TO THE EDITOR:
Fanconi anemia (FA) is a grievous inherited DNA repair disorder resulting in the inability to repair interstrand crosslinks.1,2 FA is caused by mutations in 1 of 23 different FA complementation (FANC) genes, with the most frequent mutations occurring in FANCA (∼60%-70%), FANCC (∼10%), and FANCG (∼10%).3 The increased chromosomal fragility in FA individuals leads to progressive bone marrow failure (BMF) and a systemic predisposition to malignancies. Almost all patients with FA develop BMF by young adulthood with ∼80% developing BMF by age 10 years.1,3 Additionally, all patients with FA have a high risk of developing acute myeloid leukemia and myelodysplastic syndrome throughout their lives.1,4 The only proven curative treatment for these hematologic diseases is allogeneic hematopoietic stem cell transplantation (HSCT), which is required for patients with FA who have developed BMF, myelodysplastic syndrome, and acute myeloid leukemia.5 However, the efficacy of postnatal HSCT is often complicated by the availability of HLA-matched donors, graft-versus-host disease (GVHD), and graft rejection.5 Further, the conditioning used pre-HSCT, which includes immune suppression and genotoxic chemotherapy and/or total body irradiation, poses significant comorbidity to patients with FA because it causes direct tissue injury leading to organ damage and infertility, predisposes patients to deadly infections, and increases likelihood of malignancies in these patients with hypersensitivity leading to incurable solid tumors in early adulthood in almost all patients after current HSCT.5-7 Autologous lentiviral HSC-based gene therapy is being developed as an alternative to postnatal allogeneic HSCT, which could enable preventive treatment of hematologic disease in patients with FA. However, this is challenged by the need for unique treatments for each FA complementation group, potential problematic lentiviral integration, continued risk of malignancies, and the significant resources required for personalized treatments.8,9 Early prenatal intervention via in utero (allogeneic) HSC transplantation (IUHSCT) into a FA gestational fetus may be an avenue to overcome limitations of postnatal HSCT and gene therapy: stabilizing hematopoiesis before the onset of hematologic symptoms and providing an effective intervention for all FA subtypes. Unique fetal tolerance of maternal antigens has also been observed in the prenatal setting partially due to deletion of alloreactive effector T cells.10-12 Thus, IUHSCT could potentially be conducted without any pre-HSCT conditioning, thereby eliminating current life-threatening treatment regimens.
Advances in prenatal diagnostic tools have enabled early diagnosis of many inherited diseases. Suspicion for FA in a developing fetus often arises based upon family history and/or detection of congenital abnormalities on ultrasound.13 Subsequently, FA can be verified by genetic sequencing and/or chromosomal breakage testing on fetal cells obtained by chorionic villus sampling or amniocentesis. Such diagnostic tests can be performed as early as 10-weeks’ gestation,13 before the optimal timing for IUHSCT.14 IUHSCT is most commonly conducted by transplantation of HSCs derived from donor bone marrow (BM) into the fetus through the umbilical vein via ultrasound guidance, and has been successfully performed in preclinical and clinical studies for immunodeficiencies and hemoglobinopathies without genotoxic myeloablation or immunosuppression.14,15 Despite high technical success and safety, clinical application of IUHSCT has been challenged to date due to limited engraftment below therapeutic levels for most diseases. This is likely due to donor HSC competition with the fetus’ HSCs, which cannot be overcome without HSC-ablative conditioning.16 However, in FA, we have shown that only minimal initial donor cell engraftment is necessary to stabilize the failing BM environment due to the competitive advantage of healthy cells over failing FA cells.8,17 Given this, we hypothesized that IUHSCT could stabilize the FA hematopoietic system without conventional postnatal HSCT toxicities, providing an alternative attractive early treatment option for patients with FA regardless of genotype. Specifically, IUHSCT could be a safer and easier option to prevent hematologic disease without the common complications of postnatal HSCT, including infections, mucositis, GVHD, infertility, secondary malignancies, and prolonged hospitalization/immune compromise.
