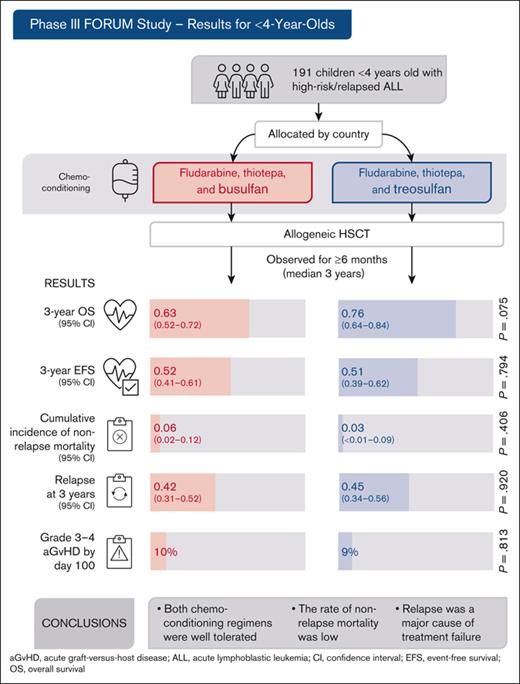
In the phase 3 FORUM trial, children aged <4 years with high-risk ALL had allogeneic HSCT after chemotherapeutic conditioning.
Chemotherapeutic conditioning allowed for HSCT with a low complication and mortality rate in this vulnerable patient cohort.
Visual Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is highly effective for treating pediatric high-risk or relapsed acute lymphoblastic leukemia (ALL). For young children, total body irradiation (TBI) is associated with severe late sequelae. In the FORUM study (NCT01949129), we assessed safety, event-free survival (EFS), and overall survival (OS) of 2 TBI-free conditioning regimens in children aged <4 years with ALL. Patients received fludarabine (Flu), thiotepa (Thio), and either busulfan (Bu) or treosulfan (Treo) before HSCT. From 2013 to 2021, 191 children received transplantation and were observed for ≥6 months (median follow-up: 3 years). The 3-year OS was 0.63 (95% confidence interval [95% CI], 0.52-0.72) and 0.76 (95% CI, 0.64-0.84) for Flu/Thio/Bu and Flu/Thio/Treo (P = .075), respectively. Three-year EFS was 0.52 (95% CI, 0.41-0.61) and 0.51 (95% CI, 0.39-0.62), respectively (P = .794). Cumulative incidence of nonrelapse mortality (NRM) and relapse at 3 years were 0.06 (95% CI, 0.02-0.12) vs 0.03 (95% CI: <0.01-0.09) (P = .406) and 0.42 (95% CI, 0.31-0.52) vs 0.45 (95% CI, 0.34-0.56) (P = .920), respectively. Grade >1 acute graft-versus-host disease (GVHD) occurred in 29% of patients receiving Flu/Thio/Bu and 17% of those receiving Flu/Thio/Treo (P = .049), whereas grade 3/4 occurred in 10% and 9%, respectively (P = .813). The 3-year incidence of chronic GVHD was 0.07 (95% CI, 0.03-0.13) vs 0.05 (95% CI, 0.02-0.11), respectively (P = .518). In conclusion, both chemotherapeutic conditioning regimens were well tolerated and NRM was low. However, relapse was the major cause of treatment failure. This trial was registered at www.clinicaltrials.gov as #NCT01949129.
Introduction
Over recent decades, tremendous progress has been made in the treatment of children and adolescents with acute lymphoblastic leukemia (ALL). Rigorous studies to optimize treatment strategies, including allogeneic hematopoietic stem cell transplantation (HSCT), have driven this considerable success, with survival rates now approaching 90%.1-4
Allogeneic HSCT is an important option for children with high-risk ALL, for whom conventional chemotherapy offers a dismal prognosis, and for infants with high-risk leukemia.5,6 Children with a slow response to initial treatment, as measured by minimal residual disease (MRD) monitoring, and children with genetic alterations associated with a poor prognosis such as hypodiploidy, KMT2A rearrangements, or TP53 mutations are candidates for allogeneic HSCT.3,5,7-9 For patients who relapse after initial treatment, the site of relapse, time from initial diagnosis to relapse, immunophenotype, and response to remission induction are the major factors determining an indication for allogeneic HSCT.10-12
Allogeneic HSCT has proven immunological antileukemic efficacy in children with ALL through the so-called graft-versus-leukemia effect.13 Allogeneic HSCT was shown to contribute to a substantial improvement in the survival probability of patients with high-risk ALL in first or second complete remission (CR1 and CR2).4,9,14 Standardization of HSCT procedures was key to this progress. The ALL-SCT-BFM-2003 trial demonstrated that the outcomes for children with ALL who received a transplant from an unrelated donor matched for at least 9 of 10 HLA loci (matched unrelated donor [MUD]) were comparable with those of children who received a transplant from a matched sibling donor (MSD).15 In that study, HSCT in children aged >4 years was performed after conditioning with total body irradiation (TBI) and etoposide (Eto), resulting in excellent rates of leukemia-free survival and overall survival (OS).15
In the past, both relapse-related mortality and nonrelapse mortality (NRM) were major causes of treatment failure in infants and young children with high-risk ALL. Because TBI has lifelong adverse effects on growth, cognitive function, and fertility and promotes the occurrence of endocrinopathies and cataracts, it is not used for young children in most countries. However, the optimal chemotherapeutic approach (chemotherapeutic conditioning) to achieve high event-free survival (EFS) and reduce NRM has not yet been defined. Thus, in the nonrandomized part of the phase 3 “For Omitting Radiation Under Majority age” (FORUM) trial we set out to compare 2 established chemotherapeutic conditioning regimens for children younger than 4 years.
Methods
Study design
The FORUM study is a prospective, international, multicenter, randomized, open-label, active-comparator controlled, phase 3 trial (EudraCT 2012-003032-22; www.clinicaltrials.gov: NCT01949129). In the randomized portion, children aged ≥4 years with high-risk ALL were randomized to receive either TBI and Eto or chemotherapeutic conditioning alone before HSCT.16 In the nonrandomized part (reported here), children aged <4 years with high-risk ALL were allocated to chemotherapeutic conditioning in 88 centers in 21 countries (supplemental Appendix 1).
Two widely established myeloablative chemotherapeutic conditioning regimens were used: fludarabine (Flu) and thiotepa (Thio) with either IV busulfan (Bu) or treosulfan (Treo). Participating countries decided before starting the trial whether patients would receive Bu- or Treo-based conditioning.
The study was designed by members of several national pediatric transplantation groups, with the contribution of experts from the ALL-Frontline and Relapse study groups. The protocol and the statistical analysis plan were approved by the international steering committee, the national competent authorities for each country, and the local ethic committees for each participating center. The trial was performed according to the Declaration of Helsinki and was registered with international trial registries.
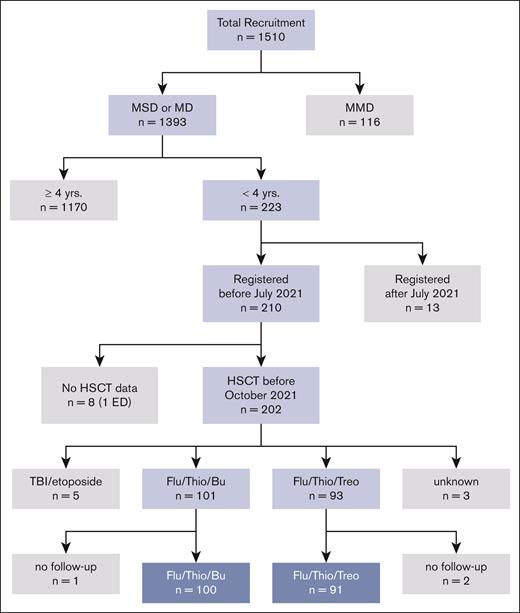
Between April 2013 and July 2021, 202 patients aged <4 years were registered for the trial and underwent HSCT before October 2021. The data cutoff was 1 February 2022, allowing a minimum observation period after HSCT of 6 months (and up to 7.2 years).
