Key Points
Health care utilization and costs of older frail and nonfrail patients with DLBCL were described from initial treatment to end-of-life.
Frailty was associated with higher adjusted health care utilization and costs in all phases except end-of-life where there was no difference.
Visual Abstract
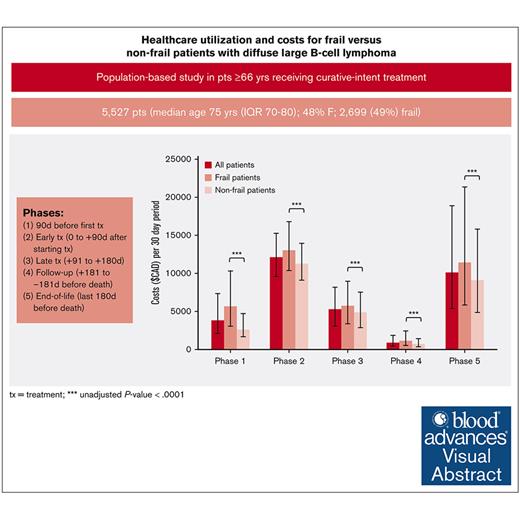
Half of older patients with diffuse large B-cell lymphoma (DLBCL) receiving curative-intent treatment are frail. Understanding the differences in health care utilization including costs between frail and nonfrail patients can inform appropriate models of care. A retrospective cohort study was conducted using population-based data in Ontario, Canada. Patients aged ≥66 years with DLBCL who received frontline curative-intent chemoimmunotherapy between 2006 and 2017 were included. Frailty was defined using a cumulative deficit–based frailty index. Health care utilization and costs were grouped into 5 phases: (1) 90 days preceding first treatment; (2) early treatment (0 to +90 days after starting treatment); (3) late treatment (+91 to +180 days); (4) follow-up (+181 to –181 days before death); and (5) end of life (last 180 days before death). Costs were standardized to 30-day intervals (2019 Canadian dollars). A total of 5527 patients were included (median age, 75 years; 48% female). A total of 2699 patients (49%) were classified as frail. The median costs for frail vs nonfrail patients per 30 days based on phase of care were (1) $5683 vs $2586 ; (2) $13 090 vs $11 256; (3) $5734 vs $4883; (4) $1138 vs $686; and (5) $11 413 vs $9089; statistically significant in all phases. In multivariable modeling, frail patients had higher rates of emergency department visits and hospitalizations and increased costs than nonfrail patients through all phases except end-of-life phase. During end-of-life phase, a substantial portion of patients (n = 2569 [84%]) required admission to hospital; 684 (27%) required intensive care unit admission. Future work could assess whether certain hospitalizations are preventable, particularly for patients identified as frail.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL), with a median age at diagnosis of 70 years.1 Older patients are at risk for frailty, a multidimensional syndrome of cumulative deficits that gives rise to vulnerability.2 We recently published a population-level analysis of frailty in older patients with DLBCL receiving frontline curative-intent therapy. Nearly half of the patients were frail, and frailty was associated with increased mortality and worse chemotherapy tolerance.3
Prior literature has examined health care utilization in patients with NHL, including DLBCL. These data have shown that patients with NHL have higher health care utilization and per-utilization spending than patients without cancer,4 and costs in patients with DLBCL are highest in the first year after treatment.5-7 However, differences between frail and nonfrail patients remain unknown.
The objectives of this study were to describe and identify predictors of lifetime health care utilization and costs of frail vs nonfrail patients with DLBCL receiving curative-intent therapy in a public health care system.
Methods
A retrospective cohort study was conducted using linked population-based health administrative data sets in Ontario, Canada, at ICES (formerly the Institute for Clinical Evaluative Sciences). These data sets were linked using unique encoded identifiers and analyzed at ICES. ICES is an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Anonymized, individual-level records are available for all 14 million residents of Ontario from ∼1990 onward. The use of data in this project is authorized under section 45 of Ontario’s Personal Health Information Protection Act and does not require review by a research ethics board.
Data sources and exposure definition
The methods for cohort creation, exposure classification using a frailty index (FI), and covariate collection have been previously described.3 Briefly, we included patients aged >65 years living in Ontario who were newly diagnosed with DLBCL (including transformed follicular lymphoma) and received frontline rituximab-containing chemoimmunotherapy between January 2006 and December 2017. To access rituximab-containing therapy for aggressive lymphoma in Ontario, the intent of therapy must be curative, although the decision on which chemoimmunotherapy regimen to select, and the dose is left to the discretion of the treating physician; in general rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone would be considered standard curative-intent therapy. We excluded patients who had a primary diagnosis of central nervous system DLBCL, HIV-associated lymphoma, and those who had a new cancer diagnosis in the 1 year before cohort entry. We defined frailty date using a modified version of a generalizable FI developed for use with Ontario data (supplemental Table 1).8 FIs sum the total amount of deficits an individual has and divide this by the total number of variables assessed to attain an FI score. Similar to previous work, we defined an FI score of <0.2 as nonfrail and ≥0.2 as frail.9 Frailty was scored at the index date (the first infusion date of frontline rituximab-containing therapy).
