TO THE EDITOR:
Histiocytoses are rare myeloid-derived clonal hematopoietic disorders in which tissue infiltrates with activated histiocytes. The subtypes are Langerhans-cell histiocytosis (LCH; L-group histiocytosis) and non-LCH, which include Rosai-Dorfman-Destombes disease (RDD; R-group histiocytosis), Erdheim-Chester disease (ECD; L-group histiocytosis), and numerous xanthogranuloma (C-group) disorders. Recently, a pivotal discovery demonstrated that disease pathophysiology is characterized by recurrent activating mutations in the MAPK pathway.1-4 This has led to a paramount therapeutic breakthrough that has resulted in long-term responses with targeted agents to these activating mutations, such as v-raf murine sarcoma viral oncogene homolog B1 (BRAF) inhibitors and MEK inhibitors.5-7
Histiocytoses are clinically heterogeneous, exhibiting as localized or diffuse, multisystem diseases. The most difficult cases to manage involve critical anatomical sites, such as ophthalmic structures, which occur in over half of the patients with histiocytosis.8 One ophthalmic site of accumulation is the orbit, which may exhibit variable frequencies depending on the histiocytosis subtype and age of the patient.
The revelation in pinpointing the genetic underpinnings that drive histiocytosis has stimulated efforts to better characterize the genotype- phenotype of the disease. Therefore, in this study, we focused on orbit-involving histiocytosis and investigated the associations between its clinical and genetic characteristics. Through this, we revealed a unique phenotype of orbital histiocytosis driven by mutations in KRAS, which is associated with the specific clinical characteristics described herein.
The study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Memorial Sloan Kettering Cancer Center (MSK). This retrospective, single-center study included 33 eligible patients recruited from MSK between January 2014 and April 2024. Consent was waived for this institutional review board–approved study (MSK 16-1604); however, all patients provided informed consent for molecular testing (MSK 12-245). Eligible patients had biopsy-confirmed histiocytosis involving orbital structures, as identified by clinical examination and/or radiologic imaging with positron emission tomography/computed tomography, computed tomography, or magnetic resonance imaging (MRI), along with molecular sequencing for the driver mutation. Patients without genetic testing were not included. Patients were subcategorized into orbit-involving multisystemic disease and isolated orbital disease (defined as orbital disease inclusive of the eyelid and conjunctiva without other sites of disease).
For evaluation of tumor mutations, genomic DNA was extracted from formalin-fixed paraffin-embedded samples and underwent mutational analysis using previously described techniques.3,9-11 The following additional data points were recorded: patient characteristics (age at diagnosis and sex of patient), tumor characteristics (histiocytosis subtype and other sites of disease), ocular structures involved, and visual findings (best-corrected Snellen visual acuity and visual symptoms).
Categorical data were compared using a 2-tailed Fisher exact test with statistical significance set at P value < 0.05.
