Key Points
After valoctocogene roxaparvovec treatment, total and encapsidated vector DNA are steadily cleared from the blood and shedding matrixes.
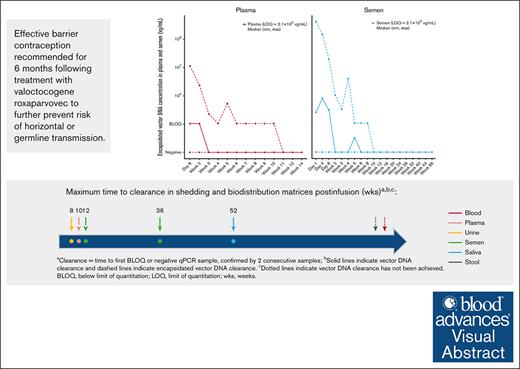
Risk of horizontal or germ line transmission following valoctocogene roxaparvovec is extremely low.
Visual Abstract
Following systemically administered adeno-associated virus gene therapy, vector particles are widely distributed, raising concerns about horizontal or germline vector transmission. Characterization of biodistribution and kinetics of vector DNA in body fluids can address these concerns and provide insights into vector behavior in accessible samples. We investigated biodistribution and vector shedding profile of valoctocogene roxaparvovec in men with severe hemophilia A enrolled in the phase 3 GENEr8-1 trial. Participants (n = 134) received a single 6 × 1013 vector genome (vg)/kg infusion and were assessed over 3 years. Vector DNA was measured using 4 different assays. Total vector DNA was evaluated in blood, saliva, stool, semen, and urine by quantitative polymerase chain reaction (qPCR). Encapsidated vector DNA was measured in plasma and semen with immunocapture-based qPCR. Contiguity of vgs and assembly of inverted terminal repeat fusions were measured in whole blood and peripheral blood mononuclear cells (PBMCs) using multicolor digital PCR. Median peak vector DNA levels observed 1 to 8 days after dosing were highest in blood, followed by saliva, semen, stool, and urine. Concentrations declined steadily. Encapsidated vector DNA cleared faster than total vector DNA, achieving clearance by ≤12 weeks in plasma and semen. Predominant vector genome forms transitioned from noncontiguous to full-length over time in whole blood and PBMCs, indicating formation of stable circularized episomes within nucleated cells. The replication-incompetent nature of valoctocogene roxaparvovec, coupled with steady clearance of total and encapsidated vector DNA from shedding matrices, indicates transmission risk is low. This trial was registered at www.ClinicalTrials.gov as #NCT03370913.
Introduction
Hemophilia A is an X-linked bleeding disorder that is caused by a deficiency in blood clotting factor VIII (FVIII). Severe hemophilia A (FVIII <1 IU/dL) is a debilitating condition that is characterized by frequent spontaneous musculoskeletal and soft tissue bleeding into joints that can lead to painful, severe, and progressive arthropathy.1-3 The standard of care for severe hemophilia A is the prophylactic administration of FVIII replacement therapy or emicizumab, a bispecific antibody that mimics activated FVIII protein.1,4,5 Gene therapy is a treatment option for people with severe hemophilia A, and a single infusion has a duration of effect of multiple years rather than the frequent, burdensome treatments required for FVIII replacement therapy and, to a lesser extent, emicizumab prophylaxis.1,6-8
Valoctocogene roxaparvovec (AAV5-hFVIII-SQ) uses an adeno-associated virus serotype 5 (AAV5) vector to deliver a B-domain–deleted human FVIII (hFVIII-SQ) coding sequence, controlled by a liver-selective promoter, that leads to steady endogenous production of FVIII protein in hepatocytes.9-14 The multicenter, open-label, single-arm, phase 3 GENEr8-1 trial (NCT03370913) was conducted in 134 adult male participants with severe hemophilia A who received a single 6 × 1013 vector genome (vg)/kg dose of valoctocogene roxaparvovec.14-16 The safety and efficacy outcomes of this gene therapy have been reported for up to 3 years after dose adminsitration.14-16
Although AAV vectors are replication incompetent and therefore pose minimal concern for horizontal transmission (ie, the spread of a transgene DNA from 1 individual to another through contact with bodily excretions containing the genetic material), assessment of potential safety risk is important. The US Food and Drug Administration (FDA) has guidelines to ensure comprehensive shedding assessment of vector DNA in secreta and excreta, including biodistribution assessments in blood. Vector shedding is likely to occur shortly after AAV administration; however, frequent safety evaluations are important, and long-term follow-up in humans is recommended to continue for a minimum (min) of 5 years when testing a potential AAV-based gene therapy product.17-19
In this study, we report data on vector DNA biodistribution and shedding over 3 years following valoctocogene roxaparvovec administration in the global phase 3 GENEr8-1 trial, to our knowledge, representing the largest comprehensive evaluation of AAV biodistribution and shedding to date. To assess biodistribution and vector shedding of uncoated, processed vector DNA, we evaluated the total vector DNA in circulation and in saliva, urine, stool, and semen over time. Furthermore, we measured the shedding of coated, unprocessed, encapsidated vector DNA in plasma and semen matrixes using a novel, ultrasensitive methodologic approach termed immunocapture immunoprecipitation–coupled quantitative polymerase chain reaction (iqPCR).20 Additional assessments of the levels and forms of vector DNA in blood were also carried out using droplet digital (dd)PCR assays throughout the observation period.
