Key Points
The presence of CLL cells delays T-cell receptor–mediated CAR T-cell activation and impairs proliferation and restimulation.
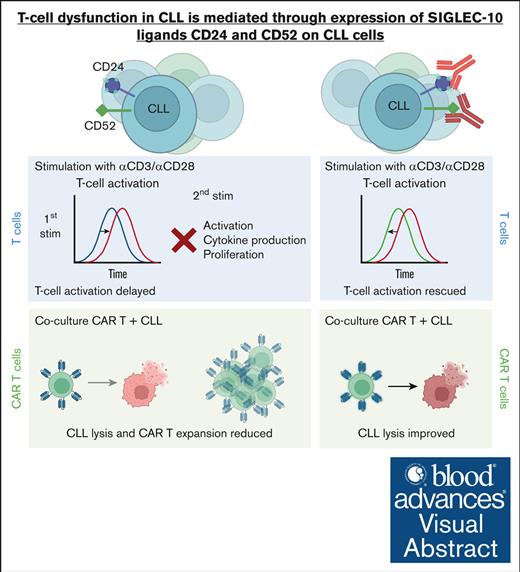
Blocking CD24 and CD52 expression on CLL cells reverses T-cell dysfunction.
Visual Abstract
Autologous T-cell–based therapies, such as chimeric antigen receptor (CAR) T-cell therapy, exhibit low success rates in chronic lymphocytic leukemia (CLL) and correlate with a dysfunctional T-cell phenotype observed in patients. Despite various proposed mechanisms of T-cell dysfunction in CLL, the specific CLL-derived factors responsible remain unidentified. This study aimed to investigate the mechanisms through which CLL cells suppress CAR T-cell activation and function. We found that CLL-derived T cells get activated, albeit in a delayed fashion, and specifically that restimulation of CAR T cells in the presence of CLL cells causes impaired cytokine production and reduced proliferation. Notably, coculture of T cells with CD40-activated CLL cells did not lead to T-cell dysfunction, and this required direct cell contact between the CD40-stimulated CLL cells and T cells. Inhibition of kinases involved in the CD40 signaling cascade revealed that the Spare Respiratory Capacity (SRC) kinase inhibitor dasatinib prevented rescue of T-cell function independent of CD40-mediated increased levels of costimulatory and adhesion ligands on CLL cells. Transcriptome profiling of CD40-stimulated CLL cells with or without dasatinib identified widespread differential gene expression. Selecting for surface receptor genes revealed CD40-mediated downregulation of the Sialic acid-binding Ig-like lectin 10 (Siglec-10) ligands CD24 and CD52, which was prevented by dasatinib, suggesting a role for these ligands in functional T-cell suppression in CLL. Indeed, blocking CD24 and/or CD52 markedly reduced CAR T-cell dysfunction upon coculture with resting CLL cells. These results demonstrated that T cells derived from CLL patients can be reinvigorated by manipulating CLL–T-cell interactions. Targeting CD24- and CD52-mediated CLL–T-cell interaction could be a promising therapeutic strategy to enhance T-cell function in CLL.
Introduction
Targeted therapy, such as inhibitors of Bruton tyrosine kinase (BTK) or B-cell lymphoma 2 (BCL2), with or without anti-CD20 monoclonal antibodies are therapies of choice for chronic lymphocytic leukemia (CLL). However, these therapies do not cure CLL with selected exceptions.1 Despite the expanding availability of treatment options, resistance to therapy remains a major problem, and patients who are refractory to both BTK and BCL2 inhibitors have a dismal outcome.2 In this regard, autologous T-cell–based therapies, such as chimeric antigen receptor (CAR) T-cell therapy, may provide a solution because these can be curative in other lymphoproliferative diseases.3 Although CAR T-cell therapy can be curative for patients with CLL, long-term remissions are only obtained in a small minority of patients.4-7 The current consensus is that acquired T-cell dysfunction underlies the dismal outcomes of these therapies. More specifically, impaired formation of the immunologic synapse,8,9 reduced metabolic plasticity,10,11 diminished proliferation, high expression of immune checkpoint molecules, and a decreased in vitro T-cell activation have all been well documented.8,9,11-13 Despite these observations, T cells are not fully incompetent in the setting of CLL, because they retain the ability to produce cytokines upon stimulation, even in presence of CLL cells.14,15 Eliminating CLL cells by cell sorting in vitro or treating patients with CLL with venetoclax reverses T-cell dysfunction,15 showing that this acquired T-cell dysfunction is, at least partly, reversible. Oligoclonal expansion of T cells during the course of CLL have been well documented,16-19 but whether those T cells get activated by CLL in an antigen-dependent manner remains uncertain.
How CLL cells impact the function of autologous T cells is not yet fully elucidated. One possibility is that circulating CLL cells that highly outnumber T cells express low to no costimulatory molecules. This lack of costimulation leads to inefficient T-cell activation.20 In contrast with resting CLL cells, CLL cells do become efficient antigen-presenting cells (APCs) upon activation through the CD40-CD40L axis.21,22 This concept was recently explored to improve CLL-derived CAR T-cell proliferation, cytokine production, and cytotoxicity and, indeed, activation of CLL cells using CD40 ligation, and interleukin-4 (IL-4) significantly enhanced CAR T-cell expansion.21
In this study, we investigated how the presence of CLL cells impacts CAR T-cell activation and function upon T-cell receptor (TCR) activation in the presence of proper costimulation. We showed that the presence of CLL cells does not fully block but delays TCR-mediated T-cell activation. Moreover, the presence of CLL cells gradually leads to a functional impairment and, subsequently, to an impaired recall response. Exploiting the CD40 preactivation system to alleviate T cells from CLL-mediated suppression, we found that CLL cells downregulate the membrane-bound Siglec-10 ligands CD24 and CD52 upon CD40 ligation in a kinase-dependent fashion. Blocking CD24 and CD52 ligation led to improved CAR T-cell activation in the presence of CLL cells, lending potential clinical relevance to this finding.
Materials and methods
Patients and HDs
Peripheral blood (PB) from age-matched healthy donors (HDs) and untreated patients with CLL (supplemental Table 1) was obtained following written and informed consent. PB mononuclear cells (PBMCs) were cryopreserved as described previously.23 Previously untreated patients with CLL were included if they had a white blood cell count >20 × 109 cells per L. The medical ethical and biobank committees at the Academic Medical Center confirmed ethical approval in accordance with the Declaration of Helsinki.
Cell culture
Primary cells and cell lines were cultured as described in the supplemental Protocol. In short, PBMCs and CAR T cells, the mantle cell lymphoma cell line JeKo-1 (CRL-3006; ATCC), and the NIH3T3 fibroblast (3T3) and CD40L-transfected NIH3T3 fibroblast (3T40) cell lines were cultured in RPMI 1640 (no. 22400089; Gibco) supplemented with 10% fetal calf serum by volume and penicillin and streptomycin (no. 15140-122; ThermoFisher Scientific) at 37°C and 5% CO2.
CAR T-cell production
CD19BBζ CAR T cells were generated as described in the supplemental Protocol. Briefly, purified T cells were stimulated for 24 hours with CD3/CD28 coated beads (11131D; ThermoFisher Scientific) and transduced with a CAR-encoding lentivirus (Tisagenlecleucel; Novartis).7 The CAR T cells were expanded for 2 weeks to obtain sufficient cells. Purified CAR T cells were subsequently obtained by cell sorting using a BD FACSAria II.
