T-cell engaging–therapies have transformed the treatment landscape of relapsed and refractory B-cell non-Hodgkin lymphomas by offering highly effective treatments for patients with historically limited therapeutic options. This review focuses on the advances in chimeric antigen receptor–modified T cells and bispecific antibodies, first providing an overview of each product type, followed by exploring the primary data for currently available products in large B-cell lymphoma, follicular lymphoma, and mantle cell lymphoma. This review also highlights key logistical and sequencing considerations across diseases and product types that can affect clinical decision-making.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common large B-cell lymphoma (LBCL), and up to 30% of patients relapse or have primary refractory disease after initial chemoimmunotherapy, which has historically been associated with poor outcomes despite intensive chemoimmunotherapy.1-3 Follicular lymphoma (FL) is the most common indolent B-cell lymphoma but remains incurable, and so, effective less-toxic therapies are needed. Mantle cell lymphoma (MCL) typically necessitates upfront chemoimmunotherapy, and outcomes for multiply relapsed/refractory (R/R) MCL are particularly disappointing. Recently, novel therapies engaging patients’ T cells via chimeric antigen receptor-modified (CAR) T cells and bispecific antibodies (BsAbs) have revolutionized the treatment of R/R LBCL, FL, and MCL (Table 1).
Summary of approved CAR T-cell and BsAb products in LBCL, FL, and MCL
| Disease . | Product . | Line . | Response∗ . | Toxicity, grade 3+ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORR, % . | CRR, % . | PFS, mo . | DOR, mo . | CRS, % . | NT, % . | Cytopenias, %† . | Infections, % . | |||
| LBCL | Axi-cel | 3+4,5 | 83 | 58 | 5.9 | 11.1 | 13 | 28 | 78 | NI |
| 26,7 | 83 | 65 | 14.7 | 41.7 | 6 | 21 | 69 | 14 | ||
| Liso-cel | 3+8,9 | 73 | 53 | 6.8 | 23.1 | 2 | 10 | 60 | 12 | |
| 210,11 | 87 | 74 | NR | NR | 1 | 4 | 80 | 15 | ||
| NTE12,13 | 80 | 54 | 9.0 | 23.3 | 2 | 5 | 48 | 7 | ||
| Tisa-cel | 3+14,15 | 53 | 39 | 2.9 | NR | 22 | 12 | 39 | 20 | |
| Epcoritamab | 3+16 | 63 | 39 | 4.4 | 12.0 | 3 | 1 | 15 | 15 | |
| Glofitamab | 3+17 | 52 | 39 | 4.9 | 18.4 | 4 | 3 | 27 | 15 | |
| FL | Axi-cel | 3+18,19,20 | 94 | 79 | 57.3 | 55.5 | 6 | 15 | 33 | 18 |
| Tisa-cel | 3+21,22 | 86 | 68 | NR | NR | 0 | 1 | 32 | 5 | |
| Liso-cel | 3+23,24 | 97 | 94 | NR | NR | 1 | 2 | 58 | 5 | |
| Mosunetuzumab | 3+25-27 | 78 | 60 | 24.0 | 35.9 | 2 | 0 | 26 | 14 | |
| Epcoritamab | 3+28,29 | 82 | 63 | 15.4 | NR | 2 | 0 | 30 | 40 | |
| MCL | Brexu-cel | 2+30,31 | 91 | 68 | 25.8 | 28.2 | 15 | 31 | 85 | 32 |
| Liso-cel | 3+32 | 83 | 72 | 15.3 | 15.7 | 1 | 9 | 56 | 15 | |
| Disease . | Product . | Line . | Response∗ . | Toxicity, grade 3+ . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ORR, % . | CRR, % . | PFS, mo . | DOR, mo . | CRS, % . | NT, % . | Cytopenias, %† . | Infections, % . | |||
| LBCL | Axi-cel | 3+4,5 | 83 | 58 | 5.9 | 11.1 | 13 | 28 | 78 | NI |
| 26,7 | 83 | 65 | 14.7 | 41.7 | 6 | 21 | 69 | 14 | ||
| Liso-cel | 3+8,9 | 73 | 53 | 6.8 | 23.1 | 2 | 10 | 60 | 12 | |
| 210,11 | 87 | 74 | NR | NR | 1 | 4 | 80 | 15 | ||
| NTE12,13 | 80 | 54 | 9.0 | 23.3 | 2 | 5 | 48 | 7 | ||
| Tisa-cel | 3+14,15 | 53 | 39 | 2.9 | NR | 22 | 12 | 39 | 20 | |
| Epcoritamab | 3+16 | 63 | 39 | 4.4 | 12.0 | 3 | 1 | 15 | 15 | |
| Glofitamab | 3+17 | 52 | 39 | 4.9 | 18.4 | 4 | 3 | 27 | 15 | |
| FL | Axi-cel | 3+18,19,20 | 94 | 79 | 57.3 | 55.5 | 6 | 15 | 33 | 18 |
| Tisa-cel | 3+21,22 | 86 | 68 | NR | NR | 0 | 1 | 32 | 5 | |
| Liso-cel | 3+23,24 | 97 | 94 | NR | NR | 1 | 2 | 58 | 5 | |
| Mosunetuzumab | 3+25-27 | 78 | 60 | 24.0 | 35.9 | 2 | 0 | 26 | 14 | |
| Epcoritamab | 3+28,29 | 82 | 63 | 15.4 | NR | 2 | 0 | 30 | 40 | |
| MCL | Brexu-cel | 2+30,31 | 91 | 68 | 25.8 | 28.2 | 15 | 31 | 85 | 32 |
| Liso-cel | 3+32 | 83 | 72 | 15.3 | 15.7 | 1 | 9 | 56 | 15 | |
NTE, non–transplant eligible; NI, not included; NT, neurologic toxicity.