Methods
Vector construct
Valoctocogene roxaparvovec gene therapy for severe hemophilia A uses a recombinant, replication-incompetent AAV5 vector capsid. The vg includes double-stranded inverted terminal repeats (ITRs) at its 5′ and 3′ ends and single-stranded DNA that encodes a hybrid human liver-selective promoter, a hFVIII-SQ coding sequence, and a synthetic polyadenylation signal.11
Clinical study design
GENEr8-1 is an open-label, single-group, multicenter, phase 3 trial in which 134 male participants, aged ≥18 years, with severe hemophilia A (FVIII, ≤1 IU/dL) were administered a single 6 × 1013 vg/kg infusion of valoctocogene roxaparvovec.14 All participants were negative for anti-AAV5 antibodies, had no history of inhibitors, and were previously receiving regular prophylaxis with exogenous FVIII. All 134 participants who received a dose of valoctocogene roxaparvovec and who were able to provide specimens of blood and the shedding matrixes were included in the presented analyses. Missing data were not imputed.
This study was conducted in accordance with the Good Clinical Practice Directive and the Declaration of Helsinki. All participants provided written informed consent, and the protocol was approved by the institutional review boards or independent ethics committees of participating sites. This trial is registered at www.ClinicalTrials.gov as #NCT03370913.
Sample collection for biodistribution and vector shedding evaluation over a 3-year period
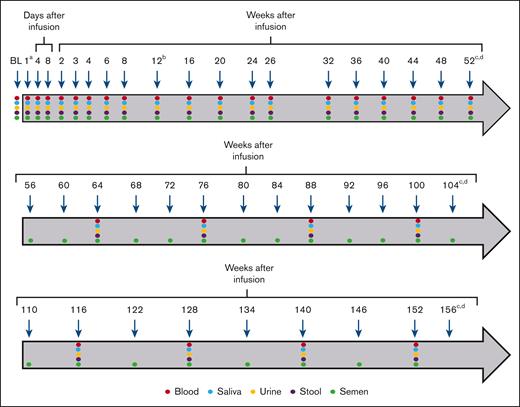
Blood, saliva, urine, stool, and semen were collected for the evaluation of biodistribution and vector shedding at baseline and periodically throughout the first 52 weeks after valoctocogene roxaparvovec administration until at least 3 consecutive negative samples were obtained via qPCR, with the exception of semen. Semen testing continued through week 12 even if 3 consecutive negative samples were previously collected. This approach was prespecified in the protocol because the risk of transmission is the highest during this time period and collecting a full data set through a complete spermatogenesis cycle21 would inform future contraceptive expectations for gene therapy studies. Participants who did not have 3 consecutive negative samples for semen by week 52 continued testing every 4 weeks during the second year and every 6 weeks thereafter. For all other samples, testing was continued every 12 weeks until 3 consecutive negative samples were recorded (Figure 1).
Blood and shedding matrix collection timeline. The baseline and follow-up sample collection regimen for blood, saliva, urine, stool, and semen are shown. Arrows indicate the timing (days to weeks) of sample collection for qPCR analyses during the first 156 weeks after infusion. Samples were collected and assessed until at least 3 consecutive negative samples were obtained. aOn the day of infusion (1), sample collection occurred between 2 and 24 hours after dosing. bSemen collection and testing continued through week 12, even if 3 consecutive negative samples were previously collected. cParticipants who did not have 3 consecutive negative samples for semen by week 52 continued testing every 4 weeks during the second year and every 6 weeks thereafter (year 3). dFor all samples other than semen, participants who did not have 3 consecutive negative samples by week 52 continued testing every 12 weeks until 3 consecutive negative samples were recorded. BL, baseline.
Blood and shedding matrix collection timeline. The baseline and follow-up sample collection regimen for blood, saliva, urine, stool, and semen are shown. Arrows indicate the timing (days to weeks) of sample collection for qPCR analyses during the first 156 weeks after infusion. Samples were collected and assessed until at least 3 consecutive negative samples were obtained. aOn the day of infusion (1), sample collection occurred between 2 and 24 hours after dosing. bSemen collection and testing continued through week 12, even if 3 consecutive negative samples were previously collected. cParticipants who did not have 3 consecutive negative samples for semen by week 52 continued testing every 4 weeks during the second year and every 6 weeks thereafter (year 3). dFor all samples other than semen, participants who did not have 3 consecutive negative samples by week 52 continued testing every 12 weeks until 3 consecutive negative samples were recorded. BL, baseline.
Detection of total vector DNA
Total vector DNA was detected and quantified using a validated qPCR assay. The qPCR method used was highly sensitive and able to detect vector DNA quantities ∼50-fold lower than the FDA-recommended limit of quantitation (LOQ).17 Briefly, 5 μL of extracted DNA was analyzed for the presence of vg DNA by qPCR with a LOQ of 50 vg copies per qPCR reaction or 50 vg per 5 μL DNA sample. The validated limit of detection (LOD) with a 95% consistency level was 7.28 copies of vg per 5 μL of DNA sample. A primer and probe set was designed to specifically bind a central region of the FVIII-SQ sequence within the vector DNA. There was no cross-reactivity of the primer and probe set with the endogenous hFVIII gene, which was validated using human genomic DNA.
