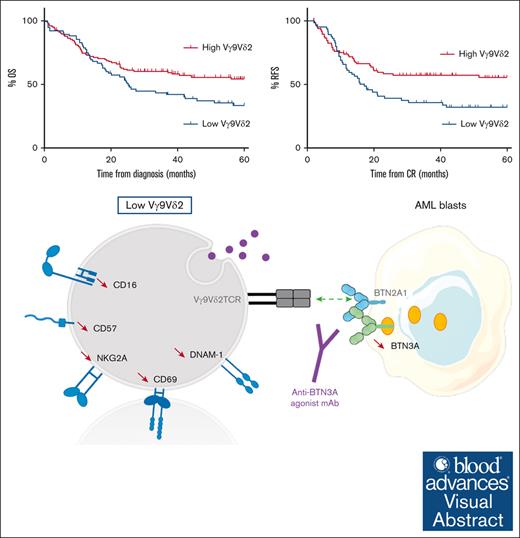
Key Points
A low frequency of Vγ9Vδ2 T cells in AML is an independent predictor of poor clinical outcome.
These data provide a strong rationale for the development of consolidation protocols aimed at enhancing Vγ9Vδ2 T-cell responses.
Visual Abstract
In several tumor subtypes, an increased infiltration of Vγ9Vδ2 T cells has been shown to have the highest prognostic value compared with other immune subsets. In acute myeloid leukemia (AML), similar findings have been based solely on the inference of transcriptomic data and have not been assessed with respect to confounding factors. This study aimed at determining, by immunophenotypic analysis (flow or mass cytometry) of peripheral blood from patients with AML at diagnosis, the prognostic impact of Vγ9Vδ2 T-cell frequency. This was adjusted for potential confounders (age at diagnosis, disease status, European LeukemiaNet classification, leukocytosis, and allogeneic hematopoietic stem cell transplantation as a time-dependent covariate). The cohort was composed of 198 patients with newly diagnosed (ND) AML. By univariate analysis, patients with lower Vγ9Vδ2 T cells at diagnosis had significantly lower 5-year overall and relapse-free survivals. These results were confirmed in multivariate analysis (hazard ratio [HR], 1.55 [95% confidence interval (CI), 1.04-2.30]; P = .030 and HR, 1.64 [95% CI, 1.06-2.53]; P = .025). Immunophenotypic alterations observed in patients with lower Vγ9Vδ2 T cells included a loss of some cytotoxic Vγ9Vδ2 T-cell subsets and a decreased expression of butyrophilin 3A on the surface of blasts. Samples expanded regardless of their Vγ9Vδ2 T-cell levels and displayed similar effector functions in vitro. This study confirms the prognostic value of elevated Vγ9Vδ2 T cells among lymphocytes in patients with ND AML. These results provide a strong rationale to consider consolidation protocols aiming at enhancing Vγ9Vδ2 T-cell responses.
Introduction
The immune landscape of acute myeloid leukemia (AML) is becoming better described, and there is increasing evidence that immune features may modulate prognosis independently of genetic markers.1-3 However, immune alterations in AML are broadly heterogeneous among patients in terms of immune infiltration and expression of immune checkpoint receptors,4-6 suggesting distinct immunoregulatory mechanisms and complicating the development of immune signatures.4,7 This complexity also led to a slow clinical translation of immunotherapeutic strategies in AML.3,8 Most studies have so far focused on conventional T cells2,5,9 or natural killer (NK) cells,10-12 but very little data are available on the impact of γδ T-cell populations in AML.
The major role of γδ T cells has emerged in infectious diseases and antitumoral immunity.13 These cells combine adaptive and innate characteristics. Their activation is not restricted by the major histocompatibility complex14,15 and depends on ligand recognition by their T-cell receptor (TCR) and other receptors such as DNAX accessory molecule-1 (DNAM-1)16 or natural killer group 2 member D (NKG2D).17 These unique recognition capabilities distinguish them from other lymphocytes. Human γδ T cells represent 1% to 10% of circulating T cells.18 Based on the TCR δ-chain, they can be classified into 4 major groups: Vδ1, Vδ2, Vδ3, and Vδ5. Vδ2+ is the major subtype in peripheral blood (PB) and usually pairs with the Vγ9 chain. Vδ1+ accounts for 25% of PB γδ T cells and Vδ3+ for <1%.19 A direct rapid effector response is exhibited by γδ T cells, which have the ability to activate other immune cells through cytokine release or through their antigen presentation capabilities.20,21
The progressive discovery of the elusive mechanism of action of γδ T cells and of their prognostic implications has evolved in parallel with γδ T-cell–based immunotherapy approaches, with promising results. The latter include allogeneic adoptive transfer of γδ T cells, from αβ T-cell–depleted products, and of ex vivo expanded γδ T-cell infusions, with optimized expansion strategies or genetic engineering.22 Adoptive transfer is currently at the forefront in AML, several studies evaluating the efficacy of ex vivo expanded allogeneic γδ T cells in refractory or relapsed (R/R) AML. In vivo stimulation strategies targeting Vγ9Vδ2 T cells were initially based on the use of aminobiphosphonates but have shown limited efficacy.23 However, new methods of autologous Vγ9Vδ2 T-cell–based immunotherapy are emerging, in particular with bispecific or monoclonal antibodies (mAbs) targeting γδ TCRs directed against tumoral antigens such as human epidermal growth factor receptor 2 (HER-2)24 or CD123.25
Vγ9Vδ2 T cells are activated by phosphoantigens (pAgs), produced by infected or transformed cells, via the mevalonate pathway during isoprenoid biosynthesis.26,27 The antitumoral activity of Vγ9Vδ2 T cells is triggered by pAgs binding to the intracellular domain of butyrophilins (BTNs) 3A1 and 2A1.28-32 Vγ9Vδ2 T cells exert pleiotropic antitumoral potential against a wide range of tumors, including hematological malignancies.33 A particular activity of Vγ9Vδ2 in AML is suspected because they exhibit a high cytotoxic potential against myeloid blasts both in vitro and in vivo.34-38 Early clinical trials of Vγ9Vδ2 T-cell–based immunotherapy have indeed led to objective responses in AML.39,40 The frequency of Vγ9Vδ2 T cells has also been observed to vary according to induction chemotherapy responses.41,42
Importantly, analysis of nearly 18 000 bulk transcriptomes from 39 human tumors has revealed the high prognostic value of high γδ T-cell infiltration, which had the best prognostic value compared with other immune subsets.43 Another study confirmed the association between Vγ9Vδ2 T-cell infiltration and favorable outcomes in a broad spectrum of cancers.44 Nevertheless, deconvolution of bulk transcriptomes using the CIBERSORT algorithm raises the issue of misclassification for some immune subsets. Besides, conflicting data have been reported with a negative role of γδ T cells in several solid tumors,45-47 possibly based on protumoral properties for some γδ T-cell subsets depending on the tumoral context.48
In AML, demonstration of the poor prognostic impact of a low frequency of Vγ9Vδ2 T cells at diagnosis is based on results extrapolated from bulk RNA sequencing analysis.44 Yet, the presence of potential confounding factors has not been assessed, preventing any solid conclusion on this matter.