Response data included from publication with longest follow-up when applicable.
Highest incidence of most common grade ≥3 cytopenia from listed rates of anemia, thrombocytopenia, and neutropenia.
CAR T cells and BsAb
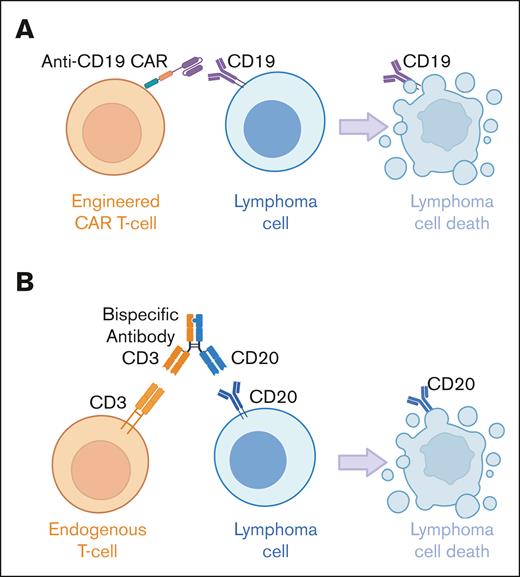
CAR T cells are manufactured by transducing autologous T cells with an inactivated viral vector to express an antigen receptor targeting the T cells to a tumor surface antigen, along with a transmembrane spacer, a costimulatory domain, and an intracellular signaling domain to drive T-cell activation. This process confers the engineered T cells with the specificity of a monoclonal antibody, resulting in major histocompatibility complex–independent lymphoma cell recognition (Figure 1). All currently approved CAR T-cell products in B-cell non-Hodgkin lymphoma (B-NHL) target CD19, which is ubiquitously expressed on mature B-cell lymphomas. The addition of costimulation to T-cell receptor–mediated signaling avoids anergy33 and improves efficacy34; however, the more rapid and higher peak expansion of CD28 costimulation is associated with increased toxicity (axicabtagene ciloleucel [axi-cel] and brexucabtagene autoleucel [brexu-cel]) vs 4-1BB costimulation (lisocabtagene maraleucel [liso-cel] and tisagenlecleucel [tisa-cel]).35
CAR T cells and BsAbs target lymphoma cells through distinct T-cell–mediated mechanisms of action. (A) Currently approved CAR T cells in B-cell lymphomas are generated from autologous T cells engineered to express an antigen receptor targeting CD19 on lymphoma cells, as well as a transmembrane spacer, a costimulatory domain, and an intracellular signaling domain, which together drive T-cell activation and ultimately lymphoma cell destruction. (B) Currently approved BsAb in B-cell lymphomas are off-the-shelf antibodies targeting CD3 on endogenous T cells and CD20 on lymphoma cells, bringing the 2 entities together to result in T-cell–mediated lymphoma cell death.
CAR T cells and BsAbs target lymphoma cells through distinct T-cell–mediated mechanisms of action. (A) Currently approved CAR T cells in B-cell lymphomas are generated from autologous T cells engineered to express an antigen receptor targeting CD19 on lymphoma cells, as well as a transmembrane spacer, a costimulatory domain, and an intracellular signaling domain, which together drive T-cell activation and ultimately lymphoma cell destruction. (B) Currently approved BsAb in B-cell lymphomas are off-the-shelf antibodies targeting CD3 on endogenous T cells and CD20 on lymphoma cells, bringing the 2 entities together to result in T-cell–mediated lymphoma cell death.
