3 (17.6) | 4 (23.5) |
| Nausea | 21 (35) | 0 (0) | 21 (35) | 3 (17.6) | 2 (11.8) | 5 (29.4) |
| Diarrhea | 13 (21.7) | 0 (0) | 13 (21.7) | 2 (11.8) | 1 (5.9) | 3 (17.6) |
| Vomiting | 6 (10) | 0 (0) | 6 (10) | 3 (17.6) | 1 (5.9) | 4 (23.5) |
| Blood bilirubin increased | 23 (38.3) | 1 (1.7) | 24 (40) | 7 (41.2) | 2 (11.8) | 9 (52.9) |
| Decreased appetite | 11 (18.3) | 1 (1.7) | 12 (20) | 2 (11.8) | 0 (0) | 2 (11.8) |
| Differentiation syndrome | 1 (1.7) | 12 (20) | 13 (21.7) | 0 (0) | 2 (11.8) | 2 (11.8) |
Only the worst grade of the same adverse event was counted for the same patient.
Seventeen patients from the phase 2 portion of the study did not achieve a CR or CRi after 5 cycles of ENA monotherapy and proceeded to the phase 1b portion of the study, in which AZA 75 mg/m2 was added to ENA on days from 1 to 7. Two dose levels of ENA (100 and 50 mg) were to be tested using a modified 3+3 design. Sixteen patients were treated at the 100 mg daily dose of ENA with AZA 75 mg/m2 from days 1 to 7, and 1 patient was treated with a reduced dose of ENA at 50 mg while on monotherapy and continued combination therapy at the reduced ENA dose level. One dose-limiting toxicity (DLT) was observed (grade 3 nausea) in the first cohort of 3 patients treated at 100 mg ENA with AZA, but no further DLTs were observed in subsequent patients. All-cause serious adverse events are reported in supplemental Table 2.
Seven of 17 patients who progressed to AZA add-on therapy achieved a CR/CRi with combination AZA/ENA (Table 2; cCR rate of 41%, 95% exact CI, 18-67). Five patients in the phase 1b cohort were not evaluated for treatment response because they discontinued treatment before finishing their first cycle of combination therapy owing to death (n = 1), treatment failure (n = 1), switch to alternative therapy (n = 1), or adverse events (n = 2). The median duration of response to combination AZA/ENA was 14.6 months, with 57% of patients without progression at 6 months and 43% of patients without progression at 24 months (Table 3; Figure 2A).
Combination therapy was tolerated, with a toxicity profile comparable to AZA monotherapy.24 As in the phase 2 cohort, hyperbilirubinemia (52.9%) was the most common treatment-related adverse event (Table 4). The addition of AZA to ENA led to higher rates of hematologic adverse events. Treatment-related grade 3 or higher hematologic adverse events in >1 patient (Tables 4 and 5) were neutropenia (41.2%), leukopenia (29.4%), thrombocytopenia (17.6%), and anemia (5.9%). Nonhematologic adverse events that were grade 3 or higher included nausea (11.8%), vomiting (5.9%), diarrhea (5.9%), and differentiation syndrome (11.8%).
ENA-related serious adverse event
| ENA-related SAE, n (%)∗ . | Phase 2 and Exp (n = 60) . | Phase 1b (n = 17) . | ||||
|---|---|---|---|---|---|---|
| Grade . | 1/2 . | 3+ . | All . | 1/2 . | 3+ . | All . |
| Differentiation syndrome | 0 (0) | 12 (20) | 12 (20) | 0 (0) | 2 (11.8) | 2 (11.8) |
| Acute respiratory failure | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Blood bilirubin increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.9) |
| Cardiomyopathy | 1 (1.7) | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Dehydration | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 1 (1.7) | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Hypoxia | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.9) |
| Peripheral embolism | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Renal failure | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Tumor lysis syndrome | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| ENA-related SAE, n (%)∗ . | Phase 2 and Exp (n = 60) . | Phase 1b (n = 17) . | ||||
|---|---|---|---|---|---|---|
| Grade . | 1/2 . | 3+ . | All . | 1/2 . | 3+ . | All . |
| Differentiation syndrome | 0 (0) | 12 (20) | 12 (20) | 0 (0) | 2 (11.8) | 2 (11.8) |
| Acute respiratory failure | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Blood bilirubin increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.9) |
| Cardiomyopathy | 1 (1.7) | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Dehydration | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Diarrhea | 1 (1.7) | 0 (0) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Hypoxia | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (5.9) | 1 (5.9) |
| Peripheral embolism | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Renal failure | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
| Tumor lysis syndrome | 0 (0) | 1 (1.7) | 1 (1.7) | 0 (0) | 0 (0) | 0 (0) |
Only the worst grade of the same adverse event was counted for the same patient. Overall, ENA-related serious adverse events occurred in 15 patients during phase 2 and 3 patients during phase 1b.