Therefore, the aim of this study was to determine the prognostic impact of the PB frequency of Vγ9Vδ2 T cells, determined by immunophenotypic analysis, in newly diagnosed (ND) treatment-naïve patients with AML, adjusting for potential confounding factors.
Methods
Study design
This study merged 4 different cohorts: 2 from the Hematology Department of Institut Paoli-Calmettes (IPC), 1 retrospective (IPC tumor bank, IPC16-007; n = 21) and 1 prospective (HEMATO-BIO-IPC 2013-015, NCT02320656, ANSM 131368B-11; n = 40); 2 cohorts from prospective multicenter randomized phase 3 trials from the French Innovative Leukemia Organization (FILO) group, AML-2006-IR cohort (NCT00860639; n = 84) and LAM-SA 2007 cohort (NCT00590837; n = 53). Patients from the IPC cohort were diagnosed between December 2002 and October 2018 and FILO patients between November 2007 and November 2012. PB mononuclear cells (PBMCs) from healthy volunteers (HVs) were obtained from the Etablissement Français du Sang (authorization number AC-2019-3428 issued by the Ministry of Higher Education, Research and Innovation).
Patients were aged from 18 to 81 years and were treated with conventional anthracycline-based induction chemotherapy. In the AML-2006-IR cohort, patients were then randomly assigned to receive a single dose of gemtuzumab ozogamicin in arm A. In the LAM-SA 2007 cohort, patients were randomized to receive lomustine or not during the induction and postinduction treatment phases.
Patients were included if PB Vγ9Vδ2 T-cell frequency could be assessed.
Patients with AML3, with unknown or adverse European LeukemiaNet (ELN) risk classification were excluded. Patient characteristics are summarized in Table 1.
Baseline patient and disease characteristics
| . | All patients . |
|---|---|
| N = 198 . | |
| Age, y | |
| Median (range) | 56.0 (18.9-81.5) |
| >60, n (%) | 75 (37.9) |
| Sex, n (%) | |
| Male | 109 (55.05) |
| Female | 89 (44.95) |
| AML etiology, n (%) | |
| De novo | 185 (93.4) |
| Secondary | 13 (6.6) |
| WBC, ×10⁹/L | |
| Median (range) | 25.7 (0.6-191.3) |
| >50, n (%) | 56 (28.3) |
| NA, n (%) | 2 (1.0) |
| FAB category, n (%) | |
| M0 | 4 (2.0) |
| M1 | 46 (23.2) |
| M2 | 43 (21.7) |
| M3 | 0 (0) |
| M4 | 51 (25.8) |
| M5 | 35 (17.7) |
| M6 | 4 (2.0) |
| M7 | 0 (0) |
| NA | 15 (7.6) |
| PB blasts, % | |
| Mean (SD) | 50.4 (32.9) |
| ELN classification, n (%) | |
| Favorable | 79 (39.9) |
| Intermediate | 119 (60.1) |
| CBF mutation, n (%) | 24 (12.1) |
| CEBPA mutation, n (%) | 20 (13.5) |
| NPM1 mutation, n (%) | 92 (53.8) |
| FLT3 mutation, n (%) | 58 (34.1) |
| Vγ9Vδ2 (% of LC) | |
| Mean (SD) | 2.5 (4.3) |
| 30-d mortality, n (%) | 3 (1.5) |
| 60-d mortality, n (%) | 11 (5.6) |
| CR after induction, n (%) | 161 (81.3) |
| HSCT, n (%) | 32 (16.3) |
| . | All patients . |
|---|---|
| N = 198 . | |
| Age, y | |
| Median (range) | 56.0 (18.9-81.5) |
| >60, n (%) | 75 (37.9) |
| Sex, n (%) | |
| Male | 109 (55.05) |
| Female | 89 (44.95) |
| AML etiology, n (%) | |
| De novo | 185 (93.4) |
| Secondary | 13 (6.6) |
| WBC, ×10⁹/L | |
| Median (range) | 25.7 (0.6-191.3) |
| >50, n (%) | 56 (28.3) |
| NA, n (%) | 2 (1.0) |
| FAB category, n (%) | |
| M0 | 4 (2.0) |
| M1 | 46 (23.2) |
| M2 | 43 (21.7) |
| M3 | 0 (0) |
| M4 | 51 (25.8) |
| M5 | 35 (17.7) |
| M6 | 4 (2.0) |
| M7 | 0 (0) |
| NA | 15 (7.6) |
| PB blasts, % | |
| Mean (SD) | 50.4 (32.9) |
| ELN classification, n (%) | |
| Favorable | 79 (39.9) |
| Intermediate | 119 (60.1) |
| CBF mutation, n (%) | 24 (12.1) |
| CEBPA mutation, n (%) | 20 (13.5) |
| NPM1 mutation, n (%) | 92 (53.8) |
| FLT3 mutation, n (%) | 58 (34.1) |
| Vγ9Vδ2 (% of LC) | |
| Mean (SD) | 2.5 (4.3) |
| 30-d mortality, n (%) | 3 (1.5) |
| 60-d mortality, n (%) | 11 (5.6) |
| CR after induction, n (%) | 161 (81.3) |
| HSCT, n (%) | 32 (16.3) |
CBF, core-bing factor; CEBPA, CCAAT enhancer binding protein alpha; FAB, French-American-British classification; FLT3, fms-like tyrosine kinase 3; LC, lymphocyte; NPM1, nucleophosmin 1; WBC, white blood cell; SD, standard deviation.
AML diagnosis was established according to the World Health Organization classification.49 Cytogenetic and molecular analyses were used for patient stratification. Response to treatment and relapse were evaluated according to ELN 2010 recommendations.50
All participants provided written informed consent in accordance with the Declaration of Helsinki.
Clinical samples
PBMCs obtained before induction chemotherapy or from HV were isolated by density gradient (Lymphoprep, Stemcell Technologies, Vancouver, Canada) and cryopreserved in 90% fetal calf serum/10% dimethyl sulfoxide (DMSO) (FILO), 90% albumin/10% DMSO (HEMATOBIO and age-matched HV), or 80% RPMI/ or 10% fetal calf serum/10% DMSO (IPC tumor bank).
Sample handling, conditioning, and storage were respectively performed by the tumor banks of the FILO group (N° BB-0033–00073) and the IPC tumor bank (AC-2007–33 granted by the French Ministry of Research).
Flow and mass cytometry analysis
Samples were included if at least 1 000 000 viable cells could be labeled. The proportion of Vγ9Vδ2 T cells among PB lymphocytes was assessed by flow or mass cytometry. Supplemental Tables 2 and 3 provide the list of mAbs used, and supplemental Figure 9 describes the gating strategy.
For flow cytometry, PMBCs were washed and incubated with human Fc Block (BD Biosciences, San Jose, CA) before immunostaining with a mix of extracellular antibodies (supplemental Table 2). A fluorescence-activated cell sorter (FACS) LSR-Fortessa or a BD FACSCanto II (BD Biosciences) were used for acquisition, and data were analyzed using DIVA 8.0.1 software (BD Biosciences).
