TO THE EDITOR:
Marginal zone lymphomas (MZLs) make up 5% to 10% of non-Hodgkin lymphomas.1,2 In the United States, extranodal MZL (EMZL) is the most common subtype (61%), followed by nodal MZL (NMZL; 30%) and splenic MZL (SMZL; 9%), with an increased incidence across all subtypes with age.2,3 MZL is characterized by an indolent course with a characteristic clinical presentation by subtype. EMZL frequently presents with localized mucosa involvement at diagnosis. SMZL commonly involves the spleen, bone marrow, and peripheral blood and less frequently the lymph nodes.4 NMZL typically presents with lymphadenopathy, occasionally involving the bone marrow or peripheral blood, and without splenic or extranodal involvement.5 The overall survival (OS) of patients with MZL is favorable with a 5-year relative survival rate of 90%.2
In a subset of patients, MZL transforms to more aggressive lymphomas, most commonly to diffuse large B-cell lymphoma (DLBCL). Transformation has been shown to be an independent risk factor for a shortened OS.6 Failure to achieve complete response (CR) after initial treatment, elevated lactate dehydrogenase, and >4 nodal sites at the time of MZL diagnosis have been associated with histologic transformation to DLBCL.6-9
Rarely, MZL can also transform to Hodgkin lymphoma (HL).10,11 This event is poorly understood with fewer than 10 cases reported in the literature.11-15 Furthermore, whether this represents transformation of MZL or co-occurrence is unknown. In this study, we report, to our knowledge, 1 of the largest cohorts of MZL transformation to HL and elucidate the clinical characteristics of this rare event.
A total of 10 patients with transformation from MZL to HL were identified at the University of Miami (5 patients), the University of Iowa (1 patient), and the Mayo Clinic (4 patients). At the University of Miami, the cases were identified by search of an MZL database containing 757 cases of MZL diagnosed from June 1996 to July 2023. Of the 757 patients, 5 had biopsy-proven transformation to HL (0.66%). At the University of Iowa and the Mayo Clinic, data were collected from 2 sources, namely the Molecular Epidemiology Resource and the Mayo Lymphoma Database.16 The Molecular Epidemiology Resource prospectively enrolled and followed 529 cases of MZL diagnosed from 2002 to 2015, and 3 of those cases subsequently transformed to HL (0.56%). The Mayo Lymphoma Database contained 347 cases of MZL diagnosed between 1995 and 2002, and 2 of those cases transformed to HL (0.57%). The clinical characteristics were collected from electronic medical records. Staging was based on imaging, which included computed tomography scans, positron-emission tomography, and bone marrow biopsy as per the discretion of the treating physicians. The study was approved by the institutional review boards. All the biopsies were reviewed at the time of diagnosis by expert hematopathologists at each institution.
Ten patients with MZL who transformed to HL were identified, including 6 patients initially diagnosed with SMZL, 3 with EMZL, and 1 with NMZL. The clinical characteristics of the patients are summarized in Table 1. The median age at MZL diagnosis was 65 years (range, 25-75) with a male to female ratio of 1:4. At the time of MZL diagnosis, clinical presentations included B symptoms, splenomegaly, lymphocytosis, autoimmune hemolytic anemia, and angioedema (the latter in patients with SMZL). The stage at presentation ranged between I and IV with 2 patients with EMZL presenting at stage I and the others presenting at an advanced stage.
