Key Points
Luspatercept improved anemia in patients with MF with/without transfusion dependence, particularly those receiving ruxolitinib.
After 3 years of minimal follow-up, luspatercept safety profile was consistent with previous studies; no new safety signals identified.
Visual Abstract
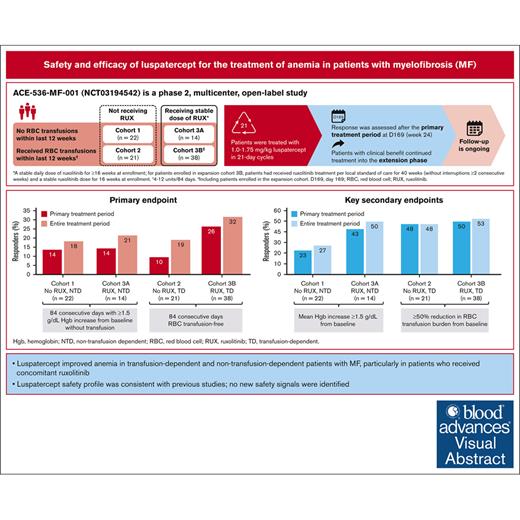
The ACE-536-MF-001 trial enrolled patients with myelofibrosis (n = 95) into 4 cohorts: patients in cohorts 1 and 3A were non–transfusion dependent (NTD) and had anemia; patients in cohorts 2 and 3B were transfusion dependent (TD); and patients in cohort 3A/3B had stable ruxolitinib treatment before and during the study. All patients received luspatercept (1.0-1.75 mg/kg, 21-day cycles). Treatment was extended if clinical benefit was observed at day 169. The primary end point was anemia response rate (NTD, ≥1.5 g/dL hemoglobin increase from baseline; TD, transfusion-independence) over any 12-week period during the primary treatment period (weeks 1-24). Overall, 14% of patients in cohorts 1 and 3A, 10% in cohort 2, and 26% in cohort 3B met the primary end point. In cohorts 1 and 3A (NTD), 27% and 50% of patients, respectively, had mean hemoglobin increase of ≥1.5 g/dL from baseline. Among TD patients, ∼50% had ≥50% reduction in transfusion burden. Reduction in total symptom score was observed in all cohorts, with the greatest response rate seen in cohort 3A. Overall, 94% of patients had ≥1 adverse event (AE); 47% had ≥1 treatment-related AE (TRAE; 11% grade ≥3), most frequently hypertension (18%), managed with medical intervention. One patient had a serious TRAE leading to luspatercept discontinuation. Nine patients died on treatment (unrelated to study drug). In most patients, ruxolitinib dose and spleen size remained stable. In patients with myelofibrosis, luspatercept improved anemia and transfusion burden across cohorts; the safety profile was consistent with previous studies. This trial was registered at www.ClinicalTrials.gov as #NCT03194542.
Introduction
Myelofibrosis (MF) is a life-threatening myeloproliferative neoplasm (MPN) characterized by excessive proliferation of clonal hematopoietic stem cells that leads to significant fibrosis in the bone marrow and progressively impaired red blood cell (RBC) production. MF can present de novo as primary MF (PMF) but can also develop from polycythemia vera or essential thrombocythemia. Patients often exhibit splenomegaly, moderate-to-severe anemia, thrombocytopenia, and constitutional symptoms.1-4
Approximately 40% of patients with MF have anemia at diagnosis, and nearly 25% are RBC transfusion dependent (TD); many will develop chronic anemia, and transfusion dependence typically increases during the disease course.5 Furthermore, patients who become RBC TD at any time have significantly worse survival compared with those who do not.6 One study reported median survival of 2.6 years in patients with PMF who received RBC transfusions at diagnosis compared with 8 years in those who did not (hazard ratio, 3.9; 95% confidence interval [CI], 2.5-6.1; P < .001).6 The Diagnostic International Prognostic Scoring System used in this trial investigates 5 risk factors, 1 of which is presence of anemia, to predict survival in patients with MF. The Diagnostic International Prognostic Scoring System Plus is a refined version of this metric that also compiles data on RBC transfusion dependence alongside presence of anemia.7
Current treatment options for MF-related anemia include erythropoiesis-stimulating agents (ESAs) and androgens, or alternatively, corticosteroids, thalidomide, and other immunomodulatory drugs.8 However, these agents are often associated with multiple adverse events (AEs) and toxicities, including increased infection risk resulting from corticosteroid use and myelosuppression with lenalidomide.8 Therefore, there is a need for therapeutic agents that are both highly effective in treating MF-related anemia and have minimal toxic effects. Poor management of anemia necessitates recurrent RBC transfusions that place a major burden on both patients and the health care system. Patients who are TD have significantly higher rates of hospitalization, emergency department visits, and outpatient visits compared with patients who are non–transfusion dependent (NTD).9 Effective management of MF-related anemia thus reduces transfusion burden and improves patient quality of life.10,11
Janus kinase inhibitors (JAKis), such as ruxolitinib and fedratinib, are among the recommended treatment options for patients with MF because they provide durable symptom relief and are generally well tolerated.12 However, 23% of patients who are JAKi naive treated with fedratinib and 38% of patients who are JAKi naive treated with ruxolitinib developed grade 3/4 anemia while on these treatments, and anemia is a leading cause of ruxolitinib discontinuation.13-15 Fedratinib therapy has also been associated with anemia in patients with MF previously treated with ruxolitinib.16 Despite the recent approval of additional JAKi therapies for the treatment of MF, such as pacritinib and momelotinib (less treatment-related anemia than other JAKis),17,18 there is still a need for further therapeutic options that help to manage MF-related anemia.
Luspatercept (ACE-536) is a first-in-class erythroid maturation agent that has been approved in the United States and Europe for the treatment of anemia in some adults with β-thalassemia and myelodysplastic syndrome (now referred to as myelodysplastic neoplasm per the 2022 World Health Organization criteria) who may require RBC transfusion.19-21 Luspatercept binds several tumor growth factor β superfamily ligands to diminish Smad2/3 signaling and enhance late-stage erythropoiesis.19,22 In recent clinical studies of patients with myelodysplastic syndromes and β-thalassemia, including in the phase 3 MEDALIST trial (www.clinicaltrials.gov identifier: NCT02631070), treatment with luspatercept resulted in transfusion independence in 38% of patients.23,24 Similarly, in the phase 3 COMMANDS trial (www.clinicaltrials.gov identifier: NCT03682536), patients who were ESA naive with myelodysplastic syndromes of very low risk, low risk, or intermediate risk who required RBC transfusions, transfusion independence for ≥12 weeks with a concurrent mean hemoglobin (Hb) increase of at least 1.5 g/dL was achieved in 86 (59%) patients treated with luspatercept. Similarly, in the BELIEVE trial (www.clinicaltrials.gov identifier: NCT02604433), which includes patients with β-thalassemia, a significantly greater percentage of patients in the luspatercept group (21.4%) compared with the placebo group (4.5%) had an erythroid response.24
Here, we report results from the phase 2, multicenter, open-label ACE-536-MF-001 trial (www.clinicaltrials.gov identifier: NCT03194542), which evaluated the efficacy and safety of luspatercept in patients with MPN-associated MF and anemia with and without both RBC transfusion dependence and concomitant JAKi treatment.
Methods
Ethics and consent
This trial was conducted in accordance with the ethical principles of the Declaration of Helsinki. The protocol, amendments, patient informed consent, and any other appropriate documents were approved by local institutional review boards or independent ethics committees. All participants provided informed consent.