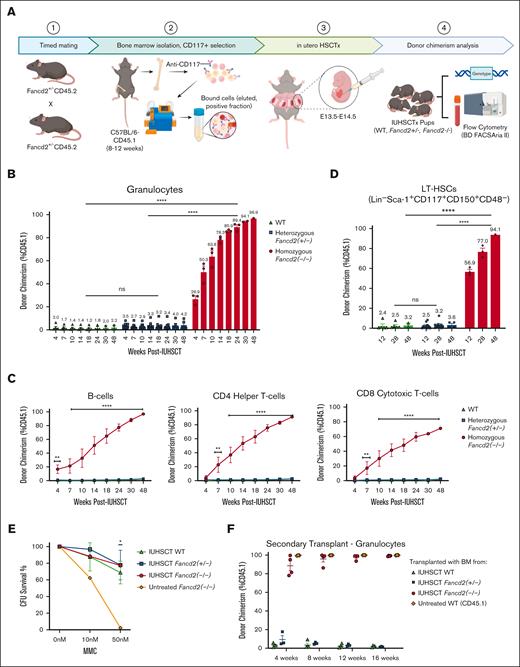
To test our hypothesis, IUHSCT was first performed in Fanconi complementation group D2 mice (Fancd2, CD45.2, mixed C57BL/6N-129/SvJ background).18,19 Due to infertility observed in Fancd2–/– mice, parental FA carriers (Fancd2+/−) were bred to generate timed FA fetuses with concurrent FA carrier and wildtype (WT) littermates. At E13.5-14.5, all fetuses were transplanted via intrahepatic injection with 1 × 106 CD117+ enriched HSCs from BM of minor-mismatched donor mice (WT, C57BL/6N-CD45.1STEM, CD45.1)20 with IUHSCT performed as previously detailed10 (Figure 1A; supplemental Figure 1). Importantly, in postnatal HSCT from similar allogeneic donors into these same Fancd2–/– mice, immunosuppression was found to be required for donor engraftment.17 After IUHSCT, peripheral blood was collected and donor chimerism (% CD45.1) was assessed by flow cytometry at periodic time points after birth (see supplemental Materials). At 4 weeks after IUHSCT with minor-mismatched donor BM–derived HSCs, Fancd2–/– mice already had significantly higher donor engraftment than heterozygous Fancd2+/− (P < .0001) and WT (P < .0001) littermates (Figure 1B; supplemental Figure 2). The average donor granulocyte chimerism was 26.9% in Fancd2–/– mice (n = 3), with only 3.5% and 2.0% in heterozygous Fancd2+/− (n = 10) and WT (n = 5) littermates, respectively.
Upon serial assessments, we excitingly observed further increasing donor chimerism, showcasing the competitive advantage of WT cells over FA cells with up to 96.9% donor chimerism (P = .0054) in granulocytes of Fancd2–/– mice 48 weeks after IUHSCT, whereas heterozygous Fancd2+/− (P = .6449) and WT (P = .8986) littermates had insignificant changes. Multilineage assessment showcased parallel increases in donor chimerism in all lineages over time with significant donor immune cell engraftment in both B-cell and T-cell compartments as well (Figure 1C). Additionally, the immune repertoire of IUHSCT-treated mice showed polyclonal repertoire in both T cells and B cells, which, after IUHSCT, more closely resembled that of untreated WT mice (supplemental Figure 3). Importantly, tissue histology at 48 weeks after IUHSCT showed no evidence of tissue inflammation or GVHD (supplemental Figure 4). To assess for true HSC engraftment, BM was also collected via aspirates at 12 and 28 weeks after IUHSCT, and terminal assessment was performed at 48 weeks after IUHSCT. Not surprisingly, the long-term (LT) HSC chimerism reflected similar robust engraftment in Fancd2–/– mice (P < .0001) that increased with time (Figure 1D; supplemental Figure 5). Although near-complete donor chimerism was observed after IUHSCT, in patients even minimal FA-cell correction across hematopoietic lineages, caused by somatic mosaicism, has been shown to be sufficient for FA disease stabilization and disease correction with resistance to DNA damage.21 Further, additional assessments were also performed on BM obtained at 48 weeks after IUHSCT, which showed resistance to DNA damaging agent mitomycin C (Figure 1E) and LT secondary reconstitution potential in lethally irradiated recipients (Figure 1F; supplemental Figure 6).