Patients
Patients eligible for the study had either relapsed or high-risk ALL as an indication for allogeneic HSCT according to frontline treatment protocols, were in morphological CR before HSCT, and had either an HLA-identical MSD or a donor matched after 4-digit high-resolution HLA typing in HLA-A, -B, -C, -DR, and -DQ in at least 9 of 10 evaluated loci (MUD). MSDs and MUDs fulfilling these criteria were defined as matched donors. The recommended stem cell source was bone marrow, peripheral blood, or cord blood from a matched donor. Written informed consent was provided by patients’ parents or their legal guardians.
Procedures
Conditioning regimens consisted of Flu 30 mg/m2 once a day over 5 days (total dose: 150 mg/m2), Thio 5 mg/kg twice a day for 1 day (total dose: 10 mg/kg), and either Treo 14 g/m2 once a day for 3 consecutive days, or Bu 4 mg/kg over 4 consecutive days. Bu was given according to local guidelines, commonly with therapeutic drug monitoring (TDM) and dose adjustments. The recommended first dose of Bu was according to the European Medicines Agency dosing nomogram, with a TDM target of daily area under the curve of 14.8 to 21.6 mg/h per L (historical target).17
The decision to use either Bu- or Treo-based regimen was decided by the participating countries before the start of the trial.
Graft-versus-host disease (GVHD) prophylaxis was performed according to donor type. Patients who received a transplant from an MSD received cyclosporine only, whereas patients with MUDs received cyclosporine, methotrexate, and either antithymocyte globulin (thymoglobulin, 2.5 mg/kg for 3 days) or anti-T-cell globulin (grafalon, 15 mg/kg for 3 days), as detailed previously.10
CR was defined as <5% blasts in the bone marrow and no evidence of extramedullary disease. Relapse was defined as ≥5% blasts and/or evidence of extramedullary disease. Adverse events (AEs), adverse reactions, serious AEs, and suspected or unexpected AEs were assessed by clinicians according to good clinical practice guidelines. Grading of AEs was performed according to the Common Terminology Criteria for Adverse Events of the National Cancer Institute, version 4.03. Life-threatening, or other medically important serious events leading to intensive care unit admission were considered serious AEs. Acute and chronic GVHD was assessed according to the Glucksberg criteria.18
Outcomes
The primary end point was OS calculated from the date of transplantation to death from any cause, which was considered the event. Patients lost to follow-up without an event were censored at last follow-up. Secondary end points included EFS, cumulative incidence of relapse (CIR), NRM, and acute GVHD on day 100, cumulative incidence of chronic GVHD, GVHD-free and relapse-free survival (GRFS), and nonhematological AEs grade 3/4 on day 100.
EFS was calculated from the date of receiving transplantation to relapse, secondary malignancy, or death from any cause, whichever occurred first. Data of patients lost to follow-up without an event were censored on the date of their last follow-up evaluation. When calculating CIR, death without relapse/progression and secondary malignancy were considered competing events. Relapse and secondary malignancy were competing events for calculating the cumulative incidence of NRM; death without malignancy was a competing event for calculating the cumulative incidence of secondary malignancy. Relapse, progression of disease, death, and secondary malignancy were competing events for calculating GRFS. Acute GVHD (grade 3/4) was considered an event at day of onset. GRFS was calculated from date of transplantation to the first event.
Statistical analysis
The data analysis was conducted by U.P., and all authors had access to the primary clinical trial data. The significance level was set at .05 for all statistical analyses. OS, EFS, and GRFS were estimated using the Kaplan-Meier method, and the difference among groups was compared using the log-rank test. Three-year estimates and 95% confidence intervals (CIs) are reported in the manuscript.
Baseline characteristics evaluated were sex, age at HSCT and diagnosis, immunophenotype of ALL, MRD before HSCT, genetic aberrations, hypodiploidy or hyperdiploidy, donor type, stem cell source, remission status, and site and time of first relapse in CR2 patients. MRD was classified as low load (<10−4) or high load (≥10−4) detected via polymerase chain reaction or flow cytometry.
The proportion of patients with grade 3/4 acute GVHD and other AEs of grade 3/4 on day 100 as well as the distribution of baseline characteristics was compared using the χ2 test. CIR, NRM, and secondary malignancy were estimated taking into account competing events and compared using the Gray test.
For multivariable analysis, Cox regression was used to explore the impact of baseline characteristics and conditioning regimen on OS and EFS. Factors evaluated were conditioning regimen, donor type, remission status, age at HSCT and diagnosis, presence of KMT2A rearrangements, and immunophenotype.
Median follow-up was estimated using the inverse Kaplan-Meier method.19
Results
Patient characteristics
Between April 2013 and July 2021, 202 patients aged <4 years from 21 countries underwent HSCT according to the FORUM protocol. Patient characteristics are shown in Table 1 for the 2 treatment arms. Eleven patients were excluded from this analysis: 5 received TBI-based conditioning, 3 had inconclusive documentation of the conditioning regimen, and 3 had no follow-up data reported. This led to 191 evaluable patients (Figure 1 [CONSORT diagram]).