Clinical characteristics at the initial MZL diagnosis, relapse, and transformation
| Median age at MZL diagnosis (range) | 65 (25-75) |
| Male sex, n (%) | 2 (20) |
| Ethnicity, n (%) | |
| Hispanic White | 1 (10) |
| Non-Hispanic White | 7 (70) |
| Non-Hispanic Black | 2 (20) |
| Ann Arbor stage MZL, n (%) | |
| I-II | 2 (20) |
| III-IV | 8 (80) |
| Type of MZL at presentation, n (%) | |
| EMZL | 3 (30) |
| SMZL | 6 (60) |
| NMZL | 1 (10) |
| Location of EMZL of subtype, n (%) | |
| Eye | 2 (66%) |
| Lung | 1 (33%) |
| MZL-directed therapies received before HT, n (%) | |
| Splenectomy | 3 (30) |
| Rituximab | 3 (30) |
| Rituximab + splenectomy | 1 (10) |
| XRT | 2 (20) |
| XRT + tositumomab | 1 (10) |
| Response to initial MZL treatment, n (%) | |
| CR | 6 (60) |
| PR | 2 (20) |
| SD | 1 (10) |
| PD | 1 (10) |
| Type of MZL in patients with CR, n (%) | |
| EMZL | 1 (16.7) |
| SMZL | 4 (66.6) |
| NMZL | 1 (16.7) |
| Relapse of MZL, n (%) | 6 (60) |
| Stage at initial MZL diagnoses of relapsed patients, n (%) | |
| IE | 1 (16.7) |
| IV | 5 (83.3) |
| Treatment of relapsed patients, n (%) | |
| No therapy | 2 (33.3) |
| Rituximab | 2 (33.3) |
| R-CVP | 1 (16.7) |
| XRT + interferon | 1 (16.7) |
| Response to treatment of relapsed patients, n (%) | |
| CR | 2 (33.3) |
| PR | 1 (16.7) |
| Unknown | 3 (50) |
| Median time to transformation, mo (range) | 93 (8-146) |
| Median time to transformation by MZL subtype, mo (range) | |
| EMZL | 92 (8-122) |
| SMZL | 94.5 (8-146) |
| NMZL | 32 (n = 1) |
| Median age at time of transformation, y (range) | 70 (28-80) |
| Pathology at transformation, n (%) | |
| Classical HL | 10 (100%) |
| Ann arbor stage HL, n (%) | |
| I-II | 2 (20%) |
| III-IV | 8 (80%) |
| Treatment of HL, n (%) | |
| ABVD or BV-AVD | 5 (50) |
| RICE + BEAM and auto-HCT | 1 (10) |
| R-AVD + Pembro-GVD | 1 (10) |
| BV-CHOP | 2 (20) |
| Rituximab | 1 (10) |
| Lost to follow-up, n (%) | 2 (20) |
| CR after treatment, n (%) | 3 (37.5) |
| Deaths, n (%)∗ | 3 (37.5) |
| Median age at MZL diagnosis (range) | 65 (25-75) |
| Male sex, n (%) | 2 (20) |
| Ethnicity, n (%) | |
| Hispanic White | 1 (10) |
| Non-Hispanic White | 7 (70) |
| Non-Hispanic Black | 2 (20) |
| Ann Arbor stage MZL, n (%) | |
| I-II | 2 (20) |
| III-IV | 8 (80) |
| Type of MZL at presentation, n (%) | |
| EMZL | 3 (30) |
| SMZL | 6 (60) |
| NMZL | 1 (10) |
| Location of EMZL of subtype, n (%) | |
| Eye | 2 (66%) |
| Lung | 1 (33%) |
| MZL-directed therapies received before HT, n (%) | |
| Splenectomy | 3 (30) |
| Rituximab | 3 (30) |
| Rituximab + splenectomy | 1 (10) |
| XRT | 2 (20) |
| XRT + tositumomab | 1 (10) |
| Response to initial MZL treatment, n (%) | |
| CR | 6 (60) |
| PR | 2 (20) |
| SD | 1 (10) |
| PD | 1 (10) |
| Type of MZL in patients with CR, n (%) | |
| EMZL | 1 (16.7) |
| SMZL | 4 (66.6) |
| NMZL | 1 (16.7) |
| Relapse of MZL, n (%) | 6 (60) |
| Stage at initial MZL diagnoses of relapsed patients, n (%) | |
| IE | 1 (16.7) |
| IV | 5 (83.3) |
| Treatment of relapsed patients, n (%) | |
| No therapy | 2 (33.3) |
| Rituximab | 2 (33.3) |
| R-CVP | 1 (16.7) |
| XRT + interferon | 1 (16.7) |
| Response to treatment of relapsed patients, n (%) | |
| CR | 2 (33.3) |
| PR | 1 (16.7) |
| Unknown | 3 (50) |
| Median time to transformation, mo (range) | 93 (8-146) |
| Median time to transformation by MZL subtype, mo (range) | |
| EMZL | 92 (8-122) |
| SMZL | 94.5 (8-146) |
| NMZL | 32 (n = 1) |
| Median age at time of transformation, y (range) | 70 (28-80) |
| Pathology at transformation, n (%) | |
| Classical HL | 10 (100%) |
| Ann arbor stage HL, n (%) | |
| I-II | 2 (20%) |
| III-IV | 8 (80%) |
| Treatment of HL, n (%) | |
| ABVD or BV-AVD | 5 (50) |
| RICE + BEAM and auto-HCT | 1 (10) |
| R-AVD + Pembro-GVD | 1 (10) |
| BV-CHOP | 2 (20) |
| Rituximab | 1 (10) |
| Lost to follow-up, n (%) | 2 (20) |
| CR after treatment, n (%) | 3 (37.5) |
| Deaths, n (%)∗ | 3 (37.5) |
ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; Auto-HCT, autologous hematopoietic cell transplantation; BEAM, carmustine, etoposide, cytarabine, melphalan; BV-AVD, brentuximab vedotin, doxorubicin, vinblastine, dacarbazine; BV-CHOP, brentuximab vedotin, cyclophosphamide, doxorubicin, prednisolone; CR, complete response; IE, stage I extranodal; Pembro-GVD, pembrolizumab + gemcitabine, vinorelbine, and doxorubicin; PD, progressive disease; PR, partial response; R-AVD, rituximab, doxorubicin, vinblastine, dacarbazine; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone; RICE, rituximab, ifosfamide, carboplatin, etoposide; SD, stable disease; XRT, radiation.