Receptor blocking experiments
Blocking antibodies specific for CD54 (1 μg/mL, 322721; BioLegend), CD58 (1 μg/mL, 330923; BioLegend), CD24 (1 μg/mL, MA5-11828; ThermoFisher Scientific, and AF5247; Bio-Techne), CD52 (1 μg/mL, MAB9889-100; Bio-Techne), or clinical-grade alemtuzumab (1-2 μg/mL; Sanofi Genzyme) were added to PBMCs for 1 hour after thawing and adjusting cell concentrations to 3.0 × 106 cells per mL. After the preincubation period, cells were either washed to remove excess alemtuzumab or left in culture (anti-CD24, and anti-CD52). The T-cell stimulating soluble antibodies, anti-CD3 clone 1XE and anti-CD28 clone 15E8, were added directly thereafter.
Proliferation assay
To measure T-cell proliferation, PBMCs were stained using CellTrace Violet (C34557; ThermoFisher Scientific) according to manufacturer’s description, and T cells were stimulated directly thereafter as previously described.11 Five days after stimulation, cells were harvested and analyzed. Analysis of proliferation was performed using the FlowJo proliferation tool. To analyze expansion of T cells for periods long than 5 days, 123count eBeads (01-1234-42; ThermoFisher Scientific) were used to according to manufacturer’s descriptions.
Cytotoxicity assay
The formula compares dead target cells in nontransduced (NT) cocultures with CAR T-cell cocultures (CAR). Dead cells were identified as those not positive for MitoTracker Orange CMTMRos and negative for TO-PRO-3 Iodide.
Flow cytometry
Cells were stained with fluorescent-labeled antibodies (see supplemental Methods), and data were acquired on an LSRFortessa (BD Biosciences). The flow cytometry data were analyzed using FlowJo (v10.9). Statistical analyses were performed using GraphPad Prism version 9.
RNA sequencing
RNA sequencing was performed as described in the supplemental Protocol. Briefly, fluorescence-activated cell sorted CLL cells were cultured for 48 hours with 3T3 or 3T40 fibroblasts or 3T40 fibroblasts in the presence or absence dasatinib (100 nM), RNA was isolated and used as input for RNA sequencing analysis. The data obtained were analyzed using R.24
Data presentation and statistical analysis
The data are presented as mean fluorescence intensity or percentages with error bars showing the standard error of the mean. Statistical analyses included unpaired or paired t tests, Mann-Whitney U tests, 1-way analysis of variance with Šídák's test, and 2-way analysis of variance with Tukey test, as appropriate. All tests were performed using GraphPad Prism version 9. Differences were considered statistically significant if P <.05.
Results
Delayed activation in CLL-derived T cells and diminished effector function after restimulation
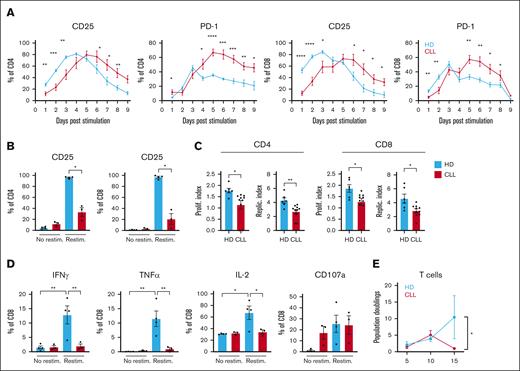
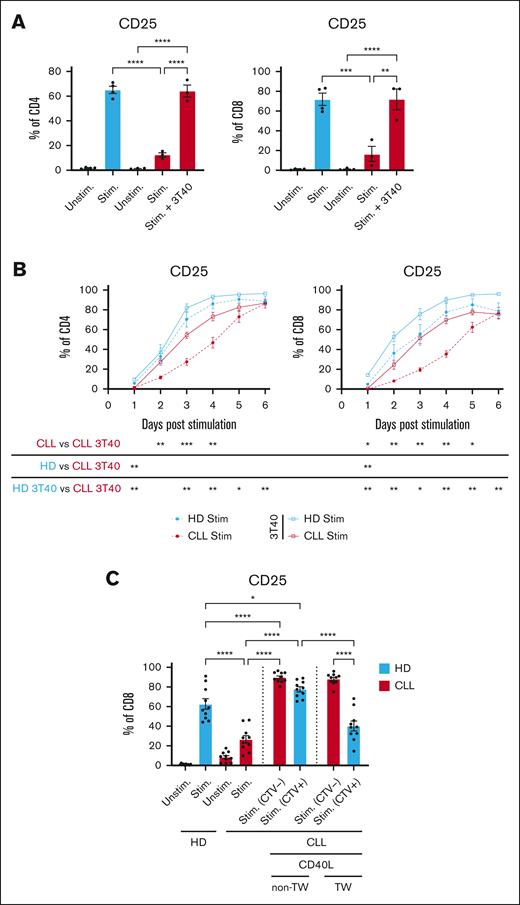
Observations by us and others who have shown that activation of T cells obtained from patients with CLL (CLL T cells) is impaired when compared with age-matched HDs were mostly made after 1 or 2 days of CD3/CD28 T-cell stimulation.11,25 We now measured the consequences of CD3/CD28 T-cell stimulation in the presence of CLL cells over time by the addition of soluble αCD3/αCD28 antibodies to PBMC and found that T cells from patients with CLL do reach similar levels of activation when compared with T cells from HDs as measured by CD25 (IL-2 receptor) expression but with significantly delayed kinetics. T cells from patients with CLL in the presence of CLL cells reached their peak of activation after 5 to 6 days as opposed to 3 to 4 days for HDs (HD, n = 6 and CLL, n = 10-12) (Figure 1A; supplemental Figure 1A). At day 9 to 14, most HD T cells returned to a resting state as indicated by downregulation of CD25 expression, which was not observed for T cells from patients with CLL (Figure 1A; supplemental Figure 1A). Similarly, peak Programmed cell death protein 1 (PD-1) expression levels were reached at day 3 and 5 in T cells from HD and those from patients with CLL, respectively. PD-1 levels remained increased at later time points in CLL T cells (Figure 1A). Restimulation of CLL T cells at day 14 to mimic recall responses revealed substantial impaired activation capacity when compared with those from HDs as measured by CD25 expression 48 hours after restimulation (Figure 1B). Expression of CD25 remained elevated in CLL T cells when compared with those from HDs at day 14 at the time of restimulation, underscoring the delayed response to T-cell stimulation (supplemental Figure 1A). Consistent with decreased activation potential, proliferative capacity upon restimulation was also strongly reduced in CLL T cells when compared with those from HDs as indicated by lower proliferation and replication indexes when compared with age-matched HDs (Figure 1C). Restimulation of previously expanded T cells revealed lower expression of interferon gamma (IFN-γ), tumor necrosis factor-α (TNF-α), and IL-2 in CLL cases, specifically in CD8+ T cells (Figure 1D; supplemental Figure 1B). Interestingly, stimulated CLL-derived T cells that returned to a preactivation state in terms of CD25 expression levels maintained a high expression of CD107a (Figure 1D; supplemental Figure 1B). We next investigated the magnitude of T-cell expansion in presence of total PBMCs after stimulation. In a repeated stimulation assay, T cells of patients with CLL and those from HDs were stimulated every 5 days for 15 days in total, and T-cell population doublings were analyzed every 5 days. Although, initially, proliferation of CLL-derived T cells was similar to those of HDs, expansion declined after day 10, whereas HD T-cell expansion continued (n = 3; Figure 1E).