Eligibility criteria and cohorts
Eligible patients were aged ≥18 years and had a diagnosis of MPN-associated MF (PMF, post–polycythemia vera MF, or post–essential thrombocythemia MF) as confirmed from the most recent local bone marrow biopsy report and according to World Health Organization 2016 criteria25; had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of ≤2; and anemia (supplemental Table 1). Patients were defined as TD (cohorts 2/3B) if they received 4 to 12 RBC units during the 12 weeks immediately preceding cycle 1 day 1 (C1D1), or NTD (cohorts 1/3A) if they had ≥3 Hb level measurements of ≤9.5 g/dL recorded on ≥3 different days with no RBC transfusions in the 12 weeks immediately preceding C1D1. Patients with peripheral blood myeloblast levels of ≥5% and patients who were taking hydroxyurea, androgens, corticosteroids, ESAs, or other drugs with potential effects on hematopoiesis within 16 weeks before enrollment were excluded.
Patients were assigned to 1 of 4 cohorts based on transfusion dependence and stable ruxolitinib treatment (supplemental Figures 1 and 2). Cohort 1/3A patients had anemia without transfusion dependence, whereas patients in cohorts 2/3B were TD. Patients in cohorts 1/2 did not receive ruxolitinib, whereas patients in cohorts 3A/3B received concomitant ruxolitinib. Cohort 3B was expanded because of early clinical benefit observed at the interim data cutoff (5 August 2019). Patients in cohort 3A/the initial cohort 3B were required to have received ≥16 weeks of a stable ruxolitinib dose immediately before enrollment; for the cohort 3B expansion, the protocol was amended to require ≥40 weeks of prior ruxolitinib treatment without interruptions exceeding 2 consecutive weeks and at least 16 weeks of stable ruxolitinib dose immediately before enrollment (supplemental Figures 1 and 2).
Treatments
During the primary treatment phase, all patients received subcutaneous luspatercept at a dose of 1.0 mg/kg with titration up to 1.75 mg/kg every 21 days for 24 weeks (1.33 mg/kg starting dose for expansion cohort; supplemental Table 2 for dose titration eligibility criteria). Disease response assessment occurred at day 169 (week 24, end of primary treatment period). If patients exhibited clinical benefit, they could continue treatment for ∼2 additional years or more (extension treatment period) until loss of response or if they experienced unacceptable toxicities, withdrew consent, or met any other treatment discontinuation criteria. Patients still receiving treatment (n = 7) or those still in the follow-up period (n = 3) at the time of trial completion are continuing treatment or follow-up in a rollover trial for at least 5 years from the first dose of luspatercept in the parent protocol, or 3 years after treatment from last dose, whichever occurs later.
End points
The primary end point was the proportion of patients who achieved anemia response during the primary treatment period (weeks 1-24). For NTD patients, anemia response was defined as 12 consecutive weeks with Hb increase of ≥1.5 g/dL from baseline without transfusion according to the Gale criteria.26 Anemia response for TD patients was defined as 12 consecutive weeks without RBC transfusion. Key secondary end points included mean Hb increase of ≥1.5 g/dL from baseline over any consecutive 12-week period without RBC transfusion (cohorts 1/3A) and ≥50% transfusion burden reduction (cohorts 2 and 3B). Additional secondary end points included anemia response during the entire treatment period (day 1 to end of treatment), time to anemia response (defined as time from first dose to first onset of anemia response during the primary treatment period), duration of anemia response (maximum duration of anemia response during the entire treatment period; assessed only for patients who achieved anemia response), frequency of RBC transfusions, and change in Hb levels from baseline.
Other secondary end points included ≥50% reduction in total symptom score or ≥50% reduction in fatigue symptom as measured by the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF). The MPN-SAF is a patient-reported outcome instrument for symptoms of MF including fatigue, splenomegaly and associated symptoms (including abdominal pain), catabolic/proliferative symptoms, and overall quality of life.27 Health-related quality of life was assessed via the mean changes in domain scores over the trial from baseline using the Functional Assessment of Cancer Therapy-Anemia scale and the European Quality of Life 5 Dimensions 5 Level.28,29 Both symptom response improvement and health-related quality of life were assessed during the primary and entire treatment periods.
Laboratory parameters
Clinical laboratory measures were assessed at screening, day 169, and at the end of treatment. Serum erythropoietin was measured either directly before RBC transfusion or 1 week after any transfusion with additional assessments on day 1 of every other cycle. On dosing days, serum ferritin samples were collected before luspatercept administration, with additional assessments on day 1 of every cycle up to end of week 24 and day 1 of every fourth cycle during the extension period.
Statistical analysis
Efficacy analyses were primarily conducted on the intent-to-treat population, consisting of all enrolled patients regardless of whether they received luspatercept. Target enrollment in cohorts 1/2 was 20 patients each, based on an estimate of 14 of 20 patients becoming efficacy evaluable. For cohorts 1/2, the probability of observing no responses among 14 patients was calculated as <.05 assuming a response probability of >20%. Target enrollment in cohort 3A was 14 patients, which would be sufficient to ensure the probability of observing no responses was <.05 if the true response probability were >20%, whereas target enrollment in cohort 3B was 46 patients based on 80% power to detect a 15% increase in response rate for luspatercept over a null response rate of 17%.30 This assumes a 1-sided z test of a binomial proportion (with a 5% significance level). CIs were presented as 2-sided 95% CIs unless specified differently in specific analyses. Safety analyses were performed on all patients who were enrolled and received at least 1 dose of luspatercept.
Results
Baseline demographics and disease characteristics
In total, 95 patients were enrolled at 21 sites across Europe and the United States (intent-to-treat population): cohort 1 (n = 22), cohort 2 (n = 21), cohort 3A (n = 14), and cohort 3B (n = 38; Table 1). Baseline demographics and clinical characteristics were similar across cohorts. Median patient age was 71 years (range, 50-89), and 61.1% of patients were male. Most (54.7%) patients had PMF, 75.8% had intermediate-2 risk score, and 74.7% were >2 years since initial diagnosis. At screening, 33.7% had an ECOG PS score of 0, 57.9% had ECOG PS score of 1, and 8.4% had an ECOG PS score of 2. Of the patients who were receiving ruxolitinib (cohorts 3A/3B), median duration of prior ruxolitinib therapy was 18.1 months (range, 5.1-150.6). In TD patients (cohorts 2/3B), 84.7% of patients had a baseline transfusion burden of 6-12 units per 12 weeks (Table 1).