IUHSCT allows for robust donor engraftment in homozygous Fancd2–/– mice with WT competitive advantage over time. (A) Generation of Fancd2–/–, Fancd2+/−, and WT fetuses and in utero transplantation schema. Parental FA carriers (Fancd2+/− CD45.2) were mated to generate time-dated FA fetuses. BM from WT (CD45.1) mice was isolated and CD117 enriched by magnetic separation. At E13.5-14.5, fetuses were transplanted via intrahepatic injection with 1 × 106 CD117+ selected HSCs. Pups were genotyped and subsequently monitored (WT, n = 5; Fancd2+/−, n = 10; and Fancd2–/–, n = 3). Peripheral blood and BM donor chimerism was assessed by flow cytometry (% CD45.1) at various time points after IUHSCT. Multilineage donor chimerism was examined in (B) granulocytes and (C) lymphocytes, including B cells, CD4 helper T cells, and CD8 cytotoxic T cells. (D) LT-HSC chimerism was also measured. (E) HSC and progenitor functionality and resistance to DNA damaging agent mitomycin C was further assessed by CFU assays (n = 3) and (F) secondary transplantation in lethally irradiated WT mice (n = 4). Data represent mean ± standard error of the mean (SEM). Comparisons were performed using analysis of variance (ANOVA) with Tukey multiple comparison test, and a P value of <.05 was considered significant. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05. CFU, colony-forming unit; ns, nonsignificant.
IUHSCT allows for robust donor engraftment in homozygous Fancd2–/– mice with WT competitive advantage over time. (A) Generation of Fancd2–/–, Fancd2+/−, and WT fetuses and in utero transplantation schema. Parental FA carriers (Fancd2+/− CD45.2) were mated to generate time-dated FA fetuses. BM from WT (CD45.1) mice was isolated and CD117 enriched by magnetic separation. At E13.5-14.5, fetuses were transplanted via intrahepatic injection with 1 × 106 CD117+ selected HSCs. Pups were genotyped and subsequently monitored (WT, n = 5; Fancd2+/−, n = 10; and Fancd2–/–, n = 3). Peripheral blood and BM donor chimerism was assessed by flow cytometry (% CD45.1) at various time points after IUHSCT. Multilineage donor chimerism was examined in (B) granulocytes and (C) lymphocytes, including B cells, CD4 helper T cells, and CD8 cytotoxic T cells. (D) LT-HSC chimerism was also measured. (E) HSC and progenitor functionality and resistance to DNA damaging agent mitomycin C was further assessed by CFU assays (n = 3) and (F) secondary transplantation in lethally irradiated WT mice (n = 4). Data represent mean ± standard error of the mean (SEM). Comparisons were performed using analysis of variance (ANOVA) with Tukey multiple comparison test, and a P value of <.05 was considered significant. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05. CFU, colony-forming unit; ns, nonsignificant.