Patient, disease and ocular structure, vision, and MRI findings for all patients are displayed in Table 1, and statistically significant P values are shown. The entire cohort of 33 patients was composed of 24 males (60%) with a median age of 54.8 years. Most of the cohorts were non-LCH (25 patients, 76%). All the patients had excellent best-corrected Snellen visual acuity.
Patient, disease and ocular structure, vision, and MRI findings for all patients with orbital histiocytosis (both nonisolated and isolated)
| Variable . | Orbit-involving multisystemic (n = 24) . | Isolated orbit (n = 9) . | P value∗ . | Isolated orbit (n = 9) . | |
|---|---|---|---|---|---|
| Non-KRAS (n = 3) . | KRAS (n = 6) . | ||||
| Age, y | 56.1 (19.2-75.1) | 51.9 (30.1-70.9) | 56.3 | 49.5 | |
| Median (range) | 56.1 (19.2-75.1) | 51.9 (30.1-70.9) | 56.3 | 49.5 | |
| Sex | 14 (58%)/10 (42%) | 6 (66%)/3 (33%) | 3 (100%) | 3 (50%)/3 (50%) | |
| Male (%)/female (%) | 14 (58%)/10 (42%) | 6 (66%)/3 (33%) | 3 (100%) | 3 (50%)/3 (50%) | |
| Disease subtype | |||||
| LCH | 0 | 2 (22%) | 2 (67%) | 0 | |
| ECD | 10 (42%) | 0 | .0003 | 0 | 0 |
| RDD | 7 (29%) | 0 | 0 | 0 | |
| Xanthogranuloma | 1 (4%) | 7 (78%) | .0001 | 1 (33%) | 6 (100%) |
| ALK-positive | 1 (4%) | 0 | 0 | 0 | |
| Mixed histiocytosis | 5 (21%) | 0 | 0 | 0 | |
| Mutational status | |||||
| BRAF V600E | 8 (33%) | 2 (22%) | 2 (67%) | 0 | |
| BRAF p.M484_P490delinsIH | 1 (4%) | 0 | 0 | 0 | |
| MAP2K1/2 | 4 (17%) | 0 | 0 | 0 | |
| KRAS | 2 (8%) | 6 (67%) | .0002 | 0 | 6 (100%) |
| Other† | 3 (13%) | 0 | 0 | 0 | |
| None identified | 6 (25%) | 1 (11%) | 1 (33%) | 0 | |
| Multisystem sites | |||||
| Neurologic‡ | 6 (25%) | 0 | 0 | 0 | |
| Brain parenchyma§ | 11 (46%) | 0 | 0 | 0 | |
| Bone | 19 (79%) | 0 | 0 | 0 | |
| Cardiovascular | 6 (25%) | 0 | 0 | 0 | |
| Pulmonary | 2 (8%) | 0 | 0 | 0 | |
| Retroperitoneum | 7 (30%) | 0 | 0 | 0 | |
| Abdomen | 5 (21%) | 0 | 0 | 0 | |
| Skin | 9 (38%) | 0 | 0 | 0 | |
| Lymph nodes | 6 (25%) | 0 | 0 | 0 | |
| Other ophthalmic sites|| | |||||
| Anterior segment | 1 episcl nodule | 1 conj infil | 0 | 1 conj infil | |
| Posterior segment | 2 choroid infil | 0 | 0 | 0 | |
| Neuroophthalmic | 4 cranial neuro | 0 | 0 | 0 | |
| Eyelid | 1 xanthelasma | 2 xanthelasma | 0 | 2 xanthelasma | |
| Visual symptoms|| | |||||
| Any symptom | 9 (50%) | 9 (100%) | .01 | ||
| Blurry vision | 6 (33%) | 3 (33%) | 0 | 3 (50%) | |
| Floaters | 1 (5%) | 1 (11%) | 0 | 1 (33%) | |
| Pain | 4 (22%) | 3 (33%) | 1 (33%) | 2 (33%) | |
| Photopsia | 1 (5%) | 0 | 0 | 0 | |
| Erythema/injections | 1 (5%) | 0 | 0 | 0 | |
| Diplopia | 3 (17%) | 2 (22%) | 1 (33%) | 1 (17%) | |
| Periorbital swelling | 2 (11%) | 6 (55%) | .006 | 1 (33%) | 5 (83%) |
| Metamorphopsia | 0 | 0 | 0 | 0 | |
| BL BC Snellen vision¶ | |||||
| >20/30 | 35 (97%) | 17 (94%) | 3 (100%) | 5 (83%) | |
| <20/200 | 1 (3%) | 1 (6%) | 0 | 1 (17%) | |
| MRI features | |||||
| Involvement adjacent cavity# | 12 (50%) | 3 (33%) | 2 (67%) | 1 (17%) | |
| Lacrimal gland involvement | 5 (21%) | 6 (55%) | .03 | 2 (67%) | 5 (83%) |
| Extraocular muscle involvement | 10 (42%) | 6 (55%) | 1 (33%) | 5 (83%) | |
| Intraconal disease | 10 (42%) | 0 | .03 | 0 (0%) | 0 (0%) |
| Unilateral | 10 (42%) | 8 (88%) | .02 | 3 (100%) | 5 (83%) |