Covariates
We gathered information on important covariates, including age, sex, treatment era, rural (<10 000 residents) vs urban residence, income quintile, education, and comorbidity burden using the Johns Hopkins Adjusted Clinical Groups (ACG) System version 10 to compute ACG system Aggregated Diagnosis Groups (ADGs).10
Phases of care
Consistent with prior work on health care utilization in patients with cancer, we divided patient trajectories into initial, follow-up, and end-of-life costing/resource utilization phases.11 We defined phases of care using a hierarchical definition. No phase was permitted to overlap. The index date was the first date of frontline rituximab-containing therapy. First, the pretreatment phase (phase 1) was defined as 90 days before the index date. Next, the end-of-life phase (phase 5) was defined as up to the last 180 days of life for patients who died. Next, the early treatment phase (phase 2) was defined as the first 90 days after the index date. Next; and the late treatment phase (phase 3) was defined as +91 days to +180 days after the index date. Finally, the follow-up phase (phase 4) was defined as the remining days between phase 3 and phase 5. Thus, the order in which observational time for each phase was assigned is as follows: phase 1, phase 5, phase 2, phase 3, and phase 4. Because the length of phases were different, and given that frail vs nonfrail patients had differential survival as previously published,3 costs were standardized to per 30-day costs in 2019 Canadian dollars for the purposes of comparison.
Outcomes
The primary outcomes were health care utilization (including emergency department [ED] visits, hospitalizations, and outpatient visits to physicians including specialists and primary care providers) and health care costs (defined as all costs to the publicly funded single-payer health care system in Ontario). Costs were calculated and disaggregated using previously published methodology (supplemental Table 2).12 Patients were censored if they received an autologous stem cell transplantation (identified through hospital diagnostic codes identifying receipt of stem cell transplant [1LZ19HHU6A, 1LZ19HHU7A, and 1LZ19HHU7A] or physician billing codes for transplant supervision [G390]), given their high costs and likely predominance among nonfrail patients, or at the end of the follow-up period (31 March 2019). Death was identified via the Registered Persons Database.
Statistical analyses
Descriptive statistics were reported using means and standard deviations, medians and interquartile ranges (IQRs), or frequencies and percentages when appropriate. Costs were reported using medians and IQRs, because mean costs may potentially be more susceptible to outliers than median costs.13 Kruskal-Wallis test was used to compare medians of continuous variables, and χ2 test was used to compare categorical variables. Poisson models were used to assess predictors of the rate of ED visits and hospitalizations (count outcomes). Quantile regression, which is used in costing analyses in which data do not typically fit the conditions of linear regression, was used to assess predictors of higher median health care costs per 30 days during each phase of care. Models were adjusted for baseline covariates differing between frail and nonfrail patients. Adjusted relative rate and increase in median total cost per 30-day period are reported alongside 95% confidence intervals. All P values were 2-sided, with <.05 used to indicate significance. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
A total of 5527 patients aged ≥66 years treated with frontline curative-intent therapy for DLBCL between 1 January 2006 and 31 December 2017 were identified. A total of 5216 patients (94%) had de novo DLBCL, and 311 patients (6%) had transformed follicular lymphoma. The median age was 75 years (IQR, 70-80), and 2672 (48%) were female (Table 1). During follow-up, 49 patients were censored due to receipt of autologous stem cell transplant.