Baseline demographics and disease characteristics in the intent-to-treat population
| . | Cohort 1 No RUX NTD (n = 22) . | Cohort 3A RUX NTD (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . | Total (N = 95) . |
|---|---|---|---|---|---|
| Age, y, median (min, max) | 69.5 (50, 89) | 65.0 (51, 81) | 75.0 (60, 88) | 72.0 (59, 83) | 71.0 (50, 89) |
| Male | 13 (59.1) | 7 (50.0) | 15 (71.4) | 23 (60.5) | 58 (61.1) |
| Race | |||||
| Asian | 2 (9.1) | 3 (21.4) | 1 (4.8) | 1 (2.6) | 7 (7.4) |
| Black or African American | 0 | 1 (7.1) | 0 | 2 (5.3) | 3 (3.2) |
| White | 16 (72.7) | 10 (71.4) | 18 (85.7) | 30 (78.9) | 74 (77.9) |
| Other | 1 (4.5) | 0 | 0 | 0 | 1 (1.1) |
| Not collected or reported | 3 (13.6) | 0 | 2 (9.5) | 5 (13.2) | 10 (10.5) |
| Ethnicity | |||||
| Hispanic or Latino | 2 (9.1) | 0 | 0 | 1 (2.6) | 3 (3.2) |
| Not Hispanic or Latino | 16 (72.7) | 14 (100.0) | 19 (90.5) | 32 (84.2) | 81 (85.3) |
| Unknown | 0 | 0 | 0 | 1 (2.6) | 1 (1.1) |
| Not reported | 4 (18.2) | 0 | 2 (9.5) | 4 (10.5) | 10 (10.5) |
| Disease diagnosis | |||||
| PMF | 15 (68.2) | 6 (42.9) | 13 (61.9) | 18 (47.4) | 52 (54.7) |
| Post-PV MF | 0 | 4 (28.6) | 2 (9.5) | 7 (18.4) | 13 (13.7) |
| Post-ET MF | 7 (31.8) | 4 (28.6) | 6 (28.6) | 13 (34.2) | 30 (31.6) |
| ECOG PS | |||||
| 0 | 9 (40.9) | 7 (50.0) | 3 (14.3) | 13 (34.2) | 32 (33.7) |
| 1 | 13 (59.1) | 7 (50.0) | 16 (76.2) | 19 (50.0) | 55 (57.9) |
| 2 | 0 | 0 | 2 (9.5) | 6 (15.8) | 8 (8.4) |
| Time since initial diagnosis to first screening | |||||
| ≤2 y | 10 (45.5) | 2 (14.3) | 6 (28.6) | 6 (15.8) | 24 (25.3) |
| >2 y | 12 (54.5) | 12 (85.7) | 15 (71.4) | 32 (84.2) | 71 (74.7) |
| Time on RUX before study, mo, median (min, max)∗ | — | 16.4 (5.1, 71.8) | — | 18.8 (6.7, 150.6) | 18.1 (5.1, 150.6) |
| DIPSS risk | |||||
| Intermediate-risk 1 | 1 (4.5) | 4 (28.6) | 1 (4.8) | 3 (7.9) | 9 (9.5) |
| Intermediate-risk 2 | 18 (81.8) | 9 (64.3) | 15 (71.4) | 30 (78.9) | 72 (75.8) |
| High-risk | 2 (9.1) | 1 (7.1) | 5 (23.8) | 5 (13.2) | 13 (13.7) |
| Unknown | 1 (4.5) | 0 | 0 | 0 | 1 (1.1) |
| Baseline transfusion burden per 12 wk† | |||||
| 4-5 units | — | — | 1 (4.8) | 6 (15.8) | 7 (11.9) |
| 6-12 units | — | — | 18 (85.7) | 32 (84.2) | 50 (84.7) |
| >12 units | — | — | 2 (9.5) | 0 | 2 (3.4) |
| Baseline hemoglobin levels (g/dL), median (min, max) | 8.7 (6.6, 9.7) | 8.3 (7.2, 9.2) | 7.3 (6.0, 9.5) | 8.2 (6.3, 9.9) | 8.3 (6.0, 9.9) |
| Baseline platelet categories (109/L) | |||||
| <100 | 6 (27.3) | 3 (21.4) | 8 (38.1) | 12 (31.6) | 29 (30.5) |
| 100-400 | 15 (68.2) | 10 (71.4) | 11 (52.4) | 24 (63.2) | 60 (63.2) |
| >400 | 1 (4.5) | 1 (7.1) | 2 (9.5) | 2 (5.3) | 6 (6.3) |
| Baseline serum erythropoietin (U/L), median (min, max) | 52.2 (7.0, 1996.6) | 157.5 (30.2, 2394.6) | 140.0 (15.9, 1594.4) | 164.6 (5.8, 1 977.2) | 133.3 (5.8, 2 394.6) |
| Baseline serum ferritin (ug/L), median (min, max) | 270.0 (27.0, 1456.7) | 136.7 (56.1, 1554.0) | 1168.2 (314.3, 4632.0) | 1 174.3 (170.2, 1 2189.0) | 759.8 (27.0, 1 2189.0) |
| Baseline spleen size,‡ cm, median (min, max)§ | 6.0 (1.0, 15.0) | 7.0 (1.0, 17.0) | 5.0 (2.0, 16.0) | 10.0 (0.0, 20.0) | — |
| . | Cohort 1 No RUX NTD (n = 22) . | Cohort 3A RUX NTD (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . | Total (N = 95) . |
|---|---|---|---|---|---|
| Age, y, median (min, max) | 69.5 (50, 89) | 65.0 (51, 81) | 75.0 (60, 88) | 72.0 (59, 83) | 71.0 (50, 89) |
| Male | 13 (59.1) | 7 (50.0) | 15 (71.4) | 23 (60.5) | 58 (61.1) |
| Race | |||||
| Asian | 2 (9.1) | 3 (21.4) | 1 (4.8) | 1 (2.6) | 7 (7.4) |
| Black or African American | 0 | 1 (7.1) | 0 | 2 (5.3) | 3 (3.2) |
| White | 16 (72.7) | 10 (71.4) | 18 (85.7) | 30 (78.9) | 74 (77.9) |
| Other | 1 (4.5) | 0 | 0 | 0 | 1 (1.1) |
| Not collected or reported | 3 (13.6) | 0 | 2 (9.5) | 5 (13.2) | 10 (10.5) |
| Ethnicity | |||||
| Hispanic or Latino | 2 (9.1) | 0 | 0 | 1 (2.6) | 3 (3.2) |
| Not Hispanic or Latino | 16 (72.7) | 14 (100.0) | 19 (90.5) | 32 (84.2) | 81 (85.3) |
| Unknown | 0 | 0 | 0 | 1 (2.6) | 1 (1.1) |
| Not reported | 4 (18.2) | 0 | 2 (9.5) | 4 (10.5) | 10 (10.5) |
| Disease diagnosis | |||||
| PMF | 15 (68.2) | 6 (42.9) | 13 (61.9) | 18 (47.4) | 52 (54.7) |
| Post-PV MF | 0 | 4 (28.6) | 2 (9.5) | 7 (18.4) | 13 (13.7) |
| Post-ET MF | 7 (31.8) | 4 (28.6) | 6 (28.6) | 13 (34.2) | 30 (31.6) |
| ECOG PS | |||||
| 0 | 9 (40.9) | 7 (50.0) | 3 (14.3) | 13 (34.2) | 32 (33.7) |
| 1 | 13 (59.1) | 7 (50.0) | 16 (76.2) | 19 (50.0) | 55 (57.9) |
| 2 | 0 | 0 | 2 (9.5) | 6 (15.8) | 8 (8.4) |
| Time since initial diagnosis to first screening | |||||
| ≤2 y | 10 (45.5) | 2 (14.3) | 6 (28.6) | 6 (15.8) | 24 (25.3) |
| >2 y | 12 (54.5) | 12 (85.7) | 15 (71.4) | 32 (84.2) | 71 (74.7) |
| Time on RUX before study, mo, median (min, max)∗ | — | 16.4 (5.1, 71.8) | — | 18.8 (6.7, 150.6) | 18.1 (5.1, 150.6) |
| DIPSS risk | |||||
| Intermediate-risk 1 | 1 (4.5) | 4 (28.6) | 1 (4.8) | 3 (7.9) | 9 (9.5) |
| Intermediate-risk 2 | 18 (81.8) | 9 (64.3) | 15 (71.4) | 30 (78.9) | 72 (75.8) |
| High-risk | 2 (9.1) | 1 (7.1) | 5 (23.8) | 5 (13.2) | 13 (13.7) |
| Unknown | 1 (4.5) | 0 | 0 | 0 | 1 (1.1) |
| Baseline transfusion burden per 12 wk† | |||||
| 4-5 units | — | — | 1 (4.8) | 6 (15.8) | 7 (11.9) |
| 6-12 units | — | — | 18 (85.7) | 32 (84.2) | 50 (84.7) |
| >12 units | — | — | 2 (9.5) | 0 | 2 (3.4) |
| Baseline hemoglobin levels (g/dL), median (min, max) | 8.7 (6.6, 9.7) | 8.3 (7.2, 9.2) | 7.3 (6.0, 9.5) | 8.2 (6.3, 9.9) | 8.3 (6.0, 9.9) |
| Baseline platelet categories (109/L) | |||||
| <100 | 6 (27.3) | 3 (21.4) | 8 (38.1) | 12 (31.6) | 29 (30.5) |
| 100-400 | 15 (68.2) | 10 (71.4) | 11 (52.4) | 24 (63.2) | 60 (63.2) |
| >400 | 1 (4.5) | 1 (7.1) | 2 (9.5) | 2 (5.3) | 6 (6.3) |
| Baseline serum erythropoietin (U/L), median (min, max) | 52.2 (7.0, 1996.6) | 157.5 (30.2, 2394.6) | 140.0 (15.9, 1594.4) | 164.6 (5.8, 1 977.2) | 133.3 (5.8, 2 394.6) |
| Baseline serum ferritin (ug/L), median (min, max) | 270.0 (27.0, 1456.7) | 136.7 (56.1, 1554.0) | 1168.2 (314.3, 4632.0) | 1 174.3 (170.2, 1 2189.0) | 759.8 (27.0, 1 2189.0) |
| Baseline spleen size,‡ cm, median (min, max)§ | 6.0 (1.0, 15.0) | 7.0 (1.0, 17.0) | 5.0 (2.0, 16.0) | 10.0 (0.0, 20.0) | — |
Baseline is defined as the last value on or before the first dose of luspatercept, or average if multiple values are present for the same date. Data are n (%) unless otherwise indicated.