| Variable . | Orbit-involving multisystemic (n = 24) . | Isolated orbit (n = 9) . | P value∗ . | Isolated orbit (n = 9) . | |
|---|---|---|---|---|---|
| Non-KRAS (n = 3) . | KRAS (n = 6) . | ||||
| Age, y | 56.1 (19.2-75.1) | 51.9 (30.1-70.9) | 56.3 | 49.5 | |
| Median (range) | 56.1 (19.2-75.1) | 51.9 (30.1-70.9) | 56.3 | 49.5 | |
| Sex | 14 (58%)/10 (42%) | 6 (66%)/3 (33%) | 3 (100%) | 3 (50%)/3 (50%) | |
| Male (%)/female (%) | 14 (58%)/10 (42%) | 6 (66%)/3 (33%) | 3 (100%) | 3 (50%)/3 (50%) | |
| Disease subtype | |||||
| LCH | 0 | 2 (22%) | 2 (67%) | 0 | |
| ECD | 10 (42%) | 0 | .0003 | 0 | 0 |
| RDD | 7 (29%) | 0 | 0 | 0 | |
| Xanthogranuloma | 1 (4%) | 7 (78%) | .0001 | 1 (33%) | 6 (100%) |
| ALK-positive | 1 (4%) | 0 | 0 | 0 | |
| Mixed histiocytosis | 5 (21%) | 0 | 0 | 0 | |
| Mutational status | |||||
| BRAF V600E | 8 (33%) | 2 (22%) | 2 (67%) | 0 | |
| BRAF p.M484_P490delinsIH | 1 (4%) | 0 | 0 | 0 | |
| MAP2K1/2 | 4 (17%) | 0 | 0 | 0 | |
| KRAS | 2 (8%) | 6 (67%) | .0002 | 0 | 6 (100%) |
| Other† | 3 (13%) | 0 | 0 | 0 | |
| None identified | 6 (25%) | 1 (11%) | 1 (33%) | 0 | |
| Multisystem sites | |||||
| Neurologic‡ | 6 (25%) | 0 | 0 | 0 | |
| Brain parenchyma§ | 11 (46%) | 0 | 0 | 0 | |
| Bone | 19 (79%) | 0 | 0 | 0 | |
| Cardiovascular | 6 (25%) | 0 | 0 | 0 | |
| Pulmonary | 2 (8%) | 0 | 0 | 0 | |
| Retroperitoneum | 7 (30%) | 0 | 0 | 0 | |
| Abdomen | 5 (21%) | 0 | 0 | 0 | |
| Skin | 9 (38%) | 0 | 0 | 0 | |
| Lymph nodes | 6 (25%) | 0 | 0 | 0 | |
| Other ophthalmic sites|| | |||||
| Anterior segment | 1 episcl nodule | 1 conj infil | 0 | 1 conj infil | |
| Posterior segment | 2 choroid infil | 0 | 0 | 0 | |
| Neuroophthalmic | 4 cranial neuro | 0 | 0 | 0 | |
| Eyelid | 1 xanthelasma | 2 xanthelasma | 0 | 2 xanthelasma | |
| Visual symptoms|| | |||||
| Any symptom | 9 (50%) | 9 (100%) | .01 | ||
| Blurry vision | 6 (33%) | 3 (33%) | 0 | 3 (50%) | |
| Floaters | 1 (5%) | 1 (11%) | 0 | 1 (33%) | |
| Pain | 4 (22%) | 3 (33%) | 1 (33%) | 2 (33%) | |
| Photopsia | 1 (5%) | 0 | 0 | 0 | |
| Erythema/injections | 1 (5%) | 0 | 0 | 0 | |
| Diplopia | 3 (17%) | 2 (22%) | 1 (33%) | 1 (17%) | |
| Periorbital swelling | 2 (11%) | 6 (55%) | .006 | 1 (33%) | 5 (83%) |
| Metamorphopsia | 0 | 0 | 0 | 0 | |
| BL BC Snellen vision¶ | |||||
| >20/30 | 35 (97%) | 17 (94%) | 3 (100%) | 5 (83%) | |
| <20/200 | 1 (3%) | 1 (6%) | 0 | 1 (17%) | |
| MRI features | |||||
| Involvement adjacent cavity# | 12 (50%) | 3 (33%) | 2 (67%) | 1 (17%) | |
| Lacrimal gland involvement | 5 (21%) | 6 (55%) | .03 | 2 (67%) | 5 (83%) |
| Extraocular muscle involvement | 10 (42%) | 6 (55%) | 1 (33%) | 5 (83%) | |
| Intraconal disease | 10 (42%) | 0 | .03 | 0 (0%) | 0 (0%) |
| Unilateral | 10 (42%) | 8 (88%) | .02 | 3 (100%) | 5 (83%) |
Bold values indicate significance. ALK, anaplastic lymphoma kinase; ARAF, serine/threonine-protein kinase A-Raf; BL BC, bilateral best corrected; Conj infil, conjunctival infiltrate; DI, diabetes inspidus; Episcl, episcleral.
Two-tailed Fisher exact test.
MAP3K6, KIF5B-ALK, and ARAF.
Neurologic = dura, orbit, DI, neuroendocrinopathy, spinal cord, and leptomeninges.
The brain parenchyma includes the cerebrum, cerebellum, and brain stem.
Available in 27 patients (18 nonisolated and 9 isolated).
Vision available in 54 eyes.
Adjacent cavity refers to the involvement of the cranial, nasal, or sinus cavity.