Baseline characteristics
| Characteristics . | Frail (n = 2699), n (%) . | Nonfrail (n = 2828), n (%) . | Total (N = 5527), n (%) . | P value . |
|---|---|---|---|---|
| Age, median (IQR), y | 76 (71-81) | 74 (70-79) | 75 (70-80) | <.001 |
| Male | 1377 (51.0%) | 1478 (52.3%) | 2855 (51.7%) | .355 |
| Female | 1322 (49.0%) | 1350 (47.7%) | 2672 (48.3%) | |
| Era of treatment | .0003 | |||
| 2006-2009 | 890 (33.0%) | 976 (34.5%) | 1866 (33.8%) | |
| 2010-2013 | 816 (30.2%) | 954 (33.7%) | 1770 (32.0%) | |
| 2014-2017 | 993 (36.8%) | 898 (31.8%) | 1891 (34.2%) | |
| Neighborhood income quintile | ||||
| Q1 (lowest) | 552 (20.5%) | 351 (12.4%) | 903 (16.3%) | <.001 |
| Q2 | 676 (25.0%) | 481 (17.0%) | 1157 (20.9%) | |
| Q3 | 581 (21.5%) | 518 (18.3%) | 1099 (19.9%) | |
| Q4 | 432 (16.0%) | 680 (24.0%) | 1112 (20.1%) | |
| Q5 (highest) | 454 (16.8%) | 791 (28.0%) | 1245 (22.5%) | |
| Neighborhood-level educational attainment (census 2006) | ||||
| Missing/unknown | 206 (7.6%) | 242 (8.6%) | 448 (8.1%) | <.001 |
| Q1 (lowest) | 418 (15.5%) | 585 (20.7%) | 1003 (18.1%) | |
| Q2 | 489 (18.1%) | 528 (18.7%) | 1017 (18.4%) | |
| Q3 | 543 (20.1%) | 535 (18.9%) | 1078 (19.5%) | |
| Q4 | 531 (19.7%) | 496 (17.5%) | 1027 (18.6%) | |
| Q5 (highest) | 512 (19.0%) | 442 (15.6%) | 954 (17.3%) | |
| Rural dwelling | ||||
| Rural | 414 (15.3%) | 359 (12.7%) | 773 (14.0%) | <.005 |
| Urban | 2285 (84.7%) | 2469 (87.3%) | 4754 (86.0%) | |
| Number of ADG comorbidities∗ | ||||
| Median ADGs (IQR) | 13 (12-15) | 10 (8-12) | 12 (9-14) | <.001 |
| Cancer type | ||||
| DLBCL | 2558 (94.8%) | 2658 (94.0%) | 5216 (94.4%) | .204 |
| Transformed FL | 141 (5.2%) | 170 (6.0%) | 311 (5.6%) | |
| Status at time of diagnosis | ||||
| Inpatient | 756 (28.0%) | 450 (15.9%) | 1206 (21.8%) | <.001 |
| Outpatient | 1943 (72.0%) | 2378 (84.1%) | 4321 (78.2%) | |
| Cancer stage | ||||
| Missing/unknown† | 1476 (54.7%) | 1420 (50.2%) | 2896 (52.4%) | <.0001 |
| I | 233 (8.6%) | 349 (12.3%) | 582 (10.5%) | |
| II | 257 (9.5%) | 348 (12.3%) | 605 (10.9%) | |
| III | 233 (8.6%) | 283 (10.0%) | 516 (9.3%) | |
| IV | 500 (18.5%) | 428 (15.1%) | 928 (16.8%) |
| Characteristics . | Frail (n = 2699), n (%) . | Nonfrail (n = 2828), n (%) . | Total (N = 5527), n (%) . | P value . |
|---|---|---|---|---|
| Age, median (IQR), y | 76 (71-81) | 74 (70-79) | 75 (70-80) | <.001 |
| Male | 1377 (51.0%) | 1478 (52.3%) | 2855 (51.7%) | .355 |
| Female | 1322 (49.0%) | 1350 (47.7%) | 2672 (48.3%) | |
| Era of treatment | .0003 | |||
| 2006-2009 | 890 (33.0%) | 976 (34.5%) | 1866 (33.8%) | |
| 2010-2013 | 816 (30.2%) | 954 (33.7%) | 1770 (32.0%) | |
| 2014-2017 | 993 (36.8%) | 898 (31.8%) | 1891 (34.2%) | |
| Neighborhood income quintile | ||||
| Q1 (lowest) | 552 (20.5%) | 351 (12.4%) | 903 (16.3%) | <.001 |
| Q2 | 676 (25.0%) | 481 (17.0%) | 1157 (20.9%) | |
| Q3 | 581 (21.5%) | 518 (18.3%) | 1099 (19.9%) | |
| Q4 | 432 (16.0%) | 680 (24.0%) | 1112 (20.1%) | |
| Q5 (highest) | 454 (16.8%) | 791 (28.0%) | 1245 (22.5%) | |
| Neighborhood-level educational attainment (census 2006) | ||||
| Missing/unknown | 206 (7.6%) | 242 (8.6%) | 448 (8.1%) | <.001 |
| Q1 (lowest) | 418 (15.5%) | 585 (20.7%) | 1003 (18.1%) | |
| Q2 | 489 (18.1%) | 528 (18.7%) | 1017 (18.4%) | |
| Q3 | 543 (20.1%) | 535 (18.9%) | 1078 (19.5%) | |
| Q4 | 531 (19.7%) | 496 (17.5%) | 1027 (18.6%) | |
| Q5 (highest) | 512 (19.0%) | 442 (15.6%) | 954 (17.3%) | |
| Rural dwelling | ||||
| Rural | 414 (15.3%) | 359 (12.7%) | 773 (14.0%) | <.005 |
| Urban | 2285 (84.7%) | 2469 (87.3%) | 4754 (86.0%) | |
| Number of ADG comorbidities∗ | ||||
| Median ADGs (IQR) | 13 (12-15) | 10 (8-12) | 12 (9-14) | <.001 |
| Cancer type | ||||
| DLBCL | 2558 (94.8%) | 2658 (94.0%) | 5216 (94.4%) | .204 |
| Transformed FL | 141 (5.2%) | 170 (6.0%) | 311 (5.6%) | |
| Status at time of diagnosis | ||||
| Inpatient | 756 (28.0%) | 450 (15.9%) | 1206 (21.8%) | <.001 |
| Outpatient | 1943 (72.0%) | 2378 (84.1%) | 4321 (78.2%) | |
| Cancer stage | ||||
| Missing/unknown† | 1476 (54.7%) | 1420 (50.2%) | 2896 (52.4%) | <.0001 |
| I | 233 (8.6%) | 349 (12.3%) | 582 (10.5%) | |
| II | 257 (9.5%) | 348 (12.3%) | 605 (10.9%) | |
| III | 233 (8.6%) | 283 (10.0%) | 516 (9.3%) | |
| IV | 500 (18.5%) | 428 (15.1%) | 928 (16.8%) |
ADG, Aggregated Diagnosis Group; FL, follicular lymphoma; Q, quintile.