Basic characteristics according to chemotherapeutic conditioning regimen
| Baseline characteristic . | Total (N = 191) . | Chemotherapeutic conditioning regimen . | P . | ||||
|---|---|---|---|---|---|---|---|
| Flu/Thio/Bu (n = 100) . | Flu/Thio/Treo (n = 91) . | ||||||
| Sex, n (%) | .564 | ||||||
| Male | 107 | 56% | 58 | 58% | 49 | 54% | |
| Female | 84 | 44% | 42 | 42% | 42 | 46% | |
| Age at HSCT, y, n (%) | |||||||
| <2 y | 90 | 47% | 42 | 42% | 48 | 53% | .137 |
| ≥2 | 101 | 53% | 58 | 58% | 43 | 47% | |
| Age at diagnosis, n (%) | |||||||
| <6 mo | 58 | 30% | 27 | 27% | 31 | 34% | .325 |
| 6 mo-1 y | 34 | 18% | 16 | 16% | 18 | 19% | |
| ≥1 y | 99 | 52% | 57 | 57% | 42 | 46% | |
| Immunophenotype | |||||||
| BCP | 156 | 82% | 86 | 86% | 70 | 77% | .140 |
| T-cell ALL | 28 | 15% | 13 | 13% | 15 | 16% | |
| Other | 6 | 3% | 1 | 1% | 5 | 5% | |
| Unknown | 1 | 1% | 0% | 1 | 1% | ||
| Donor, n (%) | |||||||
| MSD | 37 | 19% | 23 | 23% | 14 | 15% | .184 |
| MUD | 154 | 81% | 77 | 77% | 77 | 85% | |
| Remission status, n (%) | |||||||
| CR1 | 139 | 73% | 67 | 67% | 72 | 79% | .098 |
| CR2 | 50 | 26% | 31 | 31% | 19 | 21% | |
| CR3 | 2 | 1% | 2 | 2% | 0 | 0% | |
| Stem cell source, n (%) | |||||||
| Bone marrow | 131 | 69% | 63 | 64% | 68 | 75% | .041 |
| Peripheral blood | 35 | 18% | 17 | 17% | 18 | 20% | |
| Cord blood | 23 | 12% | 18 | 18% | 5 | 5% | |
| Bone marrow + peripheral blood | 1 | 1% | 1 | 1% | 0 | 0% | |
| Unknown∗ | 1 | 1% | 1 | 1% | 0 | 0% | |
| MRD before HSCT, n (%) | |||||||
| Low load (<10−4) | 105 | 70% | 62 | 77% | 43 | 61% | .044 |
| High load (≥10−4) | 46 | 30% | 19 | 23% | 27 | 39% | |
| Genetic aberration, n (%) | |||||||
| KMT2A-AFF1 | 51 | 29% | 24 | 26% | 27 | 32% | .465 |
| ETV6-RUNX1 | 6 | 3% | 4 | 4% | 2 | 2% | |
| BCR-ABL† | 13 | 7% | 9 | 10% | 4 | 5% | |
| None of the above | 108 | 61% | 56 | 60% | 52 | 61% | |
| Unknown∗ | 13 | 7% | 7 | 7% | 6 | 7% | |
| Hypodiploidy, n (%) | |||||||
| No | 169 | 99% | 88 | 99% | 81 | 99% | .954 |
| Yes | 2 | 1% | 1 | 1% | 1 | 1% | |
| Unknown∗ | 20 | 10% | 11 | 11% | 9 | 10% | |
| Hyperdiploidy, n (%) | |||||||
| No | 149 | 87% | 76 | 85% | 73 | 88% | .622 |
| Yes | 23 | 13% | 13 | 15% | 10 | 12% | |
| Unknown∗ | 19 | 10% | 11 | 11% | 8 | 9% | |
| Time of relapse, mo, n (%) | |||||||
| <18 | 32 | 67% | 18 | 60% | 14 | 78% | .390 |
| 18-30 | 15 | 31% | 11 | 37% | 4 | 22% | |
| >30 | 1 | 2% | 1 | 3% | 0 | 0% | |
| Unknown∗ | 2 | 4% | 1 | 3% | 1 | 5% | |
| Type of relapse, n (%) | |||||||
| Bone marrow | 29 | 58% | 19 | 61% | 10 | 53% | .807 |
| CNS | 10 | 20% | 6 | 19% | 4 | 21% | |
| Other | 11 | 22% | 6 | 19% | 5 | 26% | |
| Baseline characteristic . | Total (N = 191) . | Chemotherapeutic conditioning regimen . | P . | ||||
|---|---|---|---|---|---|---|---|
| Flu/Thio/Bu (n = 100) . | Flu/Thio/Treo (n = 91) . | ||||||
| Sex, n (%) | .564 | ||||||
| Male | 107 | 56% | 58 | 58% | 49 | 54% | |
| Female | 84 | 44% | 42 | 42% | 42 | 46% | |
| Age at HSCT, y, n (%) | |||||||
| <2 y | 90 | 47% | 42 | 42% | 48 | 53% | .137 |
| ≥2 | 101 | 53% | 58 | 58% | 43 | 47% | |
| Age at diagnosis, n (%) | |||||||
| <6 mo | 58 | 30% | 27 | 27% | 31 | 34% | .325 |
| 6 mo-1 y | 34 | 18% | 16 | 16% | 18 | 19% | |
| ≥1 y | 99 | 52% | 57 | 57% | 42 | 46% | |
| Immunophenotype | |||||||
| BCP | 156 | 82% | 86 | 86% | 70 | 77% | .140 |
| T-cell ALL | 28 | 15% | 13 | 13% | 15 | 16% | |
| Other | 6 | 3% | 1 | 1% | 5 | 5% | |
| Unknown | 1 | 1% | 0% | 1 | 1% | ||
| Donor, n (%) | |||||||
| MSD | 37 | 19% | 23 | 23% | 14 | 15% | .184 |
| MUD | 154 | 81% | 77 | 77% | 77 | 85% | |
| Remission status, n (%) | |||||||
| CR1 | 139 | 73% | 67 | 67% | 72 | 79% | .098 |
| CR2 | 50 | 26% | 31 | 31% | 19 | 21% | |
| CR3 | 2 | 1% | 2 | 2% | 0 | 0% | |
| Stem cell source, n (%) | |||||||
| Bone marrow | 131 | 69% | 63 | 64% | 68 | 75% | .041 |
| Peripheral blood | 35 | 18% | 17 | 17% | 18 | 20% | |
| Cord blood | 23 | 12% | 18 | 18% | 5 | 5% | |
| Bone marrow + peripheral blood | 1 | 1% | 1 | 1% | 0 | 0% | |
| Unknown∗ | 1 | 1% | 1 | 1% | 0 | 0% | |
| MRD before HSCT, n (%) | |||||||
| Low load (<10−4) | 105 | 70% | 62 | 77% | 43 | 61% | .044 |
| High load (≥10−4) | 46 | 30% | 19 | 23% | 27 | 39% | |
| Genetic aberration, n (%) | |||||||
| KMT2A-AFF1 | 51 | 29% | 24 | 26% | 27 | 32% | .465 |
| ETV6-RUNX1 | 6 | 3% | 4 | 4% | 2 | 2% | |
| BCR-ABL† | 13 | 7% | 9 | 10% | 4 | 5% | |
| None of the above | 108 | 61% | 56 | 60% | 52 | 61% | |
| Unknown∗ | 13 | 7% | 7 | 7% | 6 | 7% | |
| Hypodiploidy, n (%) | |||||||
| No | 169 | 99% | 88 | 99% | 81 | 99% | .954 |
| Yes | 2 | 1% | 1 | 1% | 1 | 1% | |
| Unknown∗ | 20 | 10% | 11 | 11% | 9 | 10% | |
| Hyperdiploidy, n (%) | |||||||
| No | 149 | 87% | 76 | 85% | 73 | 88% | .622 |
| Yes | 23 | 13% | 13 | 15% | 10 | 12% | |
| Unknown∗ | 19 | 10% | 11 | 11% | 8 | 9% | |
| Time of relapse, mo, n (%) | |||||||
| <18 | 32 | 67% | 18 | 60% | 14 | 78% | .390 |
| 18-30 | 15 | 31% | 11 | 37% | 4 | 22% | |
| >30 | 1 | 2% | 1 | 3% | 0 | 0% | |
| Unknown∗ | 2 | 4% | 1 | 3% | 1 | 5% | |
| Type of relapse, n (%) | |||||||
| Bone marrow | 29 | 58% | 19 | 61% | 10 | 53% | .807 |
| CNS | 10 | 20% | 6 | 19% | 4 | 21% | |
| Other | 11 | 22% | 6 | 19% | 5 | 26% | |
Percentages were calculated using evaluable patients as the denominator, unless otherwise stated.
BCP, B-cell precursor; CNS, central nervous system.
Percentage based on the total number of patients.
Includes 1 patient with BCR-ABL and KMT2a-AFF1 and 2 patients with BCR-ABL and ETV6-RUNX1.
Of the 191 evaluable patients, 107 (56%) were male and 84 (44%) were female. Median age at HSCT was 2.2 years (range, 0.5-4 years); 90 patients were aged <2 years (47%) and 101 were older (53%) at time of HSCT. At diagnosis, 92 patients (48%) were aged <1 year, and 99 patients (52%) were older. In total, 156 patients (82%) had B-cell precursor ALL, 28 patients (15%) had T-cell ALL, 6 patients (3%) had different phenotypes, and 1 patient (1%) had no differentiation reported. Genetic analysis at diagnosis revealed BCR-ABL in 13 patients (7%), ETV6-RUNX1 in 6 patients (3%), and KMT2A rearrangements in 51 patients (29%).