Delayed activation in CLL-derived T cells and diminished effector function after restimulation. (A-B) PBMCs from HDs and patients with CLL were thawed and T cells were stimulated once with soluble stimulating CD3/CD28 antibodies and kept in culture for 16 days. (A) Expression of CD25 and PD-1 was measured daily (HD, n = 6; CLL, n = 10-12). (B) On day 14, all cells were washed, stained with Cell Trace Violet (CTV), and resuspended in culture medium with or without a single dose of CD3/CD28 antibodies. After 48 hours, T-cell activation was measured by analyzing CD25 expression. (C) In addition, the proliferation and replication index was calculated (using FlowJo) based on CTV intensity 5 days after restimulation (HD, n = 6; CLL, n = 10). (D) In addition, after an initial 14 days of single stimulation with CD3/CD28 antibodies, T cells were restimulated and kept for 2 days, and IFN-γ, TNFα, IL-2, and degranulation (CD107a) were measured on CD8 T cells (HD, n = 4; CLL, n = 3). (E) In a repeated stimulation experiment, PBMCs from HDs and patients with CLL were thawed and at start and on day 5 and day 10, PBMCs were washed and resuspended with the addition of CD3/CD28 antibodies. Every 5 days, the proliferation (population doublings) of T cells was analyzed using counting beads (HD, n = 3; CLL, n = 3). P values were calculated using a t test (A), a Welch t test (B), a Mann-Whitney test (C), a 2-way analysis of variance (ANOVA) that corrected for multiple comparisons using a Tukey test (D), or a 1-way ANOVA (E). Data are presented as mean ± standard error of the mean (SEM); ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
Delayed activation in CLL-derived T cells and diminished effector function after restimulation. (A-B) PBMCs from HDs and patients with CLL were thawed and T cells were stimulated once with soluble stimulating CD3/CD28 antibodies and kept in culture for 16 days. (A) Expression of CD25 and PD-1 was measured daily (HD, n = 6; CLL, n = 10-12). (B) On day 14, all cells were washed, stained with Cell Trace Violet (CTV), and resuspended in culture medium with or without a single dose of CD3/CD28 antibodies. After 48 hours, T-cell activation was measured by analyzing CD25 expression. (C) In addition, the proliferation and replication index was calculated (using FlowJo) based on CTV intensity 5 days after restimulation (HD, n = 6; CLL, n = 10). (D) In addition, after an initial 14 days of single stimulation with CD3/CD28 antibodies, T cells were restimulated and kept for 2 days, and IFN-γ, TNFα, IL-2, and degranulation (CD107a) were measured on CD8 T cells (HD, n = 4; CLL, n = 3). (E) In a repeated stimulation experiment, PBMCs from HDs and patients with CLL were thawed and at start and on day 5 and day 10, PBMCs were washed and resuspended with the addition of CD3/CD28 antibodies. Every 5 days, the proliferation (population doublings) of T cells was analyzed using counting beads (HD, n = 3; CLL, n = 3). P values were calculated using a t test (A), a Welch t test (B), a Mann-Whitney test (C), a 2-way analysis of variance (ANOVA) that corrected for multiple comparisons using a Tukey test (D), or a 1-way ANOVA (E). Data are presented as mean ± standard error of the mean (SEM); ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
Together, these data show that in the presence of CLL cells, CLL-derived T cells were activated in a delayed fashion but failed to produce cytokines and to proliferate after restimulation.
Effector function of CAR T cells is reduced in the presence of CLL cells
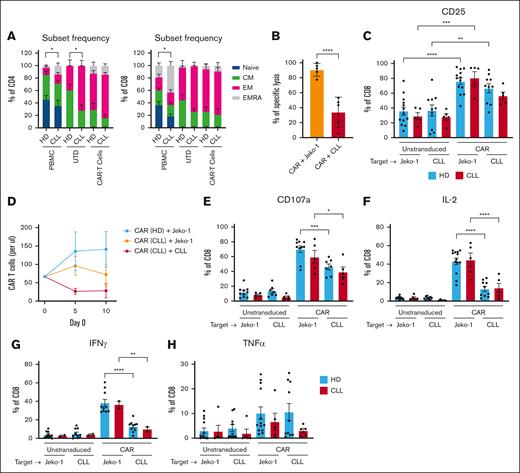
Next, we analyzed whether phenotypical and functional responses of CLL-derived CAR T cells (CD19BBζ) upon in vitro stimulation were also altered when compared with CAR T cells derived from HD T cells. Naïve (CD27+CD45RA+), memory (CD27+CD45RA−), effector memory (EM; CD27−CD45RA−), and effector memory RA+ (EMRA; CD27−CD45RA+) proportions were determined directly after thawing and at day 14 after CD19 CAR transduction and expansion. In line with previous reports, we found elevated numbers of CD4+ and CD8+ EMRA T cells from CLL cases when compared with those from HDs at baseline (n = 3-10; Figure 2A).26-28 This difference diminished in CAR-transduced CD4+ and CD8+ cells but remained in expanded, nontransduced CD4+ T cells (Figure 2A), indicating that CLL T-cell subset skewing is partly rescued by introducing the 4-1BBζ CAR. CLL-derived CAR T cells were able to kill CD19+ JeKo-1 cells more efficiently than autologous CLL cells (Figure 2B). HD-derived and CLL-derived CAR T cells were similarly activated by JeKo-1 cells based on increased expression of CD25. In contrast, only HD-derived CAR T cells were fully activated by CLL cells (Figure 2C). Repeated stimulation with JeKo-1 cells elicited a proliferative response in CLL-derived CAR T cells, albeit to a lesser extent than HD-derived CAR T cells. When repeatedly challenged with autologous CLL cells, CLL-derived CAR T cells failed to proliferate, underscoring the immune suppressive character of CLL cells in an autologous setting (n = 3; Figure 2D).
Effector function of CAR T cells is reduced in presence of CLL cells. (A) A pure population of T cells were obtained from PBMCs from HDs and patients with CLL. These purified T cells were transduced with a CD19BBζ CAR construct or left untransduced (UTD) and expanded for 14 days. The phenotype of the HD and CLL (CAR) T cells was characterized before T-cell selection (PBMC) or after transduction (UTD, transduced; CAR T cells) based on CD27 and CD45RA expression as follows: naive were CD27+CD45RA+, central memory (CM) were CD27+CD45RA−, effector memory (EM) were CD27−CD45RA−, and EM RA+ (EMRA) were CD27–CD45+ (HD, n = 3-10; CLL, n = 3-6). (B) CAR T cells were cultured with either JeKo-1 or autologous CLL cells, and after 1 day, specific lysis was calculated using TO-PRO and MitoTracker Orange (CLL, n = 5). In this same experiment, (C) UTD T cells and CAR T cells were analyzed for expression of CD25. (D) In a repeated stimulation assay, CAR T cells were cocultured in a 1:1 ratio with either JeKo-1 or autologous CLL cells, which were added every 5 days, and proliferation was measured every 5 days using counting beads (HD, n = 3; CLL, n = 3). (E-H) After 1 day of CAR T cells and (autologous) CLL or JeKo-1 coculture, expression of CD107a (E), IL-2 (F), IFN-γ (G), and TNF-α (H) was measured intracellularly (HD, n = 6-13; CLL, n = 2-5). P values were calculated using a Mann-Whitney test for panel A, a paired t test for panel B, a 1-way ANOVA for panels C,E-H, or a 2-way ANOVA for panel D. The data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
Effector function of CAR T cells is reduced in presence of CLL cells. (A) A pure population of T cells were obtained from PBMCs from HDs and patients with CLL. These purified T cells were transduced with a CD19BBζ CAR construct or left untransduced (UTD) and expanded for 14 days. The phenotype of the HD and CLL (CAR) T cells was characterized before T-cell selection (PBMC) or after transduction (UTD, transduced; CAR T cells) based on CD27 and CD45RA expression as follows: naive were CD27+CD45RA+, central memory (CM) were CD27+CD45RA−, effector memory (EM) were CD27−CD45RA−, and EM RA+ (EMRA) were CD27–CD45+ (HD, n = 3-10; CLL, n = 3-6). (B) CAR T cells were cultured with either JeKo-1 or autologous CLL cells, and after 1 day, specific lysis was calculated using TO-PRO and MitoTracker Orange (CLL, n = 5). In this same experiment, (C) UTD T cells and CAR T cells were analyzed for expression of CD25. (D) In a repeated stimulation assay, CAR T cells were cocultured in a 1:1 ratio with either JeKo-1 or autologous CLL cells, which were added every 5 days, and proliferation was measured every 5 days using counting beads (HD, n = 3; CLL, n = 3). (E-H) After 1 day of CAR T cells and (autologous) CLL or JeKo-1 coculture, expression of CD107a (E), IL-2 (F), IFN-γ (G), and TNF-α (H) was measured intracellularly (HD, n = 6-13; CLL, n = 2-5). P values were calculated using a Mann-Whitney test for panel A, a paired t test for panel B, a 1-way ANOVA for panels C,E-H, or a 2-way ANOVA for panel D. The data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001.