ADGs exclude cancer.
Staging only available from 2007 onward.
A total of 2699 patients (49%) were classified as frail (Table 1). Frail patients were older (median age, 76 years [IQR, 71-81] vs 74 years [IQR, 70-79]), more likely to be rural dwelling (15.3% vs 12.7%), and more likely to be diagnosed with DLBCL as an inpatient (28.0% vs 15.9%; Table 1). As expected based on the FI composition, patients who were frail also had greater comorbid disease burden (Aggregated Diagnosis Group score, 13 [IQR, 12-15] vs 10 [IQR, 8-12]) and tended to reside in areas with lower neighborhood income quintiles (Table 1; see FI in supplemental Materials; supplemental Table 1). The median follow-up time was 324 days (IQR, 103-1028), during which 3044 patients (55%) died (frail, n = 1673 [62%]; nonfrail, n = 1371 [48%]). The 1-year probability of survival was 67.9% ± 0.90% vs 80.5% ± 0.75% in frail vs nonfrail patients, respectively (hazard ratio, 1.8; 95% confidence interval, 1.6-2.0; P < .0001). Frail patients completed fewer chemotherapy cycles than nonfrail patients and had increased treatment toxicity. Detailed analyses have been previously published.3
Health care utilization
Health care utilization during each phase is outlined in supplemental Table 3, including outpatient visits, ED visits not resulting in hospitalization, hospitalization and intensive care unit (ICU) admission data, and average length of stay (LOS) during hospitalization or ICU admission. In the 90 days before treatment (phase 1), 1796 frail patients (67%) had at least 1 hospitalization, and 1975 (73%) had at least 1 ED visit, compared with 1042 (37%) and 1325 nonfrail patients (47%), respectively (P < .0001). During the initial phase of care (phase 2), this decreased to 48% (n = 1018) hospitalization and 55% (n = 1736) ED visit rates among frail patients vs 37% (n = 928) and 44% (n = 1430) among nonfrail patients, respectively, although the difference between the 2 patient cohorts remained statistically significant (P < .0001). Notably, during phases 1 and 2, frail patients also had longer LOS in hospital per hospitalization than nonfrail patients (supplemental Table 3). During the later phase of treatment (phase 3), the number of patients requiring hospitalization decreased to 25% of frail patients (n = 492) and 14% of nonfrail patients (n = 398; P < .0001), and LOS did not statistically differ between the groups.
The median duration of the follow-up phase (phase 4) was 1209 days (IQR, 555-2293) for frail vs 1595 days (IQR, 738-2665) for nonfrail patients (P < .0001). Both frail and nonfrail patients had ongoing need for acute care services during this follow-up phase, with 1358 (74%) and 1006 frail patients (55%) visiting the ED or being hospitalized at least once during this period, respectively, vs 1520 (65%) and 1025 nonfrail patients (45%; P < .0001).
The median duration of the end-of-life phase (phase 5) was 181 days (IQR, 95-181) for frail and 181 days (IQR, 164-181) for nonfrail patients (P < .0001). Most patients had at least 1 hospitalization (n = 2569 [84%]), and both frail and nonfrail patients were equally likely to be hospitalized. Twenty percent of patients (n = 519) had a LOS >20 days during this time. Furthermore, 27% of hospitalized patients (n = 684) were admitted to the ICU, which was equally likely between frail and nonfrail patients.