DIPSS, Dynamic International Prognostic Scoring System; max, maximum; min, minimum; post-ET MF, post-essential thrombocythemia MF; post-PV MF, post-polycythemia vera MF; RUX, ruxolitinib.
Defined as sum durations of RUX on, or before, first luspatercept dose date, with overlapping periods excluded. Data not provided for cohort 1 and cohort 2 because these patients did not receive RUX treatment.
Data not provided for cohort 1 and cohort 3A because these cohorts comprised NTD patients, meaning they could not have received any RBC transfusions within the 12-week period immediately preceding C1D1.
Spleen length from the left costal margin.
Only patients with enlarged spleen included: cohort 1 (n = 16), cohort 3A (n = 12), cohort 2 (n = 15), and cohort 3B (n = 28). Spleen size change from baseline data were summarized by cohort only.
Disposition and treatment duration
At data cutoff (19 July 2022), 100% of patients discontinued treatment (Table 2). Primary reasons for discontinuation included lack of clinical benefit after completion of the primary treatment phase (29.5%), loss of response (13.7%), withdrawal by patient (12.6%), lack of efficacy (12.6%), death (8.4%), or occurrence of an AE (8.4%); 7 (7.4%) patients transferred to the long-term follow-up trial (Table 2).
Patient disposition
| . | Cohort 1 No RUX NTD (n = 22) . | Cohort 3A RUX NTD (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . | Total (N = 95) . |
|---|---|---|---|---|---|
| Patients who discontinued treatment | 22 (100) | 14 (100) | 21 (100) | 38 (100) | 95 (100) |
| Primary reason for treatment discontinuation | |||||
| Completed primary phase∗ | 8 (36.4) | 2 (14.3) | 10 (47.6) | 8 (21.1) | 28 (29.5) |
| Death | 1 (4.5) | 0 | 2 (9.5) | 5 (13.2) | 8 (8.4) |
| AE | 1 (4.5) | 0 | 2 (9.5) | 5 (13.2) | 8 (8.4) |
| Progressive disease | 1 (4.5) | 1 (7.1) | 0 | 1 (2.6) | 3 (3.2) |
| Withdrawal by patient | 2 (9.1) | 5 (35.7) | 1 (4.8) | 4 (10.5) | 12 (12.6) |
| Lack of efficacy | 2 (9.1) | 2 (14.3) | 2 (9.5) | 6 (15.8) | 12 (12.6) |
| Physician decision | 0 | 0 | 1 (4.8) | 0 | 1 (1.1) |
| Other: loss of response | 4 (18.2) | 3 (21.4) | 2 (9.5) | 4 (10.5) | 13 (13.7) |
| Other: rollover to LTFU | 1 (4.5) | 0 | 1 (4.8) | 5 (13.2) | 7 (7.4) |
| Other: blasts of >10% | 1 (4.5) | 1 (7.1) | 0 | 0 | 2 (2.1) |
| Other: received BMT | 1 (4.5) | 0 | 0 | 0 | 1 (1.1) |
| Patients continued to follow-up | 18 (81.8) | 11 (78.6) | 14 (66.7) | 20 (52.6) | 63 (66.3) |
| . | Cohort 1 No RUX NTD (n = 22) . | Cohort 3A RUX NTD (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . | Total (N = 95) . |
|---|---|---|---|---|---|
| Patients who discontinued treatment | 22 (100) | 14 (100) | 21 (100) | 38 (100) | 95 (100) |
| Primary reason for treatment discontinuation | |||||
| Completed primary phase∗ | 8 (36.4) | 2 (14.3) | 10 (47.6) | 8 (21.1) | 28 (29.5) |
| Death | 1 (4.5) | 0 | 2 (9.5) | 5 (13.2) | 8 (8.4) |
| AE | 1 (4.5) | 0 | 2 (9.5) | 5 (13.2) | 8 (8.4) |
| Progressive disease | 1 (4.5) | 1 (7.1) | 0 | 1 (2.6) | 3 (3.2) |
| Withdrawal by patient | 2 (9.1) | 5 (35.7) | 1 (4.8) | 4 (10.5) | 12 (12.6) |
| Lack of efficacy | 2 (9.1) | 2 (14.3) | 2 (9.5) | 6 (15.8) | 12 (12.6) |
| Physician decision | 0 | 0 | 1 (4.8) | 0 | 1 (1.1) |
| Other: loss of response | 4 (18.2) | 3 (21.4) | 2 (9.5) | 4 (10.5) | 13 (13.7) |
| Other: rollover to LTFU | 1 (4.5) | 0 | 1 (4.8) | 5 (13.2) | 7 (7.4) |
| Other: blasts of >10% | 1 (4.5) | 1 (7.1) | 0 | 0 | 2 (2.1) |
| Other: received BMT | 1 (4.5) | 0 | 0 | 0 | 1 (1.1) |
| Patients continued to follow-up | 18 (81.8) | 11 (78.6) | 14 (66.7) | 20 (52.6) | 63 (66.3) |
Data are n (%).
BMT, bone marrow transplant; LTFU, long term follow-up; RUX, ruxolitinib.
Completed primary treatment phase without clinical benefit.
In the safety population, median treatment duration was 24.0 weeks; most patients remained on luspatercept treatment up to the response assessment on day 169 (after 8 cycles of treatment). Patients who did not show benefit at this time point discontinued luspatercept treatment. As a result, all cohorts had a median of 8.0 doses per patient (supplemental Table 3 for cumulative luspatercept dose by cohort). Median length of cycles between doses was 21.0 days (range, 15-52) and 71 (74.7%) patients received the maximum dose of 1.75 mg/kg luspatercept.
Efficacy
During the primary treatment period (weeks 1-24), the anemia response rate (primary end point) in NTD patients was 13.6% in cohort 1 and 14.3% in cohort 3A. The entire treatment period spanned day 1 through to treatment end (treatment end varied among patients, with the duration of follow-up averaging 2 years for patients with clinical benefit); during this period, anemia response was 18.2% and 21.4% in cohort 1 and cohort 3A, respectively (Figure 1). Among TD patients, the anemia response rate in the primary treatment period was 9.5% in cohort 2 and 26.3% in cohort 3B (Figure 1), and 19.0% and 31.6%, respectively, in the entire treatment period.