We next studied whether CAR T cells derived from patients with CLL displayed altered cytokine production and degranulation capacity. CLL- and HD-derived CAR T cells degranulated (as measured by CD107a) to equal levels when challenged with JeKo-1 but did so significantly less upon challenge with primary CLL cells (Figure 2E). Similar observations were made regarding the production of IL-2 (Figure 2F) and IFN-γ (Figure 2G) but not TNF-α (Figure 2H), confirming previously published observations that CLL cells elicited an aborted CAR T-cell response.21 No differences in the expression of CD107a, IL-2, IFN-γ, or TNF-α were observed between CLL- and HD-derived CAR T cells regardless of whether the target cells were JeKo-1 cells or primary CLL cells (Figure 2E-H). This suggests that CAR T cells generated from patients with CLL or HDs are equally hyporesponsive when stimulated with CLL cells.
Together, these results show that CLL-derived CAR T cells are functional but that effector functions, especially proliferation, is diminished when CLL cells are presented as target cells.
CD40 ligation of CLL cells alleviates CLL-mediated T-cell suppression in a contact-dependent manner
Results thus far imply that CLL T cells are not intrinsically dysfunctional but that they become suppressed in the presence of CLL cells. To better understand how CLL cells modulate T-cell activation, our next experiments were aimed at manipulating the CLL cells before coculture with T cells. CD40 stimulation of CLL cells is a well-known system to induce the expression of costimulatory ligands, adhesion molecules, and major histocompatibility complex (MHC)–class molecules on CLL cells,21,29-31 thereby improving the APC function of CLL cells. Because T-cell activation in the presence of CD40-activated CLL cells is not impaired when compared with activation in presence of resting CLL cells,21,30 we compared resting vs CD40-activated CLL cells to decipher which CLL-derived factor(s) affects T-cell function upon activation.
CLL cells that were primed with CD40 stimulation by coculture with CD40L-expressing fibroblasts improved autologous T-cell activation, as measured by CD25, to the level of HD T cells (Figure 3A). In addition, the previously demonstrated delayed activation of T cells in the presence of CLL cells was not observed upon coculturing with CD40-stimulated CLL cells (n = 4-6; Figure 3B). Restored T-cell activation occurred only when direct cell contact between CD40-primed CLL cells and T cells was possible and was not observed when CD40-primed CLL cells and T cells were physically separated by a porous membrane (Figure 3C; supplemental Figure 2). These results indicate that CLL cells that are activated through the CD40-CD40L axis lose the ability to suppress T-cell activation, which requires direct cell contact between the CD40-stimulated CLL cells and T cells.
CD40 ligation of CLL cells alleviates CLL-mediated T-cell suppression in a contact-dependent manner. PBMCs derived from patients with CLL were thawed and cultured for 2 days on a layer of CD40L expressing fibroblasts. Two days after CD40 stimulation, CLL cells were harvested and fresh PBMCs derived from patients with CLL and HDs were thawed, CTV labeled and cocultured with or without the CD40-stimulated CLL cells in a 1:1 ratio. (A) T cells in presence or absence of CD40-stimulated CLL cells were stimulated for 2 days with soluble stimulatory CD3/28 antibodies, after which expression of CD25 was measured (HD, n = 4; CLL, n = 3). (B) In a different experiment, PBMCs from HDs and patients with CLL were thawed and stimulated with CD3/28 antibodies in the presence of CD40L expressing fibroblasts. Fibroblasts were removed after 24 hours, and T-cell activation (CD25) was analyzed for 6 days (HD, n = 4; CLL, n = 6). (C) To analyze cell-cell contact dependency, PBMCs derived from patients with CLL were first cultured on a layer of CD40L-expressing fibroblasts and harvested after 2 days and were plated either in the transwell insert or not with autologous PBMCs (schematic overview: supplemental Figure 2). After plating the cells in the transwell or together, T cells were stimulated for 2 days with CD3/28 antibodies after which CD25 expression was analyzed (HD, n = 10; CLL, n = 10). Red bars indicate T cells present from the start of the experiment and blue bars indicate T cells that were added during the transwell set up. P values were calculated using a 1-way ANOVA for panels A,C or a t test for panel B. The data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. Stim, stimulated.
CD40 ligation of CLL cells alleviates CLL-mediated T-cell suppression in a contact-dependent manner. PBMCs derived from patients with CLL were thawed and cultured for 2 days on a layer of CD40L expressing fibroblasts. Two days after CD40 stimulation, CLL cells were harvested and fresh PBMCs derived from patients with CLL and HDs were thawed, CTV labeled and cocultured with or without the CD40-stimulated CLL cells in a 1:1 ratio. (A) T cells in presence or absence of CD40-stimulated CLL cells were stimulated for 2 days with soluble stimulatory CD3/28 antibodies, after which expression of CD25 was measured (HD, n = 4; CLL, n = 3). (B) In a different experiment, PBMCs from HDs and patients with CLL were thawed and stimulated with CD3/28 antibodies in the presence of CD40L expressing fibroblasts. Fibroblasts were removed after 24 hours, and T-cell activation (CD25) was analyzed for 6 days (HD, n = 4; CLL, n = 6). (C) To analyze cell-cell contact dependency, PBMCs derived from patients with CLL were first cultured on a layer of CD40L-expressing fibroblasts and harvested after 2 days and were plated either in the transwell insert or not with autologous PBMCs (schematic overview: supplemental Figure 2). After plating the cells in the transwell or together, T cells were stimulated for 2 days with CD3/28 antibodies after which CD25 expression was analyzed (HD, n = 10; CLL, n = 10). Red bars indicate T cells present from the start of the experiment and blue bars indicate T cells that were added during the transwell set up. P values were calculated using a 1-way ANOVA for panels A,C or a t test for panel B. The data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. Stim, stimulated.