Costs
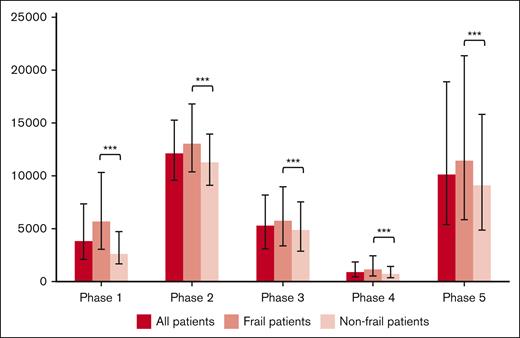
Health care utilization costs standardized to per 30-day period are shown in Figure 1 and Table 2. The greatest health care utilization costs per 30-day period occurred in the initial treatment and end-of-life phases. Disaggregated costs (supplemental Table 4) reveal that the majority of these costs were associated with inpatient hospitalization and chemo-immunotherapy costs. In all phases, frail patients had significantly increased unadjusted health care costs compared with nonfrail patients.
Median costs standardized to a 30-day period during each phase, with error bars depicting the IQR. During all phases, frail patients had significantly greater health care costs than nonfrail patients (∗∗∗P < .0001).
Median costs standardized to a 30-day period during each phase, with error bars depicting the IQR. During all phases, frail patients had significantly greater health care costs than nonfrail patients (∗∗∗P < .0001).
Health care costs per phase of life
| . | Frail patients (n = 2 699), median cost (IQR) . | Nonfrail patients (n = 2 828), median cost (IQR) . | Total (N = 5 527), median cost (IQR) . | P value . |
|---|---|---|---|---|
| By phase, costs standardized to per 30-d period | ||||
| Phase 1: pretreatment phase (90 d before the start of frontline treatment) | 5 683 (3 065-10 322) | 2 586 (1 656-4 721) | 3 794 (2 073-7 355) | <.0001 |
| Phase 2: initial treatment phase (start of frontline treatment plus 90 d) | 13 090 (10 385-16 809) | 11 256 (9 107-13 976) | 12 077 (9 576-15 248) | <.0001 |
| Phase 3: late treatment phase (90 d from start of frontline treatment to 180 d from start of frontline treatment) | 5 734 (3 347-8 904) | 4 883 (2 845-7 543) | 5 256 (3 059-8 168) | <.0001 |
| Phase 4: follow-up phase (end of treatment phase to the beginning of the end-of-life phase) | 1 138 (552-2 397) | 686 (350-1 425) | 856 (425-1 850) | <.0001 |
| Phase 5: end-of-life phase (final 180 d of life) | 11 413 (5 845-21 381) | 9 089 (4 844-15 793) | 10 099 (5 384-18 874) | <.0001 |
| . | Frail patients (n = 2 699), median cost (IQR) . | Nonfrail patients (n = 2 828), median cost (IQR) . | Total (N = 5 527), median cost (IQR) . | P value . |
|---|---|---|---|---|
| By phase, costs standardized to per 30-d period | ||||
| Phase 1: pretreatment phase (90 d before the start of frontline treatment) | 5 683 (3 065-10 322) | 2 586 (1 656-4 721) | 3 794 (2 073-7 355) | <.0001 |
| Phase 2: initial treatment phase (start of frontline treatment plus 90 d) | 13 090 (10 385-16 809) | 11 256 (9 107-13 976) | 12 077 (9 576-15 248) | <.0001 |
| Phase 3: late treatment phase (90 d from start of frontline treatment to 180 d from start of frontline treatment) | 5 734 (3 347-8 904) | 4 883 (2 845-7 543) | 5 256 (3 059-8 168) | <.0001 |
| Phase 4: follow-up phase (end of treatment phase to the beginning of the end-of-life phase) | 1 138 (552-2 397) | 686 (350-1 425) | 856 (425-1 850) | <.0001 |
| Phase 5: end-of-life phase (final 180 d of life) | 11 413 (5 845-21 381) | 9 089 (4 844-15 793) | 10 099 (5 384-18 874) | <.0001 |
Phase 1, the median duration was 90 days (IQR, 90-90; N = 5527). Phase 2, the median duration was 91 days (IQR, 91-91; n = 4631). Phase 3, the median duration was 90 days (IQR, 90-90; n = 4358). Phase 4, the median duration was 1412 days (IQR, 631-2513; n = 4112). Phase 5, the median duration was 181 days (IQR, 122-181; n = 3044).
Predictors of hospitalization and ED utilization
In modified Poisson regression models considering the relative rate of ED visit or hospitalization during each phase, frailty was significantly associated with an increased rate of ED visit or hospitalization during each phase in both unadjusted and adjusted models, except the end-of-life phase (Table 3; supplemental Table 5 for details of adjusted model). Other consistent predictors across phases for an increased rate for ED visit and hospitalization included rural vs urban status and increased comorbidity burden.
