In this issue of Blood Advances, Ragni et al1 have written a timely article highlighting the importance of liver health both before and after gene therapy for hemophilia A. Since the first liver-directed adeno-associated virus (AAV) gene therapy for hemophilia B,2 transaminitis has occurred in a proportion of patients in all systemically delivered AAV gene therapies. The phenotypic heterogeneity of a population of people with hemophilia with prior or current infection to numerous blood-borne viral pathogens, coupled with an epidemic of metabolic dysfunction–associated steatotic liver disease (MASLD), has been well described, and procedures for screening potential gene therapy candidates are a welcome addition.1 Candidates with risk factors, including advanced fibrosis, use of liver-toxic drugs (including alcohol), and advanced MASLD, have been excluded from clinical trials and will need thoughtful screening and judgment now that gene therapy is commercially available.3
The much greater enigma is what is happening after AAV gene therapy? Since the original publication of transient transaminitis in 2006,2 some hepatologists have told hematologists not to worry about it. But hematologists (and the patient population) are worried for 2 reasons. First, in the AAV-factor IX (FIX) studies,2,4 the transaminitis was coincident with a loss of FIX expression, and in a later trial, steroid treatment appeared to stop or slow the loss. These investigations suggested cytotoxic T lymphocytes mediated the destruction of FIX transgene–containing hepatocytes based upon AAV capsid peptides displayed in the context of HLA class 1 on the hepatocyte cell surface.2 However, the story for FVIII, and perhaps FIX as well, is not that straightforward because AAV clinical doses have escalated logarithmically, as described by Ragni et al and in a recent comprehensive review.1,5 With escalating AAV doses, a far more complex host response is generated.
The second reason for concern relates to the natural history of hepatitis C infection in persons with hemophilia. The epidemic started with the introduction of pooled clotting factor concentrates derived from tens of thousands of donors in the late 1960s, with most individuals with severe hemophilia infected until viral inactivation procedures for the plasma-derived clotting factors were introduced in the mid-1980s. Transaminase levels waxed and waned, being 2 to 3 times the upper limit of normal, then normal on the next test, and so on, for decades. However, during this prolonged time, the liver was being destroyed, leading to liver transplants for some and death for many due to cirrhosis and/or hepatocellular carcinoma.6 HIV, which coinfected thousands of people with hemophilia, accelerated the time to cirrhosis. Therefore, although these generally small transaminitis elevations in valoctocogene roxaparvovec–treated patients, which can persist at least into year 3 after treatment, may be benign, that has yet to be proven.
Thus, administration of 6E13 AAV-FVIII vector genomes per kg, 150 to 200 times greater than all the cells and bacteria in a 50-kg person, plus a variable number of empty or incomplete capsids, unleashes a spectrum of immune responses as if it were a viral infection that needs to be contained.7 It is unclear whether steroids are helpful and, if they are, which relevant immunological and metabolic circuits they modulate. Although the causes of transaminitis are likely multifactorial and individualized, as are the efficacy and durability responses,8 there is general agreement among hepatologists that leakage of transaminases from hepatocytes constitute intracellular damage that can lead to hepatocyte death.