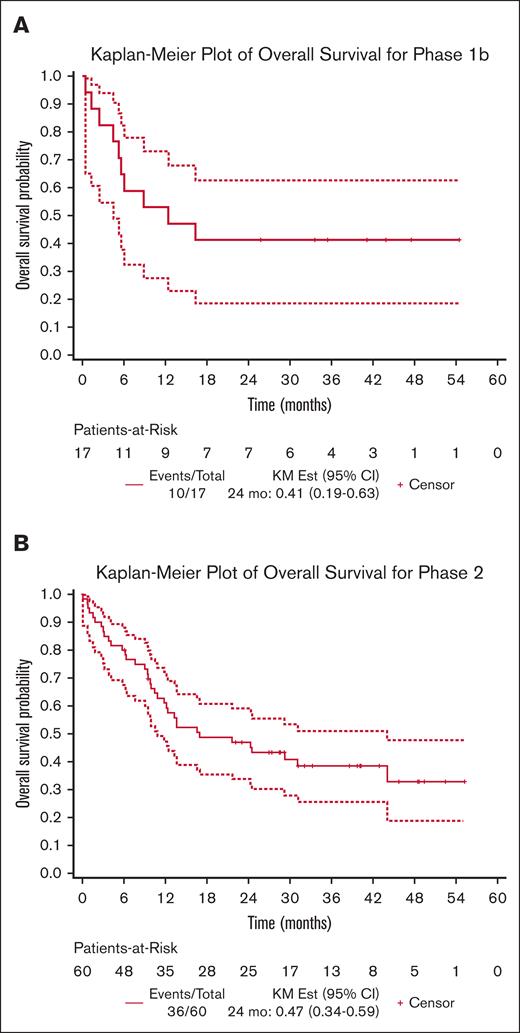
Overall survival (Table 6) was calculated from the time of initiation of ENA during phase 2 and of ENA plus AZA during phase 1b. With a median follow-up time of 36 months and 41.1 months for the phase 2 and 1b cohorts, respectively, the median survival was 17.1 months (95% CI, 11.0-44.2 months) for the phase 2 cohort (Figure 3B) and 12.5 months (95% CI, 4.5 to not reached) for the phase 1b cohort (Figure 3A). In the phase 2 cohort, 6 of the 60 patients (10%) proceeded to allogeneic hematopoetic stem cell transplantation (HSCT), at which point, they discontinued treatment but were still followed for survival. (supplemental Table 3). Similarly, in the phase 1b cohort, 2 of 17 patients (13%) proceeded to HSCT.