The mass cytometry experiments were performed as previously described.51 Extracellular and intracellular antibodies are listed in supplemental Table 3. Cells were acquired on a Helios mass cytometer (Fluidigm), and samples were further analyzed using FlowJo V10.6.2 (BD Biosciences). Statistical analyses of markers expressed by Vγ9Vδ2 T cells were performed when >30 or >50 viable Vγ9Vδ2 T cells were analyzed in mass or flow cytometric cytometry panels, respectively.
Vγ9Vδ2 T cells were manually gated and exported using FlowJo V10.6.2. Consensus files were generated using the OMIQ software from Dotmatics with a downsampling to a total number of 10 000 Vγ9Vδ2 T cells for each group. Vγ9Vδ2 T-cell subpopulations and markers were described using the t-distributed stochastic neighbor embedding (t-SNE) dimensionality reduction algorithm.
Generation of anti-human BTN3A mAbs
To generate anti-BTN3A 20.1 and anti-BTN3A 108.5 mAbs, BALB/c mice were immunized with a soluble BT3.1-Ig fusion protein, as previously described.52 The relative surface expression of BTN3A (mAbs 20.1 and 108.5) on AML blasts was then assessed by flow cytometry using calibrator beads, allowing for the quantification of antigen density (Quantum Simply Cellular, Bangs Laboratories, Fishers, IN). Cells were acquired on a BD FACSCanto II and analyzed using Flowjo V10.6.2.
Expansion of Vγ9Vδ2 T cells
For the establishment of allogeneic Vγ9Vδ2 T cells, fresh PBMCs from HV were stimulated with zoledronate (ZOL) 1 μM (Sigma-Aldrich, Saint Louis, MO) and recombinant human interleukin-2 (rhIL-2; Miltenyi Biotec, Bergisch Gladbach, Germany) at day 0. From day 5, rhIL-2 (100 IU/mL) was renewed every 2 days, and cells were kept at 1.5 × 106/mL until day 14. Only cell cultures that reached >80% of Vγ9Vδ2 T-cell purity on day 14 were selected and frozen until use. Allogeneic Vγ9Vδ2 T cells were thawed and cultured with rhIL-2 (200 UI/mL) overnight before functional assays.
For the establishment of autologous Vγ9Vδ2 T cells, thawed PBMCs from patients with AML were treated similarly, but rhIL-15 (10 ng/mL) was also added to enhance their proliferative capacities.53-55 Fresh autologous expanded cells were then used for functional assays, depending on the quantity of Vγ9Vδ2 T cells. Fold increases of viable Vɣ9Vδ2 T cells were calculated according to the following formula: (day-14 %Vɣ9Vδ2 × day-14 total cell number)/(day-0 %Vγ9Vδ2 × day-0 total cell number). Cells were acquired on a BD FACSCanto II and analyzed using DIVA 8.0.1 software.
Degranulation assays
To analyze CD107 expression, autologous Vγ9Vδ2 T cells and primary AML blasts were cocultured in an effector-to-target ratio of 1:1 with anti-CD107a, anti-CD107b, GolgiStop, and anti-BTN3A 20.1 agonist mAb or isotype control (1 μg/mL). Primary AML blasts preincubated overnight with zoledronate (45 μM) were used as positive control. After 4 hours, cells were collected and analyzed by flow cytometry. Cells were acquired on a BD FACSCanto II and analyzed using DIVA 8.0.1 software.
Statistics
Statistical analyses were carried out using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA), Statistical Package for the Social Sciences (SPSS software, Chicago, IL), and R software (www.rproject.org).
Normality of distributions was assessed using the d’Agostino-Pearson normality test.
Comparisons of categorial variables were performed using χ2 or Fisher exact tests. For continuous variables, comparisons between 2 groups used 2-tailed Mann-Whitney U test or unpaired t test. For multiple comparisons of paired values, a Friedman test was performed followed by a Dunn's test. For multiple comparisons of independent samples, a Kruskal-Wallis test was performed followed by a Dunn's test. Patient groups were defined according to the frequency of Vγ9Vδ2 T cells among lymphocytes. The threshold was defined based on optimized cutoff using maximally selected log-rank statistics (maxstat package, R software V 3.6.2).56
A multivariate Cox regression model was used to assess the prognostic value of Vγ9Vδ2 T-cell frequency, adjusting for other prognostic factors: age at diagnosis, disease status, ELN classification, and allogeneic hematopoietic stem cell transplantation (HSCT) as time-dependent covariate.
Results
Patients with AML with a low frequency of Vγ9Vδ2 T cells have poorer survival
A total of 198 patients were included. Their mean age was 56 years (range, 18.9-81.5); 79 (39.9%) had favorable ELN risk and 119 (60.1%) an intermediate ELN risk. The mean complete remission (CR) rate was 81.3% (Table 1). Analysis of overall survival (OS) and relapse-free survival (RFS), based on the frequency of Vγ9Vδ2 T cells, showed the 0.75% threshold to be the most significantly discriminant (Figure 1A-B) and identified the high and low frequencies referred to thereupon.
Univariate survival analysis according to the frequency of Vγ9Vδ2 T cells. (A-B) Threshold determination of Vγ9Vδ2 T cells among viable lymphocytes is determined using the optimal cut points, performed using the maximally selected log-rank statistics using the maxstat R package. For a given prognostic parameter, maxstat identifies the optimal cut point that best discriminates 2 patient groups (https://cran.r-project.org/web/packages/maxstat/index.html). The threshold of 0.75 % of Vγ9Vδ2 T cells among viable lymphocytes was selected for this study. (C-D) 5-y OS and 5-y RFS (n = 198 and 161, respectively) were estimated using the Kaplan-Meier method and compared between groups using the log-rank test.
Univariate survival analysis according to the frequency of Vγ9Vδ2 T cells. (A-B) Threshold determination of Vγ9Vδ2 T cells among viable lymphocytes is determined using the optimal cut points, performed using the maximally selected log-rank statistics using the maxstat R package. For a given prognostic parameter, maxstat identifies the optimal cut point that best discriminates 2 patient groups (https://cran.r-project.org/web/packages/maxstat/index.html). The threshold of 0.75 % of Vγ9Vδ2 T cells among viable lymphocytes was selected for this study. (C-D) 5-y OS and 5-y RFS (n = 198 and 161, respectively) were estimated using the Kaplan-Meier method and compared between groups using the log-rank test.
As summarized in Table 2, patients with AML with lower Vγ9Vδ2 T cells tended to be older (56.9 vs 54.5 years; P = .058). No difference was observed in Vγ9Vδ2 T-cell frequency group according to the French-American-British classification,49 response to induction in all cohorts (Table 1; supplemental Figure 1A); however, patients with favorable ELN risk had a lower Vγ9Vδ2 T-cell frequency (supplemental Figure 1B). In the FILO cohorts, patients who received lomustine in the LAM-SA 2007 trial (n = 21) were more often in the low Vγ9Vδ2 T cells group (supplemental Table 1). As Vγ9Vδ2 T cells are also critical effectors against infection,57 the proportion of infectious complications was compared after induction in the FILO cohorts depending on Vγ9Vδ2 T cells, but no difference was observed in terms of incidence of septic shock nor G3/4 infectious adverse events.