Primary and key secondary end points across all cohorts in the primary and entire treatment periods. TD patients: anemia response defined as 12 consecutive weeks without RBC transfusion; NTD patients: anemia response defined as 12 consecutive weeks with ≥1.5 g/dL Hb increase from baseline without transfusion. Values above bars show % patients; top panel shows % patients (95% CI). RUX, ruxolitinib.
Primary and key secondary end points across all cohorts in the primary and entire treatment periods. TD patients: anemia response defined as 12 consecutive weeks without RBC transfusion; NTD patients: anemia response defined as 12 consecutive weeks with ≥1.5 g/dL Hb increase from baseline without transfusion. Values above bars show % patients; top panel shows % patients (95% CI). RUX, ruxolitinib.
Median time to anemia response was 63.0 days (range, 41-68) and 63.5 days (range, 42-85) in cohort 1 and cohort 3A, respectively (Table 3). Among TD patients, 2 patients in cohort 2 became NTD starting 2 days after the first dose of luspatercept (calculated from the administration of the first luspatercept dose to the first day of the 12-week transfusion independence period); median time to transfusion independence in cohort 3B was 37.0 days (range, 2-71; Table 3). The longest duration of anemia response varied between cohorts, with the longest median duration of response seen in cohort 3B: 448 days (Table 3). Median duration of response was not calculable for cohort 2 because of the small sample size. Median increase in Hb levels for NTD patients during the entire treatment period was 0.8g/dL in cohort 1, and 1.2g/dL in cohort 3A (Table 3). During the entire treatment period, 27.3% of patients in cohort 1 and 50.0% of patients in cohort 3A had mean Hb increase ≥1.5 g/dL from baseline, and approximately half of TD patients had ≥50% reduction in transfusion burden (cohort 2, 47.6%; cohort 3B, 52.6%; Figure 1). The proportion of patients with ≥50% reduction in MPN-SAF total system score and/or fatigue scores was higher in patients who received ruxolitinib (cohort 3A/3B) than those who did not (cohort 1/2; Table 3).
Secondary end points
| . | Cohort 1 No RUX NTD (n = 22) . | Cohort 3A RUX NTD (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . |
|---|---|---|---|---|
| Time to, and duration of, response | ||||
| Evaluable patients, n | 3 | 2 | 2 | 10 |
| Time to anemia response,∗ d; median (min, max) | 63.0 (41, 68) | 63.5 (42, 85) | 2.0 (2, 2) | 37.0 (2, 71) |
| Longest duration of anemia response,† d; median (min, max) | 126.0 (84, 1163) | 88.5 (84, 93) | NA (602, 644) | 448.0 (85, 1582) |
| Transfusion burden reduction‡ | ||||
| Evaluable patients, n | 21 | 38 | ||
| Patients with transfusion burden reduction during primary treatment period, n (%) | ||||
| Primary treatment period | – | – | 10 (47.6) | 19 (50.0) |
| Entire treatment period | – | – | 10 (47.6) | 20 (52.6) |
| Hb levels§ | ||||
| Evaluable patients, n | 22 | 14 | 21 | 38 |
| Hb increase, g/dL; median (range) | ||||
| Primary treatment period | 0.8 (−1.0, 2.6) | 1.2 (0.2, 2.0) | 0.4 (−1.1, 1.9) | 0.2 (−1.7, 2.5) |
| Entire treatment period | 0.8 (−1.0, 3.0) | 1.2 (0.2, 2.4) | 0.4 (−1.1, 2.7) | 0.3 (−1.8, 2.6) |
| Symptom and fatigue reduction | ||||
| Symptom evaluable patients, n | 22 | 14 | 21 | 38 |
| Patients with mean MPN-SAF TSS of ≥50% reduction from baseline, n (%) | ||||
| Primary treatment period | 2 (9.1) | 3 (21.4) | 2 (9.5) | 6 (15.8) |
| Entire treatment period | 2 (9.1) | 3 (21.4) | 2 (9.5) | 6 (15.8) |
| Patients with mean MPN-SAF fatigue of ≥50% reduction from baseline, n (%) | ||||
| Primary treatment period | 5 (22.7) | 3 (21.4) | 1 (4.8) | 5 (13.2) |
| Entire treatment period | 4 (18.2) | 3 (21.4) | 0 | 5 (13.2) |
| . | Cohort 1 No RUX NTD (n = 22) . | Cohort 3A RUX NTD (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . |
|---|---|---|---|---|
| Time to, and duration of, response | ||||
| Evaluable patients, n | 3 | 2 | 2 | 10 |
| Time to anemia response,∗ d; median (min, max) | 63.0 (41, 68) | 63.5 (42, 85) | 2.0 (2, 2) | 37.0 (2, 71) |
| Longest duration of anemia response,† d; median (min, max) | 126.0 (84, 1163) | 88.5 (84, 93) | NA (602, 644) | 448.0 (85, 1582) |
| Transfusion burden reduction‡ | ||||
| Evaluable patients, n | 21 | 38 | ||
| Patients with transfusion burden reduction during primary treatment period, n (%) | ||||
| Primary treatment period | – | – | 10 (47.6) | 19 (50.0) |
| Entire treatment period | – | – | 10 (47.6) | 20 (52.6) |
| Hb levels§ | ||||
| Evaluable patients, n | 22 | 14 | 21 | 38 |
| Hb increase, g/dL; median (range) | ||||
| Primary treatment period | 0.8 (−1.0, 2.6) | 1.2 (0.2, 2.0) | 0.4 (−1.1, 1.9) | 0.2 (−1.7, 2.5) |
| Entire treatment period | 0.8 (−1.0, 3.0) | 1.2 (0.2, 2.4) | 0.4 (−1.1, 2.7) | 0.3 (−1.8, 2.6) |
| Symptom and fatigue reduction | ||||
| Symptom evaluable patients, n | 22 | 14 | 21 | 38 |
| Patients with mean MPN-SAF TSS of ≥50% reduction from baseline, n (%) | ||||
| Primary treatment period | 2 (9.1) | 3 (21.4) | 2 (9.5) | 6 (15.8) |
| Entire treatment period | 2 (9.1) | 3 (21.4) | 2 (9.5) | 6 (15.8) |
| Patients with mean MPN-SAF fatigue of ≥50% reduction from baseline, n (%) | ||||
| Primary treatment period | 5 (22.7) | 3 (21.4) | 1 (4.8) | 5 (13.2) |
| Entire treatment period | 4 (18.2) | 3 (21.4) | 0 | 5 (13.2) |
Data are n (%) unless otherwise indicated.
TSS, total symptom score; max, maximum; min, minimum; NA, not applicable; RUX, ruxolitinib.
Time to anemia response for cohorts 1 and 3A defined as time between first administration of luspatercept and the first Hb increase of ≥1.5 g/dL from a baseline that starts a consecutive 84-day period of consecutive increase ≥1.5 g/dL without RBC transfusions; for cohorts 2 and 3B defined as time between first administration of LUSPA and the first day of an RBC transfusion–free period of 12 weeks between day 1 and day 168 (primary treatment period). Assessed only in evaluable patients with anemia response.
Duration of anemia response defined as last day of longest response, first date of longest response +1, assessed from day 1 to end of treatment (entire treatment period). Median duration of anemia response was not calculable for cohort 2 because of cohort 2 only containing 2 patients.
Patients achieving ≥50% RBC transfusion burden reduction over any 12-week period.