Overall survival
| Survival . | Phase 2 and Exp (n = 60)∗ . | Phase 1b (n = 17)† . |
|---|---|---|
| Overall survival | ||
| No. of events | 36 | 10 |
| Median in mo (95% CI) | 17.1 (11.0-44.2) | 12.5 (4.5-NE) |
| % alive at 7 d (95% CI) | 98 (89-100) | 100 |
| % alive at 30 d (95% CI) | 95 (85-98) | 94 (65-99) |
| % alive at 60 d (95% CI) | 90 (79-95) | 88 (61-97) |
| % alive at 6 mo (95% CI) | 80 (67-88) | 65 (38-82) |
| % alive at 24 mo (95% CI) | 47 (34-59) | 41 (19-63) |
| Median follow-up in months (range) | 36.0 (6.2-55.4) | 41.1 (25.8-54.5) |
| Death, n (%)‡ | ||
| Within 7 d | 1 (2) | 0 (0) |
| Within 30 d | 3 (5) | 1 (6) |
| Within 60 d | 6 (10) | 2 (12) |
| Survival . | Phase 2 and Exp (n = 60)∗ . | Phase 1b (n = 17)† . |
|---|---|---|
| Overall survival | ||
| No. of events | 36 | 10 |
| Median in mo (95% CI) | 17.1 (11.0-44.2) | 12.5 (4.5-NE) |
| % alive at 7 d (95% CI) | 98 (89-100) | 100 |
| % alive at 30 d (95% CI) | 95 (85-98) | 94 (65-99) |
| % alive at 60 d (95% CI) | 90 (79-95) | 88 (61-97) |
| % alive at 6 mo (95% CI) | 80 (67-88) | 65 (38-82) |
| % alive at 24 mo (95% CI) | 47 (34-59) | 41 (19-63) |
| Median follow-up in months (range) | 36.0 (6.2-55.4) | 41.1 (25.8-54.5) |
| Death, n (%)‡ | ||
| Within 7 d | 1 (2) | 0 (0) |
| Within 30 d | 3 (5) | 1 (6) |
| Within 60 d | 6 (10) | 2 (12) |
Overall survival in phase 2 and phase 2 expansion cohort was calculated from time of ENA start date to the date of death owing to any reason; patients who were alive were censored at the time of last follow-up.
Overall survival in phase 1b was calculated from time of combination therapy to the date of death owing to any reason; patients who were alive were censored at the time of last follow-up.
For phase 2 and expansion, deaths within 7, 30, or 60 days of the monotherapy are provided. For phase 1b, deaths within 7, 30, or 60 days of the combination therapy are provided. One death is included in both phase 2 and phase 1b, a patient who died 34 days after initiation of monotherapy and 13 days after the start of combination therapy.
Survival assessments for patients enrolled on study. Overall survival estimated by the Kaplan-Meier method for phase 1b (A) and phase 2 and Exp (B).
Survival assessments for patients enrolled on study. Overall survival estimated by the Kaplan-Meier method for phase 1b (A) and phase 2 and Exp (B).
The presence of mutations in the tumor suppressor gene TP53 is known to be correlated with therapeutic resistance and adverse outcomes in AML.25 Although most patients with TP53-mutant AML enrolled on the BEAT-AML master protocol were assigned to other substudies, 9 patients with TP53 + IDH2 comutations were assigned to this substudy. Across both phase 2 monotherapy and phase 1b combination therapy arms, 6 (67%) of these patients achieved a CR or CRh, whereas 3 (33%) patients did not (supplemental Table 4). Although these patient numbers are small, this suggests that the presence of TP53 mutations did not affect response rates, though a larger sample size will be required to confirm this finding. When we further examined variables that may have affected the cCR rate using a univariate logistic regression model, only IDH2 R140 mutations when compared with R172 mutations were associated with a higher rate of response to ENA monotherapy (supplemental Tables 5 and 6).