Baseline patient and disease characteristics according to the frequency of Vγ9Vδ2 T cells
| . | Low Vγ9Vδ2 T cells (≤0.75%/LC) . | High Vγ9Vδ2 T cells (> 0.75%/LC) . | P value . |
|---|---|---|---|
| n = 75 (37.9%) . | n = 123 (62.1%) . | ||
| Age, y | |||
| Median (range) | 56.9 (22.4-80.4) | 54.5 (18.9-81.5) | .058 |
| >60 | 30 (40) | 45 (36.6) | .652 |
| Sex, n (%) | |||
| Male | 38 (50.7) | 71 (57.7) | .378 |
| Female | 37 (49.3) | 52 (42.3) | |
| AML etiology, n (%) | |||
| De novo | 69 (92) | 116 (94.3) | .562 |
| Secondary | 6 (8) | 7 (5.7) | |
| WBC, ×10⁹/L | |||
| Median (range) | 30.2 (0.6-191.3) | 24.6 (1.1-154.9) | .451 |
| >50, n (%) | 23 (31.1) | 33 (26.8) | .423 |
| NA, n (%) | 1 (1.3) | 1 (0.8) | |
| FAB category, n (%) | |||
| M0 | 2 (2.7) | 2 (1.6) | .397 |
| M1 | 15 (20.0) | 31 (25.2) | |
| M2 | 14 (18.7) | 29 (23.6) | |
| M3 | 0 (0) | 0 (0) | |
| M4 | 22 (29.3) | 29 (23.6) | |
| M5 | 16 (21.3) | 19 (15.4) | |
| M6 | 0 (0) | 4 (3.3) | |
| M7 | 0 (0) | 0 (0) | |
| NA | 6 (8.0) | 9 (7.3) | |
| PB blasts, % | |||
| Mean (SD) | 49.5 (31.1) | 50.9 (34.1) | .828 |
| ELN classification, n (%) | |||
| Favorable | 33 (44.0) | 46 (37.4) | .373 |
| Intermediate | 42 (56.0) | 77 (62.6) | |
| CBF mutation, n (%) | 10 (13.3) | 14 (11.4) | .823 |
| CEBPA mutation, n (%) | 7 (13.0) | 13 (13.8) | >.999 |
| NPM1 mutation, n (%) | 37 (56.9) | 55 (51.9) | .532 |
| FLT3 mutation, n (%) | 22 (34.4) | 36 (34.0) | >.999 |
| Vγ9Vδ2 T cells among LC, % | |||
| Mean (SD) | 0.4 (0.2) | 3.9 (5.1) | <.0001 |
| 30-d mortality, n (%) | 1 (1.3) | 2 (1.6) | >.999 |
| 60-d mortality, n (%) | 6 (8.0) | 5 (4.1) | .339 |
| CR after induction, n (%) | 64 (85.3) | 97 (78.9) | .347 |
| HSCT, n (%) | 12 (16.0) | 20 (16.5) | >.999 |
| . | Low Vγ9Vδ2 T cells (≤0.75%/LC) . | High Vγ9Vδ2 T cells (> 0.75%/LC) . | P value . |
|---|---|---|---|
| n = 75 (37.9%) . | n = 123 (62.1%) . | ||
| Age, y | |||
| Median (range) | 56.9 (22.4-80.4) | 54.5 (18.9-81.5) | .058 |
| >60 | 30 (40) | 45 (36.6) | .652 |
| Sex, n (%) | |||
| Male | 38 (50.7) | 71 (57.7) | .378 |
| Female | 37 (49.3) | 52 (42.3) | |
| AML etiology, n (%) | |||
| De novo | 69 (92) | 116 (94.3) | .562 |
| Secondary | 6 (8) | 7 (5.7) | |
| WBC, ×10⁹/L | |||
| Median (range) | 30.2 (0.6-191.3) | 24.6 (1.1-154.9) | .451 |
| >50, n (%) | 23 (31.1) | 33 (26.8) | .423 |
| NA, n (%) | 1 (1.3) | 1 (0.8) | |
| FAB category, n (%) | |||
| M0 | 2 (2.7) | 2 (1.6) | .397 |
| M1 | 15 (20.0) | 31 (25.2) | |
| M2 | 14 (18.7) | 29 (23.6) | |
| M3 | 0 (0) | 0 (0) | |
| M4 | 22 (29.3) | 29 (23.6) | |
| M5 | 16 (21.3) | 19 (15.4) | |
| M6 | 0 (0) | 4 (3.3) | |
| M7 | 0 (0) | 0 (0) | |
| NA | 6 (8.0) | 9 (7.3) | |
| PB blasts, % | |||
| Mean (SD) | 49.5 (31.1) | 50.9 (34.1) | .828 |
| ELN classification, n (%) | |||
| Favorable | 33 (44.0) | 46 (37.4) | .373 |
| Intermediate | 42 (56.0) | 77 (62.6) | |
| CBF mutation, n (%) | 10 (13.3) | 14 (11.4) | .823 |
| CEBPA mutation, n (%) | 7 (13.0) | 13 (13.8) | >.999 |
| NPM1 mutation, n (%) | 37 (56.9) | 55 (51.9) | .532 |
| FLT3 mutation, n (%) | 22 (34.4) | 36 (34.0) | >.999 |
| Vγ9Vδ2 T cells among LC, % | |||
| Mean (SD) | 0.4 (0.2) | 3.9 (5.1) | <.0001 |
| 30-d mortality, n (%) | 1 (1.3) | 2 (1.6) | >.999 |
| 60-d mortality, n (%) | 6 (8.0) | 5 (4.1) | .339 |
| CR after induction, n (%) | 64 (85.3) | 97 (78.9) | .347 |
| HSCT, n (%) | 12 (16.0) | 20 (16.5) | >.999 |
CBF, core-bing factor; CEBPA, CCAAT enhancer binding protein alpha; FAB, French-American-British; FLT3, fms-like tyrosine kinase 3; LC, lymphocyte; NPM1, nucleophosmin 1; WBC, white blood cell; SD, standard deviation.
In univariate analysis, patients with a low frequency of Vγ9Vδ2 T cells had significantly lower 5-year OS and 5-year RFS (43.1% vs 64%; hazard ratio [HR], 1.54 [95% confidence interval (CI), 1.03-2.31]; P = .028 and 42.3% vs 67.2%; HR, 1.69 [95% CI, 1.09-2.62]; P = .015, respectively). These results were confirmed in multivariate analyses (HR, 1.55 [95% CI, 1.04-2.30]; P = .030 and HR, 1.64 [95% CI, 1.06, 2.53]; P = .025; Table 3). CR and early mortality rates were similar in both groups of Vγ9Vδ2 T-cell frequency (Table 2).