All deaths were due to lymphoma.
Initial treatment varied by diagnosis and presentation. Patients with SMZL were treated with splenectomy (n = 3) or rituximab (n = 3), and 1 of the latter patients subsequently also underwent a splenectomy for resistant autoimmune hemolytic anemia. Patients with localized EMZL received radiation (n = 2, both stage I) and tositumomab (n = 1, multiple mucosal sites, including conjunctiva and lung). The patient with NMZL (n = 1) received radiation. Following initial treatment, CR was achieved in 6 patients, partial response in 2 (SMZL and EMZL treated with tositumomab), and stable or progressive disease each observed in 1 patient. Before transformation, 6 patients relapsed. Their characteristics, treatment of relapse, and response are summarized in Table 1.
Transformation to classical HL was confirmed by biopsy in all patients (Table 1) based on the presence of Hodgkin/Reed-Sternberg (HRS) cells in a polymorphous background, uniformly positive for CD30 and Paired box protein 5 (Pax5), most positive for CD15, and all negative for BOB1 and/or organic cation transporter 2 (OCT2) expression. CD20 was expressed in 2 cases, and Epstein-Barr virus (EBV)–encoded small RNA was detected in 1 of the 4 analyzed tumors. Composite MZL was not detected in any of these biopsies. The median time to transformation was 93 months (range, 8-146). The median age at the time of transformation was 70 years (range, 28-80), and 6 patients were in CR from their underlying MZL at that time. Most presented with lymphadenopathy with or without abdominal pain, shortness of breath, fatigue, night sweats, and chills. Two patients presented with early-stage HL (I-II) and 8 patients with advance stage (III-IV) disease. Unfortunately, at the time of this analysis, none of the patients had accessible paired biopsies to confirm the clonality relation between the MZL and classical HL.
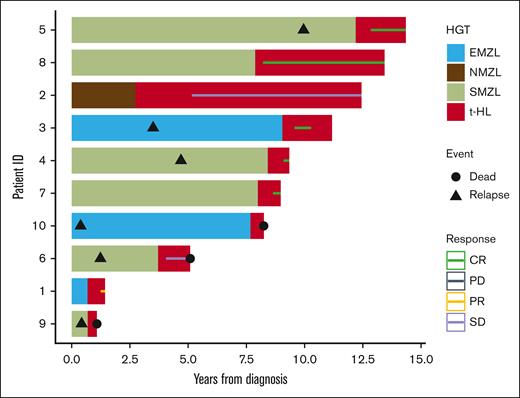
Treatment for HL transformation was given at the discretion of the treating physicians and are summarized in Table 1. Of note, 1 patient initially received single-agent rituximab because the patient’s biopsy was initially thought to be MZL, and later, upon review at the academic center, the diagnosis was changed to classical HL. Two patients were lost to follow-up; the median follow-up after transformation for remaining 8 patients was 62.5 months (range, 9-116). Three achieved CR following treatment with RICE (rituximab, ifosfamide, carboplatin, etoposide)/BEAM (carmustine, etoposide, cytarabine, melphalan) autologous hematopoietic cell transplantation (auto-HCT; n = 1), BV-AVD (brentuximab vedotin, doxorubicin, vinblastine, dacarbazine; n = 1), and ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine; n = 1). The median OS from the time of transformation was 12 months (range, 4.6-67) with median progression-free survival from transformation of 9.7 months (range, 2-67). The median OS from initial diagnosis was 102.5 months (range, 19-161). None of the patients had MZL relapse after transformation to HL. Five patients were alive and 3 were deceased (all 3 died due to lymphoma, depicted in the swimmer plot; Figure 1).2
Swimmer plot of clinical course of patients with MZL who transformed to classical HL.