The primary qPCR results, expressed as vg grams per 5 μL of DNA sample, were interpolated against a linear 8-point standard curve consisting of linearized FVIII-SQ plasmid DNA with a range of quantitation from 50 to 5 × 108 vg per 5 μL. The primary results were then back-calculated and reported as copies of vg per volume (mL) or mass (mg) of the original specimen using specimen-specific conversion factors that were based on the measured starting quantities of the specimen and any volume changes performed during or after DNA extraction. The qPCR results were also back calculated and reported as copies of vg per microgram of total DNA when applicable.
For each biologic sample, DNA extraction efficiency was assessed during method validation. In summary, the extraction efficiency ranges for blood, stool, saliva, semen, and urine were 32.7% to 65.0%, 57.2% to 160.3%, 4.6% to 80.3%, 124.5% to 140.9%, and 29.0% to 105.7%, respectively. Samples that were positive for vector DNA above the LOQ were reported with the numerical copy number concentration values (vg/mL, vg/mg, or vg/μg DNA), and positive samples below the LOQ were reported as being below the limit of quantification (BLOQ). Samples were only reported as negative if no fluorescent signal was detected after 40 qPCR cycles. The back-calculated LOQ values for blood, stool, saliva, semen, and urine were 6000 vg/mL, 16 vg/mg, 7000 vg/mL, 24 000 vg/mL, and 15 400 vg/mL, respectively.
We defined vector clearance as 3 consecutive BLOQ or negative qPCR samples. In addition to vector clearance (reported as a percentage), we assessed the percentage of participants with detectable vector DNA, time to first detectable sample (week), peak concentration (vg/mL), time to peak concentration (week), time to first BLOQ or negative sample (week), time to first negative sample (using FDA definition; [week]), and the percentage of participants with 3 negative consecutive samples (using FDA definition).
Encapsidated vector DNA detection
Encapsidated vector DNA that was potentially capable of cell transduction was detected and quantified in plasma and semen using an iqPCR assay.20 AAV5 vector capsids were first immunoprecipitated from plasma or liquefied semen and subsequently treated with Benzonase (Millipore Sigma, Merck, Darmstadt, Germany) to digest any external DNA. Samples were then heat treated to denature the capsid and to release the encapsidated vector DNA. The levels of encapsidated vector DNA were quantified using qPCR according to the same methods described above. The LOQ was 2.1 × 105 vg/mL for both plasma and semen, and the LOD was 1.2 × 104 vg/mL for plasma and 2.3 × 104 vg/mL for semen.
Assessment of vg contiguity and circular episome formation
The contiguity of vg was measured in whole blood and peripheral blood mononuclear cells (PBMCs) using a multiplexed ddPCR assay (Bio-Rad).12 This method uses different fluorescently labeled PCR probes to simultaneously detect 2 independent target sequences to quantify the percentage of PCR target sites that are linked (ie, present on the same contiguous DNA strand). Specifically, the ddPCR software (QuantaSoft) uses an algorithm for quantitation that corrects for the number of double-positive droplets generated by random coincidence from unlinked anchor and reference sites to yield the final number of double-positive droplets produced by truly contiguous (linked) target sites. The contiguity results are expressed as the fraction of linked sites relative to the total anchor sites of the most 3′ amplicon on the vector. vg ITR fusions were measured in whole blood and PBMCs by fusion-specific ddPCR using primers directed outward from the 5′ and 3′ ends of the linear vg. Because the primer set was designed with nonoverlapping directionality of a linear vg, exponential amplification of a PCR product was not possible and therefore does not create any amplification product from the linear vector sequence. However, in the context of a circularized vg, the primer set will face each other and form a productive amplicon, triggering exponential amplification and detection of the ITR fusion. ITR fusions were assumed to represent circularized episomal vectors10; however, it is possible that a small portion represent head-to-tail tandem integrated vector DNA.22 The ITR results are expressed as the fraction of successful cross-ITR amplifications relative to the same anchor site on the 3′ end of the vector.
Results
Patient characteristics
In the GENEr8-1 trial, 134 participants received a single 6 × 1013 vg/kg dose of valoctocogene roxaparvovec. As published previously, 72% of the participants in the trial population were White, and the median age at baseline was 30 years.14 During year 2, 2 participants discontinued the study; 1 participant was lost to follow-up at week 66, and 1 participant died at week 96 by suicide, which was considered by study investigators to be unrelated to treatment.15 All 134 participants enrolled in this study were assessed for vector DNA in saliva, urine, stool, and blood and encapsidated vector DNA in plasma, and 133 participants were assessed for vector DNA and encapsidated DNA in semen (Tables 1 and 2).