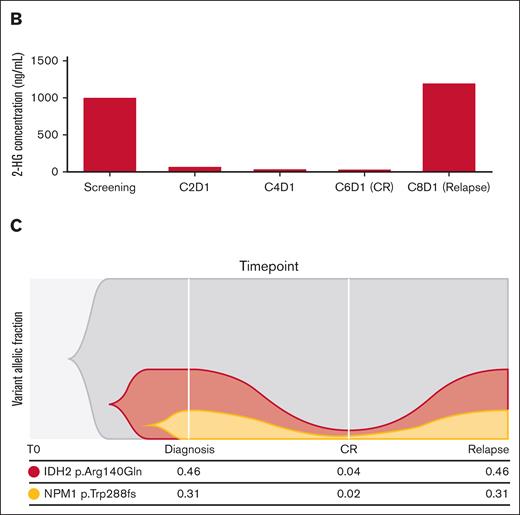
Exploratory correlative studies were performed to understand immunophenotypic and clonal dynamics underlying relapse and therapeutic resistance. We examined serial clonal cell subset changes by flow cytometry as well as mutational clearance by next-generation sequencing in bone marrow aspirates or peripheral blood (Figure 4A). Serial flow cytometric studies from a patient treated with ENA monotherapy who achieved a CR after 5 cycles but ultimately relapsed are shown with data represented as a 2-dimensional t-distributed Stochastic Neighbor Embedding plot. Markers for a primitive blast population were characterized by initial high expression of CD34, CD123, and CD117. This population contracted over the course of therapy until CR was achieved but recurred at cycle 8 when fulminant relapse was observed. In contrast, a differentiated CD11b myeloid population was preserved during monotherapy through cycle 6, but at the time of relapse, this mature myeloid population was no longer detected. These findings were correlated with response and pharmacodynamic changes in 2-HG, with full restoration of 2-HG production in the setting of continued ENA therapy at the time of relapse (Figure 4B). This is mirrored by the known baseline pathogenic mutations in NPM1 and IDH2. Although VAF of these 2 mutations reached 0.04 and 0.02, respectively, at the time of CR, they remained detectable, as seen in other studies.19 At relapse, the leukemic clone harbored the same mutations at VAF similar to those that were present at diagnosis. Mutations detected at diagnosis of AML were typical for IDH2-mutated AML (Figure 5). Interestingly, no patients with AML harboring mutations in NRAS, KRAS, or PTPN11 achieved a CR, consistent with prior studies establishing a functional link between RAS-receptor tyrosine kinase pathway gene mutations and resistance to IDH inhibition.26 Finally, the pharmacokinetic analysis of ENA (AG-221) and its active metabolite (AGI-0016903) in the plasma was as expected and revealed nothing remarkable (supplemental Table 7).
Correlates of treatment response and resistance. (A) Serial bone marrow aspirate samples analyzed using multiparameter flow cytometry visualized using t-distributed Stochastic Neighbor Embedding (t-SNE) plots generated by Cytobank. (B) Correlating 2-HG data from serial plasma samples. (C) Serial bone marrow aspirate samples taken at baseline, CR and relapse were analyzed using the ArcherDX’s FusionPlex Myeloid and VariantPlex Myeloid panels.
Correlates of treatment response and resistance. (A) Serial bone marrow aspirate samples analyzed using multiparameter flow cytometry visualized using t-distributed Stochastic Neighbor Embedding (t-SNE) plots generated by Cytobank. (B) Correlating 2-HG data from serial plasma samples. (C) Serial bone marrow aspirate samples taken at baseline, CR and relapse were analyzed using the ArcherDX’s FusionPlex Myeloid and VariantPlex Myeloid panels.
Molecular correlates of response. Baseline mutations identified using the FoundationOne Heme panel and grouped by responses during phase 2 (A) or phase 1b (B). CRMRD, CR without minimal residual disease; MLFS, morphologic leukemia-free state; NE, not evaluated; PD, progressive disease; PR, partial remission; SD, stable disease; TF, treatment failure.
Molecular correlates of response. Baseline mutations identified using the FoundationOne Heme panel and grouped by responses during phase 2 (A) or phase 1b (B). CRMRD, CR without minimal residual disease; MLFS, morphologic leukemia-free state; NE, not evaluated; PD, progressive disease; PR, partial remission; SD, stable disease; TF, treatment failure.