Overall, 139 patients (73%) received transplantation in CR1, and 52 patients (27%) were in ≥CR2. In 154 patients (81%), the donor was an MUD, whereas 37 (19%) patients had an MSD. The stem cell source was bone marrow for 131 (69%) patients, peripheral blood for 35 (18%) patients, and cord blood for 23 (12%) of patients; 1 patient (∼0.5%) had a combination of bone marrow and cord blood, and 1 (∼0.5%) patient had no available details on stem cell source.
Survival
For the whole study population, the 3-year probabilities of EFS and OS were .52 (95% CI, 0.44-0.59) and .69 (95% CI, 0.61-0.76), respectively. In the univariable analysis, there was no difference in probability of EFS or OS for patients aged <2 years vs those aged ≥2 but <4 years, according to the immunophenotype or genetic aberration (Table 2). The probability of EFS was .40 (95% CI, 0.29-0.50) in patients aged <1 year at diagnosis (n = 92) and .63 (95% CI, 0.52-0.72) for patients aged ≥1 year at diagnosis (n = 99; P = .002), whereas the probability of OS did not differ between these age groups (.64 [95% CI, 0.52-0.74] vs .73 [95% CI, 0.63-0.81], respectively; P = .220).
Univariable analysis of the 3-year EFS and OS
| . | N . | EFS . | OS . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Events . | 3-y EFS . | P . | Deaths . | 3-y OS . | P . | ||
| Total | 191 | 87 | 0.52 (0.44-0.59) | 56 | 0.69 (0.61-0.76) | |||
| Sex | ||||||||
| Male | 107 | 48 | 0.52 (0.41-0.61) | .989 | 31 | 0.69 (0.58-0.78) | .902 | |
| Female | 84 | 39 | 0.52 (0.40-0.62) | 25 | 0.69 (0.57-0.78) | |||
| Age at HSCT, y | ||||||||
| <2 | 90 | 44 | 0.48 (0.37-0.59) | .328 | 26 | 0.71 (0.59-0.80) | .793 | |
| ≥2 | 101 | 43 | 0.55 (0.44-0.64) | 30 | 0.68 (0.57-0.76) | |||
| Age at diagnosis, y | ||||||||
| <1 | 92 | 52 | 0.40 (0.29-0.50) | .002 | 30 | 0.64 (0.52-0.74) | .220 | |
| ≥1 | 99 | 35 | 0.63 (0.52-0.72) | 26 | 0.73 (0.63-0.81) | |||
| Immunophenotype | ||||||||
| BCP | 156 | 78 | 0.47 (0.38-0.55) | .038 | 50 | 0.66 (0.57-0.73) | .160 | |
| T-cell ALL | 28 | 9 | 0.67 (0.46-0.81) | 6 | 0.78 (0.57-0.89) | |||
| Other | 6 | 0 | 1.00 (1.00-1.00) | 0 | 1.00 (1.00-1.00) | |||
| MRD before HSCT | ||||||||
| Cutoff ≥10−4 vs <10−4 | ||||||||
| Low | 105 | 49 | 0.50 (0.40-0.60) | .172 | 32 | 0.68 (0.57-0.77) | .384 | |
| High | 46 | 27 | 0.39 (0.24-0.53) | 19 | 0.58 (0.42-0.71) | |||
| Genetic aberration | ||||||||
| KMT2A-AFF1 | 51 | 28 | 0.41 (0.26-0.55) | .349 | 21 | 0.55 (0.39-0.68) | .164 | |
| ETV6-RUNX1 | 6 | 2 | 0.67 (0.19-0.90) | 1 | 1.00 (1.00-1.00) | |||
| BCR-ABL∗ | 13 | 5 | 0.59 (0.28-0.81) | 3 | 0.77 (0.44-0.92) | |||
| None of the above | 108 | 46 | 0.55 (0.45-0.64) | 29 | 0.71 (0.60-0.79) | |||
| Hypodiploidy | ||||||||
| No | 169 | 78 | 0.51 (0.43-0.59) | .962 | 49 | 0.70 (0.61-0.77) | .761 | |
| Yes | 2 | 1 | 0.50 (0.01-0.91) | 1 | 0.50 (0.01-0.91) | |||
| Hyperdiploidy | ||||||||
| No | 149 | 71 | 0.50 (0.41-0.58) | .181 | 46 | 0.68 (0.59-0.75) | .195 | |
| Yes | 23 | 8 | 0.65 (0.42-0.81) | 4 | 0.81 (0.56-0.92) | |||
| Donor | ||||||||
| MSD | 37 | 14 | 0.58 (0.39-0.73) | .382 | 10 | 0.73 (0.54-0.85) | .883 | |
| MUD | 154 | 73 | 0.50 (0.42-0.58) | 46 | 0.68 (0.59-0.75) | |||
| Remission status | ||||||||
| CR1 | 139 | 55 | 0.58 (0.49-0.66) | .035 | 34 | 0.74 (0.65-0.81) | .091 | |
| CR2 | 50 | 31 | 0.36 (0.22-0.49) | 21 | 0.57 (0.41-0.70) | |||
| CR3 | 2 | 1 | 0.50 (0.01-0.91) | 1 | 0.50 (0.01-0.91) | |||
| Remission status | .029 | |||||||
| CR1 | 139 | 55 | 0.58 (0.49-0.66) | .011 | 34 | 0.74 (0.65-0.81) | ||
| ≥CR2 | 52 | 32 | 0.36 (0.23-0.50) | 22 | 0.57 (0.41-0.69) | |||
| Stem cell source | ||||||||
| Bone marrow | 131 | 55 | 0.54 (0.45-0.63) | .264 | 37 | 0.70 (0.61-0.78) | .429 | |
| Peripheral blood | 35 | 21 | 0.39 (0.23-0.55) | 9 | 0.71 (0.52-0.84) | |||
| Cord blood | 23 | 11 | 0.52 (0.31-0.70) | 10 | 0.58 (0.34-0.76) | |||
| Bone marrow + peripheral blood | 1 | 0 | 1.00 (1.00-1.00) | 0 | 1.00 (1.00-1.00) | |||
| Time of relapse, mo | ||||||||
| <18 | 32 | 21 | 0.30 (0.14-0.47) | .577 | 13 | 0.60 (0.40-0.75) | .569 | |
| 18-30 | 15 | 9 | 0.40 (0.16-0.63) | 8 | 0.42 (0.16-0.66) | |||
| >30 | 1 | 0 | 1.00 (1.00-1.00) | 0 | 1.00 (1.00-1.00) | |||
| Type of relapse | ||||||||
| Bone marrow | 29 | 15 | 0.45 (0.26-0.63) | .017 | 9 | 0.66 (0.44-0.81) | .080 | |
| CNS | 10 | 7 | 0.27 (0.05-0.56) | 4 | 0.53 (0.17-0.80) | |||
| Other | 11 | 9 | 0.18 (0.03-0.44) | 8 | 0.36 (0.11-0.63) | |||
| . | N . | EFS . | OS . | |||||
|---|---|---|---|---|---|---|---|---|
| . | Events . | 3-y EFS . | P . | Deaths . | 3-y OS . | P . | ||