Swimmer plot of clinical course of patients with MZL who transformed to classical HL.
MZL transformation to HL is rare, and data on this event are erratic. Among our patients, most (8/10) initially presented with advanced (stage III-IV) MZL disease and 6 of the patients relapsed before transformation. Of the 10 patients, 6 (60%) had SMZL at initial diagnosis, representing a higher incidence of SMZL than in the general MZL population (∼9%). In several studies, SMZL has been shown to have the highest risk for transformation to DLBCL of all MZL subtypes.17-19 A similar phenomenon is potentially applicable in the transformation to HL.
Transformation occurred within 10 years in most patients (90%) at a median of 93 months. This is similar to the reported transformation of MZL to DLBCL that occur within 10 years in 89% of patients and is similar to the transformation of chronic lymphocytic leukemia to HL with a median time of 5.5 years.17,20
At the time of transformation to HL, most patients presented with advanced disease. Three patients died, all due to lymphoma. One patient was treated with RICE, followed by BEAM and auto-HCT. Unfortunately, this patient was lost to follow-up, and it is unknown if salvage treatment, followed by BMT, may be a more efficient treatment.
To our knowledge, this is the largest reported cohort of transformation of MZL to HL. MZL transformation to HL was reported in only 1 of 13 transformation cases in a cohort of 340 patients with MZL (0.29%); the remaining 12 cases transformed to DLBCL.11 In a SMZL cohort of 105 patients, 36 had transformation, and only 1 of the 36 had transformation to HL (0.9%).14 There are also a few case reports, including transformation of laryngeal EMZL to HL and another of transformed SMZL to HL.12,15 The mechanism of transformation is unknown, and it was not evaluated in most cases if these lymphomas were clonally related, including in ours, likely because of a lack of paired tissue samples. In only 1 case report of SMZL transformation to classical HL, the paired tumors expressed immunoglobulin D, evaluated using immunohistochemistry. Because classical de novo HL lack immunoglobulin D expression, this finding may suggest clonal relations between the initial SMZL and the transformed HL.13 Given the absence of molecular clonality studies, it cannot be excluded that the cases of HL may be de novo primary malignancies. However, a previous report on 12 cases of composite MZL (mostly EMZL) and HL at diagnosis may suggest a common origin and/or shared biological triggers.21 Furthermore, it is possible that some but not all cases of MZL transformation to HL are clonally related. Indeed, in Richter transformation of chronic lymphocytic leukemia to HL, only 14 of the 33 analyzed cases (42%) were clonally related.22 Furthermore, in this study, 71% of HRS cells were positive for EBV, including in clonally related cases. Therefore, detection of EBV positivity in HRS cells in 1 of our tested cases does not indicate that this case was clonally unrelated. In a study of follicular lymphoma transformation to HL, polymerase chain reaction analysis showed t(14;18) in only 2 of 32 HL cases (6%), indicating a clonal relationship; however, this study showed expression of B-cell lymphoma 2 (BCL2) protein in 7 cases (22%).23
Therefore, many outstanding questions remain in terms of the biology of this rare event, including further investigation into genomic and clonality analyses. Although we and others did not demonstrate a definitive clonal link between MZL and HL, the latter occurs in patients with MZL like in other indolent B-cell lymphoproliferative disorders and is confirmed to be clonally related in a fraction of these cases. Overall, this report provides the most comprehensive clinical data on this event in MZL.
Acknowledgments: I.S.L. is supported by the National Cancer Institute (grant 1RO1CA233945-01), the Institute for Follicular Lymphoma Innovation, the Dwoskin and Anthony Rizzo Families Foundations, and the University of Miami Sylvester Comprehensive Cancer Center. This work was supported by the National Institutes of Health (NIH), National Cancer Institute (grant NIH U01 CA195568 [multiple authors]).
Contribution: I.S.L. conceptualized the study, collected and analyzed the data, and wrote the manuscript; and K.N.M., J.P.A., C.B., T.H., B.K.L., J.R.C., and J.F. collected and analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Izidore S. Lossos, Division of Hematology, Sylvester Comprehensive Cancer Center, University of Miami, 1475 NW 12th Ave (D8-4), Miami, FL 33136; email: ilossos@med.miami.edu.
References
Author notes
Data are not available, but specific questions regarding the data can be sent to the corresponding author, Izidore S. Lossos (ilossos@med.miami.edu).