BsAb simultaneously engage T cells and lymphoma cells to result in T-cell–mediated cytotoxicity independent of Fcγ receptor and major histocompatibility complex (Figure 1). All currently approved BsAb in B-NHL target the broadly expressed CD20 antigen on B cells and CD3, which is ubiquitously expressed on T cells. BsAbs contain a heavy chain (immunoglobulin G1: mosunetuzumab, glofitamab, and epcoritamab; immunoglobulin G4: odronextamab) and CD20 and CD3 binding domains, which are present in either a 1:1 ratio (mosunetuzumab, epcoritamab, and odronextamab) or a 2:1 ratio (glofitamab).36 Although each BsAb is manufactured uniquely and contains a distinct CD20 binding domain, all incorporate Fc-silencing mutations to avoid antigen-independent and/or fratricidal T-cell activation and cytotoxicity.36
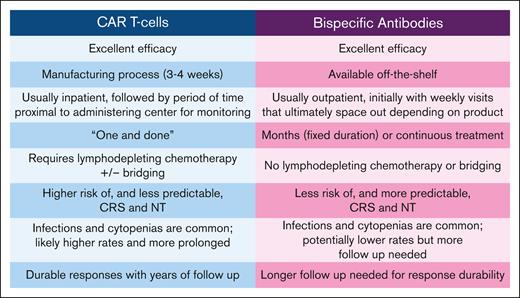
CAR T cells and BsAbs have overlapping toxicities but with several differences (Figure 2). Both classes are associated with side effects arising from T-cell activation and proliferation, including cytokine release syndrome (CRS) and immune effector cell–associated neurologic syndrome (ICANS). CAR T cells can expand exponentially which can lead to rapidly escalating and potentially unpredictable CRS/ICANS, whereas the BsAbs have a fixed half-life, and step up dosing results in predictable CRS/ICANS kinetics. CRS and neurotoxicity management guidelines for CAR T cells37,38 and BsAb39 highlight that class differences in toxicity profiles warrant unique considerations. Some BsAbs (eg, epcoritamab) recommend prophylactic steroids during the highest CRS/ICANS risk, whereas others (eg, glofitamab, mosunetuzumab) do not. Although prophylactic steroids with CAR T cells can decrease CRS and neurotoxicity rates and may not compromise outcomes,40 questions remain regarding the effects on long-term efficacy, and thus, prophylactic steroids are typically used quite sparingly. Although both CAR Tcells and BsAb are associated with cytopenias and infections, infection risk is likely higher with CAR Tcells, with which cytopenias and hypogammaglobulinemia can be prolonged in part due to lymphodepleting chemotherapy (LDC) and CRS, although emerging data also demonstrate an ongoing infection risk with BsAb,41,42 which may be of particular concern with prolonged dosing schedules.
Comparison of CAR T-cell and BsAb characteristics. CAR T cells and BsAbs are associated with unique considerations regarding logistics and toxicity. NT, neurologic toxicity.
Comparison of CAR T-cell and BsAb characteristics. CAR T cells and BsAbs are associated with unique considerations regarding logistics and toxicity. NT, neurologic toxicity.
Logistics also differ (Figure 2). CAR T-cell manufacturing typically requires 3 to 4 weeks, and thus, “bridging” therapy is often needed for disease control, which may be ineffective and can increase cytopenia and infection risk. Before CAR T-cell infusion, LDC is given to prevent rejection of the modified T cells and to promote CAR T-cell expansion.43 CAR T cells also typically include a 1 to 2 week hospitalization for administration and monitoring, followed by a requirement for patients to remain proximal to the administering center for at least several more weeks. Increasingly, centers are administering selected CAR T-cell products in the outpatient setting, but patients must remain in the immediate vicinity of the treating center. BsAbs, in contrast, are available off-the-shelf, obviating treatment delays and need for bridging. Although a single 24-hour hospitalization is currently recommended during the highest CRS/ICANS risk with epcoritamab and glofitamab, this is expected to change with validation of effective prophylactic and outpatient monitoring, and thus, BsAbs will likely move to community administration long before CAR T cells, allowing for patients to receive treatment close to home with their primary oncology team. On the contrary, CAR T-cell therapy has the attractive quality of being a “one and done” treatment, in contrast to BsAbs that are administered over several months to indefinitely. Of note, bendamustine should be avoided before leukophresis when possible due to potential deleterious effects on CAR T-cell function,18,30,44 but its effects remain to be seen with BsAbs. Finally, most people still lack access to CAR T-cell therapy, given that it is only available at highly specialized referral centers and referral barriers exist,45,46 whereas BsAb access is likely to be broader.