| Total | 191 | 87 | 0.52 (0.44-0.59) | 56 | 0.69 (0.61-0.76) | |||
| Sex | ||||||||
| Male | 107 | 48 | 0.52 (0.41-0.61) | .989 | 31 | 0.69 (0.58-0.78) | .902 | |
| Female | 84 | 39 | 0.52 (0.40-0.62) | 25 | 0.69 (0.57-0.78) | |||
| Age at HSCT, y | ||||||||
| <2 | 90 | 44 | 0.48 (0.37-0.59) | .328 | 26 | 0.71 (0.59-0.80) | .793 | |
| ≥2 | 101 | 43 | 0.55 (0.44-0.64) | 30 | 0.68 (0.57-0.76) | |||
| Age at diagnosis, y | ||||||||
| <1 | 92 | 52 | 0.40 (0.29-0.50) | .002 | 30 | 0.64 (0.52-0.74) | .220 | |
| ≥1 | 99 | 35 | 0.63 (0.52-0.72) | 26 | 0.73 (0.63-0.81) | |||
| Immunophenotype | ||||||||
| BCP | 156 | 78 | 0.47 (0.38-0.55) | .038 | 50 | 0.66 (0.57-0.73) | .160 | |
| T-cell ALL | 28 | 9 | 0.67 (0.46-0.81) | 6 | 0.78 (0.57-0.89) | |||
| Other | 6 | 0 | 1.00 (1.00-1.00) | 0 | 1.00 (1.00-1.00) | |||
| MRD before HSCT | ||||||||
| Cutoff ≥10−4 vs <10−4 | ||||||||
| Low | 105 | 49 | 0.50 (0.40-0.60) | .172 | 32 | 0.68 (0.57-0.77) | .384 | |
| High | 46 | 27 | 0.39 (0.24-0.53) | 19 | 0.58 (0.42-0.71) | |||
| Genetic aberration | ||||||||
| KMT2A-AFF1 | 51 | 28 | 0.41 (0.26-0.55) | .349 | 21 | 0.55 (0.39-0.68) | .164 | |
| ETV6-RUNX1 | 6 | 2 | 0.67 (0.19-0.90) | 1 | 1.00 (1.00-1.00) | |||
| BCR-ABL∗ | 13 | 5 | 0.59 (0.28-0.81) | 3 | 0.77 (0.44-0.92) | |||
| None of the above | 108 | 46 | 0.55 (0.45-0.64) | 29 | 0.71 (0.60-0.79) | |||
| Hypodiploidy | ||||||||
| No | 169 | 78 | 0.51 (0.43-0.59) | .962 | 49 | 0.70 (0.61-0.77) | .761 | |
| Yes | 2 | 1 | 0.50 (0.01-0.91) | 1 | 0.50 (0.01-0.91) | |||
| Hyperdiploidy | ||||||||
| No | 149 | 71 | 0.50 (0.41-0.58) | .181 | 46 | 0.68 (0.59-0.75) | .195 | |
| Yes | 23 | 8 | 0.65 (0.42-0.81) | 4 | 0.81 (0.56-0.92) | |||
| Donor | ||||||||
| MSD | 37 | 14 | 0.58 (0.39-0.73) | .382 | 10 | 0.73 (0.54-0.85) | .883 | |
| MUD | 154 | 73 | 0.50 (0.42-0.58) | 46 | 0.68 (0.59-0.75) | |||
| Remission status | ||||||||
| CR1 | 139 | 55 | 0.58 (0.49-0.66) | .035 | 34 | 0.74 (0.65-0.81) | .091 | |
| CR2 | 50 | 31 | 0.36 (0.22-0.49) | 21 | 0.57 (0.41-0.70) | |||
| CR3 | 2 | 1 | 0.50 (0.01-0.91) | 1 | 0.50 (0.01-0.91) | |||
| Remission status | .029 | |||||||
| CR1 | 139 | 55 | 0.58 (0.49-0.66) | .011 | 34 | 0.74 (0.65-0.81) | ||
| ≥CR2 | 52 | 32 | 0.36 (0.23-0.50) | 22 | 0.57 (0.41-0.69) | |||
| Stem cell source | ||||||||
| Bone marrow | 131 | 55 | 0.54 (0.45-0.63) | .264 | 37 | 0.70 (0.61-0.78) | .429 | |
| Peripheral blood | 35 | 21 | 0.39 (0.23-0.55) | 9 | 0.71 (0.52-0.84) | |||
| Cord blood | 23 | 11 | 0.52 (0.31-0.70) | 10 | 0.58 (0.34-0.76) | |||
| Bone marrow + peripheral blood | 1 | 0 | 1.00 (1.00-1.00) | 0 | 1.00 (1.00-1.00) | |||
| Time of relapse, mo | ||||||||
| <18 | 32 | 21 | 0.30 (0.14-0.47) | .577 | 13 | 0.60 (0.40-0.75) | .569 | |
| 18-30 | 15 | 9 | 0.40 (0.16-0.63) | 8 | 0.42 (0.16-0.66) | |||
| >30 | 1 | 0 | 1.00 (1.00-1.00) | 0 | 1.00 (1.00-1.00) | |||
| Type of relapse | ||||||||
| Bone marrow | 29 | 15 | 0.45 (0.26-0.63) | .017 | 9 | 0.66 (0.44-0.81) | .080 | |
| CNS | 10 | 7 | 0.27 (0.05-0.56) | 4 | 0.53 (0.17-0.80) | |||
| Other | 11 | 9 | 0.18 (0.03-0.44) | 8 | 0.36 (0.11-0.63) | |||
Data presented are probabilities and 95% CIs. BCP, B-cell precursor; CNS, central nervous system.
Includes 1 patient with BCR-ABL and KMT2A-AFF1 and 2 patients with BCR-ABL and ETV6-RUNX1.
The 139 patients who received transplantation in CR1 had a significantly higher probability of EFS (.58 [95% CI, 0.49-0.66]) and OS (.74 [95% CI, 0.65-0.81]) compared with the 52 patients who received transplantation in ≥CR2 (.36 [95% CI, 0.23-0.50] and .57 [95% CI, 0.41-0.69], respectively; P = .011 and P = .029). These differences were due to a lower CIR among patients who received transplantation in CR1 vs patients who received transplantation in ≥CR2, whereas there was no difference in the NRM. Donor type, stem cell source, genetic alterations, immunophenotype, and hypodiploidy or hyperdiploidy did not significantly affect the probability of EFS or OS (Table 2).
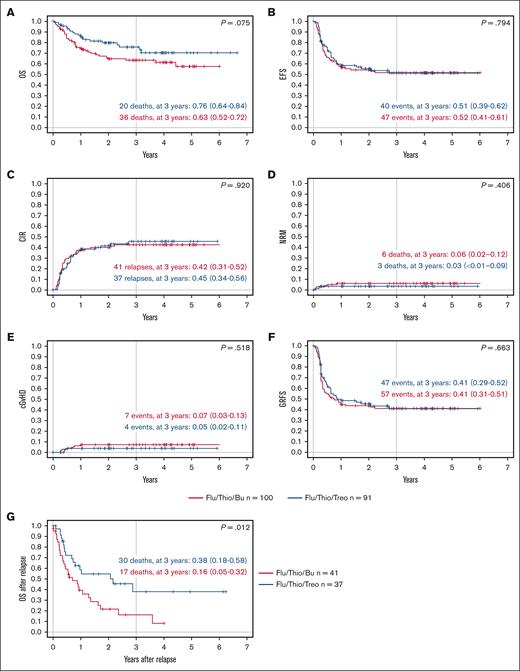
When assessed based on the conditioning regimen, patients who received transplantation after conditioning with Flu/Thio/Bu and Flu/Thio/Treo had a comparable probability of EFS at 3 years (.52 [95% CI, 0.41-0.61] vs .51 [95% CI, 0.39-0.62], respectively; P = .794; Table 3; Figure 2). Probability of OS at 3 years was numerically lower for patients receiving Flu/Thio/Bu compared with patients receiving Flu/Thio/Treo (.63 [95% CI, 0.52-0.72] vs .76 [95% CI, 0.64-0.84], respectively; P = .075; Table 3; Figure 2).