Total vector DNA biodistribution and shedding following 6 × 1013 vg/kg valoctocogene roxaparvovec infusion
| . | Participants with detectable vector DNA, n (%) . | Time to first detectable sample∗, wk . | Peak concentration†, vg/mL . | Time to peak concentration, wk . | Time to first BLOQ/negative sample‡, wk . | Participants with 3 consecutive BLOQ/negative samples, n (%) . | Time to first negative sample‡,§, wk . | Participants with 3 consecutive negative samples, n (%) . |
|---|---|---|---|---|---|---|---|---|
| Blood | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 18 | 18 (13.4) | 0 | 0 (0) |
| Median | 0.1 | 4.7 × 1010 | 0.1 | 123.0 | 0 | |||
| Min | 0.1 | 1.6 × 108 | 0.1 | 31.9 | 0 | |||
| Max | 1.0 | 2.0 × 1011 | 1.0 | 182.0 | 0 | |||
| Saliva | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 134 | 134 (100) | 111 | 111 (82.8) |
| Median | 0.1 | 6.6 × 107 | 0.1 | 6.6 | 48.0 | |||
| Min | 0.1 | 1.3 × 106 | 0.1 | 3.1 | 7.9 | |||
| Max | 1.1 | 4.3×109 | 2.3 | 52.0 | 122.0 | |||
| Semen | ||||||||
| n | 133‖ (100) | 133 | 133 | 133 | 133 | 133 (100) | 122 | 122 (91.7) |
| Median | 0.1 | 1.8 × 106 | 1.0 | 6.1 | 20.6 | |||
| Min | 0.1 | 1.2 × 104 | 0.1 | 0.6 | 6.1 | |||
| Max | 2.1 | 1.0 × 1010 | 12.1 | 36.1 | 94.0 | |||
| Stool | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 119 | 119 (88.8) | 7 | 7 (5.2) |
| Median | 0.1 | 2.7 × 105 | 1.1 | 44.1 | 105 | |||
| Min | 0.1 | 2.1 × 102 | 0.1 | 12.0 | 68.4 | |||
| Max | 6.3 | 5.7 × 106 | 6.3 | 131.0 | 144.0 | |||
| Urine | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 134 | 134 (100) | 132 | 132¶ (98.5) |
| Median | 0.1 | 8.9 × 104 | 0.2 | 2.3 | 12.1 | |||
| Min | 0.1 | 7.7 × 103 | 0.1 | 0.3 | 3.0 | |||
| Max | 0.9 | 3.7 × 107 | 2.3 | 8.1 | 48.0 |
| . | Participants with detectable vector DNA, n (%) . | Time to first detectable sample∗, wk . | Peak concentration†, vg/mL . | Time to peak concentration, wk . | Time to first BLOQ/negative sample‡, wk . | Participants with 3 consecutive BLOQ/negative samples, n (%) . | Time to first negative sample‡,§, wk . | Participants with 3 consecutive negative samples, n (%) . |
|---|---|---|---|---|---|---|---|---|
| Blood | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 18 | 18 (13.4) | 0 | 0 (0) |
| Median | 0.1 | 4.7 × 1010 | 0.1 | 123.0 | 0 | |||
| Min | 0.1 | 1.6 × 108 | 0.1 | 31.9 | 0 | |||
| Max | 1.0 | 2.0 × 1011 | 1.0 | 182.0 | 0 | |||
| Saliva | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 134 | 134 (100) | 111 | 111 (82.8) |
| Median | 0.1 | 6.6 × 107 | 0.1 | 6.6 | 48.0 | |||
| Min | 0.1 | 1.3 × 106 | 0.1 | 3.1 | 7.9 | |||
| Max | 1.1 | 4.3×109 | 2.3 | 52.0 | 122.0 | |||
| Semen | ||||||||
| n | 133‖ (100) | 133 | 133 | 133 | 133 | 133 (100) | 122 | 122 (91.7) |
| Median | 0.1 | 1.8 × 106 | 1.0 | 6.1 | 20.6 | |||
| Min | 0.1 | 1.2 × 104 | 0.1 | 0.6 | 6.1 | |||
| Max | 2.1 | 1.0 × 1010 | 12.1 | 36.1 | 94.0 | |||
| Stool | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 119 | 119 (88.8) | 7 | 7 (5.2) |
| Median | 0.1 | 2.7 × 105 | 1.1 | 44.1 | 105 | |||
| Min | 0.1 | 2.1 × 102 | 0.1 | 12.0 | 68.4 | |||
| Max | 6.3 | 5.7 × 106 | 6.3 | 131.0 | 144.0 | |||
| Urine | ||||||||
| n | 134 (100) | 134 | 134 | 134 | 134 | 134 (100) | 132 | 132¶ (98.5) |
| Median | 0.1 | 8.9 × 104 | 0.2 | 2.3 | 12.1 | |||
| Min | 0.1 | 7.7 × 103 | 0.1 | 0.3 | 3.0 | |||
| Max | 0.9 | 3.7 × 107 | 2.3 | 8.1 | 48.0 |
Defined as time to first positive shedding sample.
Units for stool reported as vg/mg.
Confirmed by 2 consecutive samples.
FDA definition of time to clearance.
One participant did not have available semen shedding assessments.
Three consecutive negatives were confirmed by week 24 in 1 participant after the data snapshot, and another participant achieved clearance defined by 3 consecutive BLOD and/or negative samples by week 32.