LBCLs
Until recently, patients with LBCL refractory to or relapsed after initial response to upfront chemoimmunotherapy and deemed “transplant eligible” received second-line (2L) platinum-based chemoimmunotherapy and, if responding, proceeded to curative-intent high-dose chemotherapy and autologous stem cell transplant (ASCT). Patients considered ineligible for this approach, however, received palliative-intent regimens. For patients requiring 3L or later LBCL treatment, anti-CD19 CAR T-cells were approved beginning in 2017 (axi-cel, liso-cel, and tisa-cel),4,8,14 making curative treatments available in multiply relapsed LBCL. Recent studies have also led to the approval of axi-cel and liso-cel for primary refractory or early disease relapse.6,7,10,11 Liso-cel is also approved in non–transplant-eligible patients after at least 1 therapy regardless of initial response duration.12
Approval for CAR T cells in 2L LBCL come from randomized phase 3 studies comparing CAR T cells with standard of care (SOC; platinum-based 2L chemotherapy followed by high-dose chemotherapy and ASCT if chemosensitive) in primary refractory or early relapsed disease (<1 year after initial chemoimmunotherapy). In ZUMA-7, the complete response rate (CRR) for axi-cel (n = 180) was 65% vs 32% for SOC (n = 179), and the study met its primary end point of an improved median event-free survival (mEFS) for axi-cel vs SOC (8.3 months vs 2.0 months; hazard ratio [HR], 0.40).6,7 CRS occurred in 92% and was mostly grade 1 to 2 (6% grade 3+), and neurologic events (NE) occurred in 60%, with 21% experiencing grade 3+ events. With additional follow-up (median, 47.2 months), axi-cel demonstrated an overall survival (OS) benefit (median, not reached [NR] vs 31.1 months; HR, 0.73).7 In TRANSFORM, the CRR for liso-cel (n = 92) was 74% vs 42% for SOC (n = 92), and the study met its primary end point with improved mEFS favoring liso-cel (NR vs 2.4 months; HR, 0.356).10,11 CRS of any grade occurred in 49% and was predominantly grade 1 to 2 (1% grade 3), whereas NEs occurred in 12% and were generally low grade (grade 1, 5%; grade 2, 2%; grade 3, 4%), with no grade 4+ CRS or NEs. More than two-third of patients in TRANSFORM crossed over from SOC to receive liso-cel at a median of 14 days; however, the CRR (53%) and mEFS (5.9 months) were inferior in crossover patients compared with those randomized to receive 2L liso-cel, suggesting that 2L CAR T-cell administration should be preferred. With 17.5 months of median follow-up, median OS was NR for liso-cel and 29.9 months for SOC; when adjusted for crossover, 18-month OS was 73% for liso-cel and 54% for SOC (HR, 0.415).10 Notably, the BELINDA study failed to meet its primary end point and demonstrate that tisa-cel was superior to platinum-based salvage and ASCT.47
Liso-cel is also approved for transplant ineligible R/R LBCL regardless of time from initial therapy based on the phase 2 PILOT study.12,13 In the 61 liso-cel–treated patients (median age, 74 years), overall response rate (ORR) was 80%, CRR was 54%, and grade 3 CRS or NEs were uncommon (1.6% and 4.9%, respectively), with no grade 4+ events. At a median follow-up of 18.2 months, median progression-free survival (mPFS) was 9.0 months, and responses were durable, with a median duration of response (mDOR) of 23.3 months; mDOR for those in complete response (CR) was NR, and the median OS was NR.13
CAR T cells are approved in 3L+ R/R LBCL based on 3 pivotal phase 2 studies.4,5,8,9,14,15 In ZUMA-1, axi-cel demonstrated a CRR of 58%, and after a median follow-up of 63.1 months, mPFS was 5.9 months, mDOR was 11.1 months with 31% of patients in an ongoing response, and the median OS was 25.8 months.4,5 CRS occurred in 93% and was mostly grade 1 to 2 (37% grade 1, 44% grade 2, and 13% grade 3+); NEs occurred in 64%, with 28% experiencing grade 3+ events. In JULIET, tisa-cel demonstrated a CRR of 39%, and after a median follow-up of 40.3 months, mPFS was 2.9 months, the median DOR was NR with 60.4% in an ongoing response 3 years later, and the median OS was 11.1 months.14,15 CRS occurred in 58% (36% grade 1-2; 22% grade 3-4), and NEs occurred in 21% (9% grade 1-2; 12% grade 3-4). In TRANSCEND NHL 001, liso-cel demonstrated a CRR of 53%, and after a median follow-up of 19.9 months, mPFS was 6.8 months, mDOR was 23.1 months with 49.5% of patients in an ongoing response at 2 years, and the median OS was 27.3 months.8,9 CRS occurred in 42% and was mostly grade 1 to 2 (40%), with grade 3 to 4 occurring in 2%, whereas NEs occurred in 30% and were mostly grade 1 to 2 (20%), with 10% of patients experiencing grade 3 to 4 events.
Retrospective analyses have validated the results of the pivotal 3L+ R/R LBCL CAR T-cell trials despite including trial-ineligible patients48-51 and highlight that a “CAR T-cell–eligible patient” can likely be defined relatively broadly beyond age and comorbidities, and ultimately, the decision to proceed with CAR T cells requires a patient-tailored discussion of the potential risks/benefits. It should also be noted that although different CAR T-cell products have not been compared prospectively head-to-head, real-world analyses comparing commercial axi-cel– and tisa-cel–treated patients with R/R LBCL suggest inferior efficacy for tisa-cel relative to axi-cel,52-55 which are consistent with efficacy results in the pivotal trials.
Practically, when selecting between axi-cel and liso-cel in 2L LBCL,6,7,10,11 both products appear similarly effective, whereas liso-cel is less toxic, which is an important consideration. Axi-cel’s faster turnaround time and more reliable manufacturing6,7 may be of particular importance for a patient in urgent need of therapy, whereas the pivotal liso-cel studies permitted broader lymphoma subtypes (eg, primary mediastinal B-cell lymphoma, T-cell histiocyte-rich LBCL, grade 3B FL, and transformed LBCL from any indolent histology), secondary central nervous system lymphoma, and no minimum absolute lymphocyte count,10,11 which may influence product choice. Ultimately product selection is highly personalized to the patient.