Univariate and multivariate analysis of factors influencing survival
| Variable . | 5-y OS . | 5-y RFS . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age, y | ||||||||||||
| >60 vs ≤60 | 2.21 | (1.46-3.34) | <.0001 | 1.90 | (1.27-2.83) | .002 | 1.98 | (1.25-3.13) | .001 | 1.57 | (1.01-2.46) | .045 |
| WBC, ×10⁹/L | ||||||||||||
| >50 vs ≤50 | 0.64 | (0.4-1.01) | .093 | NE∗ | 0.99 | (0.6-1.59) | .977 | NE∗ | ||||
| ELN classification | ||||||||||||
| Intermediate vs favorable | 1.80 | (1.22-2.67) | .005 | 1.95 | (1.26-3.00) | .003 | 1.77 | (1.15-2.71) | .012 | 2.04 | (1.28-3.25) | .003 |
| Status | ||||||||||||
| Secondary vs de novo | 1.35 | (0.62-2.92) | .393 | NE∗ | 1.67 | (0.71-3.92) | .142 | 1.21 | (0.59-2.47) | .595 | ||
| Vγ9Vδ2 T cells among LC, % | ||||||||||||
| ≤0.75 vs >0.75 | 1.54 | (1.03-2.31) | .028 | 1.55 | (1.04-2.30) | .030 | 1.69 | (1.09-2.62) | .015 | 1.64 | (1.06-2.53) | .025 |
| HSCT | ||||||||||||
| Yes vs no | 0.46 | (0.28-0.76) | .017 | 0.59 | (0.30-1.16) | .127 | 0.53 | (0.31-0.88) | .045 | 0.38 | (0.17-0.86) | .019 |
| Variable . | 5-y OS . | 5-y RFS . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate analysis . | Multivariate analysis . | |||||||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | |
| Age, y | ||||||||||||
| >60 vs ≤60 | 2.21 | (1.46-3.34) | <.0001 | 1.90 | (1.27-2.83) | .002 | 1.98 | (1.25-3.13) | .001 | 1.57 | (1.01-2.46) | .045 |
| WBC, ×10⁹/L | ||||||||||||
| >50 vs ≤50 | 0.64 | (0.4-1.01) | .093 | NE∗ | 0.99 | (0.6-1.59) | .977 | NE∗ | ||||
| ELN classification | ||||||||||||
| Intermediate vs favorable | 1.80 | (1.22-2.67) | .005 | 1.95 | (1.26-3.00) | .003 | 1.77 | (1.15-2.71) | .012 | 2.04 | (1.28-3.25) | .003 |
| Status | ||||||||||||
| Secondary vs de novo | 1.35 | (0.62-2.92) | .393 | NE∗ | 1.67 | (0.71-3.92) | .142 | 1.21 | (0.59-2.47) | .595 | ||
| Vγ9Vδ2 T cells among LC, % | ||||||||||||
| ≤0.75 vs >0.75 | 1.54 | (1.03-2.31) | .028 | 1.55 | (1.04-2.30) | .030 | 1.69 | (1.09-2.62) | .015 | 1.64 | (1.06-2.53) | .025 |
| HSCT | ||||||||||||
| Yes vs no | 0.46 | (0.28-0.76) | .017 | 0.59 | (0.30-1.16) | .127 | 0.53 | (0.31-0.88) | .045 | 0.38 | (0.17-0.86) | .019 |
5-y OS and 5-y RFS were estimated using the Kaplan-Meier method and compared between groups using the log-rank test (n = 198 and 161, respectively). Among the usual AML prognostic factors (age, WBC, ELN 2010 risk category, de novo vs secondary AML, and HSCT), those with a P value <.15 were included in the multivariate logistic regression, with HSCT as a time-dependent covariate (n = 196 and 159 respectively for 5-y OS and 5-y RFS).
LC, lymphocyte; NE, not entered; WBC, white blood cell.
Not entered in the model.
Relapsed patients were found to have a significantly lower frequency of Vγ9Vδ2 T cells than those with sustained CR (supplemental Figure 2A). However, the proportions of NK cells and other lymphocyte subsets were similar regardless of outcome in the FILO cohorts (supplemental Figure 2B-C), as well as CD4+ and CD8+ T cells in the HEMATOBIO cohort (supplemental Figure 2D-E). Additionally, Vγ9Vδ2 T cells were found to contribute the most to the prognostic effect of γδ T cells, suggesting that Vδ1 subtype predominance is not involved (supplemental Figure 2F-I).
Interestingly, the impact of a low frequency of Vγ9Vδ2 T cells was independent of ELN risk (supplemental Figure 3), allogeneic HSCT (supplemental Figure 4), or age (supplemental Figure 5).
Hence, a low frequency of Vγ9Vδ2 T cells was independently associated with poor outcomes. This adverse impact was likely related to an increased risk of relapse, as evidenced by the similar CR or early mortality rates.
Low Vγ9Vδ2 T-cell levels are associated with immunophenotypic shifts of the Vγ9Vδ2 T-cell compartment
The immunophenotype of Vγ9Vδ2 T cells was then examined according to their frequency. In the FILO cohorts, we observed a great heterogeneity in the expression of Vγ9Vδ2 T-cell coactivating receptors, with a significant decrease in the expression of NKG2A, CD57, and DNAM-1 in patients with low Vγ9Vδ2 T-cell frequencies. At variance, there was no difference in NKG2D or CD56 levels (Figure 2A). Moreover, no association was observed between the expression of these 5 molecules and clinical outcomes (supplemental Figure 6).