Encapsidated vector DNA biodistribution and shedding following 6 × 1013 vg/kg valoctocogene roxaparvovec infusion
| . | Participants with detectable vector DNA, n (%) . | Peak concentration, vg/mL . | Time to peak concentration, wk . | Time to last detectable sample, wk . | Participants with 3 consecutive negative samples, n (%) . | Time to first negative sample∗,†, wk . |
|---|---|---|---|---|---|---|
| Plasma (n = 134) | ||||||
| Median | 130 (97.0) | BLOQ | 1.1 | 2.2 | 134 (100) | 3.3 |
| Min | BLOQ | 0.9 | 0.9 | 1.3 | ||
| Max | 1.1 × 107 | 4.1 | 10.0 | 10.1 | ||
| Semen (n = 133)‡ | ||||||
| Median | 131 (98.5) | 9.3 × 105 | 0.6 | 1.9 | 131§ (98.5) | 3.0 |
| Min | BLOQ | 0.1 | 0.1 | 0.4 | ||
| Max | 3.8 × 108 | 4.0 | 8.1 | 12.1 |
| . | Participants with detectable vector DNA, n (%) . | Peak concentration, vg/mL . | Time to peak concentration, wk . | Time to last detectable sample, wk . | Participants with 3 consecutive negative samples, n (%) . | Time to first negative sample∗,†, wk . |
|---|---|---|---|---|---|---|
| Plasma (n = 134) | ||||||
| Median | 130 (97.0) | BLOQ | 1.1 | 2.2 | 134 (100) | 3.3 |
| Min | BLOQ | 0.9 | 0.9 | 1.3 | ||
| Max | 1.1 × 107 | 4.1 | 10.0 | 10.1 | ||
| Semen (n = 133)‡ | ||||||
| Median | 131 (98.5) | 9.3 × 105 | 0.6 | 1.9 | 131§ (98.5) | 3.0 |
| Min | BLOQ | 0.1 | 0.1 | 0.4 | ||
| Max | 3.8 × 108 | 4.0 | 8.1 | 12.1 |
Confirmed by 2 consecutive negative or BLOQ samples.
FDA definition of time to clearance.
One participant did not provide semen shedding assessments.
Two participants had insufficient sample quantity for vector DNA assessment by iqPCR.
Biodistribution and vector shedding of valoctocogene roxaparvovec
Vector DNA
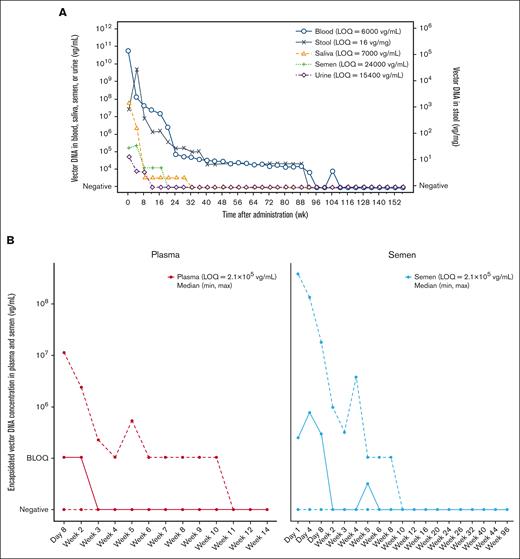
The median peak vector DNA levels were observed 1 to 8 days after the infusion of a single 6 × 1013 vg/kg dose of valoctocogene roxaparvovec and were followed by a steady decline in vector DNA levels (Figure 2A). The median peak vector DNA levels assessed in blood were 4.7 × 1010 vg/mL. Because valoctocogene roxaparvovec was administered directly into the bloodstream via IV infusion, initial high levels of vector DNA in blood are unsurprising. The median peak vector DNA levels, measured using qPCR, were second highest in saliva (6.6 × 107 vg/mL), followed by semen (1.8 × 106 vg/mL), stool (2.7 × 105 vg/mg), and urine (8.9 × 104 vg/mL; Table 1).
Biodistribution and shedding profiles of each collected sample. (A) Median vector DNA biodistribution and shedding profiles in blood, saliva, semen, stool, and urine, and (B) median (min, max) encapsidated vector DNA biodistribution and shedding profiles in plasma and semen following an infusion of 6 × 1013 vg/kg valoctocogene roxaparvovec. Data were binned in 4-week intervals. Lines represent the median concentration of vector DNA (A). Solid lines represent the median concentration of encapsidated vector DNA. Dashed lines represent the min and max concentration levels of encapsidated vector DNA (B). wk, week.
Biodistribution and shedding profiles of each collected sample. (A) Median vector DNA biodistribution and shedding profiles in blood, saliva, semen, stool, and urine, and (B) median (min, max) encapsidated vector DNA biodistribution and shedding profiles in plasma and semen following an infusion of 6 × 1013 vg/kg valoctocogene roxaparvovec. Data were binned in 4-week intervals. Lines represent the median concentration of vector DNA (A). Solid lines represent the median concentration of encapsidated vector DNA. Dashed lines represent the min and max concentration levels of encapsidated vector DNA (B). wk, week.