Discussion
In this study, we show that ENA monotherapy is a safe and well-tolerated upfront therapy for newly diagnosed patients with IDH2-mutant AML aged ≥60 years with significant clinical activity. Responses were sufficiently deep, durable, and in some cases allowed for consolidation HSCT. The cCR rate of 48% (adjusted 95% CI, 30.3-60.5), inclusive of CR and CRi, achieved with ENA monotherapy is comparable with the cCR rate achieved with combination ENA plus AZA in AG221-AML-005 (66% cCR rate), the phase 2 randomized study comparing ENA plus AZA with AZA alone in a population of newly diagnosed patients with IDH2-mutant AML similar to those evaluated in our study.18 Furthermore, a higher proportion of patients enrolled in this study had adverse-risk disease (53%) relative to the same cohort in AG221-AML-005 (29%). Importantly, ENA monotherapy was associated with less myelosuppression compared with ENA/AZA combination therapy; this myelosuppression was predominantly mediated by AZA in AG221-AML-005. These response rates are also comparable with the upfront experience of ENA monotherapy in newly diagnosed patients with IDH2-mutant AML, demonstrating an overall response rate of 30.8% (95% CI, 17.0-47.6), including 18% who achieved a CR with a 21% rate of grade 3 to 4 cytopenias.27
This study also sought to evaluate whether a risk-adapted approach in which AZA was added to ENA if ENA monotherapy failed to achieve a sufficient treatment response could safely be used in clinical practice. Although a single DLT was observed in the first cohort of 3 patients treated at 100 mg ENA, no further DLTs were observed in subsequent patients. The presence of differentiation syndrome, which was observed in all cohorts, suggests an on-target effect of ENA and supports close monitoring and prompt administration of corticosteroids in patients showing early signs of this manifestation. Furthermore, a cCR rate of 41% with a true CR rate of 24% after adding HMA to ENA demonstrated that patients who did not achieve a remission with monotherapy could do so with combination therapy and, in some cases (13%), proceed to consolidation HSCT after combination AZA/ENA therapy. Although this study is insufficiently powered for superiority or noninferiority analyses, these findings demonstrate the feasibility of this risk-adapted approach to treat IDH-mutant AML to mitigate the toxicity conferred by HMA/venetoclax.
The success of HMA/venetoclax combination therapy in the treatment of newly diagnosed patients with AML raises the potential of adding IDH inhibitors to this backbone as a novel triplet that may not only deepen and/or accelerate clinical responses but may also worsen cytopenias. Our study provides proof-of-principle that ENA monotherapy can be initiated upfront in patients with IDH2-mutant AML and induce CRs that allow some patients to proceed to consolidation HSCT with curative intent while avoiding adverse reactions associated with combination regimens. Although some patients were able to achieve CR/CRi with the addition of AZA, correlative studies revealed that some patients relapsed with IDH-mutant disease and aberrant 2-HG production. Notably, this patient population may not be amenable to further IDH-directed differentiation therapy. These data suggest that ENA monotherapy with risk-adapted AZA combination therapy is an active clinical regimen for newly diagnosed IDH2-mutant AML and should be compared with venetoclax-based regimens in comparative studies in older adults with newly diagnosed IDH2-mutant AML.
Acknowledgments
S.F.C. was supported by a Young Investigator Award from the Edward P. Evans Foundation and a Career Development Award from the National Cancer Institute (K08 CA241371-01A1). Studies supported by Memorial Sloan Kettering core facilities were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core grant P30 15 CA008748. R.L.L. is supported by National Cancer Institute grants P01 CA108671 and R35197594, as well as a Leukemia & Lymphoma Society Specialized Center of Research grant. Sequencing efforts were funded and performed by ArcherDx.