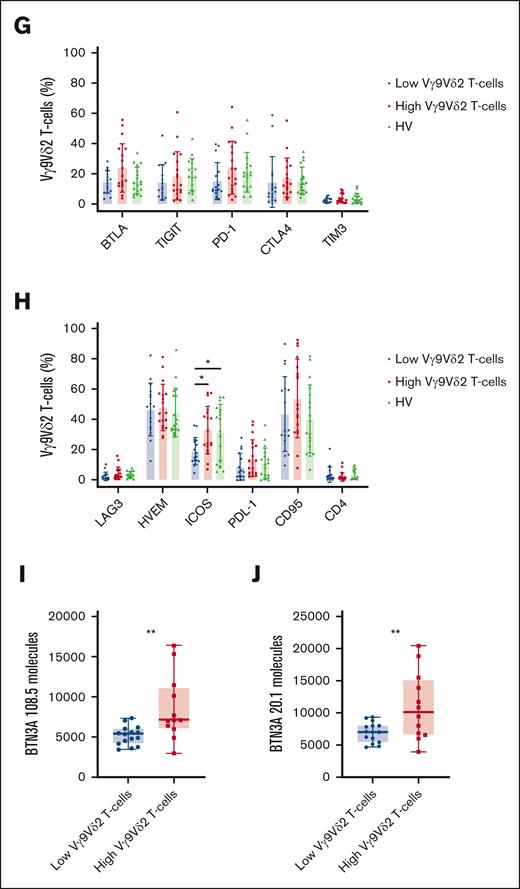
Low Vγ9Vδ2 T-cell phenotype is associated with phenotypical changes. (A) Expression of surface Vγ9Vδ2 T-cell coreceptors in the FILO cohorts according to the frequency group of Vγ9Vδ2 T cells: percentage of cells positive for CD57 (n = 123), NKG2A (n = 116), DNAM-1 (n = 116), NKG2D (n = 115), and CD56 (n = 123). (B) Vγ9Vδ2 T cells from PBMCs of HVs and patients with AML from the HEMATOBIO cohort were manually pregated and exported in OMIQ for t-SNE analysis. Subsampling was performed for HVs and for each patient group with a fixed number of 100 000 Vγ9Vδ2 T cells for each group (HV, n = 8; patients with AML with low Vγ9Vδ2 T cells, n = 12; patients with AML with high Vγ9Vδ2 T cells, n = 7). Vγ9Vδ2 T cells were analyzed using t-the t-SNE dimensionality reduction algorithm. In the left panel, the density of Vγ9Vδ2 T-cell subsets in each patient group is projected (blue, low cell density; red, high cell density). The expression of markers of Vγ9Vδ2 T cells is projected onto t-SNE maps in the right panel (blue, low expression; red, high expression). (C-H) Maturation phenotype and expression of surface Vγ9Vδ2 T-cell coreceptors from PBMCs of HVs (n = 18) and patients with AML from the HEMATOBIO cohort (n = 40) according to the Vγ9Vδ2 T-cell frequency group (n = 20 in each group): (C) percentage of Vγ9Vδ2 T cells positive for NKG2A, CD57, DNAM-1, NKG2D, and CD56; (D) percentage of Vγ9Vδ2 T cells positive for CD16, CD69, CD8, CD28, and CD25; (E) maturation phenotype of Vγ9Vδ2 T cells according to the expression of CD45RA and CD27; (F) percentage of Vγ9Vδ2 T cells positive for OX40, 4-1BB, CD44, CCR7, and CD127; (G) percentage of Vγ9Vδ2 T cells positive for BTLA, TIGIT, PD-1, CTLA4, and TIM3; (H) percentage of Vγ9Vδ2 T cells positive for LAG3, HVEM, ICOS, PDL-1, CD95, and CD4. (I-J) BTN3A expression was assessed by flow cytometry on the surface of primary blasts from patients with AML (n = 27), the relative number of BTN3A molecules was quantified with MESF (molecules of equivalent soluble fluorochrome) method, using the anti-BTN3A 108.5 (I) and 20.1 epitopes (J). Results are presented as mean ± standard deviation (SD) (A, C-H) or as box plots (I-J), and statistical significance was established using a Mann-Whitney test (A), or a Kruskal-Wallis test followed by a Dunn's test (C-H), or a t test (I-J). ∗P < .5; ∗∗P < .01.
Low Vγ9Vδ2 T-cell phenotype is associated with phenotypical changes. (A) Expression of surface Vγ9Vδ2 T-cell coreceptors in the FILO cohorts according to the frequency group of Vγ9Vδ2 T cells: percentage of cells positive for CD57 (n = 123), NKG2A (n = 116), DNAM-1 (n = 116), NKG2D (n = 115), and CD56 (n = 123). (B) Vγ9Vδ2 T cells from PBMCs of HVs and patients with AML from the HEMATOBIO cohort were manually pregated and exported in OMIQ for t-SNE analysis. Subsampling was performed for HVs and for each patient group with a fixed number of 100 000 Vγ9Vδ2 T cells for each group (HV, n = 8; patients with AML with low Vγ9Vδ2 T cells, n = 12; patients with AML with high Vγ9Vδ2 T cells, n = 7). Vγ9Vδ2 T cells were analyzed using t-the t-SNE dimensionality reduction algorithm. In the left panel, the density of Vγ9Vδ2 T-cell subsets in each patient group is projected (blue, low cell density; red, high cell density). The expression of markers of Vγ9Vδ2 T cells is projected onto t-SNE maps in the right panel (blue, low expression; red, high expression). (C-H) Maturation phenotype and expression of surface Vγ9Vδ2 T-cell coreceptors from PBMCs of HVs (n = 18) and patients with AML from the HEMATOBIO cohort (n = 40) according to the Vγ9Vδ2 T-cell frequency group (n = 20 in each group): (C) percentage of Vγ9Vδ2 T cells positive for NKG2A, CD57, DNAM-1, NKG2D, and CD56; (D) percentage of Vγ9Vδ2 T cells positive for CD16, CD69, CD8, CD28, and CD25; (E) maturation phenotype of Vγ9Vδ2 T cells according to the expression of CD45RA and CD27; (F) percentage of Vγ9Vδ2 T cells positive for OX40, 4-1BB, CD44, CCR7, and CD127; (G) percentage of Vγ9Vδ2 T cells positive for BTLA, TIGIT, PD-1, CTLA4, and TIM3; (H) percentage of Vγ9Vδ2 T cells positive for LAG3, HVEM, ICOS, PDL-1, CD95, and CD4. (I-J) BTN3A expression was assessed by flow cytometry on the surface of primary blasts from patients with AML (n = 27), the relative number of BTN3A molecules was quantified with MESF (molecules of equivalent soluble fluorochrome) method, using the anti-BTN3A 108.5 (I) and 20.1 epitopes (J). Results are presented as mean ± standard deviation (SD) (A, C-H) or as box plots (I-J), and statistical significance was established using a Mann-Whitney test (A), or a Kruskal-Wallis test followed by a Dunn's test (C-H), or a t test (I-J). ∗P < .5; ∗∗P < .01.
In mass cytometry, a much broader panel was used to detect the various differentiation states and cytotoxic T-cell subsets in a subgroup of patients from the HEMATOBIO cohort and HVs. This disclosed no difference between patients with a high frequency of Vγ9Vδ2 T cells and HVs (Figure 2B-H). In this cohort, we confirm decreased levels of NKG2A and DNAM-1 in patients with low Vγ9Vδ2 T-cell frequencies (Figure 2C). Patients with low Vγ9Vδ2 T-cell frequencies expressed lower levels of CD69 and CD16 than HVs and patients with high Vγ9Vδ2 T-cell frequencies (Figure 2D), indicating a less activated TCR state and a lower antibody-dependent cell cytotoxicity potential.58 In contrast to the FILO cohorts, we observed higher CD56 levels and similar CD57 expression in patients with low Vγ9Vδ2 T-cell frequencies. These discrepancies are relative, because CD56 and CD57 levels followed the same trend in both cohorts, and could be related to the heterogeneity of these markers on Vγ9Vδ2 T cells.59-61 We did not find any differences in terms of maturation states (Figure 2E) or expression of triggering or coinhibiting molecules (Figure 2F-H), apart from a lower expression of inducible T-cell costimulator (ICOS) in patients with low Vγ9Vδ2 T-cell frequencies than HVs and patients with high Vγ9Vδ2 T-cell frequencies.
Analysis of surface ligand expression on the surface of AML blasts disclosed that patients with fewer Vγ9Vδ2 T cells had a lower expression of the BTN3A molecule (Figure 2I-J).