Until recently, the array of available treatments in R/R LBCL after CAR T cells or for patients who decline, lack access to, or are ineligible for CAR T cells and/or ASCT was associated with low response rates and durations. Rituximab, gemcitabine, and oxaliplatin resulted in a 12.8% 5-year PFS in a phase 2 study56 and an 18% 2-year PFS in a real-world analysis.57 Polatuzumab vedotin with bendamustine and rituximab has an mPFS of 9.5 months.58 Tafasitamab with lenalidomide demonstrated an 18-month PFS of 46%, however, this study excluded high-risk disease,59 and in a real-world cohort, the mPFS was only 1.9 months.60 Loncastuximab tesirine demonstrated an mPFS of 4.9 months,61 and in a real-world analysis, the mPFS was 2.1 months.62
Two CD20/CD3 BsAb are now approved in 3L+ R/R LBCL. In the phase 2 EPCORE NHL-1 study, subcutaneous epcoritamab was administered to patients with R/R DLBCL or other aggressive LBCL (n = 157) after at least 2 prior therapies (median 3) and continued until progression or intolerance.16 At a median follow-up of 10.7 months, ORR was 63.1% (CRR, 38.9%), with an mPFS of 4.4 months and an mDOR of 12.0 months. CRS occurred in 49.7% and was generally low grade (32% grade 1, 15% grade 2, and 2.5% grade 3), and ICANS occurred in 6.4% (4% grade 1, 1% grade 2, and 1% grade 5). In a phase 2 study, glofitamab was administered IV to patients with R/R DLBCL (n = 154) after 2+ lines of therapy (median, 3) with fixed-duration dosing (12 cycles).17 ORR was 52% (CRR, 39%), and at a median follow-up of 12.6 months, mPFS was 4.9 months, and mDOR was 18.4 months. CRS occurred in 63% and was mostly low grade (47% grade 1, 12% grade 2, 3% grade 3, and 1% grade 4), whereas ICANS occurred in 8% (6% grade 1-2 and 3% grade 3+). Finally, odronextamab is in late-stage development in R/R DLBCL and demonstrated an ORR and CRR of 52% and 31%, respectively, in the 141 patients in the phase 2 ELM-2 study.63 The median DOR was 10.2 months, and the median duration of CR was 17.9 months. CRS occurred in 55% (all grade 1-2 except for 1 grade 3 event), and there was no ICANS. Practically, both epcoritamab and glofitamab have similar response and toxicity rates, so differences in administration may influence choice; with epcoritamab being subcutaneous yet continuous and with higher prophylactic steroid doses; and glofitamab being IV yet time-limited with a more convenient dosing schedule and with lower prophylactic steroid doses but also with an obinutuzumab pretreatment step.
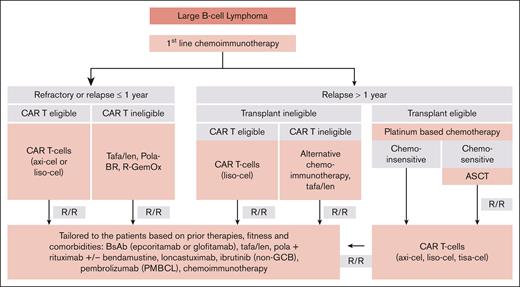
An ongoing question is how best to sequence the now multiple T-cell–directed therapies in R/R LBCL (Figure 3). Ultimately, when determining whether a patient is “eligible” for CAR T cells and/or BsAbs, potential product-specific toxicities must be weighed with patient comorbidities, but with careful monitoring and appropriate supportive care, most patients including those historically considered transplant ineligible can safely receive these therapies. For patients who qualify for and have access to 2L CAR T cells, we encourage this approach, given the axi-cel6,7 and liso-cel10,11 data with relatively long follow-up demonstrating durable responses consistent with cure, coupled with an OS benefit over platinum-based salvage chemotherapy and ASCT consolidation. In later lines, when both CAR T cells and BsAbs are available, our practice is to offer CAR T cells first in CAR-naïve patients, based on years of post–CAR T-cell follow-up with evidence of cure,4,5,8,9,14,15 whereas longer follow-up is needed with BsAbs, which do not obviously demonstrate a plateau on the PFS curves (eg, cure) with limited follow-up. It is clear from retrospective studies before BsAbs were widely available that relapse after CAR T-cells in LBCL is associated with poor outcomes.64-66 There has been theoretical concern of T-cell exhaustion compromising efficacy of subsequent T-cell therapies, however, the pivotal studies of epcoritamab and glofitamab in R/R LBCL demonstrate similar response rates for patients with and without prior CAR T cells: 35% vs 42% with epcoritamab16 and 34.4% vs 41.7% with glofitamab,17 respectively. Moreover, retrospective outcomes for patients with BsAb exposure before CAR T cells compared to those without demonstrated no differences in PFS, OS, and toxicity.67,68 Thus, although preliminary T-cell exhaustion data are reassuring, further studies are warranted. Finally, as CAR T cells and/or BsAbs potentially develop indications in 1L LBCL (eg, ongoing studies with axi-cel7 and glofitamab/chemoimmunotherapy69 in high-risk disease), sequencing considerations will need recalibration.