Effect of frailty status on health care utilization and costs
| Predictors of health care utilization . | |||||||
|---|---|---|---|---|---|---|---|
| RR of ED visits . | |||||||
| Unadjusted model . | Adjusted model∗ . | ||||||
| Phase . | Predictor . | RR . | 95% CI . | P value . | RR . | 95% CI . | P value . |
| 1 | Frailty | 1.91 | 1.80-2.03 | <.0001 | 1.50 | 1.40-1.62 | <.0001 |
| 2 | Frailty | 1.41 | 1.30-1.53 | <.0001 | 1.11 | 1.01-1.22 | .03 |
| 3 | Frailty | 1.62 | 1.44-1.82 | <.0001 | 1.27 | 1.10-1.46 | .0009 |
| 4 | Frailty | 1.86 | 1.72-2.01 | <.0001 | 1.29 | 1.18-1.42 | <.0001 |
| 5 | Frailty | 1.12 | 1.05-1.19 | .0004 | 1.01 | 0.94-1.09 | .74 |
| Predictors of health care utilization . | |||||||
|---|---|---|---|---|---|---|---|
| RR of ED visits . | |||||||
| Unadjusted model . | Adjusted model∗ . | ||||||
| Phase . | Predictor . | RR . | 95% CI . | P value . | RR . | 95% CI . | P value . |
| 1 | Frailty | 1.91 | 1.80-2.03 | <.0001 | 1.50 | 1.40-1.62 | <.0001 |
| 2 | Frailty | 1.41 | 1.30-1.53 | <.0001 | 1.11 | 1.01-1.22 | .03 |
| 3 | Frailty | 1.62 | 1.44-1.82 | <.0001 | 1.27 | 1.10-1.46 | .0009 |
| 4 | Frailty | 1.86 | 1.72-2.01 | <.0001 | 1.29 | 1.18-1.42 | <.0001 |
| 5 | Frailty | 1.12 | 1.05-1.19 | .0004 | 1.01 | 0.94-1.09 | .74 |
| RR of hospitalizations . | |||||||
|---|---|---|---|---|---|---|---|
| Unadjusted model . | Adjusted model∗ . | ||||||
| 1 | Frailty | 2.16 | 2.03-2.31 | <.0001 | 1.84 | 1.70-1.99 | <.0001 |
| 2 | Frailty | 1.35 | 1.24-1.47 | <.0001 | 1.19 | 1.07-1.32 | .0009 |
| 3 | Frailty | 1.61 | 1.40-1.84 | <.0001 | 1.43 | 1.21-1.69 | <.0001 |
| 4 | Frailty | 1.68 | 1.52-1.85 | <.0001 | 1.30 | 1.16-1.46 | <.0001 |
| 5 | Frailty | 1.10 | 1.03-1.17 | .0027 | 1.03 | 0.96-1.11 | .39 |
| RR of hospitalizations . | |||||||
|---|---|---|---|---|---|---|---|
| Unadjusted model . | Adjusted model∗ . | ||||||
| 1 | Frailty | 2.16 | 2.03-2.31 | <.0001 | 1.84 | 1.70-1.99 | <.0001 |
| 2 | Frailty | 1.35 | 1.24-1.47 | <.0001 | 1.19 | 1.07-1.32 | .0009 |
| 3 | Frailty | 1.61 | 1.40-1.84 | <.0001 | 1.43 | 1.21-1.69 | <.0001 |
| 4 | Frailty | 1.68 | 1.52-1.85 | <.0001 | 1.30 | 1.16-1.46 | <.0001 |
| 5 | Frailty | 1.10 | 1.03-1.17 | .0027 | 1.03 | 0.96-1.11 | .39 |
| Increase in median total cost per 30-d period . | ||||
|---|---|---|---|---|
| Adjusted model∗ . | ||||
| Phase . | Predictor . | Increase in median cost (CAD) . | 95% CI . | P value . |
| 1 | Frailty | 1866 | 1603-2088 | <.0001 |
| 2 | Frailty | 983 | 671-1296 | <.0001 |
| 3 | Frailty | 683 | 340-1027 | <.0001 |
| 4 | Frailty | 271 | 182-360 | <.0001 |
| 5 | Frailty | 1036 | –40 to 2111 | .06 |
| Increase in median total cost per 30-d period . | ||||
|---|---|---|---|---|
| Adjusted model∗ . | ||||
| Phase . | Predictor . | Increase in median cost (CAD) . | 95% CI . | P value . |
| 1 | Frailty | 1866 | 1603-2088 | <.0001 |
| 2 | Frailty | 983 | 671-1296 | <.0001 |
| 3 | Frailty | 683 | 340-1027 | <.0001 |
| 4 | Frailty | 271 | 182-360 | <.0001 |
| 5 | Frailty | 1036 | –40 to 2111 | .06 |
CAD, Canadian dollars; CI, confidence interval; RR, relative rate.