Dasatinib inhibits effects of CD40 ligation but does not restrict the expression of accessory molecules on CLL cells
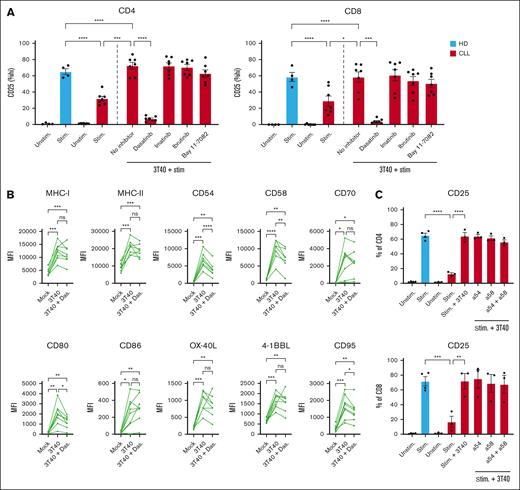
We earlier described the intertwined cross talk between CD40, cytokine, and B cell receptor (BCR) signaling in CLL in which case inhibition of key kinases (BTK, c-Abl, JAK, and SRC) differentially affected CD40-mediated functional alterations and signaling.20,31-33 To study which signaling cascade downstream of CD40 in CLL cells mediates improved T-cell activation, we added the kinase inhibitor dasatinib (1 μM/mL and 100 nM/mL) during coculture of CLL cells with CD40 ligand–expressing 3T3 cells. At these concentrations, dasatinib should not affect survival or CD40L expression of the fibroblast.32,33 After 2 days, CLL cells were harvested and, to exclude the direct effects of the drug on T cells,20,34,35 a thorough washing protocol was applied before coculture with T cells. Dasatinib targets multiple kinases reported to be activated in CLL cells upon interaction with the microenvironment, including SRC family members, Tyrosine-protein kinase ABL1 (c-Abl), Lck/Yes novel tyrosine kinase (LYN), BTK, and downstream NF-kB,36 and inhibits the phenotypical changes of CLL cells upon CD40 activation, including the acquisition of an antiapoptotic profile.33,37,38 Treatment of CLL cells with dasatinib during CD40 stimulation of the CLL cells completely reversed the effects of enhanced T-cell activation in the subsequent coculture seen during coculture with untreated CD40-activated CLL cells (Figure 4A; supplemental Figure 3A). We next tested whether more specific kinase inhibitors had similar effects as dasatinib. Treatment of CLL cells with either imatinib (c-abl; 1 μM/mL),33 ibrutinib (BTK; 1 μM/mL), or the IkappaB kinase (IKK) inhibitor Bay 11-7082 (0.1 μM/mL) during CD40 stimulation of the CLL cells did not lead to reversal of the enhanced T-cell activation seen during subsequent coculture with CD40-activated CLL cells (Figure 4A).
Dasatinib inhibits the effects of CD40 ligation but does not restrict the expression of accessory molecules on CLL cells. (A) PBMCs from patients with CLL were first cultured on a layer of CD40L expressing fibroblasts with or without dasatinib (1 μM/mL), imatinib (1 μM/mL), ibrutinib (1 μM/mL), or Bay 11-7082 (0.1 μM/mL) for 2 days, after which the CLL cells were thoroughly washed to remove the inhibitors. The untreated and treated CLL cells were resuspended in fresh media and cultured in a 1:1 ratio with autologous PBMCs for 2 days with soluble stimulatory CD3/CD28 antibodies after which expression of CD25 was measured on T cells (HD, n = 4; CLL, n = 7). In a similar experiment, expression of (B) MHC-I, MHC-II, CD54, CD58, CD70, CD80, CD86, 4-1BBL, OX40L, and CD95 were measured on CLL cells after incubation with 1 μM dasatinib for 2 days using the 3T40 system (CLL, n = 8). (C) Before culturing CD40-stimulated CLL cells with autologous T cells, CLL cells were preincubated for 1 hour with CD54 and CD58 blocking antibodies after which T cells were stimulated for 2 days and analyzed for expression of CD25 (HD, n = 4; CLL, n = 2-3). P values were calculated using a 1-way ANOVA for panels A-C. Data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. MFI, mean fluorescence intensity; Stim, stimulated.
Dasatinib inhibits the effects of CD40 ligation but does not restrict the expression of accessory molecules on CLL cells. (A) PBMCs from patients with CLL were first cultured on a layer of CD40L expressing fibroblasts with or without dasatinib (1 μM/mL), imatinib (1 μM/mL), ibrutinib (1 μM/mL), or Bay 11-7082 (0.1 μM/mL) for 2 days, after which the CLL cells were thoroughly washed to remove the inhibitors. The untreated and treated CLL cells were resuspended in fresh media and cultured in a 1:1 ratio with autologous PBMCs for 2 days with soluble stimulatory CD3/CD28 antibodies after which expression of CD25 was measured on T cells (HD, n = 4; CLL, n = 7). In a similar experiment, expression of (B) MHC-I, MHC-II, CD54, CD58, CD70, CD80, CD86, 4-1BBL, OX40L, and CD95 were measured on CLL cells after incubation with 1 μM dasatinib for 2 days using the 3T40 system (CLL, n = 8). (C) Before culturing CD40-stimulated CLL cells with autologous T cells, CLL cells were preincubated for 1 hour with CD54 and CD58 blocking antibodies after which T cells were stimulated for 2 days and analyzed for expression of CD25 (HD, n = 4; CLL, n = 2-3). P values were calculated using a 1-way ANOVA for panels A-C. Data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. MFI, mean fluorescence intensity; Stim, stimulated.
Dasatinib blocked the effects of CD40 stimulation at 1 μM and also at the more clinical relevant 100 nM concentration39 (supplemental Figure 3A). Various membrane-bound costimulatory and adhesion molecules that are strongly enhanced by CD40 stimulation were reduced by dasatinib treatment, such as CD95, CD54, CD58, and CD80 (Figure 4B). However, the CD40-mediated enhanced expression of MHC-I, MHC-II, CD70, CD86, OX-40L, or 4-1BBL was not affected by dasatinib. No differences in the expression of PD-L1 were observed after CD40 stimulation with or without dasatinib (supplemental Figure 3B).
Because the adhesion molecules CD54 (ICAM-1; Intercellular Adhesion Molecule 1) and CD58 (LFA-3; lymphocyte function-associated antigen 3) were significantly affected by dasatinib and are described in literature to play a role in CLL–T-cell cross talk,20,40 we questioned whether these molecules could be involved in the enhanced T-cell activation by CD40-primed CLL cells. These molecules were efficiently blocked by antibodies (supplemental Figure 3C),41,42 however, blocking did not lead to the inhibition of T-cell activation in the 3T40 system (Figure 4C). CD28 ligation is instrumental for proper HD- and CLL-derived T-cell activation.43,44 Although CD40-mediated expression of CD80 (the ligand of CD28) on CLL cells was also affected by dasatinib (Figure 4B), we argued that the lack of CD28 ligation was not the main driver of the inability of T cells to fully become activated in the presence of dasatinib-treated CD40–stimulated CLL cells because abundant activating αCD28 antibodies were added.
These data showed that enhanced costimulation or adhesion-ligand interactions were not the sole reasons for restored T-cell function upon coculture with CD40-activated vs resting CLL cells and suggest that mechanisms other than the poor APC function of CLL cells underlie the CLL-mediated T-cell dysfunction.