Not surprisingly, we are left with more questions than answers with this new and different therapeutic modality.5 The findings in hemophilia are not unique and occur, sometimes with more serious consequences, in other monogenic diseases such as spinal muscular atrophy and X-linked myotubular myopathy. Acute liver failure and death have been seen at AAV doses of 1E14 per kg and above in these diseases, although underlying pathology may be a contributing factor.3 Thus, the questions revolving around the interaction of this complex entity, AAV, with a considerably more complex life form need focus and attention if AAV is to become a mainstream therapeutic modality. Patient-specific genetic and epigenetic contributors to AAV toxicity are important to discover, as the unknown determinants of efficacy and toxicity are unraveled, and benefit/risk is better quantified.5
With a multiplicity of infection of >40 000 vector genomes per hepatocyte, AAV might not be considered an efficient vector for transduction. A considerably more efficient AAV that widens the therapeutic window, thus decreasing the risk of liver damage, may provide a solution. Increasing liver tropism for the capsid or increasing endosomal escape or other aspects of nuclear entry and subsequent nuclear events to form episomes may offer possibilities to decrease toxicity while increasing efficacy. However, this line of experimentation may not help if FVIII itself is contributing to hepatocyte damage due to an induced endoplasmic reticulum–unfolded protein stress response, as noted by Ragni et al and discovered by Kaufman et al.1,5,9 Solutions include developing known variants that increase FVIII secretion from cells and/or increase the specific activity of FVIII. FIX Padua is an example of sixfold to eightfold increased specific activity from 1 amino acid change that has enabled AAV-FIX gene therapy.10 Another alternative would be to express FVIII in a different cell, because it is the liver sinusoidal endothelial cell and not the hepatocyte that normally makes FVIII. These efforts require more scientific research, alternative delivery mechanisms, and the ability to test FVIII variant hypotheses in nonhuman and human primates.
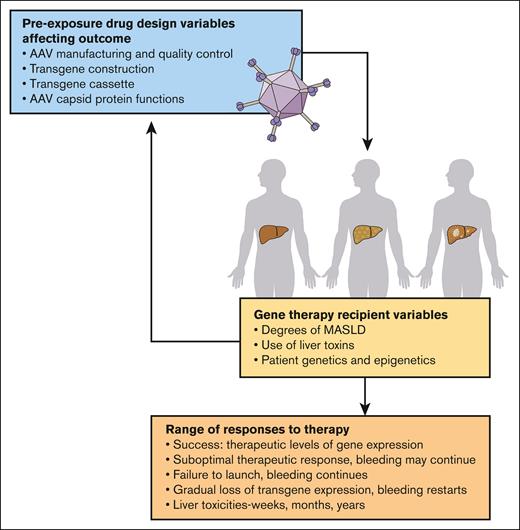
Ragni et al have provided an important road map for managing persons with hemophilia A before and after this first-generation gene therapy, valoctocogene roxaparvovec. Deeper understanding of the complexity of AAV, transgene, and host interactions at the subcellular and molecular levels, which may come in part from informative liver biopsies,8 is required to improve the efficacy and safety of this important treatment modality for hemophilia as well as many other monogenic diseases (see figure).
Multifarious variables causing unpredictable responses to AAV gene therapy. Many variables, known and unknown, contribute to the success of AAV gene therapy and define the benefit-risk balance for individuals and for disease populations.3,5 The manufacturing process remains poorly defined, with lot-to-lot variability and inconsistent scale-up. Each recipient has a unique phenotype based upon their genetics and epigenetic influences. Collectively, these variables dictate the range of individual response to gene therapy, from durable therapeutically beneficial effects to suboptimal responses and durability. Predictability of individual responses is not yet possible because biomarkers for host hepatocyte condition and host defenses are not well established. Professional illustration by Patrick Lane, ScEYEnce Studios.
Multifarious variables causing unpredictable responses to AAV gene therapy. Many variables, known and unknown, contribute to the success of AAV gene therapy and define the benefit-risk balance for individuals and for disease populations.3,5 The manufacturing process remains poorly defined, with lot-to-lot variability and inconsistent scale-up. Each recipient has a unique phenotype based upon their genetics and epigenetic influences. Collectively, these variables dictate the range of individual response to gene therapy, from durable therapeutically beneficial effects to suboptimal responses and durability. Predictability of individual responses is not yet possible because biomarkers for host hepatocyte condition and host defenses are not well established. Professional illustration by Patrick Lane, ScEYEnce Studios.
Conflict-of-interest disclosure: G.F.P. reports consultantcy fees from BioMarin, Novo Nordisk, Regeneron, Spark, and St. Jude Children’s Hospital; is a member of the scientific advisory boards of Be Biopharma, Frontera, Metagenomi, and hC Bioscience; and serves on the board of Voyager Therapeutics and the World Federation of Hemophilia.