Outcome according to conditioning regimen
| . | Flu/Thio/Bu . | Flu/Thio/Treo . | P . |
|---|---|---|---|
| Evaluable patients, n | 100 | 91 | |
| Primary end point | |||
| Death | |||
| Death, n | 36 | 20 | |
| 3-y OS (95% CI) | 0.63 (0.52-0.72) | 0.76 (0.64-0.84) | .075 |
| Secondary end points | |||
| Any failure | |||
| Relapse, secondary malignancy, or death, n | 47 | 40 | |
| 3-y EFS (95% CI) | 0.52 (0.41-0.61) | 0.51 (0.39-0.62) | .794 |
| Relapse | |||
| Relapse, n | 41 | 37 | |
| 3-y CIR (95% CI) | 0.42 (0.31-0.52) | 0.45 (0.34-0.56) | .920 |
| NRM | |||
| Death in CR, n | 6 | 3 | |
| 3-y NRM (95% CI) | 0.06 (0.02-0.12) | 0.03 (<0.01-0.09) | .406 |
| Acute GVHD by day 100 | |||
| Evaluable, n | 100 | 89 | |
| Grade 0/1, n (%) | 71 (71%) | 74 (83%) | |
| Grade 2, n (%) | 19 (19%) | 7 (8%) | .049∗ |
| Grade 3/4, n (%) | 10 (10%) | 8 (9%) | .813† |
| cGVHD by 3 y | |||
| cGVHD/events without cGVHD/patients, n | 7/45/100 | 4/39/90 | |
| 3-y cumulative incidence of cGVHD (95% CI) | 0.07 (0.03-0.13) | 0.05 (0.02-0.11) | .518 |
| 3-y cumulative incidence of event without cGVHD (95% CI) | 0.46 (0.36-0.56) | 0.48 (0.36-0.59) | .902 |
| GVHD and relapse | |||
| Events/patients, n | 57/99 | 47/88 | |
| 3-y GRFS (95% CI) | 0.41 (0.31-0.51) | 0.41 (0.29-0.52) | .663 |
| Nonhematologic AEs of grade 3/4 at day 100 | |||
| Evaluable, n | 98 | 91 | |
| Grade 3/4, n (%) | 76 (78%) | 63 (69%) | .195 |
| . | Flu/Thio/Bu . | Flu/Thio/Treo . | P . |
|---|---|---|---|
| Evaluable patients, n | 100 | 91 | |
| Primary end point | |||
| Death | |||
| Death, n | 36 | 20 | |
| 3-y OS (95% CI) | 0.63 (0.52-0.72) | 0.76 (0.64-0.84) | .075 |
| Secondary end points | |||
| Any failure | |||
| Relapse, secondary malignancy, or death, n | 47 | 40 | |
| 3-y EFS (95% CI) | 0.52 (0.41-0.61) | 0.51 (0.39-0.62) | .794 |
| Relapse | |||
| Relapse, n | 41 | 37 | |
| 3-y CIR (95% CI) | 0.42 (0.31-0.52) | 0.45 (0.34-0.56) | .920 |
| NRM | |||
| Death in CR, n | 6 | 3 | |
| 3-y NRM (95% CI) | 0.06 (0.02-0.12) | 0.03 (<0.01-0.09) | .406 |
| Acute GVHD by day 100 | |||
| Evaluable, n | 100 | 89 | |
| Grade 0/1, n (%) | 71 (71%) | 74 (83%) | |
| Grade 2, n (%) | 19 (19%) | 7 (8%) | .049∗ |
| Grade 3/4, n (%) | 10 (10%) | 8 (9%) | .813† |
| cGVHD by 3 y | |||
| cGVHD/events without cGVHD/patients, n | 7/45/100 | 4/39/90 | |
| 3-y cumulative incidence of cGVHD (95% CI) | 0.07 (0.03-0.13) | 0.05 (0.02-0.11) | .518 |
| 3-y cumulative incidence of event without cGVHD (95% CI) | 0.46 (0.36-0.56) | 0.48 (0.36-0.59) | .902 |
| GVHD and relapse | |||
| Events/patients, n | 57/99 | 47/88 | |
| 3-y GRFS (95% CI) | 0.41 (0.31-0.51) | 0.41 (0.29-0.52) | .663 |
| Nonhematologic AEs of grade 3/4 at day 100 | |||
| Evaluable, n | 98 | 91 | |
| Grade 3/4, n (%) | 76 (78%) | 63 (69%) | .195 |
cGVHD, chronic GVHD.
Grade 1 to 4 acute GVHD.
Grade 3 to 4 acute GVHD.
Key outcomes according to conditioning regimen. (A) Probability of OS; (B) probability of EFS over time; (C) CIR; (D) NRM; (E) probability of cGVHD; (F) probability of GRFS over time; and (G) probability of OS after relapse. Flu/Thio/Bu is shown in red and Flu/Thio/Treo in blue. n = 100 for Flu/Thio/Bu, and n = 91 for Flu/Thio/Treo for panels A to F; n = 41 for Flu/Thio/Bu, and n = 37 for Flu/Thio/Treo for panel G; 95% CIs are shown in parentheses. cGVHD, chronic GVHD.
Key outcomes according to conditioning regimen. (A) Probability of OS; (B) probability of EFS over time; (C) CIR; (D) NRM; (E) probability of cGVHD; (F) probability of GRFS over time; and (G) probability of OS after relapse. Flu/Thio/Bu is shown in red and Flu/Thio/Treo in blue. n = 100 for Flu/Thio/Bu, and n = 91 for Flu/Thio/Treo for panels A to F; n = 41 for Flu/Thio/Bu, and n = 37 for Flu/Thio/Treo for panel G; 95% CIs are shown in parentheses. cGVHD, chronic GVHD.
Relapse
No difference was observed in the CIR or NRM between treatment arms. Importantly, of those patients who relapsed after HSCT (n = 78), those receiving Flu/Thio/Treo (n = 37) had a better OS at 3 years after relapse compared with those receiving Flu/Thio/Bu (n = 41; 0.38 [95% CI, 0.18-0.58] vs 0.16 [95% CI, 0.05-0.32], respectively; P = .012; Figure 2).
GVHD
The percentage of patients with severe acute GVHD was low, and there was no significant difference between the 2 treatment arms through day 100. Of the patients evaluable on day 100, 29 of 100 (29%) receiving Flu/Thio/Bu developed grade 2 to 4 acute GVHD and 10 of 100 (10%) developed grade 3/4 acute GVHD, whereas 15 of 89 (17%) receiving Flu/Thio/Treo developed grade 2 to 4 acute GVHD and 8 of 89 (9%) experienced grade 3/4 acute GVHD (Table 3).
At 3 years, the cumulative incidence of chronic GVHD among patients receiving Flu/Thio/Bu and Flu/Thio/Treo was 0.07 (95% CI, 0.03-0.13) and 0.05 (95% CI, 0.02-0.11), respectively (P = .518; Table 3). GRFS at 3 years was 0.41 (95% CI, 0.31-0.51) in the Flu/Thio/Bu group compared with 0.41 (95% CI, 0.29-0.52) in the Flu/Thio/Treo group (P = .663; Figure 2).