Adjusted for covariates including age, era of diagnosis, income quintile, educational quintile, rural vs urban status, comorbidity burden, status at time of diagnosis (inpatient vs outpatient). See supplemental Table 5 for full multivariable model including all predictors.
Predictors of health care costs
Predictors of health care costs were analyzed using multivariable quantile regression (Table 3; supplemental Table 6). Significant predictors of high costs across phases included frailty (except in phase 5), older age, diagnosis of DLBCL as an inpatient, increased comorbidity burden, and older eras of treatment than more recent years.
Discussion
This population-based study provides a longitudinal overview of the health care utilization and costs of older patients with DLBCL receiving curative-intent frontline chemoimmunotherapy over their life span after diagnosis, comparing frail vs nonfrail patients. Nearly half of patients were frail. All patients had high health care utilization and costs, particularly surrounding the initial treatment phase (over $12 000 per 30-day period) and end-of-life phase (over $10 000 per 30-day period). In multivariable modeling controlling for other factors such as age and comorbidities, frailty consistently associated with increased health care utilization (up to 45% higher rate of ED visit and 66% higher rate of hospitalizations) and costs (up to $1846 higher per 30 days) across all phases except the end-of-life phase.
Prior analyses using claims or administrative databases in the United States and Japan found that costs were highest during the first year after the diagnosis of DLBCL, mainly driven by outpatient costs and inpatient hospitalization,5-7 which aligns with our findings in which costs were highest during the treatment phase and mainly driven by outpatient chemoimmunotherapy drug costs and hospitalization.
In our study, building on our prior work,3 frailty was measured using a modified version of the generalizable FI, developed for use with health administrative data in Ontario.8 Although this method is not a clinical frailty assessment, FIs have high agreement with a clinical frailty phenotype; however, FIs are considered to discriminate better at the lower end of the frailty spectrum.14
To our knowledge, our study is the first to compare frail vs nonfrail patients with lymphoma with respect to health care utilization and costs. Previous studies of general medical populations have shown that frailty was significantly associated with health care utilization and costs across multiple settings, including patients with cancer.15-18 We similarly found significant differences in utilization and costs through the life span. Specifically, frail patients had more inpatient hospitalizations and longer LOS than patients who were not frail. Potential explanations for the difference in pretreatment hospitalization rates between frail and nonfrail patients could relate to enhanced vulnerability to lymphoma symptoms or could reflect more aggressive lymphoma disease biology leading to complications requiring hospitalization. During follow-up, frail patients may have increased vulnerability to therapy-related adverse effects and potential increased severity of these complications, exacerbation of underlying chronic health conditions, increased risk of nosocomial infections and other hospital-related complications such as pressure ulcers, increased risk of delirium, and lower functional status and delayed recovery, among other possibilities.16 Although this study was not designed to understand whether hospitalizations were preventable, it does raise potential questions for future work, such as whether comprehensive geriatric consultation or supportive care strategies may help to prevent hospitalizations.
By the end-of-life phase, frail and nonfrail patients had more similarity among their health care utilization with respect to hospitalization and ICU admission, during which 84% of patients were admitted to hospital at least once, and 27% of hospitalized patients required ICU admission, suggesting that care needs surrounding the end-of-life phase become a dominating factor. This warrants further analysis that may be most appropriate through mixed-methods studies. The majority of deaths among patients with DLBCL are attributable to lymphoma.19 The high risks of hospitalization close to death may reflect lymphoma complications such as infection or progression that may be otherwise difficult to manage in the outpatient setting. The high risk of hospitalizations among patients who died also illustrates the challenges with predicting end-of-life trajectory among patients with aggressive cancers, because death can be sudden and unexpected, related to infection or other treatment toxicity. Previous work has shown that even among hospitalized patients with DLBCL, the use of palliative care consultation was low (7%), including only 36% among patients who died.20 Thus, for certain patients, development of tools to predict imminent end of life, strategies to improve goals of care discussions, and early palliative care involvement may help with preventing hospitalization for patients who wish to die at home or in hospice or reduce aggressive interventions near the end-of-life phase such as ICU admission.