Isolated orbital disease was significantly characterized by unilateral disease (P = 0.02), xanthogranuloma subtype (P = .0001), KRAS mutations (P = .0002), periorbital swelling (P = .006), and extraconal disease, particularly lacrimal gland involvement, on MRI (P = .03).
Within the isolated orbital disease group alone, 6 patients (67%) had KRAS-mutant disease. All patients with KRAS-mutant had xanthogranuloma compared with 1 patient in the non-KRAS–mutant group. In addition to the differences in mutational status, there were no other significant differences between the groups.
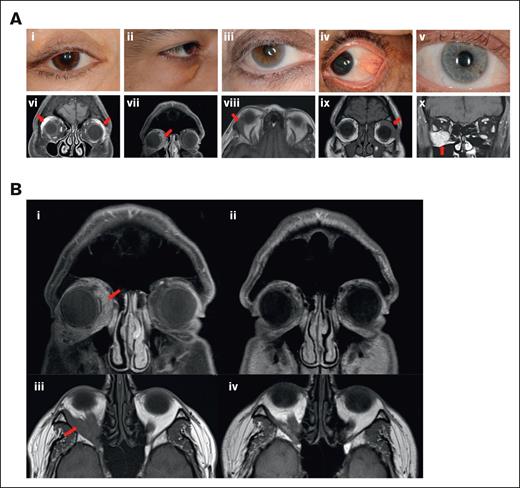
Figure 1A shows representative images of 5 patients with KRAS-mutant isolated orbital disease. Mutation, treatment, and response data for the 9 patients with isolated orbital histiocytosis are shown in supplemental Table 1. Representative images of disease responses are shown in Figure 1B. A summary of findings is displayed in supplemental Table 2.
Representative patient images. (A) Representative images of patients 1 to 5 with KRAS-mutant isolated orbital histiocytosis. External photographs of the external adnexa and involved eye: eyelid edema and ptosis (i), conjunctival histiocytic infiltration (ii), exophthalmos, and scleral show (iii), lower eyelid swelling with xanthelasma (iv), exophthalmos and scleral show (v). MRI of representative patients: arrows showing bilateral diffusely enlarged lacrimal glands (vi), arrow showing thickening of the left lateral rectus (vii), arrow showing mass lesion involving the right lateral rectus muscle (viii), arrow showing infiltration and involvement of the lacrimal gland, superior muscle complex and lateral rectus (ix), and arrow showing bulky disease in the inferior orbit with diffuse involvement of the inferior rectus muscle (x). (B) Representative patient images demonstrating disease response to treatment in KRAS-mutant isolated orbital disease. T1-weighted MRI coronal image of patient 2 demonstrating predominantly inferior and medial histiocytosis infiltration of the right orbit before treatment (i) and marked response after cobimetinib treatment (ii). T1-weighted MRI axial image of patient 5 showing right infiltration of the inferolateral orbit with exophthalmos (iii), with marked improvement following intra-arterial chemotherapy with melphalan (iv).
Representative patient images. (A) Representative images of patients 1 to 5 with KRAS-mutant isolated orbital histiocytosis. External photographs of the external adnexa and involved eye: eyelid edema and ptosis (i), conjunctival histiocytic infiltration (ii), exophthalmos, and scleral show (iii), lower eyelid swelling with xanthelasma (iv), exophthalmos and scleral show (v). MRI of representative patients: arrows showing bilateral diffusely enlarged lacrimal glands (vi), arrow showing thickening of the left lateral rectus (vii), arrow showing mass lesion involving the right lateral rectus muscle (viii), arrow showing infiltration and involvement of the lacrimal gland, superior muscle complex and lateral rectus (ix), and arrow showing bulky disease in the inferior orbit with diffuse involvement of the inferior rectus muscle (x). (B) Representative patient images demonstrating disease response to treatment in KRAS-mutant isolated orbital disease. T1-weighted MRI coronal image of patient 2 demonstrating predominantly inferior and medial histiocytosis infiltration of the right orbit before treatment (i) and marked response after cobimetinib treatment (ii). T1-weighted MRI axial image of patient 5 showing right infiltration of the inferolateral orbit with exophthalmos (iii), with marked improvement following intra-arterial chemotherapy with melphalan (iv).
Orbital infiltration is a potential feature of histiocytosis, found in a fifth of our predominantly adult histiocytosis cohort.8 Orbital disease has varying levels of clinical severity; it may be a presenting sign of histiocytosis or has the potential to remain clinically occult and only detectable via radiographic imaging, and it may occur in isolation or has the potential to be part of multisystem histiocytosis. Other blood neoplasms may infiltrate the orbit, and it is standard to consider eyelid and conjunctival infiltration as manifestations of orbital disease. Given that histiocytosis is also a blood neoplasm, we defined concomitant eyelid and conjunctival infiltration as part of the orbital disease.