Multivariable analysis
In the multivariable analysis of EFS (which considered conditioning regimen, donor source, remission status, immunophenotype, age at HSCT, and KMT2A gene rearrangement), we found only age < 1 year at diagnosis to be significantly associated with a reduced outcome (hazard ratio [HR], 0.49 for age ≥ 1 year). For OS, only the presence of KMT2A gene rearrangement was found to be negatively associated with outcome, with an HR of 1.96 (Table 4).
Multivariable analysis of OS and EFS
| . | . | EFS . | OS . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Conditioning regimen | Flu/Thio/Bu | 1 | 1 | ||
| Flu/Thio/Treo | 0.94 (0.60-1.46) | .769 | 0.64 (0.37-1.13) | .124 | |
| Donor | MSD | 1 | 1 | ||
| MUD | 1.31 (0.71-2.42) | .388 | 3 (0.51-2.10) | .928 | |
| Remission status | CR1 | 1 | 1 | ||
| ≥CR2 | 1.49 (0.88-2.52) | .136 | 1.44 (0.75-2.76) | .271 | |
| Immunophenotype | BCP | 1 | 1 | ||
| T-cell ALL | 0.81 (0.37-1.78) | .603 | 0.98 (0.38-2.49) | .964 | |
| Other | NA∗ | NA∗ | NA∗ | NA∗ | |
| Age at HSCT, y | <2 | 1 | 1 | ||
| >2 to ≥4 | 1.23 (0.74-2.08) | .403 | 1.42 (0.62-3.22) | .405 | |
| KMT2A-AFF1 | No | 1 | 1 | ||
| Yes | 1.25 (0.74-2.08) | .403 | 1.96 (1.05-3.68) | .036 | |
| Age at diagnosis, y | <1 | 1 | 1 | ||
| ≥1 | 0.49 (0.25-0.99) | .046 | 0.70 (0.31-1.59) | .397 | |
| . | . | EFS . | OS . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Conditioning regimen | Flu/Thio/Bu | 1 | 1 | ||
| Flu/Thio/Treo | 0.94 (0.60-1.46) | .769 | 0.64 (0.37-1.13) | .124 | |
| Donor | MSD | 1 | 1 | ||
| MUD | 1.31 (0.71-2.42) | .388 | 3 (0.51-2.10) | .928 | |
| Remission status | CR1 | 1 | 1 | ||
| ≥CR2 | 1.49 (0.88-2.52) | .136 | 1.44 (0.75-2.76) | .271 | |
| Immunophenotype | BCP | 1 | 1 | ||
| T-cell ALL | 0.81 (0.37-1.78) | .603 | 0.98 (0.38-2.49) | .964 | |
| Other | NA∗ | NA∗ | NA∗ | NA∗ | |
| Age at HSCT, y | <2 | 1 | 1 | ||
| >2 to ≥4 | 1.23 (0.74-2.08) | .403 | 1.42 (0.62-3.22) | .405 | |
| KMT2A-AFF1 | No | 1 | 1 | ||
| Yes | 1.25 (0.74-2.08) | .403 | 1.96 (1.05-3.68) | .036 | |
| Age at diagnosis, y | <1 | 1 | 1 | ||
| ≥1 | 0.49 (0.25-0.99) | .046 | 0.70 (0.31-1.59) | .397 | |
No estimates or P values are given because of limited sample size in this subgroup (6 patients and 0 events).
Toxicity and safety
Both regimens were well tolerated, with very favorable toxicity and safety. In supplemental Table 1 (supplemental Appendix 2) all AEs are listed. When comparing the Flu/Thio/Bu and Flu/Thio/Treo groups, the most frequently reported nonhematological toxicities were stomatitis (grade 3/4: 33% and 41%, respectively), infection (grade 3/4: 42% and 44%, respectively), and nausea (grade 3/4: 31% and 26%, respectively). Grade 3/4 veno-occlusive disease (VOD) of the liver occurred in 12% of patients in the Flu/Thio/Bu group compared with 0% in the Flu/Thio/Treo group. Secondary malignancies were not observed (supplemental Table 1).
Historical comparison
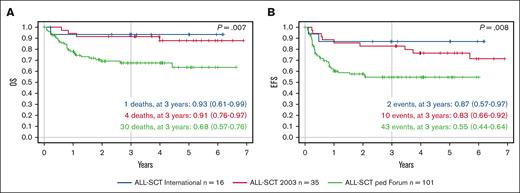
In the previously reported studies ALL-SCT BFM 2003 (NCT01423747)15 and ALL-SCT BFM International (NCT01423500),20 35 and 16 children, respectively, aged 2 to 4 years received TBI/Eto conditioning. We performed a retrospective analysis to compare these 2 cohorts with the 101 children aged 2 to 4 years in this FORUM trial. In the ALL-SCT BFM 2003 trial, 3-year probability of EFS and OS was 0.83 (95% CI, 0.66-0.92) and 0.91 (95% CI, 0.76-0.97), respectively, for children aged 2 to 4 years,15 whereas in the ALL-SCT BFM International trial, the 3-year probabilities of EFS and OS were .87 (95% CI, 0.57-0-97) and .93 (95% CI, 0.61-0.99), respectively.20 In comparison, in this FORUM trial, 3-year probabilities of EFS and OS for children aged 2 to 4 years receiving either chemotherapeutic conditioning regimen were .55 (95% CI, 0.44-0.64) and .68 (95% CI, 0.57-0.76), respectively (Figure 3).
Outcomes in comparison with those of historical cohorts of children aged ≥2 but <4 years with high-risk ALL undergoing HSCT. (A) Probability of OS over time and (B) probability of EFS over time, showing the ALL-SCT BFM International trial of TBI/Eto (blue; n = 16), the ALL-SCT BFM 2003 trial of TBI/Eto (red; n = 35), and children aged >2 but ≤4 years in the FORUM study of chemotherapeutic conditioning (green; n = 101).
Outcomes in comparison with those of historical cohorts of children aged ≥2 but <4 years with high-risk ALL undergoing HSCT. (A) Probability of OS over time and (B) probability of EFS over time, showing the ALL-SCT BFM International trial of TBI/Eto (blue; n = 16), the ALL-SCT BFM 2003 trial of TBI/Eto (red; n = 35), and children aged >2 but ≤4 years in the FORUM study of chemotherapeutic conditioning (green; n = 101).
Discussion
Allogeneic HSCT is an important treatment option for pediatric ALL. When initiating the FORUM trial, international experts had reached consensus to raise the age at which children with ALL could receive TBI from 2 to 4 years. The aim was to prevent the severe late effects of radiation therapy in younger children.6,21 As a consequence, 2 chemotherapeutic conditioning regimens were used for children aged <4 years in the trial. Morbidity was generally low, and the NRM rates at 3 years were 6% with Flu/Thio/Bu and 3% with Flu/Thio/Treo. This is a remarkably low NRM rate for such a high-risk group, considering that it was obtained in a multicenter, international trial in which 81% of patients received a transplant from an MUD.
We did not find statistically significant differences in the NRM rates between patients who received transplantation with low MRD and those with high MRD; however, that might be because of small patient numbers. Patients with aberrations in the KMT2A-AFF1 gene had a nearly twofold higher HR for death than patients without this aberration. Children who were diagnosed with ALL at an age <1 year had poorer EFS than those diagnosed when older. However, allogeneic HSCT could rescue them if they received transplantation in CR1.