Change from baseline calculated based on average Hb measurements collected during the period, Hb measures followed the 14/3 rule (only Hb values >14 days after transfusion used unless there is another transfusion within 3 days after Hb assessment).
In cohort 3A, patients received ruxolitinib at a mean dose of 20.7 mg per day; the initial dose was maintained in all patients. In cohort 3B, patients started with a mean dose of 20.2 mg per day; 25 (65.8%) patients maintained their dose, 6 (15.8%) patients had ≥1 dose increase, 4 (10.5%) had ≥1 dose decrease, and 3 (7.9%) had dose interruption.
Among patients with evaluable measurements for spleen enlargement at baseline and day 169 (cohort 1 [n = 12], cohort 3A [n = 5], cohort 2 [n = 10], and cohort 3B [n = 15]), spleen size by palpation remained stable with no clinically meaningful variation across all 4 cohorts at day 169.
Safety
In the safety population, 89 (93.7%) patients had an AE and 45 (47.4%) patients had a treatment-related AE (TRAE; Table 4). In total, 23 (24.2%) patients had hypertension (grade ≥3 in 7 patients); 17 (17.9%) patients had treatment-related hypertension (grade ≥3 in 5 patients). Hypertension was managed with medical intervention and no instances led to luspatercept discontinuation. Other frequently reported AEs included diarrhea (24.2%), dyspnea (22.1%), thrombocytopenia (18.9%), and fatigue (17.9%).
TRAEs in the safety population
| Preferred term . | Cohort 1 No RUX anemia (n = 22) . | Cohort 3A RUX anemia (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . | Total (N = 95) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Patients with ≥1 TRAEs∗ | 14 (63.6) | 0 | 7 (50.0) | 2 (14.3) | 5 (23.8) | 1 (4.8) | 19 (50.0) | 7 (18.4) | 45 (47.4) | 10 (10.5) |
| Hypertension | 4 (18.2) | 0 | 4 (28.6) | 2 (14.3) | 1 (4.8) | 0 | 8 (21.1) | 3 (7.9) | 17 (17.9) | 5 (5.3) |
| Bone pain | 2 (9.1) | 0 | 3 (21.4) | 0 | 2 (9.5) | 0 | 0 | 0 | 7 (7.4) | 0 |
| Diarrhea | 1 (4.5) | 0 | 0 | 0 | 2 (9.5) | 1 (4.8) | 3 (7.9) | 1 (2.6) | 6 (6.3) | 2 (2.1) |
| Fatigue | 1 (4.5) | 0 | 0 | 0 | 0 | 0 | 3 (7.9) | 0 | 4 (4.2) | 0 |
| Hyperuricemia | 2 (9.1) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.6) | 0 | 4 (4.2) | 0 |
| Dizziness | 1 (4.5) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.6) | 0 | 3 (3.2) | 0 |
| Headache | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.3) | 2 (5.3) | 2 (2.1) | 2 (2.1) |
| Back pain | 0 | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Myalgia | 1 (4.5) | 0 | 0 | 0 | 0 | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Abdominal pain | 2 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (2.1) | 0 |
| Nausea | 1 (4.5) | 0 | 0 | 0 | 1 (4.8) | 0 | 0 | 0 | 2 (2.1) | 0 |
| Peripheral edema | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.3) | 0 | 2 (2.1) | 0 |
| Alanine aminotransferase increased | 0 | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Blood alkaline phosphatase increased | 0 | 0 | 1 (7.1) | 0 | 1 (4.8) | 0 | 0 | 0 | 2 (2.1) | 0 |
| Preferred term . | Cohort 1 No RUX anemia (n = 22) . | Cohort 3A RUX anemia (n = 14) . | Cohort 2 No RUX TD (n = 21) . | Cohort 3B RUX TD (n = 38) . | Total (N = 95) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Patients with ≥1 TRAEs∗ | 14 (63.6) | 0 | 7 (50.0) | 2 (14.3) | 5 (23.8) | 1 (4.8) | 19 (50.0) | 7 (18.4) | 45 (47.4) | 10 (10.5) |
| Hypertension | 4 (18.2) | 0 | 4 (28.6) | 2 (14.3) | 1 (4.8) | 0 | 8 (21.1) | 3 (7.9) | 17 (17.9) | 5 (5.3) |
| Bone pain | 2 (9.1) | 0 | 3 (21.4) | 0 | 2 (9.5) | 0 | 0 | 0 | 7 (7.4) | 0 |
| Diarrhea | 1 (4.5) | 0 | 0 | 0 | 2 (9.5) | 1 (4.8) | 3 (7.9) | 1 (2.6) | 6 (6.3) | 2 (2.1) |
| Fatigue | 1 (4.5) | 0 | 0 | 0 | 0 | 0 | 3 (7.9) | 0 | 4 (4.2) | 0 |
| Hyperuricemia | 2 (9.1) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.6) | 0 | 4 (4.2) | 0 |
| Dizziness | 1 (4.5) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.6) | 0 | 3 (3.2) | 0 |
| Headache | 0 | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.3) | 2 (5.3) | 2 (2.1) | 2 (2.1) |
| Back pain | 0 | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Myalgia | 1 (4.5) | 0 | 0 | 0 | 0 | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Abdominal pain | 2 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (2.1) | 0 |
| Nausea | 1 (4.5) | 0 | 0 | 0 | 1 (4.8) | 0 | 0 | 0 | 2 (2.1) | 0 |
| Peripheral edema | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5.3) | 0 | 2 (2.1) | 0 |
| Alanine aminotransferase increased | 0 | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (2.6) | 0 | 2 (2.1) | 0 |
| Blood alkaline phosphatase increased | 0 | 0 | 1 (7.1) | 0 | 1 (4.8) | 0 | 0 | 0 | 2 (2.1) | 0 |
Data are n (%) unless otherwise indicated.
RUX, ruxolitinib.
Only preferred terms occurring in ≥2 patients shown; therefore, overall total may not match values shown below.
In total, 37 patients had ≥1 serious AE, and 3 patients had serious TRAEs: cerebral toxoplasmosis, urinary tract infection, and diarrhea (1 patient each). Across all patients, 2 (2.1%) in cohort 2 had transformation to blast phase, which were not considered related to treatment. There were 5 thromboembolic events (aortic embolus, cerebrovascular accident [followed by ischemic stroke], pulmonary embolism, acute myocardial infarction, and splenic infarction, occurring in 1 patient each). None of these events resulted in luspatercept discontinuation, nor were any events considered related to luspatercept. Nine patients had ≥1 TRAE that resulted in study drug withdrawal. Events included COVID-19, cerebral toxoplasmosis, sepsis, urinary tract infection, fatigue, general physical health deterioration, splenomegaly, diverticular perforation, and hemorrhage (in 1 patient each).
In total, 24 (25.3%) patients had ≥1 dose delay; 7 (7.4%) patients had ≥1 TRAE that led to luspatercept dose interruption, the most common of which was hypertension (3.2%). Overall, 7 (7.4%) patients had ≥1 dose reduction; 5 (5.3%) patients had ≥1 TRAE that led to luspatercept dose reduction including hypertension (3.2%), diarrhea (1.1%), and injection site rash (1.1%). Nine patients died while on treatment with death due to pneumonia, septic shock, intracranial hemorrhage, ischemic stroke, multiple organ dysfunction syndrome, post-procedural hemorrhage, MF, renal failure, and pneumonitis (n = 1 patient each). No deaths were considered related to treatment with luspatercept.