Authorship
Contribution: E.M.S. and S.F.C. designed and performed the experiments, analyzed the data, and wrote the manuscript; Y.H. and A.S. designed the experiments and performed the statistical analysis; J.R.L., C.M., A.J.D., S.N.M., T.D., Y.L., J.C., J.-A.V., and N.A.H. performed the experiments and analyzed data; M.R.B., W.S., T.K., W.G.B., G.J.S., R.L.O., J.M.F., M.L., T.L., P.P., M.C.F., M.B., and R.H.C. performed the experiments; L.R., T.L.C., A.O.Y., F.D., S.M., and M.S. designed and performed the experiments and analyzed the data; B.J.D., A.S.M., U.B., A.B., J.C.B., and R.L.L. designed and performed the experiments and supervised the study; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: S.F.C. is a consultant for and holds equity interest in Imago BioSciences. G.J.S. has commercial interests in Bristol Myers Squibb (BMS), Amgen, and Johnson & Johnson (J&J); has received fees from AbbVie, Agios, Amgen, Astellas, BMS, Incyte, Janssen, Jazz, Karyopharm, Kite, Pharmacyclics, Sanofi/Genzyme, and Stemline; and has received research funding from AbbVie, Actinium, Actuate, Arog, Astellas, AltruBio, AVM Bio, BMS/Celgene, Celator, Constellation, Daiichi-Sankyo, Deciphera, Delta-Fly, Forma, FujiFilm, Gamida, Genentech-Roche, GlycoMimetics, Geron, Incyte, Karyopharm, Kiadis, Kite/Gilead, Kura, Marker, Mateon, Onconova, Pfizer, PrECOG, Regimmune, Samus, Sangamo, Sellas, Stemline, Syros, Takeda, Tolero, Trovagene, Agios, Amgen, Jazz, Orca, Ono-UK, and Novartis. J.M.F. has received research funding from Takeda/Millennium. P.P. has served on the advisory board of Agios Pharmaceuticals and is currently an employee of Servier. J.-A.V. is an employee of Foundation Medicine Inc with equity in Roche. B.J.D. reports serving on the scientific advisory board of Adela Bio, Aileron Therapeutics, and Therapy Architects/ALLCRON (inactive) as well as Cepheid, Celgene, DNA SEQ, Nemucore Medical Innovations, Novartis, RUNX1 Research Program, and Vivid Biosciences (inactive); reports serving on scientific advisory board and owning stocks in Aptose Biosciences, Blueprint Medicines, Enliven Therapeutics, Iterion Therapeutics, Grail, and Recludix Pharma; serving on the board of directors and owning stocks in Amgen and Vincerx Pharma; serving on the board of directors in Burroughs Wellcome Fund and CureOne; being a member on the joint steering committee of Beat AML LLS; being a member on the advisory committee of Multicancer Early Detection Consortium; is the founder of VB Therapeutics; reports sponsored research agreements with Enliven Therapeutics and Recludix Pharma; clinical trial funding from Novartis and AstraZeneca; royalties from patent 6958335 (Novartis exclusive license) and Oregon Health and Science University (OHSU) and Dana-Farber Cancer Institute (1 Merck exclusive license, 1 CytoImage, Inc exclusive license, and 1 Sun Pharma Advanced Research Company nonexclusive license); and holds US patents 4326534, 6958335, 7416873, 7592142, 10473667, 10664967, and 11049247. A.S.M. has served on the advisory boards of Jazz Pharmaceuticals, AbbVie/Genentech, Astellas Pharma, PTC Therapeutics, Novartis, Agios Pharmaceuticals and Syndax Pharmaceuticals. U.B. has been a consultant for Genentech, Daiichi Sankyo, Takeda, Pfizer, AbbVie/Genentech, and Novartis. J.C.B. is a current equity holder in Vincerx Pharma Inc (a publicly traded company); holds membership on the board of directors or advisory committees of Vincerx, Newave, and Orange Grove Bio; and reports being a consultant or receiving honoraria from Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, and Syndax. R.L.L. is on the supervisory board of Qiagen; is a scientific adviser to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics, and Isoplexis; receives research support from and has consulted for Celgene and Roche; has consulted for Incyte, Janssen, Astellas, MorphoSys, and Novartis; and has received honoraria from AstraZeneca, Roche, Lilly, and Amgen for invited lectures and from Gilead for grant reviews. E.M.S. has served on the advisory boards of Astellas Pharma, AbbVie, Genentech, Daiichi Sankyo, Novartis, Amgen, Seattle Genetics, Syros Pharmaceuticals, Syndax Pharmaceuticals, Agios Pharmaceuticals, and Celgene, and is an equity holder in Auron Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Eytan M. Stein, Department of Medicine, Leukemia Service, Memorial Sloan Kettering Cancer Center, 545 East 73rd St, New York, NY 10021; email: steine@mskcc.org.
References
Author notes
For original deidentified data, please contact the corresponding author, Eytan Stein (steine@mskcc.org). In general, individual participant data will not be shared but may be available depending on the request.
The full-text version of this article contains a data supplement.