Transcriptome analysis reveals expression of CD24 and CD52 as drivers of T-cell dysfunction
To investigate the impact of CD40 stimulation and the influence of dasatinib in CLL cells at the transcriptional level in an unbiased manner, RNA sequencing was performed on CD40-stimulated CLL cells treated with or without dasatinib (100 nM) for 2 days. Widespread transcriptional changes were observed upon CD40 stimulation by culture on 3T40 fibroblasts when compared with culturing with control 3T3 fibroblasts (Figure 5A), which is in line with previous findings.45-47 A comparison of CD40-stimulated CLL cells treated with or without dasatinib led to the identification of 807 differentially expressed genes (Figure 5B). We were specifically interested in the inversely correlated genes, for example, genes that were either increased upon CD40-stimulation and decreased after dasatinib treatment or vice versa. This analysis led to the identification of 666 genes. Because we determined earlier that improved T-cell activation by CD40 activation was contact dependent (Figure 3C), only genes reported to be involved in direct cell-cell interaction were selected (supplemental Figure 4).48,49 This selection revealed that, among others, the expression levels of CD24 and CD52 (supplemental Figure 4) decreased after CD40 stimulation but not when CD40 stimulation occurred in presence of dasatinib (Figure 5C), which was confirmed at the protein level using flow cytometry (Figure 5D-E). Furthermore, we analyzed CD24 and CD52 membrane expression on resting CLL cells and healthy B cells and found elevated expression of CD24 but lower CD52 expression on CLL cells when compared with normal B cells of age-matched HDs (Figure 5D). Further analyses of CD24 expression on CLL cells revealed trends toward decreased variability in expression and increased intensity in patients with elevated total lymphocyte counts, suggesting a potential association between disease burden and CD24 expression patterns (supplemental Figure 4B-C).
RNA sequencing reveals expression of CD24 and CD52 as drivers of T-cell dysfunction. PBMCs from 4 patients with CLL were thawed and cultured on a layer of CD40L expressing fibroblasts for 2 days with or without 1 μM dasatinib. (A) Volcano plot depicting gene expression changes between CLL cells cultured on 3T40 vs those cultured on 3T3 (control). The black dots depict genes that were significantly differentially expressed (false discovery rate [FDR] <0.05; fold change >2), green dots represent known genes induced by CD40 signaling, and red dots are known genes that are down regulated after CD40 signaling (retrieved from Molecular Signatures Database; MSigDB [down = M1899 and up = M8493, CLL, n = 3-4]). (B) Volcano plot depicting gene expression changes between CLL cells cultured on 3T40 in the presence or absence of dasatinib. In black are genes that were significantly differentially expressed (FDR <0.05; fold change >2; CLL, n = 3-4). (C) Bar graph depicting the fold change of CD24 and CD52 transcription in CD40 stimulated CLL cells treated with or without dasatinib. (D) PBMCs from HDs or patients with CLL were cultured on a layer of mock or CD40L expressing fibroblasts for 2 days, and CD52 and CD24 expression was measured (HD, n = 4; CLL, n = 4). (E) PBMCs from patients with CLL were cultured on a layer of CD40L-expressing fibroblasts for 2 days with or without dasatinib, and CD24 and CD52 expression was measured (CLL, n = 4). (F) Expression of Siglec-10, CD24, and CD52 was measured on freshly thawed T cells from HD and patients with CLL ex vivo (HD, n = 4; CLL, n = 4). P values were calculated using a 1-way ANOVA (D) or a paired t test (E) or a Welch t test (F). Data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. MFI, mean fluorescence intensity.
RNA sequencing reveals expression of CD24 and CD52 as drivers of T-cell dysfunction. PBMCs from 4 patients with CLL were thawed and cultured on a layer of CD40L expressing fibroblasts for 2 days with or without 1 μM dasatinib. (A) Volcano plot depicting gene expression changes between CLL cells cultured on 3T40 vs those cultured on 3T3 (control). The black dots depict genes that were significantly differentially expressed (false discovery rate [FDR] <0.05; fold change >2), green dots represent known genes induced by CD40 signaling, and red dots are known genes that are down regulated after CD40 signaling (retrieved from Molecular Signatures Database; MSigDB [down = M1899 and up = M8493, CLL, n = 3-4]). (B) Volcano plot depicting gene expression changes between CLL cells cultured on 3T40 in the presence or absence of dasatinib. In black are genes that were significantly differentially expressed (FDR <0.05; fold change >2; CLL, n = 3-4). (C) Bar graph depicting the fold change of CD24 and CD52 transcription in CD40 stimulated CLL cells treated with or without dasatinib. (D) PBMCs from HDs or patients with CLL were cultured on a layer of mock or CD40L expressing fibroblasts for 2 days, and CD52 and CD24 expression was measured (HD, n = 4; CLL, n = 4). (E) PBMCs from patients with CLL were cultured on a layer of CD40L-expressing fibroblasts for 2 days with or without dasatinib, and CD24 and CD52 expression was measured (CLL, n = 4). (F) Expression of Siglec-10, CD24, and CD52 was measured on freshly thawed T cells from HD and patients with CLL ex vivo (HD, n = 4; CLL, n = 4). P values were calculated using a 1-way ANOVA (D) or a paired t test (E) or a Welch t test (F). Data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. MFI, mean fluorescence intensity.
CD52 and CD24, which are both expressed on CLL cells, bind to Siglec-10, a sialic acid–binding immunoglobulin-like lectin that recruits the Src homology region 2 domain-containing phosphatase-1 and 2 (SHP-1 and SHP-2) and thereby inhibits TCR signaling intracellularly.50-52 When analyzing the T cells, we found that Siglec-10 was elevated on CD8+ T cells in CLL samples. CD24 was not expressed by HD-derived T cells, whereas a small proportion of CLL T cells did express CD24 (Figure 5F). CD52 expression was comparable between CLL and HD samples (Figure 5F).
In conclusion, transcriptome analysis identified CD24 and CD52 as potential ligands that interfere with T-cell function. Expression levels of these ligands on the surface of CLL cells can be modulated via CD40 stimulation, which makes them straightforward targets for intervention.
Inhibition of CD24 and CD52 signaling rescues T-cell function in CLL
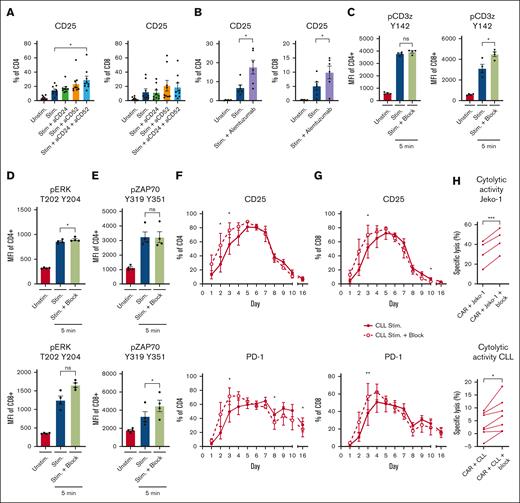
The interactions of CD24 and CD52 were blocked to test whether ligation of these molecules altered the suppressive effect of CLL on T-cell stimulation. Blocking CD24 and/or CD52 on PBMCs enhanced T-cell activation in the presence of CLL cells, which was specifically seen in CD4+ cells (Figure 6A-B; supplemental Figure 5A). Alemtuzumab, a clinically approved CD52 antibody for the treatment of CLL,53 similarly enhanced both CD4+ and CD8+ cell activation in patients with CLL (Figure 6B), but not in HDs (supplemental Figure 5A). In addition, we observed a trend toward increased phosphorylation of key kinases downstream of the TCR, such as CD3ζ, extracellular signal-regulated kinase (ERK), and Zeta-chain-associated protein kinase 70 (ZAP70), when blocking CD24 and CD52 in CLL-derived T cells (Figure 6C-E; supplemental Figure 5B-D). A time course similar to that described in Figure 1A showed that blockade of CD24 and CD52 normalized the kinetics of CD25 expression in the presence of CLL cells and expedited the transition to the resting phase (n = 3; Figure 6F-G). In addition, PD-1 expression levels were significantly lower on day 8 and 16 after blocking CD24 and CD52 in CLL-derived CD4+ T cells (Figure 6F). Spiking the cell culture media with CD24 and CD52 blocking antibodies every 2 days did not improve T-cell activation further (CD25, CD69, and CD71) or reduce PD-1 expression after cells returned to the resting phase (supplemental Figure 5E-H). Blocking CD24 and CD52 in the setting of CAR T-cell cocultures increased specific lysis of JeKo-1 cells and autologous CLL cells by CAR T cells from patients with CLL, indicating a therapeutic window (Figure 6H). From these experiments we can, however, not determine whether the blocking antibodies worked solely on the CLL cells or whether they also inhibited a cis-interaction on the T cells. Together, these results demonstrate that CD24 and CD52 can be exploited to benefit autologous-based therapies in CLL.