Laboratory parameters
Among NTD patients with anemia response and TD patients who became NTD, erythropoietin generally increased from the measurement obtained at baseline compared with day 169. The greatest increase was observed in cohort 3B, with a mean absolute increase from baseline of 1322.2 IU/L (standard deviation [SD]: 2024.1 IU/L) among responders (n = 11) and 2465.8 IU/L (SD: 2654.9 IU/L) among nonresponders (n = 11; supplemental Figure 2). Ferritin levels generally remained stable during the primary treatment period for anemia responders. Among TD patients who became NTD, ferritin levels decreased from the measurement obtained at baseline compared with day 169, with a mean change of −293.4 μg/L (SD: 188.6 μg/L) in cohort 2 (n = 4) and −61.2 μg/L (SD: 827.2 μg/L) in cohort 3B (n = 11; supplemental Figure 3). In total, 10 patients received iron chelation therapy for ≥30 days during the trial, 9 of whom started before the trial. Patients receiving iron chelation therapy had a mean decrease in ferritin levels of −355.6 μg/L (SD: 634.5 μg/L) from baseline over the entire treatment period.
Discussion
In this phase 2 trial comprising TD and NTD patients with MF-related anemia, luspatercept demonstrated anemia improvement (primary end point) across all cohorts regardless of transfusion dependence and use of ruxolitinib, with the greatest number of anemia responses seen among patients receiving concomitant ruxolitinib (cohorts 3A/3B). During the primary treatment period (weeks 1-24), the anemia response rate (primary end point) among NTD patients was 13.6% in cohort 1 and 14.3% in cohort 3A. Among TD patients, the anemia response rate in the primary treatment period was 9.5% in cohort 2 and 26.3% in cohort 3B. During the entire treatment period, 27.3% of patients in cohort 1 and 50.0% of patients in cohort 3A had mean Hb increase of ≥1.5 g/dL from baseline, and approximately half of TD patients had ≥50% reduction in transfusion burden (cohort 2, 47.6%; cohort 3B, 52.6%). The effect of luspatercept on transfusion independence was more robust in TD patients who received concomitant JAKi therapy (cohort 3B). The higher transfusion-independence rate in cohort 3B compared with cohort 2 may be related to concomitant use of ruxolitinib in cohort 3B, because it is thought that the combined effects of JAK1 and JAK2 inhibition by ruxolitinib and the erythroid maturation activity of luspatercept cause a greater improvement in erythropoiesis and, eventually, lead to a reduction in the transfusion burden. Time to achieve anemia response was ∼63 days in NTD patients; across all cohorts, duration of response ranged from 84 to 1582 days. The assessment of time to response and duration of response was limited by low patient numbers and warrants long-term follow-up. Patients in all cohorts also showed reduced fatigue scores with the greatest improvement in fatigue seen in patients who received concomitant ruxolitinib (cohorts 3A/3B).
The safety profile of luspatercept was expected and consistent with previous studies, with no new safety signals identified.23,24,31 Hypertension was the most common TRAE, but cases were generally low grade and manageable, and did not result in luspatercept discontinuation. None of 9 deaths that occurred during the trial were considered related to luspatercept treatment. Among NTD patients with anemia response and TD patients who became NTD, erythropoietin generally increased from baseline to day 169. Ferritin levels were generally stable or decreased among anemia responders.
Stable ruxolitinib dosing is associated with improved spleen volume reduction and symptom control32; however, ruxolitinib dose modifications are commonly used to help manage AEs including JAKi-associated anemia. To assess the treatment effect of luspatercept appropriately, the ACE-536-MF-001 trial required 16 weeks of stable ruxolitinib dosing before luspatercept treatment in cohorts 3A/3B to minimize confounding variables. Interestingly, in this trial, patients receiving luspatercept plus concomitant ruxolitinib (cohort 3A/3B) had the highest rates of anemia response. Additionally, for most patients, ruxolitinib dose was maintained or increased, whereas spleen size remained stable across all cohorts, suggesting that luspatercept was effective in the management of anemia and enabled continued stable ruxolitinib dosing. The anemia-remitting activity of luspatercept may mitigate potential ruxolitinib-induced anemia and diminish the need for ruxolitinib dose reduction in select patients.
The results of ACE-536-MF-001 must be considered in the context of its different design vs other trials. Anemia among NTD patients was defined as ≥3 Hb level measurements of ≤9.5 g/dL on ≥3 different days in the 12 weeks immediately preceding C1D1. Transfusion independence was defined as 12 weeks transfusion free per Gale criteria (TD cohorts)26 and anemia response defined as ≥1.5 g/dL Hb increase from baseline for 12 consecutive weeks (NTD cohorts). There is no universal standard definition for transfusion independence or anemia response, and clinical trials have used heterogeneous definitions, limiting the ability to compare anemia benefit received from different therapies.33
As a result of varied response criteria, clinical trials for approved and investigational agents have reported a range of anemia response rates. One study with ESAs in MF reported that 37% and 23% of NTD and TD patients, respectively, had anemia response, defined as Hb increase of ≥2.0 g/dL, and transfusion independence of ≥1 month,34 whereas another reported a 57%/29% NTD/TD anemia response rate with a longer requirement of ≥3 months.35 However, ESAs have been linked to vascular complications and can also exacerbate splenomegaly.8,34,35 In a phase 3, randomized, double-blind trial evaluating pomalidomide vs placebo in TD patients with MF not receiving JAKis, anemia response rate (≥12 weeks RBC transfusion free) was 16% in both treatment arms (pomalidomide: 95% CI, 11-23; placebo: 95% CI, 8-26; P = 1.00).36 Other recently approved JAKis for MF, pacritinib and momelotinib,17,18 aim to reduce JAKi-associated cytopenias and transfusion burden in select patient populations. In PERSIST-2 (pacritinib vs best available therapy including ruxolitinib, in patients with thrombocytopenia), a significantly greater proportion of patients who were TD at baseline became transfusion independent (per Gale criteria)26 on pacritinib vs those treated with best available therapy (37% vs 7%, P = .001).37 However, in the United States, pacritinib is limited to the treatment of patients with thrombocytopenia (platelet count of <50 × 109/L).18 Momelotinib is approved for the treatment of patients with MF and anemia regardless of prior MF therapy.17 In the phase 3 Momelotinib versus Danazol in Symptomatic Patients with Anemia and Myelofibrosis trial, 26% of patients treated with momelotinib who were TD at baseline became transfusion independent (RBC transfusion free with no Hb levels of <8 g/dL for ≥12 weeks) at the end of week 24 vs 15% of patients receiving danazol.38 Although momelotinib has been shown to improve transfusion dependency in patients with MF regardless of prior MF therapy, splenic response rate (≥35% reduction) occurred in just 22% of patients treated with momelotinib,38 highlighting the need for treatment options that reduce both transfusion dependence and spleen volume. There was a modest trend toward improved overall survival with momelotinib vs danazol in the Momelotinib versus Danazol in Symptomatic Patients with Anemia and Myelofibrosis trial.38
In the context of these results, this study demonstrates improvement in anemia for all cohorts treated with luspatercept. Improved anemia and consequent reduction in transfusion burden for TD patients with MF is notable given that the requirement for RBC transfusions is associated with poor survival and places a burden on both patients and the health care system. Thus, reductions in transfusion frequency could positively affect both long-term survival and health care resource use.6,39
To conclude, these results emphasize the meaningful anemia improvement in MF-associated anemia in both NTD and TD patients as well as an expected safety profile associated with luspatercept. Furthermore, the effect of luspatercept as an erythroid maturation agent appears to be consistent in patients with MF with that seen in other approved indications.24,31 In addition to benefits in anemia, most patients receiving ruxolitinib were able to maintain or increase their ruxolitinib dose while using luspatercept, supporting maintenance of JAKi therapy for adequate disease control of MF.40 Overall, these results support continued study of luspatercept in MF-associated anemia in TD patients receiving concomitant JAKi therapy, which is currently underway in the phase 3 INDEPENDENCE trial (ACE-536-MF-002).