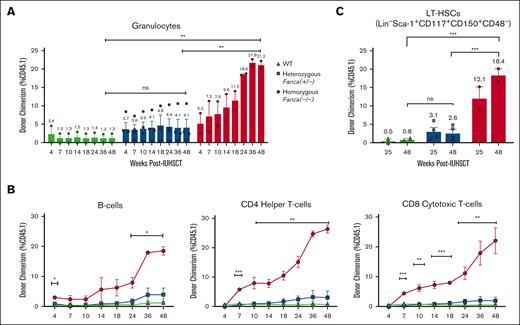
To explore whether similar findings could be observed across diverse FA complementation groups, including the most common FA subtype, we further conducted IUHSCT in Fanconi complementation group A mice (Fanca, C57BL/6J, CD45.2). FA fetuses were generated, transplanted, and analyzed as previously stated for Fancd2–/– mice. Similarly, homozygous Fanca–/– mice had more robust multilineage donor engraftment than heterozygous Fanca+/− (P = .0062) and WT (P = .0060) littermates with comparable increased donor granulocyte (Figure 2A) and lymphocyte chimerism (Figure 2B). BM was also collected via aspirate at 25 and 48 weeks after IUHSCT and analyzed for LT-HSC chimerism, which was similarly significantly increased in Fanca–/– mice (P = .007) compared to WT (Figure 2C). There appeared to be a slight trend toward increased engraftment in Fanca+/− over WT littermates, though this was not statistically significant, and additional experiments to increase the sample size were challenging to perform due to poor breeding efficiencies of these mice. Although robust engraftment was importantly observed in both Fanca–/– and Fancd2–/– mice, the increased engraftment in the Fancd2–/– model is likely due to the more severe hematopoietic phenotype previously noted in this FA subtype,19,22 which consequently results in a greater competitive advantage of the WT donor cells. However, further experimentation would be necessary to understand the clonality of the initial engraftment and changes over time.
IUHSCT similarly allows for robust donor engraftment in homozygous Fanca–/– mice with WT competitive advantage over time.Fanca–/–, Fanca+/−, and WT fetuses were generated as previously described for Fancd2–/– mice. At E13.5-14.5, fetuses were transplanted via intrahepatic injection with 1 × 106 CD117+ selected HSCs. Pups were genotyped and followed (WT, n = 2; Fanca+/−, n = 4; and Fanca–/–, n = 2). Peripheral blood and BM donor chimerism was assessed by flow cytometry (% CD45.1) at various time points after IUHSCT. (A) Multilineage donor chimerism was examined in (A) granulocytes and (B) lymphocytes, including B cells, CD4 helper T cells, and CD8 cytotoxic T cells. (C) LT-HSC chimerism was also determined. Data represent mean ± SEM. Comparisons were performed using ANOVA with Tukey multiple comparison test, and a P value of <.05 was considered significant. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05. ns, nonsignificant.
IUHSCT similarly allows for robust donor engraftment in homozygous Fanca–/– mice with WT competitive advantage over time.Fanca–/–, Fanca+/−, and WT fetuses were generated as previously described for Fancd2–/– mice. At E13.5-14.5, fetuses were transplanted via intrahepatic injection with 1 × 106 CD117+ selected HSCs. Pups were genotyped and followed (WT, n = 2; Fanca+/−, n = 4; and Fanca–/–, n = 2). Peripheral blood and BM donor chimerism was assessed by flow cytometry (% CD45.1) at various time points after IUHSCT. (A) Multilineage donor chimerism was examined in (A) granulocytes and (B) lymphocytes, including B cells, CD4 helper T cells, and CD8 cytotoxic T cells. (C) LT-HSC chimerism was also determined. Data represent mean ± SEM. Comparisons were performed using ANOVA with Tukey multiple comparison test, and a P value of <.05 was considered significant. ∗∗∗∗P < .0001; ∗∗∗P < .001; ∗∗P < .01; ∗P < .05. ns, nonsignificant.