Inhibition of CD24 and CD52 signaling rescues T-cell function in CLL. (A-B) PBMCs from HDs and patients with CLL were thawed and preincubated for 1 hour with 1 μg/mL CD24 and 1 μg/mL CD52 antibodies (A) or alemtuzumab (1 μg/mL) (B) and subsequently stimulated with soluble stimulatory CD3/28 antibodies for 2 days. T cells were analyzed for expression of CD25 afterwards (n = 6-9). (C-E) CLL-derived T cells were stimulated with soluble CD3/28 antibodies in the presence or absence of CD24 and CD52 blocking antibodies. Five minutes after activation, the T cells were stained and analyzed for phosphorylation of CD3ζ (C; n = 4), ERK (D; n = 4), and ZAP70 (E; n = 4). (F-G) PBMCs from patients with CLL were cultured for 16 days in the presence of a single dose of CD3/28 antibodies with or without CD24 and CD52 blocking antibodies. During the culturing period, T-cell activation (CD25) and the expression of PD-1 was measured on CD4 (F) and CD8 (G) T cells. (H) 19BBζ CAR T cells were generated from T cells derived from patients with CLL and cocultured in a 1:1 ratio with either JeKo-1 cells or primary CLL cells, a single dose of blocking antibodies for CD24 and CD52 was added to the culture, and, after 1 day, the cell death of JeKo-1 cells or CLL cells was analyzed and specific lysis was calculated. P values were calculated using a t test for panels A,C,E, a paired t test for panel H, or a 1-way ANOVA for panel B. Data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001. MFI, mean fluorescence intensity.
Inhibition of CD24 and CD52 signaling rescues T-cell function in CLL. (A-B) PBMCs from HDs and patients with CLL were thawed and preincubated for 1 hour with 1 μg/mL CD24 and 1 μg/mL CD52 antibodies (A) or alemtuzumab (1 μg/mL) (B) and subsequently stimulated with soluble stimulatory CD3/28 antibodies for 2 days. T cells were analyzed for expression of CD25 afterwards (n = 6-9). (C-E) CLL-derived T cells were stimulated with soluble CD3/28 antibodies in the presence or absence of CD24 and CD52 blocking antibodies. Five minutes after activation, the T cells were stained and analyzed for phosphorylation of CD3ζ (C; n = 4), ERK (D; n = 4), and ZAP70 (E; n = 4). (F-G) PBMCs from patients with CLL were cultured for 16 days in the presence of a single dose of CD3/28 antibodies with or without CD24 and CD52 blocking antibodies. During the culturing period, T-cell activation (CD25) and the expression of PD-1 was measured on CD4 (F) and CD8 (G) T cells. (H) 19BBζ CAR T cells were generated from T cells derived from patients with CLL and cocultured in a 1:1 ratio with either JeKo-1 cells or primary CLL cells, a single dose of blocking antibodies for CD24 and CD52 was added to the culture, and, after 1 day, the cell death of JeKo-1 cells or CLL cells was analyzed and specific lysis was calculated. P values were calculated using a t test for panels A,C,E, a paired t test for panel H, or a 1-way ANOVA for panel B. Data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001. MFI, mean fluorescence intensity.
Discussion
Our current observations add new insights to T-cell dysfunction and its potential reversal in CLL. First, we showed that T-cell activation in CLL is not fully impaired but that it is delayed. Second, T-cell dysfunction in CLL-derived T cells is especially evident after restimulation. In addition, we showed that CLL-derived T cells transduced with a CD19BBζ CAR construct were able to execute effector functions and to proliferate. However, both proliferation and the killing capacity were abrogated in the presence of CLL cells, indicating a direct suppressive effect by CLL cells. Using the CD40 stimulation model with CLL cells revealed the mechanisms behind the diminished suppressive effects on T cells and specifically highlighted the potential role of the CD24-CD52 regulatory axis. Inhibition of CD24 and/or CD52 ligation in CLL–T-cell cultures improved T-cell activation over time, thereby offering potential targets for future therapeutic strategies.
Our finding that CLL-derived T cells transduced with a CD19BBζ CAR construct can proliferate and kill target cells but that the presence of CLL cells abrogates these key effector functions is clinically relevant. CAR T-cell therapy in CLL has been tested primarily in relapsed and refractory disease settings with overall response rates close to 50% and complete response rates below 20%. Achieving a complete response is crucial for meaningful response durations,54 thereby necessitating improved efficacy. The underperformance of T cells in active disease is well documented with allogeneic stem cell transplantation studies in CLL showing a negative correlation between disease burden at transplant and the outcomes.55,56 Enhancing CAR T-cell efficacy may involve altering the therapy setting or mitigating the inhibitory effects of CLL cells on autologous T cells. Currently, CAR T-cell therapy is mainly explored during the induction phase of refractory disease. Efficacy might increase if used as consolidation treatment after induction with targeted agents like BTK inhibitors and venetoclax, which have been shown to improve T-cell function.57
It has been well established that T-cell activation in the presence of CD40-stimulated CLL cells enhances T-cell activation when compared with coculture with resting CLL cells.21,30 Manipulation of CLL cells to increase their APC capacity has been studied widely. Previous studies demonstrated that CD40 stimulation of CLL cells increases the expression of costimulatory molecules.21,22,58 In the pre-CAR era, this concept was tested in the clinic by infusions of autologous CLL cells that were ex vivo transduced with an adenovirus expressing the CD40 ligand gene. This led to the expansion of CLL-reactive T cells, which killed and reduced the CLL cell numbers.59,60 A potential disadvantage of adopting CD40 stimulation of CLL cells in the therapeutic setting is that CD40 ligation also increases resistance to apoptosis through the induction of expression of antiapoptotic proteins, such as Myeloid cell leukemia-1 (MCL1) and B-cell lymphoma-extra large (BCL-xL),61,62 which might counteract a potentially increased killing capacity of the CLL-derived T cells. Despite this, CAR T cells are capable of killing CD40-stimulated CLL cells even more efficiently than non-CD40 stimulated CLL.21
Our data argue against the lack of adequate costimulation of CLL cells as the main and foremost cause of impaired T-cell function in this disease.11,21,63 First, isolated CLL-derived T cells responded well to stimulation without the need for bystander cells to express costimulatory ligands.11 Second, T-cell activation in this study combined both CD3 and CD28 ligation, which did not improve CLL T-cell activation whenever CLL cells were present in culture. In addition, although CD40-stimulated CLL cells improved T-cell activation, blocking CD54 and CD58 on CD40-stimulated CLL cells did not lead to the reversal of improved T-cell activation. It could therefore be that in addition to increased expression of costimulatory molecules, suppression of CD24 and CD52 are instrumental in normalizing T-cell function in CLL upon coculture with CD40 stimulated CLL cells.