One of the most important findings of this part of the FORUM trial is that remission status at the time of HSCT affects the prognosis of children with ALL aged <4 years. Children aged <4 years who underwent HSCT in CR1 achieved higher EFS and OS than those who received transplantation in CR2 or CR3, comparable with children aged >4 years who underwent chemotherapeutic conditioning in the randomized part of FORUM.16
We found similar outcomes for patients who received transplantation from either an MUD or an MSD. As demonstrated in our previous studies, this outcome for MUD HSCT is achieved using a stringent transplant protocol with in vivo T-cell depletion/modulation using antithymocyte globulin or anti–T-cell globulin.15,16 The use of serotherapy most likely also contributed to the very low incidence of severe acute GVHD and chronic GVHD.
In this vulnerable age group of patients with ALL, the composition of the conditioning regimen plays an important role with regard to toxicity and mortality. We observed a very low rate of NRM of only 0.06 (95% CI, 0.02-0.12) in the Flu/Thio/Bu cohort and 0.03 (95% CI, <0.01-0.09) in the Flu/Thio/Treo cohort. VOD occurred in only 12% of patients receiving Bu. Our results are superior to those observed in other trials, such as the Japanese Pediatric Leukemia/Lymphoma Study Group MLL-10 trial, which used conditioning with Bu, cyclophosphamide, and Eto, and reported VOD and pulmonary toxicities in 21.9% and 28.1% of patients, respectively.5 Although TDM was used in that trial to guide Bu concentration, these complication rates were high. In the Interfant-06 trial, the conditioning regimen was Bu, cyclophosphamide, and melphalan between 2006 and 2011: 13 of 50 children (26%) died because of HSCT-related complications. Thereafter, the conditioning regimen was changed to Flu/Thio/Bu, and between 2012 and 2016 only 3 of 61 patients (5%) died in remission after HSCT.22
No difference was observed with regard to the probability of EFS, CIR, or NRM between the 2 regimens used in the FORUM trial. It is to be acknowledged, however, that in the Treo group, slightly more patients were treated in CR1 and fewer patients received cord blood grafts than in the Bu group.
Interestingly, patients conditioned with Flu/Thio/Treo with subsequent relapse had a better probability of OS than patients receiving Flu/Thio/Bu. Because data on posttransplant treatment of relapse were not available, one can only speculate on the reason for this finding. It might be that patients relapsing after HSCT conditioned with Treo-based regimens have another chance of rescue with either a second allograft or chimeric antigen receptor (CAR) T-cell therapy.23-25 In fact, in a recent retrospective study of European real-world data of patients with ALL who relapsed after HSCT and were treated with the anti-CD19 CAR T-cell therapy tisagenlecleucel, patients had superior EFS and OS when the conditioning for HSCT was based on Treo not Bu.26 Importantly, in our study, Bu-based conditioning was chosen and administered in countries with access to anti-CD19 CAR T-cell therapies.
Although our study protocol provided a robust platform for safe allogeneic HSCT in young children with high-risk ALL and achieved low NRM rates, the 3-year EFS and OS rates were low vs historical cohorts of patients aged 2 to 4 years receiving TBI/Eto (from the ALL-SCT BFM 2003 trial and the ALL-SCT BFM International trial).
Because no observed increase in fatal complication rates has been observed in trials comparing TBI with chemotherapy in childhood ALL, the ideal age threshold below which non–TBI-containing regimens should be used is yet to be determined.16,27,28 Previous long-term studies have demonstrated that younger age at HSCT is a risk factor for compromised cognitive development in a dose-dependent manner, particularly when conditioning regimens involve myeloablative doses of TBI.27,29 Furthermore, younger children who undergo TBI are susceptible to enduring long-term toxicities such as gonadal and thyroid dysfunction, growth impairment, and secondary malignancies, but this does happen with Bu-based regimens as well.
In the ALL-SCT BFM 2003 trial, 4 of 55 children aged 2 to 4 years developed a secondary malignancy within 10 years after transplantation, all of whom were treated with TBI.30 Secondary malignancies have been reported with Bu-containing regimens too and other long-term toxicities are reported as well,31,32 whereas data on the long-term effects of Treo-containing regimens are currently lacking.
Further prospective studies are required to determine the optimal age for TBI conditioning in pediatric patients with ALL. In light of this, the planned FORUM-2 trial will assign children aged >2 years to TBI/Eto conditioning before HSCT. In children aged <2 years, upfront treatment with immunotherapy using blinatumomab or inotuzumab could be helpful in reducing disease recurrence.33 Deciding upon the best choice of conditioning, and weighing the possible lifelong consequences of sequelae against the risk of relapse is a very challenging decision. Addressing and acknowledging the individual risks and fears of each child and their family should remain an important part of the shared decision process.
In conclusion, this nonrandomized part of the international, multicenter FORUM trial showed that chemotherapeutic conditioning with either Flu/Thio/Bu or Flu/Thio/Treo allows for HSCT for children with high-risk ALL aged <4 years with a low complication rate. Further prospective trials are required and planned to reduce the risk of leukemia recurrence and to improve survival in this vulnerable young population.
Acknowledgments
The authors acknowledge the Fédération Enfants et Santé and Association Hubert Gouin for support of the French study team. The St. Anna Children’s Research Institute supported the FORUM study and editorial manuscript preparation. Editorial support in the preparation of this manuscript was provided by Hannah Bridges of HB Health Comms Limited, funded by the St. Anna Children’s Research Institute. A list of site investigators is given in the supplemental Material.
This work was supported by a grant from the Deutsche Knochenmarkspenderdatei and unrestricted grants from Neovii, Riemser, and Medac to the German study team. The Austrian team was supported by grants from Neovii, Medac, and Jazz.
Authorship
Contribution: All authors contributed to study conception and design, the collection and assembly of data, data analysis, manuscript writing, and final approval of the manuscript, and are accountable for all aspects of the work; and all authors other than U.P. provided study materials or recruited patients.
Conflict-of-interest disclosure: P.B. declares research grants from Neovii, Riemser, Medac, and Bristol Myers Squibb (to the institution); is a member of advisory boards for Novartis, Celgene, Amgen, Medac, and Servier (personal and institutional); has received speaker fees from Miltenyi, Jazz, Riemser, Novartis, and Amgen (to the institution); and declares a patent with and royalties from Medac. M.A. has received speaker and traveling fees from Jazz. J.B. has participated in advisory boards for Amgen, Novartis, Pfizer, and Janssen (to the institution), and has received speaker fees from Novartis. T.G. has received research grants from Jazz. M.I. has received honoraria for an advisory board role from Novartis. H.P. declares travel grants from Neovii. T.T. has received honoraria for consultancy and advisory board roles in Servier and Jazz. F.L. is a member of advisory boards for Amgen, Novartis, Bellicum Pharmaceuticals, Neovii, and Vertex, and has received speaker fees from Amgen, Novartis, Miltenyi, Medac, Jazz Pharmaceuticals, and Takeda, outside the submitted work. C.P. declares research grants from Amgen, Neovii, Riemser, Medac, and Jazz; is a member of advisory boards for Amgen, Neovii, Jazz, and Novartis; and has received speaker fees from Amgen, Neovii, Novartis, Medac, and Riemser. The remaining authors declare no competing financial interests.
Correspondence: Peter Bader, Goethe University, University Hospital, Department of Pediatrics, Division for Stem Cell Transplantation, Immunology and Intensive Care Medicine, Theodor-Stern Kai 7, 60590 Frankfurt am Main, Germany; email: p.bader@em.uni-frankfurt.de.
References
Author notes
∗B.P., U.P., and J.-H.D. are joint first authors.
†F.L. and C.P. are joint last authors.
Data are available on request from the corresponding author, Peter Bader (p.bader@em.uni-frankfurt.de).
The full-text version of this article contains a data supplement.