Sequencing treatments in LBCL. Flowchart outlines a proposed sequencing of therapies in LBCL based on current approvals and highlighting when to consider CAR T cells and/or BsAbs. GCB, germinal center B cell; Lonca, loncastuximab tesirine; PMBCL (primary mediastinal B-cell lymphoma); Pola-BR, polatuzumab vedotin, bendamustine rituximab; R-GemOx, rituximab, gemcitabine, oxaliplatin; Tafa/len, tafasitamab/lenalidomide.
Sequencing treatments in LBCL. Flowchart outlines a proposed sequencing of therapies in LBCL based on current approvals and highlighting when to consider CAR T cells and/or BsAbs. GCB, germinal center B cell; Lonca, loncastuximab tesirine; PMBCL (primary mediastinal B-cell lymphoma); Pola-BR, polatuzumab vedotin, bendamustine rituximab; R-GemOx, rituximab, gemcitabine, oxaliplatin; Tafa/len, tafasitamab/lenalidomide.
FL
FL is incurable and often requires multiple treatments over a lifetime, highlighting the need for therapies capable of inducing durable remissions with minimal toxicity. Upfront FL treatment depends on disease stage and symptoms, and advanced stage symptomatic/bulky disease is typically treated with chemoimmunotherapy. In 2L, we generally favor lenalidomide with an anti-CD20 antibody.70,71 Until recently, 3L R/R FL treatment options were limited and included additional chemoimmunotherapy or the EZH2 inhibitor tazemetostat, which has an mPFS of 13.8 months in 20% of EZH2-mutated FLs.72 Treatment options in FL after 2+ therapies have since evolved rapidly, with CAR T cells and BsAbs now available.
Axi-cel, tisa-cel, and, very recently, liso-cel are approved in R/R FL. The pivotal phase 2 ZUMA-5 study treated patients with R/R FL after 2+ lines with axi-cel (median of 3 prior therapies), with 55% of patients classified as POD24 (progression of disease within 24 months of initial chemoimmunotherapy).18,19,20 The ORR was 94%, CRR was 79%, and mPFS was 57.3 months, with a median follow-up of 53.7 months. The mDOR was 55.5 months, and the median OS was NR. Most patients (78%) experienced CRS, although mostly grade 1 to 2 (72%), whereas 6% were grade 3+, and there was 1 fatal event. NEs occurred in 56% and approximately one-third of events were high grade (41% grades 1-2, 15% grade 3-4, and no fatal events). Axi-cel is also under investigation in 2L FL.73 The pivotal ELARA study of tisa-cel in R/R FL included more heavily pretreated and high-risk patients (median 4 prior lines and 62.9% with POD24).21,22 The ORR was 86.2%, CRR was 68.1%, and the median DOR, PFS, and OS were NR, with a median follow-up of 29 months; the estimated 24-month DOR was 66.4%. CRS occurred in 49% (all events grade 1-2), whereas NEs occurred in 37.1% (4.1% with any ICANS; 1% with grade 3+ ICANS). Importantly, with both axi-cel and tisa-cel, there has been a continual gradual pattern of relapse, suggesting that, in contrast to LBCL, CAR T cells are unlikely to be curative in FL but rather capable of inducing deep and long-lasting remissions. Notably, FLs re-treated on ZUMA-5 with axi-cel (n = 13) demonstrated an ORR of 100%, CRR 69%, and an mDOR 5.0 months (23 months of median follow-up),18,74 whereas CAR T-cell re-treatment has typically not been effective in LBCL.75
Liso-cel was recently approved in R/R 3L+ FL based on the phase 2 TRANSCEND FL study23,24,76 in 2L patients with high-risk disease (POD24 with treatment ≤6 months from original FL diagnosis and/or high tumor burden by modified GELF; n = 23) and 3L+ regardless of risk (n = 107). In 3L+ patients, the median prior treatments was 3, and 43% had POD24. In the efficacy analysis of 3L+ FL, the ORR was 97%, CRR was 94%, and mDOR and mPFS were NR at a median follow-up of 16.6 and 17.6 months, respectively. The rates of CRS and NEs were 58% and 15%, respectively, with rare grade 3 events (1% and 2%, respectively) and no grade 4+ events. In the 23 patients with high-risk 2L FL treated with liso-cel, the ORR was 96% (all complete), and mDOR and mPFS were NR at a median follow-up of 16.8 and 17.8 months, respectively; CRS occurred in 52% (all grade 1-2), and NEs occurred in 17% (1% grade 3 and no grade 4+).