Supplemental Table 3 summarizes the differences between isolated orbital- and orbit-involving multisystem diseases. In our study, most patients (72%) had orbit-involving multisystem histiocytosis. Compared with isolated orbital disease, multisystemic orbital disease was more predominantly an ECD or RDD subtype, bilateral, and had a trend toward involving other structures of the eye beyond the orbital and soft tissue adnexa, specifically, the choroid and cranial nerves. Based on the MRI characteristics, orbit-involving multisystem histiocytosis significantly occupied the intraoconal space and spared the lacrimal glands. The findings of the orbit-involving multisystemic group are consistent with those of published cohorts; for instance, in a cohort of 45 patients with ECD, the orbital disease was predominantly bilateral (84%), sparing the lacrimal glands (75%), and intraconal (84%).12
In comparison, isolated orbital disease was predominantly driven by KRAS mutations, unilateral in nature, a xanthogranuloma subtype, with lacrimal gland infiltration, and extraconal involvement on MRI. Isolated disease was significantly more symptomatic, with complaints of periorbital swelling being the most common symptom. Even though our cohort demonstrated that isolated orbital disease is statistically driven by KRAS mutations, other genetic factors may be implicated, including BRAFV600E in 2 patients and nonidentifiable mutations in 1 other. Isolated orbital disease driven by KRAS mutations vs other genetics were similar in most clinical features (age, sex, other ophthalmic sites of disease, MRI features, and visual symptoms).
Notably, KRAS-mutant orbit-involving histiocytosis was not exclusively isolated from the orbit in all cases; 2 KRAS-mutant patients had multisystem orbital disease. Interestingly, these 2 patients differed from KRAS-mutant isolated orbital disease in other ways and were more in keeping with multisystemic characteristics; they both had an RDD subtype and possessed intraconal disease. The only clinical feature in keeping with isolated orbital disease was unilaterality in both patients. Finally, the difference in KRAS-mutant isolated orbital disease compared with orbit-involving multisystem disease cannot be explained by the specific KRAS mutation alone, at least in this small cohort, because both KRAS K117N and A146P occurred in both groups.
In this small cohort, patients with non-KRAS mutant isolated orbital disease responded well to either observation or radiation. However, most patients with KRAS mutant isolated orbital disease required multiple lines of treatment, including conventional treatment with methotrexate, intra-arterial melphalan, and the targeted agent cobimetinib. This suggests a variable clinical behavior of isolated orbital histiocytosis and an informal observation that the KRAS phenotype is more aggressive or requires more treatment(s).
In summary, isolated orbital histiocytosis has significantly distinct clinical features compared with orbit-involving multisystemic disease, namely, a predominance of KRAS mutations, unilaterality, xanthogranuloma subtype, extraconal disease, and lacrimal gland infiltration. KRAS-driven isolated orbital disease may necessitate more aggressive treatment. To our knowledge, we present these new findings and look forward to other groups and larger cohorts providing confirmation, in addition to other genotype-phenotype connections.
Acknowledgments: This study was supported by The Fund for Ophthalmic Knowledge (J.H.F.), Research to Prevent Blindness (J.H.F.), the Histiocytosis Association (J.H.F.), and National Cancer Institute Cancer Center support grant P30 CA008748 (all authors). This work was also supported by the National Cancer Institute (R37CA259260 [E.L.D.]), Frame Family Fund (E.L.D.), Joy Family West Foundation (E.L.D.), and the Applebaum Foundation (E.L.D.).
The sponsor or funding organization had no role in the design or conduct of this research.
Contribution: J.H.F. and E.L.D. designed the research; J.H.F., R.F.S., J.C., D.B., D.D.R., and E.L.D. contributed to data acquisition/research execution; J.H.F., V.H., D.H.A., and E.L.D. contributed to data analysis/interpretation; and J.H.F. and E.L.D. prepared the manuscript.
Conflict-of-interest disclosure: E.L.D. receives unpaid editorial support from Pfizer Inc and serves on advisory boards for Day One Biopharmaceuticals, SpringWorks Therapeutics, and Opna Bio, all outside of the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Jasmine H. Francis, Ophthalmic Oncology Service, Department of Surgery, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: francij1@mskcc.org.
References
Author notes
Data are available on request from the corresponding author, Jasmine H. Francis (francij1@mskcc.org).
The full-text version of this article contains a data supplement.