Acknowledgments
The authors thank the patients, families, and caregivers who made the trial possible, and the clinical study teams who participated. Study drugs were provided by Bristol Myers Squibb (Princeton, NJ). The sponsor did not supply ruxolitinib for this trial. Instead, ruxolitinib was obtained by the sites according to local clinical study agreements and in accordance with local guidelines. Writing and editorial assistance were provided by Niamh Hunnisett, of Caudex, funded by Bristol Myers Squibb.
This trial was sponsored by Bristol Myers Squibb.
Authorship
Contribution: A.T.G. performed research, analyzed and interpreted data, and collected data; C.H. designed research, performed research, and wrote/provided feedback and/or reviewed the manuscript; J.-J.K. designed research, performed research, analyzed and interpreted data, and wrote/provided feedback and/or reviewed the manuscript; R.M. designed research, performed research, analyzed and interpreted data, and wrote/provided feedback and/or reviewed the manuscript; A.M.V. performed research and collected data; R.K. performed research, collected data, and wrote/provided feedback and/or reviewed the manuscript; P.B. wrote/provided feedback and/or reviewed the manuscript; M.K. performed research, collected data, and wrote/provided feedback and/or reviewed the manuscript; A.J.M. performed research, analyzed and interpreted data, and wrote/provided feedback and/or reviewed the manuscript; J.G. performed research, collected data, and wrote/provided feedback and/or reviewed the manuscript; S.R. performed research, analyzed and interpreted data, collected data, and wrote/provided feedback and/or reviewed the manuscript; F.S. performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript and/or reviewed the manuscript; N.M. performed research and analyzed and interpreted data; A.C.G. analyzed and interpreted data, and wrote/provided feedback and/or reviewed the manuscript; H.J. designed research, performed research, analyzed and interpreted data, collected data, and wrote/provided feedback and/or reviewed the manuscript; J.M.P. collected data, analyzed and interpreted data, and wrote/provided feedback and/or reviewed the manuscript; K.M. performed research, collected data, and wrote/provided feedback and/or reviewed the manuscript; V.R. performed research, analyzed and interpreted data, and wrote/provided feedback and/or reviewed the manuscript; F.P. designed research, performed research, analyzed and interpreted data, collected data, and wrote/provided feedback and/or reviewed the manuscript; and all authors contributed to the manuscript development and approved the final manuscript.
Conflict-of-interest disclosure: A.T.G. has received consulting fees from AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Kartos Therapeutics, MorphoSys, Sierra Oncology, and Telios Pharma, and has participated in advisory boards for AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Kartos Therapeutics, MorphoSys, Sierra Oncology, and Telios Pharma. C.H. has received research grants (to institution) from Constellation, MorphoSys, and Novartis; has received consulting fees from Galecto Biotech, Geron, and GlaxoSmithKline; has received honoraria from AbbVie, AOP Health, Bristol Myers Squibb, Celgene, CTI Biopharma, Galecto Biotech, Geron, Gilead, Imago, Janssen, Keros Therapeutics, Novartis, Promedior, Roche, Shire and Sierra Oncology; has participated in advisory boards for Galecto Biotech and Keros Therapeutics; and has a leadership role with the European Hematology Association. J.-J.K. has received consulting fees from AbbVie and GlaxoSmithKline; received honoraria from AOP Health and Novartis; and has participated in advisory boards for Bristol Myers Squibb and Incyte. R.M. has received consulting fees from AbbVie, Blueprint, Bristol Myers Squibb, CTI Biopharma, Genetech, Geron, GlaxoSmithKline, Incyte, MorphoSys, Novartis, Sierra Oncology, and Telios Pharma. A.M.V. has received honoraria from AbbVie, AOP Health, Blueprint, Bristol Myers Squibb, GlaxoSmithKline, Incyte, and Novartis; and has participated in advisory boards for AbbVie, Bristol Myers Squibb, GlaxoSmithKline, and Incyte. R.K. has received consulting fees from Geron; has received research funding from Bristol Myers Squibb; has participated in advisory boards for AbbVie, Bristol Myers Squibb, CTI Biopharma, Jazz Pharmaceuticals, Novartis, PharmaEssentia, Rigel, Servio, and Taiho; and has received payment for speakers bureaus from AbbVie, CTI Biopharma, Jazz Pharmaceuticals, PharmaEssentia, and Servio. P.B. has received research grants (to institution) from Blueprint, Cogent, CTI Biopharma, Disc, Geron, Incyte, Ionis, Janssen, Kartos Therapeutics, MorphoSys, Sumitomo, and Telios Pharma; has received consulting fees from AbbVie, Blueprint, Bristol Myers Squibb, Cogent, CTI Biopharma, GlaxoSmithKline, Incyte, Jubilant, Karyopharm, MorphoSys, Novartis, and PharmaEssentia; has received honoraria from AbbVie, Blueprint, CTI Biopharma, GlaxoSmithKline, Incyte, and PharmaEssentia; has participated in study steering committees for Blueprint; and has participated in advisory boards for GlaxoSmithKline. M.K. has received research grants from Bristol Myers Squibb; has received consulting fees from AbbVie, CTI Biopharma, Incyte, MorphoSys, and Protagonist; and has received honoraria from AbbVie, CTI Biopharma, Incyte, MorphoSys, and Protagonist. A.J.M. has received royalties from Alethiomics; has received consulting fees from AbbVie, Bristol Myers Squibb/Celgene, CTI Biopharma, Galecto Biotech, Gilead, GlaxoSmithKline, Incyte, Karyopharm, Novartis, Pfizer, Relay Therapeutics, Sensyn Robotics, and Sierra Oncology; has received honoraria from AbbVie, Bristol Myers Squibb, Novartis, and Pfizer; has received travel support from Bristol Myers Squibb and Novartis; has patents with Alethiomics; has participated in advisory boards for AbbVie; and owns stock or stock options in Alethiomics. J.G. has received research grants (to institution) from Incyte and Kartos Therapeutics; has received honoraria from Incyte; and has participated in advisory boards for Incyte. S.R. is an employee of Bristol Myers Squibb and owns stock or stock options in Bristol Myers Squibb and Celgene. F.S. is an employee of Bristol Myers Squibb and owns stock or stock options in Bristol Myers Squibb and F Hoffmann La Roche. N.M. is an employee of, has received travel support from, and owns stock or stock options in Bristol Myers Squibb. A.C.G. is an employee of, and owns stock or stock options in, Bristol Myers Squibb. J.M.P. has participated in advisory boards for MorphoSys. V.R. has received research grants from Astex and GlaxoSmithKline; has received honoraria from AbbVie, Bristol Myers Squibb, and Gilead; has received travel support from AstraZeneca; and has participated in advisory boards for AstraZeneca. F.P. has received research grants from Bristol Myers Squibb/Celgene; has received consulting fees from AbbVie, AOP Orphan, Bristol Myers Squibb/Celgene, Kartos Therapeutics, Karyopharm, Kyowa Kirin, MEI Pharma, Novartis, Roche, Sierra Oncology, and Sumitomo; and has received honoraria from AbbVie, AOP Orphan, Bristol Myers Squibb/Celgene, Novartis, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Aaron T. Gerds, Hematology and Medical Oncology, Cleveland Clinic Taussig Cancer Institute, 9500 Euclid Ave #CA-60, Cleveland, OH 44195; email: gerdsa@ccf.org.
References
Author notes
The Bristol Myers Squibb policy on data sharing can be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The full-text version of this article contains a data supplement.