This study has important limitations. Because this was a retrospective study, we may have incomplete or misclassified information. For example, we did not have information on cell of origin, molecular data (such a cmyc rearrangement), international prognostic index score for patients, and specific information on the rituximab-containing chemoimmunotherapy regimen selected nor the doses used. Given that this was a population-based study, we did not have granular information regarding the rationale for why physicians selected patients for rituximab-containing curative-intent therapy nor details on subsequent line(s) of therapy or decision for palliative-intent care. We also censored patients at the time of autologous stem cell transplant, although this was a small number of patients (n = 49); thus, costs related to the posttransplant period are not described in this study. Additionally, the time period of this study precedes the availability of CD19 chimeric antigen receptor T-cell therapy for patients with DLBCL and therefore does not capture these costs and also may have led to a palliative care approach for non–transplant-eligible patients on the basis of age, who may have otherwise been chimeric antigen receptor T-cell therapy candidates. Notably, as expected based on the FI composition, frail patients were more likely to live in rural areas with lower neighborhood income quintiles. These socioeconomic factors variably influenced health care utilization and costs in modeling. Finally, multiple statistical tests were conducted comparing frail vs nonfrail patients, without adjusting for multiple comparisons. However, we did not intend to test any specific hypotheses and intended to be descriptive in our analyses. Despite the limitations of this study, we feel that its population-based nature, large sample size, and comparison of frail vs nonfrail patients from a health care utilization and cost perspective provides added value to the literature.
Notably, we do not intend for these analyses to suggest that frail patients should be excluded from curative-intent therapy; as previously reported, frail patients in this analysis has a median overall survival of 3.5 years.3 Thus, although they may benefit from added supports including geriatric assessment and comanagement or modulation of therapies to improve treatment tolerance, frailty itself should not necessarily preclude a patient from curative therapy if felt to be appropriate by their clinician.
In conclusion, this study examined health care utilization and costs among frail vs nonfrail older patients with DLBCL in a public health care system and highlights the significant costs and health care utilization through the life span among all patients. Nearly half of patients in this study were frail, and we demonstrated that frail patients had higher utilization and costs during all phases of care except the end-of-life phase, largely driven by inpatient hospitalization and cancer drug costs. The estimates from our analyses could be used to inform policy makers and clinicians on the costs of managing patients with DLBCL through the life span and provide estimates for cost-effectiveness analyses of publicly funded health care systems. This study highlights that consideration of frailty in future health care policy and prevention strategies for patients with DLBCL is important. Future prospective work could seek to identify whether identification and optimization of frailty helps to reduce health care utilization, particularly inpatient hospitalizations.
Acknowledgments
The authors thank the Lymphoma Clinical Research Mentoring Program (LCRMP) for mentorship and feedback regarding this project, particularly Christopher Flowers, Michael Williams, and Matthew Maurer. This study used data adapted from the Statistics Canada Postal Code Conversion File, which is based on data licensed from Canada Post Corporation and/or data adapted from the Ontario Ministry of Health (MOH) Postal Code Conversion File. These files contain data copied under license from Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by the Ontario MOH, Ontario Health, Cancer Care Ontario, the Canadian Institute for Health Information (CIHI), and the Ontario Registrar General information on deaths, the original source of which is ServiceOntario. The authors thank IQVIA Solutions Canada Inc for use of their Drug Information File.
The authors acknowledge the funding supports for this study, including the Conquer Cancer Foundation of the American Society of Clinical Oncology Young Investigator Award, the LCRMP, the Hold’Em for Life Oncology Clinician Scientist Award, and the Ontario MOH and Long-Term Care Clinician Investigator Program. This study was also supported by ICES, which is funded by an annual grant from the Ontario MOH and the Ministry of Long-Term Care.
The opinions results, view, and conclusions reported in this paper are those of the author and do not necessarily reflect those of Ontario Health (OH), funding or other data sources. No endorsement by OH, funding or other data sources should be inferred.
Authorship
Contribution: A.V. conceptualized the study, developed methodology, acquired funding, and wrote the original draft of the manuscript, including reviewing and editing; A.C., C.N., and N.L. contributed to methodology, data curation, formal analysis, and writing, including reviewing and editing; Y.K. contributed to data curation, formal analysis, and writing, including review and editing; D.B. contributed to data curation and writing, including reviewing and editing; S.A. contributed to conceptualization, supervision, and writing, including reviewing and editing; A.P. and M.C.C. contributed to conceptualization, methodology, funding acquisition, supervision, and writing including reviewing and editing; and L.M. participated in conceptualization, methodology, supervision, and writing, including reviewing and editing.
Conflict-of-interest disclosure: A.P. reports honoraria from Kite-Gilead, AbbVie, and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Lee Mozessohn, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada; email: lee.mozessohn@sunnybrook.ca.
References
Author notes
The data set from this study is held securely in coded form at ICES. Although legal data sharing agreements between ICES and data providers (eg, health care organizations and government) prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca).
The full data set creation plan and underlying analytic code are available upon request from the authors, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and thus may be inaccessible or require modification.
The full-text version of this article contains a data supplement.