Altogether, these findings revealed that a “low Vγ9Vδ2 T-cell profile” is related to a loss of mature and cytotoxic subsets. This finding could explain the poor prognostic influence of a low frequency of Vγ9Vδ2 T cells, related to a loss of the most immunocompetent Vγ9Vδ2 T cells. This also suggests an important role for BTN3A in modulating the maturation and the proliferation of Vγ9Vδ2 T cells.
The nonproliferating low Vγ9Vδ2 T-cell immunophenotype can be reversed
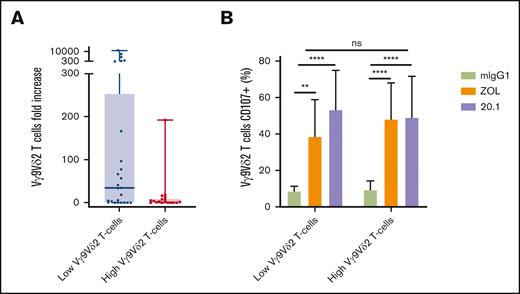
The expansion capacities of Vγ9Vδ2 T cells were then explored with respect to their frequency. Using ZOL-stimulation combined to IL-2 plus IL-15 for 14 days, Vγ9Vδ2 T cells from PBMCs of 43 patients with AML at diagnosis were expanded. Cells from patients with a lower frequency of Vγ9Vδ2 T cells tended to expand better (low vs high Vγ9Vδ2 T cells; P = .072; Figure 3A).
Autologous expansion rates and functional capacities according to Vγ9Vδ2 T-cell phenotype. (A) PBMCs from patients with AML (n = 43) were cultured with 1 μM zoledronate (ZOL) plus 200 U/mL rhIL-2 and 10 μg/mL rhIL-15 for 14 days. (B) Degranulation of Vγ9Vδ2 T cells expanded from PBMCs of patients with AML (n = 23) against autologous blasts pretreated or not with ZOL 50 μM and with or without the agonist anti-BTN3A 20.1 mAb. Results are presented as box plots (A) or as mean ± SD (B), and the statistical significance was established using a Mann-Whitney test (A-B) or a Friedman test followed by a Dunn's test (B). ∗∗P < .01; ∗∗∗∗P < .0001.
Autologous expansion rates and functional capacities according to Vγ9Vδ2 T-cell phenotype. (A) PBMCs from patients with AML (n = 43) were cultured with 1 μM zoledronate (ZOL) plus 200 U/mL rhIL-2 and 10 μg/mL rhIL-15 for 14 days. (B) Degranulation of Vγ9Vδ2 T cells expanded from PBMCs of patients with AML (n = 23) against autologous blasts pretreated or not with ZOL 50 μM and with or without the agonist anti-BTN3A 20.1 mAb. Results are presented as box plots (A) or as mean ± SD (B), and the statistical significance was established using a Mann-Whitney test (A-B) or a Friedman test followed by a Dunn's test (B). ∗∗P < .01; ∗∗∗∗P < .0001.
Some of the samples were also functionally tested by measuring the degranulation of Vγ9Vδ2 T cells. Surprisingly, there was no difference in degranulation capacities between Vγ9Vδ2 T-cell frequency groups whatever the tested conditions. As previously described by our team, pAg-stimulation resulted in an increased degranulation of expanded autologous Vγ9Vδ2 T cells.34 Furthermore, BTN3A targeting with the 20.1 agonist mAb led to an increase in degranulation compared with controls.
This demonstrated that the use of an anti-BTN3A agonists empower degranulation capacities of autologous Vγ9Vδ2 T cells against primary AML blasts, confirming results that had only been obtained by using allogeneic Vγ9Vδ2 T cells expanded from HVs.36 Interestingly, it was also observed that ages of patients with AML did not influence the ability of Vγ9Vδ2 T cells to expand and degranulate (supplemental Figure 7).
Overall, these expansion and degranulation experiments demonstrated that the functional impact of the immunophenotype associated with a low frequency of Vγ9Vδ2 T cells was reversible in vitro, even in older patients.
Discussion
Probably because of their major histocompatibility complex–unrestricted mechanism of activation, the prognostic impact of γδ T cells in AML has been mostly studied after HSCT. Here, a reduced frequency of circulating Vγ9Vδ2 T cells at AML diagnosis was shown to be independently associated with worse outcome.
These findings support results suggesting the prognostic role of Vγ9Vδ2 T-cell abundance assessed by deconvolution of bulk tumor transcriptomes using algorithms such as CIBERSORT-LM2243 or CIBERSORT-LM7.44 However, such approaches based on bulk transcriptomic data carry a risk of erroneous results, especially when the analysis is focused on such a rare immune subset as that of Vγ9Vδ2 T cells, in particular at AML diagnosis. In addition, transcriptome analysis is difficult to apply in clinical routine. Moreover, the presence of confounding factors had not been tested in these studies, thus preventing solid conclusions regarding the prognostic value of PB Vγ9Vδ2 T-cell frequency in AML.
The results reported here are also consistent with studies evaluating the good prognostic impact of reconstitution of a high frequency of γδ T cells on OS and RFS after HSCT.62-64 However, the distinction between Vδ2+ and other γδ T-cell subsets had only been occasionally analyzed, and the prognostic value of this reconstitution in AML remained controversial.64,65
In contrast to the many immunomonitoring studies available after HSCT,66 immunophenotypic approaches to evaluate Vγ9Vδ2 T cells at diagnosis have been limited to very small cohorts42,67 and evidenced no impact of Vγ9Vδ2 T-cell immunophenotype according to clinical outcomes. It was shown that the PB frequency of Vγ9Vδ2 T cells varies according to the quality of the response to induction41 and is lower after induction in R/R patients than patients in sustained CR.42 By contrast, in the study reported here, patients with lower Vγ9Vδ2 T cells at diagnosis responded similarly to induction but had a higher risk of relapse. Therefore, it could be hypothesized that the frequency of Vγ9Vδ2 T cells at diagnosis rather represents their proliferative capabilities, which could be further inhibited in R/R patients.