When choosing a CAR T-cell product in 3L+ FL, axi-cel, tisa-cel, and liso-cel all demonstrate excellent efficacy.18,19,21,22,23 The more favorable toxicity profiles of tisa-cel and liso-cel may more strongly influence product selection for an indolent disease such as FL, in which the shorter turnaround time for axi-cel is usually less relevant than in LBCL.
The IV-administered BsAb mosunetuzumab is approved as fixed-duration therapy (8-17 cycles) in R/R FL based on a phase 2 study in patients with 3L+ FL (n = 90) with a median of 3 prior therapies and 52% with POD24.25-27 The ORR was 78%, CRR was 60%, mPFS was 24 months, median DOR was 35.9 months, and median DOCR was not reached (median follow up of 37.4 months). CRS occurred in 44% (42% grade 1-2, 1% grade 3, and 1% grade 4), and NEs occurred in 5% (all grade 1-2). The subcutaneously administered BsAb epcoritamab has also been approved in R/R FL as continuous therapy based on the Epcore NHL-1 FL in patients with R/R FL 3L+ (n = 128; median of 3 prior therapies; POD24 in 42%).28,29 After a median follow-up of 17.4 months, ORR was 82%, CRR was 63%, mPFS was 15.4 months, and the median DOR, duration of CR, and OS were NR. CRS occurred in 67% (65% grade 1-2 and 2% grade 3), and ICANS occurred in 6% (all grade 1-2). Finally, IV odronextamab, although not yet approved in FL, was studied in the ELM-2 study in patients with 3L+ R/R classic FL and demonstrated favorable results.77,78
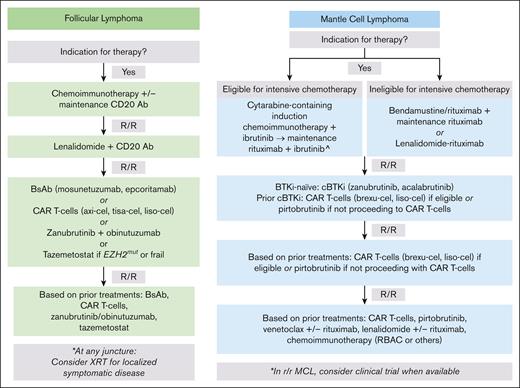
In R/R FL, neither CAR T cells nor BsAbs appear curative, and therefore, shared patient-physician decision-making in product sequencing is imperative (Figure 4). Generally, we suggest consideration of a BsAb before CAR T cells, given the high response rates, low incidence of immune-related toxicities, avoidance of LDC, and preliminary data suggesting durable responses; however, the true durability of BsAb responses in R/R FL needs longer follow-up. At the same time, FL is highly heterogenous, and a CAR might be preferred for higher-risk disease, including POD24 followed by rapid relapse after 2L therapy. CARs are also attractive for a young patient with minimal medical comorbidities who values a “one and done” approach to maximize time off therapy. Finally, clinical concern about occult high-grade transformation warrants consideration of a CAR T cell before BsAb, given proven curability of CAR in transformed FL,4-11,14,15 whereas longer follow-up is needed for BsAbs.
Sequencing treatments in FL and MCL. Flow charts outline a proposed sequencing of therapies in FL and MCL based on current approvals and highlighting when to consider CAR T-cells and/or BsAb. In R/R FL, patients should again have an indication for therapy to proceed to next line of treatment. ^This approach is based on the TRIANGLE study.79 In situations in which 1L ibrutinib is not available, recommend treatment with cytarabine-containing induction chemoimmunotherapy followed by maintenance rituximab and replacement of ibrutinib with another covalent BTKi if possible. BR, bendamustine rituximab; CD20 Ab, CD20 antibody; RBAC, rituximab, bendamustine, cytarabine.
Sequencing treatments in FL and MCL. Flow charts outline a proposed sequencing of therapies in FL and MCL based on current approvals and highlighting when to consider CAR T-cells and/or BsAb. In R/R FL, patients should again have an indication for therapy to proceed to next line of treatment. ^This approach is based on the TRIANGLE study.79 In situations in which 1L ibrutinib is not available, recommend treatment with cytarabine-containing induction chemoimmunotherapy followed by maintenance rituximab and replacement of ibrutinib with another covalent BTKi if possible. BR, bendamustine rituximab; CD20 Ab, CD20 antibody; RBAC, rituximab, bendamustine, cytarabine.