At the 3-year data cutoff, 3 consecutive BLOQ or negative samples were achieved in 134 (100%), 134 (100%), 133 (100%), and 119 (88.8%) participants in urine, saliva, semen, and stool, respectively (Table 1). Median (min, maximum [max]) time to the first BLOQ or negative measurement, confirmed by 2 additional consecutive measurements for each respective shedding matrix, was 2.3 (0.3, 8.1) weeks, 6.1 (0.6, 36.1) weeks, 6.6 (3.1, 52.0) weeks, and 44.1 (12.0, 131.0) weeks for urine, semen, saliva, and stool, respectively. In contrast with these shedding matrixes, only 18 (13.4%) participants achieved 3 consecutive BLOQ or negative samples in blood at the data cutoff, and the median (min, max) time to the first BLOQ or negative measurement confirmed by 2 additional consecutive measurements was 123.0 (31.9, 182.0) weeks. Overall, these data demonstrate that vector DNA is shed at different levels and rates in secreta and excreta over a 3-year period, and vector DNA cleared more rapidly in urine and took the longest to clear in stool.
Encapsidated vector DNA
To better understand the biodistribution and shedding of potentially transmissible vector DNA, plasma and semen samples were further evaluated for encapsidated vector DNA concentrations using iqPCR. Given that the iqPCR method has been optimized for encapsidated vector DNA detection in plasma and semen,20 these 2 biological fluids were chosen for assessment over blood and the remaining shedding matrixes. Following administration of valoctocogene roxaparvovec, encapsidated vector DNA was detectable in plasma from 130 of 134 participants (97.0%) evaluated and in semen from 131 of the 133 participants (98.5%) evaluated (Table 2). The median peak encapsidated vector DNA concentrations were BLOQ and 9.3 × 105 vg/mL in plasma and semen, respectively, with the LOQ being 2.1 × 105 vg/mL for both plasma and semen. Three consecutive negative samples were observed in plasma for all 134 participants with a median (min, max) time to the first of 3 consecutive negatives of 3.3 (1.3, 10.1) weeks. In semen, 3 consecutive negative samples were observed for 131 of the 133 participants evaluated with a median (min, max) time to the first of 3 consecutive negatives of 3.0 (0.4, 12.1) weeks. Encapsidated vector DNA was cleared more rapidly than total vector DNA when considering that complete clearance was achieved by all participants with evaluable samples in both plasma (134 participants) and semen (131 evaluable participants) by ≤12 weeks after AAV administration (Figure 2B).
Contiguity of vg and formulation of circularized episomes
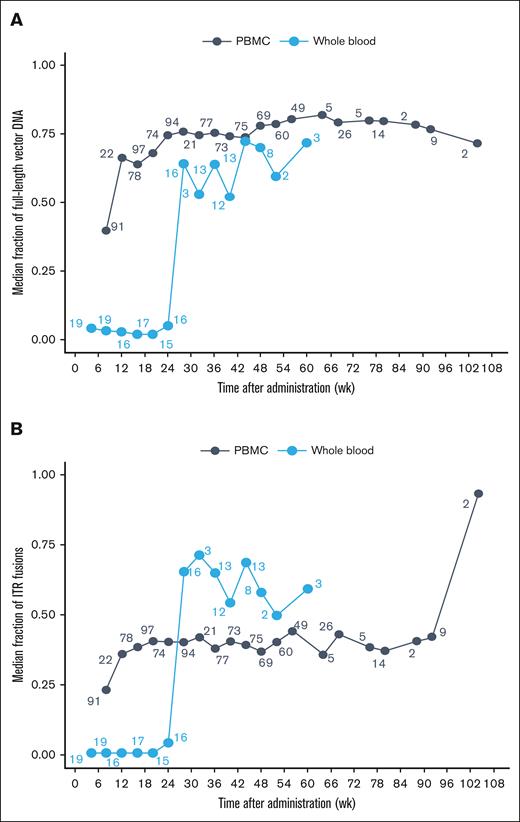
A subset of whole blood and PBMC samples were used to assess the contiguity of vg using ddPCR. Unlike qPCR, which only measures a small nucleotide region (150 nucleotides), ddPCR evaluates the complete valoctocogene roxaparvovec vector DNA as it transitions from truncated vector DNA into a full-length transgene within transduced cells. By week 52, the median fraction of full-length vector DNA in whole blood was ∼0.75. PBMC samples at weeks 28 to 48 had a similar fraction of full-length DNA as whole blood after week 28 (Figure 3A). These data are consistent with the conclusion that, after red blood cells expire, the residual vector DNA in whole blood resides as an assembled, complete transgene within the PBMC fraction.
Median fraction of vector DNA in whole blood and PBMCs. Median fraction of (A) full-length vg and (B) ITR fusions in blood matrices following 6 × 1013 vg/kg valoctocogene roxaparvovec infusion. The average of each participant’s fractions within each interval was calculated and the median fraction across participants for each interval is presented. The number next to each point represents the number included in the median calculation. Bins with n = 1 were excluded from median calculations. wk, week.
Median fraction of vector DNA in whole blood and PBMCs. Median fraction of (A) full-length vg and (B) ITR fusions in blood matrices following 6 × 1013 vg/kg valoctocogene roxaparvovec infusion. The average of each participant’s fractions within each interval was calculated and the median fraction across participants for each interval is presented. The number next to each point represents the number included in the median calculation. Bins with n = 1 were excluded from median calculations. wk, week.