Importantly, a decreased frequency of Vγ9Vδ2 T cells was associated with pronounced immunophenotypic alterations that affect predominantly a decreased expression of cytotoxicity and/or activation markers, including NKG2A, CD57, DNAM-1, and CD16. Our results are consistent with previous natural killer receptor blockade assays of autologous Vγ9Vδ2 T cells from patients with AML that showed that masking of DNAM-1 decreased the cytotoxic functions of Vγ9Vδ2 T cells against autologous blasts, whereas masking of NKG2D did not.34 In addition, CD16 plays a key role in effector functions of Vγ9Vδ2 T-cell functions,58,68 including against acute lymphoblastic leukemia or chronic lymphocytic leukemia cells.69,70 Meanwhile, downregulation of NKG2A was also demonstrated on Vγ9Vδ2 T cells from patients with AML with lower Vγ9Vδ2 T cells, and NKG2A has recently been reported to characterize a subset of “educated” circulating Vγ9Vδ2 T cells that exert great antitumor functions.71 CD57 is thought to be a marker of replicative senescence in αβT cells,72 but its significance in Vγ9Vδ2 T cells is debated.73 Indeed, CD57+ Vγ9Vδ2 T cells proliferate less74 but do not accumulate with age.59-61 Regarding coinhibitory molecules, a low frequency of Vγ9Vδ2 T cells was only associated with an upregulation of ICOS on Vγ9Vδ2 T cells, suggesting that these Vγ9Vδ2 T cells are neither exhausted nor senescent at diagnosis, in contrast to the signature of terminal senescence often observed in αβ T cells of patients with ND AML.42 Interestingly, ICOS/ICOS-ligand (ICOS-L) pathway has been involved in NK-cell activation by pAg–activated Vγ9Vδ2 T cells and may lead to the acquisition of DC editing function by NK cells.75
However, further investigations are needed to understand the respective qualitative42,67,76 vs quantitative41,43,44 contribution of Vγ9Vδ2 T cells in ND AML, as well as the associated alterations in other immune subtypes and their dynamic changes over the course of treatment. Interestingly, although phenotypically altered and/or nonproliferating, Vγ9Vδ2 T cells retained their ability to kill autologous blasts, as we have recently shown in acute lymphoblastic leukemia.77 These data confirm the persistent functional plasticity of Vγ9Vδ2 T cells leading to an adaptive response in leukemia.
Finally, it was shown here that surface BTN3A levels on AML blasts could influence the frequency of Vγ9Vδ2 T cells. We were limited by the number of cells needed to perform other coculture assays, but it could be hypothesized that more BTN3A molecules on the blast surface might make them more “visible” to Vγ9Vδ2 T cells, with a subsequent impact on their proliferation. This is consistent with a recent transcriptomic study showing a positive association between γδ T-cell infiltration and BTN3A expression in head and neck squamous cell carcinoma.78 Alternatively, Vγ9Vδ2 T-cell proliferation could be inhibited by a specific mechanism such as defective mevalonate metabolism in AML blasts38 or by more general immune escape processes such as enhanced inhibitory ligand expression, reduced expression, or shedding of activating ligands.3 For instance, baseline plasmatic levels of soluble BTN3A and BTN2A1 have been associated with outcomes in several solid tumors.79-81 The underlying mechanisms of BTN3A level expression are still unknown but have been investigated in a recent CRISPR screen that showed a multilayered regulation dependent on a metabolic crisis in tumor cells,82 and further studies are warranted to understand BTN3A-specific regulation in AML blasts. In addition, targeting BTN3A on AML blasts improved the cytotoxic functions of autologous Vγ9Vδ2 T cells, demonstrating that BTN3A could be a reliable target to “awaken” Vγ9Vδ2 T cells from patients with AML who have decreased frequency of this subset. These observations warrant further investigation of the efficacy of anti-BTN3A agonist antibodies in AML, such as ICT01, which is currently being evaluated in the multicenter phase 1/2 EVICTION trial.83
Age-related immune alterations in AML are closely associated with poor clinical outcomes with a higher proportion of late effector T cells,4 limiting the individualization of an independent effect of immune alterations in older patients with AML. In the present cohorts, age-related changes appeared to be similar to the previously described decreased frequency of circulating Vγ9Vδ2 T cells in older patients.84-86 Interestingly, a low PB Vγ9Vδ2 T-cell frequency was found to yield a similar prognostic impact whatever the age of the patients. Moreover, older patients had similar expansion and degranulation capacities as younger ones, indicating a persistent Vγ9Vδ2 T-cell plasticity with aging, as already described in HVs.60,74 Similarly, it was confirmed that Vγ9Vδ2 T cells adopt a more resilient behavior and are more resistant to senescence than most immune effectors.60,74,85,87 Collectively, these results demonstrate that Vγ9Vδ2 T-cell levels retain a prognostic impact in older patients, and immunotherapeutic approaches to harness their functions in this population could be developed to reduce the risk of relapse. In this regard, recent findings suggest that the use of 5-azacitidine or venetoclax may potentiate the antileukemic functions of Vγ9Vδ2 T cells, as demonstrated by encouraging results in combination with ICT01.88 It would be also interesting to evaluate the prognostic contribution of Vγ9Vδ2 T cells in a cohort of patients with AML treated with hypomethylating agents and/or venetoclax.
In summary, evidence is reported here that a low PB frequency of Vγ9Vδ2 T cells in ND AML is independently associated with poor prognosis. Although this reduced frequency of Vγ9Vδ2 T cells is associated with major phenotypic alterations, the remaining Vγ9Vδ2 T cells can proliferate and exert cytotoxic functions against autologous blasts. Altogether, these findings confirm that Vγ9Vδ2 T cells act as critical stress-sensors in AML and provide a strong rationale for harnessing and potentiating these immune effectors in the early treatment of AML.
Acknowledgments
The authors thank the FILO and FILOtheque (no. BB-0033-00073) for their involvement in this study. The authors thank the IPC Immunomonitoring platform and IPC Tumor Bank (authorization number AC-2007-33, granted by the French Ministry of Research. The “Immunity and Cancer” team was labeled “FRM DEQ20180339209” (D.O.).
This work has been financially supported by the Institut National Du Cancer (grant 2012-064/2019-038; D.O.), the Fondation de France (grant 00076207; A.-S.C.), the Sites de Recherche Intégrée sur le Cancer of Marseille (grant INCa-DGOS-INSERM 6038), and the Groupement d’intérêt scientifique -Infrastructures pour la Biologie, la Santé et l’Agronomie, ITMO cancer (grant 2014-P032760; A.-S.C.), the Association Laurette Fugain (grant ALF2020/05; D.O.), and the Agence Nationale de la Recherche (French National Infrastructure for Mouse Phenogenomics [PHENOMIN] project).
A.-C.L.F. was funded by the Fondation pour la Recherche Médicale (grant DEA 40820) from November 2016 to October 2017 and a Poste d’Accueil INSERM grant from November 2019 to October 2021.
Authorship
Contribution: A.-C.L.F., A.-S.C., and D.O. conceptualized and designed the study; A.-C.L.F., F.O., A.B.A., M.-S.R., N.S., C.C., C.D., A.-S.C., and A.L.R. performed experiments and interpreted the data; N.V. treated patients and coordinated the study; J.-F.H., N.I., P.C.-L., J.D., and E.D. coordinated the research; A.-C.L.F. and D.O. coordinated the research and wrote the manuscript; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: D.O. is a cofounder and shareholder of ImCheck Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Daniel Olive, Centre de Recherche en Cancérologie de Marseille, INSERM UMR1068, CNRS UMR7258, Aix-Marseille Université U105, Institut Paoli-Calmettes, 27 Bd Leï Roure, CS 30059, 13273 Marseille Cedex 13009, France; email: daniel.olive@inserm.fr.
References
Author notes
Presented in poster form at the 27th annual meeting of the European Hematology Association, Vienna, Austria, 9-12 June 2022.
The data sets used and/or analyzed during this study are available on reasonable request from the corresponding author, Daniel Olive (daniel.olive@inserm.fr).
The full-text version of this article contains a data supplement.