MCL
Chemoimmunotherapy is the standard initial treatment for MCL, often followed by maintenance rituximab.80,81 The role of ASCT in upfront MCL is evolving and may be obviated by the addition of a covalent Bruton tyrosine kinase inhibitor (cBTKi) to induction and maintenance.79 However, chemoimmunotherapy is typically not curative for MCL, and at first relapse, acalabrutinib82,83 and zanubrutinib84 are available in the United States for BTKi-naïve patients with an mPFS of 20 and 33 months, respectively. Notably, survival after progression on cBTKi is poor, with a median OS of only 2.9 months in a global retrospective analysis.85 Until recently, subsequent therapies were limited and included lenalidomide (ORR, 28%; mPFS, 4.0 months)86 and venetoclax (ORR, 53%; mPFS, 3.2 months).87 The noncovalent BTKi pirtobrutinib is the most recent addition to the R/R MCL armamentarium, and in patients with prior cBTKi, it demonstrates an ORR of 49.3% (CRR, 15.8%), mPFS of 5.6 months, and mDOR of 21.6 months.88
Brexu-cel was the first CAR approved for R/R MCL. In the pivotal phase 2 ZUMA-2 study, patients with R/R MCL after 2+ prior lines including cBTKi received brexu-cel, which demonstrated an ORR of 91%, CRR 68%, mDOR 28.2 months, mPFS 25.8 months, and OS of 46.6 months (median follow-up, 35.6 months).30,31 Almost all patients had CRS (91%), mostly grade 1 to 2 (76%) and 15% grade 3 to 4; and 63% of patients had NEs, of which 32% were grade 1-2 and 31% were grade 3-4. Real-world data for brexu-cel demonstrate similar response rates and toxicity, including in ZUMA-2–ineligible patients.89-91 Very recently, liso-cel was approved in R/R MCL based on the TRANSCEND NHL 001 study in which patients after 2+ prior lines including cBTKi received liso-cel and had a CRR of 72.3%, with mDOR of 15.7 months and mPFS of 15.3 months.32 Grade 3 or 4 CRS and NEs occurred in 1% and 9% of patients, respectively, with no grade 5 events. Although the long-term durability of liso-cel responses remains to be seen, notable differences in side effects will likely lead to liso-cel being favored over brexu-cel for many patients when both products are available.
BsAbs are in development in R/R MCL and, once approved, will expand therapeutic offerings including for high-risk disease (eg, TP53 mutations and blastoid morphology), in which CAR T-cell responses may be inferior. The largest initial cohort of BsAb-treated patients with MCL (n = 37) received glofitamab after a median of 3 prior lines of therapy (including 64.9% with prior BTKi) preceded by 2 different doses of obinutuzumab pretreatment.92 With a median follow-up of 8 months, ORR was 83.8%, CRR was 73.0%, and mDOR was 12.6 months. Most CRS was low grade, and incidence of grade 3+ CRS appeared reduced with the higher obinutuzumab dose (2000 mg; 9.5% grade 3 CRS; vs 1000 mg; 12.5% grade 3 and 12.5% grade 4 CRS); ICANS occurred in 13.5% across cohorts and was all grade 1 or 2. A phase 3 study randomizing patients with 2L+ R/R MCL to glofitamab or investigator’s choice of bendamustine-rituximab or rituximab-lenalidomide93 is ongoing. Epcoritamab94 and odronextamab95 also demonstrate promising response rates and manageable toxicity in MCL, albeit with limited numbers.
When sequencing novel therapies in MCL (Figure 4), current options for multiply relapsed disease after a cBTKi include brexu-cel, liso-cel, and pirtobrutinib, which have a mPFS of 25.8 months,30,31 15.3 months,32 and 5.6 months,88 respectively. Thus, although CAR T cells may not be curative in MCL, they do offer the best chance at a prolonged remission among the current options and therefore should be prioritized after cBTKi. Pirtobrutinib remains appealing in patients without access to CAR or after CAR. We also eagerly await BsAb approvals in MCL.
Future directions
CAR T cells and BsAbs have revolutionized the treatment landscape of R/R B-NHL (Table 1; Figures 3 and 4). Ongoing studies will evaluate clinical questions including agents in combination with BsAbs or CAR T cells, BsAb and CAR T-cell therapy in earlier lines (eg, BsAb monotherapy in patients with 2L LBCL not proceeding with CAR T cells and CAR T cells in 1L high-risk LBCL), and BsAbs in bridging and maintenance after CAR. In the future, we anticipate BsAbs will ultimately have a role in frontline treatment, including with chemotherapy in LBCL and potentially in lieu of chemotherapy in treatment-naïve FL. At the same time, ongoing investigations into CAR optimization include combinatorial approaches to enhance efficacy and development of novel CAR constructs and allogeneic products. Ultimately, it will be crucial to ensure all eligible patients have access to these potentially life-saving T-cell–mediated therapies.
Authorship
Contribution: J.E.H. and J.S.A. planned and wrote the manuscript.
Conflict-of-interest disclosure: J.E.H. reports consulting for Genmab and research funding (to institution) from Genmab. J.S.A. reports consulting for AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), Cellectar, Caribou Biosciences, Celgene, Genentech, Gilead, Incyte, Interius, Janssen, Lilly, Novartis, Roche, Seagen, and Takeda, and research support (to institution) from BMS, Celgene, Cellectis, Genentech, Merck, Mustang Bio, Regeneron, Seagen, and Takeda.
Correspondence: J. Erika Haydu, Lymphoma Program, Mass General Cancer Center, 55 Fruit St, Yawkey 9A, Boston, MA 02114; email: julie_haydu@mgh.harvard.edu.