To understand the structural characteristics of full-length transgenes following valoctocogene roxaparvovec infusion, we assessed whether the ITR region of the vector fused to form a circularized episome structure. Using fusion-specific ddPCR, head-to-tail ITR analyses were conducted on a subset of whole blood and PBMC samples. Following AAV treatment, the fraction of ITR fusions in PBMCs increased from a median value of 0.3 at week 8 to 0.4 during weeks 28 to 48, indicating that circularized episomes formed in PBMCs (Figure 3B). Again, whole blood ITR fusions also increased after week 24 at the same time that the initial population of red blood cells would be expected to expire. This transition to stable, full-length episomes in long-lived PBMCs likely contributes to the duration of detection in blood by qPCR and provides mechanistic evidence for the assembly of a stable and functional transgene from transduced vector DNA.
Discussion
Over a 3-year period, we investigated the biodistribution and vector shedding characteristics of valoctocogene roxaparvovec in participants with severe hemophilia A from the phase 3 GENEr8-1 clinical trial. Although the risk for horizontal transmission or environmental release after valoctocogene roxaparvovec administration is low, characterization of vector shedding is an important component of the safety evaluations necessary to fully assess the long-term impact of AAV gene therapy. Our novel analysis not only included quantities of total vector DNA but also discriminated the molecular form in several matrixes, thereby providing deeper insight into the complex pharmacokinetics of vector DNA and a more refined analysis of the residual risk of transmission of transduction-competent vector particles.
In summary, total and encapsidated vector DNA were detected in all biodistribution and shedding matrixes investigated here. The rate of vector clearance across sample types varied with vector DNA clearance being achieved the fastest in urine, followed by semen, saliva, and stool. The prolonged time to achieve clearance of vector DNA from stool samples could be the consequence of immune cells trafficking the vector to the intestines or through the lysing of transduced hepatocytes; however, additional exploration is required to establish a mechanism. Encapsidated vector DNA in semen was cleared by participants by at least 12 weeks after AAV infusion. The marginally higher levels of peak encapsidated vector DNA in semen than in plasma can be explained by the difference in the earliest sample evaluated between the 2 matrixes (day 1 for semen and day 8 for plasma). By the 3-year cutoff date, >88% of the participants met the requirements for vector clearance for each shedding matrix.
In contrast to the shedding matrixes, persistence of vector DNA in blood was consistently longer with only 13.4% of participants reaching 3 consecutive BLOQ or negative samples by year 3; however, encapsidated vector DNA rapidly cleared from plasma by week 10. Blood is not traditionally considered true excreta relative to the other shedding matrixes19,23; however, many studies, including this report, incorporate it into shedding assessments.23-26 The inclusion of blood in our study provided insight into the assembly of stable vector forms in whole blood and PBMCs and illustrated how encapsidated transmissible forms decrease. In this study, we observed low levels of residual assembled vector DNA that have ITR fusions remaining within PBMCs. These structures are not present in the DNA packaged into capsids but are the expected results of uncoating of the DNA during the process of capsid removal, nuclear uptake, and assembly. This persistent episomal DNA in long-lived lymphocytes represents a set of genomes that are free of capsid and no longer capable of transducing to other cells. These complementary data sets support pharmacodynamic clearance of the initial transduction-competent AAV vector particles and the presence of low levels of long-lived residual DNA in nontransmissible forms.
Previous clinical studies that investigated the biodistribution and vector shedding profiles of AAV-mediated gene therapies suggest that the rate of vector clearance may be dose dependent.24,25,27 During follow-up for a phase 1/2 clinical trial of SPK-8011 for hemophilia A (NCT03003533/NCT03432520), assessments of vector clearance after a range of lower administration doses (5 × 1011 to 2 × 1012 vg/kg) revealed that vg concentrations were BLOQ in saliva, semen, serum, and urine for all participants, regardless of dose, by 3 weeks (range, 1-3) after treatment (qPCR information unavailable).27
However, higher doses of AAV-mediated gene therapy take longer to achieve clearance. Specifically, during a phase 1/2 trial that investigated AAV5 treatment for hemophilia B (NCT02396342), 5 × 1012 and 2 × 1013 genome copies per kg doses led to relatively longer periods of vector clearance in saliva, semen, urine, nasal secretions, and feces; 3 consecutive measurements of zero or below the LOD (BLOD) were achieved by week 90 (range, 3-90 weeks) and 64 (range, 7-64 weeks), respectively.25 Assay LODs of 10 copies per reaction for shedding matrixes were as follows: <400 copies/mL for saliva and semen, <571 copies per mL for urine and nasal secretions (<571 copies per swab), and ∼1 copy per mg for stool.24 Time to semen clearance was notably different between the 2 cohorts considering that clearance took longer in the 5 × 1012 genome copies per kg cohort, which was previously speculated to be the cause of an age-dependent factor between the 2 cohorts.24 In line with our report, blood (LOD of 10 copies/reaction, <571 copies per mL)24 took the longest to achieve clearance with 100% of the participants in the low-dose group and 80% of the participants in the high-dose group meeting the clearance criteria by 3 and by 2.5 years, respectively.25 This result is most likely because of assembled vg in long-lived PBMCs.
Importantly, our clearance criteria, which have been used for other AAV5 gene therapy trials,27 were defined as 3 consecutive BLOQ or negative qPCR samples, which were slightly different from the technical definition in the FDA guidance (3 consecutive BLOD or negative samples).19 Under the FDA definition, shedding matrixes took consistently longer to fulfill the clearance criteria, which would have extended the need to continue sample collection. Using a highly sensitive qPCR method enabled us to accurately measure and assess the timing of vector clearance while also minimizing the burden of continued sample collection for participants. The impact qPCR assay sensitivity and clearance criteria have on the interpretation of vector clearance should be considered when comparing data across different studies, because this work revealed that the outcomes are not the same.