We used dasatinib and more specific inhibitors to block CD40-mediated signaling in CLL cells before coculture with autologous T cells. This enabled us to study the potentially relevant changes in CLL cells that may underlie enhanced T-cell activation in the presence of these CLL cells. Because dasatinib, but not the inhibitors of BTK, c-Abl, or NF-kB, reversed enhanced T-cell activation in the presence of CD40-stimulated CLL cells, these data suggest a role for SRC kinase in this effect, although we cannot rule out that other dasatinib-sensitive kinases are at play.64 Although we did not study direct effects of dasatinib on acquired T-cell dysfunction in the setting of CLL, it is interesting to note that dasatinib also has direct immune regulatory activity on T cells, which led to studies that used in vitro dasatinib treatment to improve memory formation and longevity in CAR T cells.65-68
In healthy T cells, recognition of MHC-class proteins by the TCR leads to phosphorylation of SRC family kinases, such as CD3ζ and ZAP-70, which subsequently leads to T-cell activation.69 CD24 and CD52 can modulate immunity by interacting with Siglec-10 on immune cells, a mechanism exploited in solid oncology settings.50-52 Siglec-10 recruits phosphatases like SHP1 via immunoreceptor tyrosine–based inhibitory motif domains,70 thereby causing dephosphorylation of ZAP-70 and Lck, which impairs T-cell activation.50,70 High phosphatase activity in CLL-derived T cells may raise the activation threshold, thereby explaining the delayed T-cell activation in CLL. Although we did not directly link Siglec-10 interactions with CD24 or CD52 to dampened TCR signaling, our data suggest increased phosphorylation in CLL T cells when CD24 and CD52 are blocked. It is debated if Siglec ligands can inhibit immune cells in cis,71 but we have shown that T-cell function is restored in the absence of CLL cells, making cis-interaction less likely.
Our findings suggest that CD24 and CD52 represent promising therapeutic targets in CLL. Although intervention with blocking antibodies enhanced CLL cell killing in CAR T-cell coculture experiments, complete cytolysis was not achieved. In vivo studies are warranted to definitively evaluate the clinical potential of this approach and to validate its therapeutic efficacy in a physiological context.
Targeting CD52 to treat CLL has been suggested in the past. Treatment with the CD52 antibody alemtuzumab was able to deplete CLL cells from the blood in 95% of the patients.53 However, targeting CD52 in vivo also depleted T cells and is therefore unsuitable to combine with autologous-based therapies.72,73 Alternatively, CAR vectors can be designed to simultaneous allow downmodulation of Siglec-10 via small interfering RNA and expression of the CAR itself on T cells, making these CAR T cells resistant to interactions with CD24 and CD52 on CLL cells. Lastly, CD24 has already been suggested as a targetable molecule in the context of cancer immunotherapy.74
In conclusion, these new insights demonstrate that T-cell dysfunction is reversible via CD40 stimulation of CLL cells, which leads to reduced expression of CD24 and CD52 on the CLL cells. Therefore, CD24 and CD52 may be interesting targets for therapy regarding autologous-based therapies in CLL and possibly including other hematologic malignancies as well.
Acknowledgments
The authors thank the patients and healthy donors for their blood donations. The authors also thank Julie Dubois, Bianca van Driel, Dennis de Rooij, Ilse Ligtenberg, and Inoka Twickler from the Amsterdam B-cell biobank.
This work was supported by European Research Council Consolidator grants (BOOTCAMP [864815] and Lymph and Co [2018-LYCo-008]).
The figures were created with BioRender.com.
Authorship
Contributions: J.A.C.v.B., F.S.P., G.J.W.v.d.W., E.E., and A.P.K. designed the research; J.A.C.v.B., F.S.P., M.M., J.M.R., E.C., G.C., and R.v.K. conducted the research; J.A.C.v.B., F.S.P., P.D.M., and A.J. analyzed the data; and J.A.C.v.B., F.S. P., J.J.M., E.E., and A.P.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arnon P. Kater, Amsterdam University Medical Centers, de Boelelaan 1117, 1081HV, Amsterdam, The Netherlands; email: a.p.kater@amsterdamumc.nl.
References
Author notes
J.A.C.v.B. and F.S.P. are joint first authors.
RNA sequencing data have been deposited in the Gene Expression Omnibus database (accession numbers GSE201341 and GSE264554).
The full-text version of this article contains a data supplement.

![RNA sequencing reveals expression of CD24 and CD52 as drivers of T-cell dysfunction. PBMCs from 4 patients with CLL were thawed and cultured on a layer of CD40L expressing fibroblasts for 2 days with or without 1 μM dasatinib. (A) Volcano plot depicting gene expression changes between CLL cells cultured on 3T40 vs those cultured on 3T3 (control). The black dots depict genes that were significantly differentially expressed (false discovery rate [FDR] <0.05; fold change >2), green dots represent known genes induced by CD40 signaling, and red dots are known genes that are down regulated after CD40 signaling (retrieved from Molecular Signatures Database; MSigDB [down = M1899 and up = M8493, CLL, n = 3-4]). (B) Volcano plot depicting gene expression changes between CLL cells cultured on 3T40 in the presence or absence of dasatinib. In black are genes that were significantly differentially expressed (FDR <0.05; fold change >2; CLL, n = 3-4). (C) Bar graph depicting the fold change of CD24 and CD52 transcription in CD40 stimulated CLL cells treated with or without dasatinib. (D) PBMCs from HDs or patients with CLL were cultured on a layer of mock or CD40L expressing fibroblasts for 2 days, and CD52 and CD24 expression was measured (HD, n = 4; CLL, n = 4). (E) PBMCs from patients with CLL were cultured on a layer of CD40L-expressing fibroblasts for 2 days with or without dasatinib, and CD24 and CD52 expression was measured (CLL, n = 4). (F) Expression of Siglec-10, CD24, and CD52 was measured on freshly thawed T cells from HD and patients with CLL ex vivo (HD, n = 4; CLL, n = 4). P values were calculated using a 1-way ANOVA (D) or a paired t test (E) or a Welch t test (F). Data are presented as mean ± SEM; ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, ∗∗∗∗P ≤ .0001. MFI, mean fluorescence intensity.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/17/10.1182_bloodadvances.2023011934/2/m_blooda_adv-2023-011934-gr5.jpeg?Expires=1767699456&Signature=ZM4UFvEkc1edpfy3WeEktZTV65eQFO3OHTrKHIMfhCGhvAqX0hm-dZQxvt6B1mvWim-1QSHyH0SqiVZBC35j-WC2ItxjBo6NO6AS33ITQnsfpoygUxjRRlsS5xsg95-VTTRZ0ghcFGmPwCL3fGZyUHvCMBNeVQ8xhLlu1PygBWrQ65aqnijRI1FADBDkpReJMtlcrHGFxQAPqthuMecO4hOczfjB5KKdSW0a0eE8bVvlLtsJxZT01Km4wyHKwth5SBt7EBXyQES8mVbUQguQY5wsBjaWLv5KDgYNVuwxgobgj5jvlgGZDP5M4oVGpJOc-M-gFjtXU2LxQJ9Qn5DqJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)