Despite the relatively small sample size in the homozygous FA mice due to breeding challenges of these animals, our results in 2 separate FA mouse models show impressive ∼20% to 95% donor multilineage engraftment through unconditioned IUHSCT, highlighting that this approach could be an effective curative prenatal treatment for all subtypes of FA. Specifically, this could be a safe, 1-time outpatient procedure performed before birth to facilitate hematolymphoid stabilization without any immune suppression or genotoxic conditioning toxicities. Such an approach could become a preventive treatment for the hematologic and immune manifestations of FA, which could subsequently avert the need for other hematologic treatments such as postnatal HSCT, which currently has troubling short-term and LT side effects that dramatically limit health outcomes and life span for patients with FA. Although not tested directly in these studies, IUHSCT using haploidentical parental HSCs without conditioning has been tested in parallel efforts in both preclinical and clinical settings. Given our data showing the selective advantage of WT HSCs in FA settings, IUHSCT is likely to provide a curative treatment option to FA fetuses diagnosed in utero, which is now being developed by our group. Importantly, further headway into such therapies is also expected to enhance FA screening efforts and bring greater awareness of FA disease among the scientific and clinical community.
Experimental protocols involving animals were approved by the administrative panel on laboratory animal care (APLAC) at Stanford University School of Medicine (APLAC number 32459).
Acknowledgments: The authors thank Kenneth I. Weinberg, Stanford University, for his generous gift of the Fancd2–/– mice, which originated from Marcus Grompe at Oregon Health and Science University; Irving L. Weissman at Stanford University and Jinyi Xiang within his laboratory for their collaboration to generate the Fanca–/– mice; David Scadden at Massachusetts General Hospital/Harvard University for his generous gift of the donor C57BL/6N-CD45.1STEM mice; Amelia Scheck and Cynthia Klein at Stanford University for their help with laboratory management; and the Stanford University Stem Cell Institute Fluorescence-Activated Cell Sorting Core for flow cytometry access and assistance. Additionally, the authors also thank the Stanford University School of Medicine Dunlevie Maternal-Fetal Medicine Center for Discovery, Innovation and Clinical Impact and the laboratory of T.C.M. at University of California San Francisco, including Marisa Schwab and Maria Clarke within her laboratory, for their collaboration and guidance of this project.
This project was funded by a grant to A.D.C. from the Fanconi Cancer Foundation (formerly Fanconi Anemia Research Fund) and the Stanford University School of Medicine Dunlevie Maternal-Fetal Medicine Center for Discovery, Innovation and Clinical Impact.
Figures were created with BioRender.com and GraphPad.
Contribution: L.S., A.G., Y.J.B., Y.Y.E.-S., M.G.R., T.C.M., and A.D.C. conceptualized and designed the studies; L.S., H.W., M.D., C.D., and M.R.K. performed the research, collected results, and analyzed data; E.H. and K.H. managed animal colonies and provided technical assistance; J.H., W.P., M.S., and B.B. conducted immune repertoire analysis; L.S., M.G.R., T.C.M., M.R.K., and A.D.C. interpreted data; L.S. and A.D.C. wrote the manuscript; and A.D.C. provided mentorship to the first author.
Conflict-of-interest disclosure: A.D.C. discloses financial interests in the following entities working in the rare genetic disease space: Beam Therapeutics, Editas Medicine, GV, Inograft Biotherapeutics, Magenta Therapeutics, Prime Medicine, and Spotlight Therapeutics. M.G.R. is a cofounder and has equity in Tr1X Inc and serves on the board of directors of Atara Biotherapeutics and Cosmo Pharmaceuticals and receives compensation for those activities. T.C.M. has received grant funding from companies in the rare disease space, including Novartis, BioMarin, Biogen, and Ultragenyx. The remaining authors declare no competing financial interests.
Correspondence: Agnieszka D. Czechowicz, Stanford University School of Medicine, 240 Pasteur Dr, Biomedical Innovations Building, Rm 2351, Stanford, CA 94304; email: aneeshka@stanford.edu.
References
Author notes
Data that support the findings of this study are available on reasonable request from the corresponding author, Agnieszka D. Czechowicz (aneeshka@stanford.edu).
The full-text version of this article contains a data supplement.