Similar to the FDA guidelines, we controlled for underestimated or false-negative shedding results by determining vector DNA clearance with 3 consecutive qPCR measurements. Biologic components of shedding matrixes, such as urine, saliva, and stool, are rich in proteases, nucleases, ions, and salts. These components can affect the amplification process of PCR, which can reduce the overall detection of vector shedding.19 Therefore, we maintained this standard practice.
To better understand the molecular forms of the detected vector DNA, we quantified amounts of encapsidated vector DNA in plasma and semen. The iqPCR method revealed that clearance of potentially transmissible encapsidated vector DNA was achieved at ≤12 weeks after AAV administration, which was longer than previously reported for this particular gene therapy for hemophilia A.20 In a phase 1/2 dose-escalation study (NCT02576795) that investigated valoctocogene roxaparvovec in men with severe hemophilia A, encapsidated vector DNA in both plasma and semen met the clearance criteria, which were defined as 3 consecutive BLOD or negative results, by ≤9 weeks after AAV administration. The difference in the reported persistence of encapsidated vector DNA may be because of the increased sample size and individual variability within the larger phase 3 trial population relative to the phase 1/2 trial (2022; n = 134 vs 15).20
Based on the replication-incompetent nature of AAV gene therapies and the very low vector and encapsidated vector DNA levels in vector shedding assessments, valoctocogene roxaparvovec poses minimal risk for horizontal transmission to untreated individuals over an extended period. Furthermore, our iqPCR findings suggest that no additional cell transduction that leads to the transfer of vector DNA to the next generation through incorporation into germ cells (ie, germ line transmission) could occur. Preclinical work in mice also suggests that the risk for germ line transmission after valoctocogene roxaparvovec treatment is inherently low. Mating AAV-treated male mice with naïve females did not lead to transgene expression in their offspring, and the estimated risk for germ line transmission of the AAV therapy was <5% with a 99.2% confidence level.28
Although there is low risk, we recommend that individuals who received valoctocogene roxaparvovec treatment still take precaution and abstain from certain activities like donating blood or tissues. The use of effective barrier contraception is also recommended for 6 months following treatment with valoctocogene roxaparvovec to further prevent the risk for horizontal transmission and to avoid the theoretical risk of germ line transmission.29 This recommendation is based primarily on the time to clearance in semen and plasma of potentially transduction-competent encapsidated vector DNA measured by iqPCR plus a 3-month washout period and the time to clearance in semen of all other residual vector DNA.
Altogether, free and encapsidated vector DNA and vector capsids were steadily cleared from the blood and shedding matrixes of GENEr8-1 participants treated with valoctocogene roxaparvovec, and the risk for horizontal and germ line transmission seems to be low. We will continue to evaluate the long-term safety of valoctocogene roxaparvovec as the duration of follow-up from clinical trials and registries increases.
Acknowledgments
The authors thank all of the study participants and their families, the trial principal investigators, and the study site personnel. The authors also thank members of all valoctocogene roxaparvovec development teams at BioMarin Pharmaceutical Inc.
Primary funding for the study was provided by BioMarin Pharmaceutical Inc. Medical writing support was provided by Taryn Bosquez-Berger of AlphaBioCom, a Red Nucleus company, and was funded by BioMarin Pharmaceutical Inc. Jonathan Morton, of BioMarin Pharmaceutical Inc, provided project management support.
Authorship
Contribution: T.M.R. oversaw conduct of the trial; K.J. provided input on safety assessments; S.A., K.S., K.O.M., M.V., A.B., B.B., J. Holcomb, C.B.R., S.Z., C.V., and J. Henshaw performed data analyses; and all authors contributed to interpretation of the data, provided critical input during manuscript drafting, and approved the final version for submission.
Conflict-of-interest disclosure: S.A., K.O.M., M.V., T.M.R., K.J., C.B.R., S.Z., and J. Henshaw report being employees and shareholders of BioMarin Pharmaceutical Inc. K.S., A.B., B.B., J. Holcomb, and C.V. report being former employees of BioMarin Pharmaceutical Inc and may hold stocks.
Correspondence: Suresh Agarwal, Translational Sciences, BioMarin Pharmaceutical Inc, 105 Digital Dr, Novato, CA 94949; email: Suresh.Agarwal@bmrn.com.
References
Author notes
The deidentified individual participant data that underlie the results reported in this article (including text, tables, figures, and appendixes) will be made available, together with the research protocol and data dictionaries, for noncommercial, academic purposes. Additional supporting documents may be available upon request. Investigators will be able to request access to these data and supporting documents via a data sharing portal beginning 6 months and ending 2 years after publication. Data associated with any ongoing development program will be made available within 6 months after approval of the relevant product. Requests must include a research proposal that clarifies how the data will be used, including proposed analysis methodology. Research proposals will be evaluated in terms of publicly available criteria available at www.BioMarin.com/patients/publication-data-request/ to determine if access will be given, contingent upon the execution of a data access agreement with BioMarin Pharmaceutical Inc.