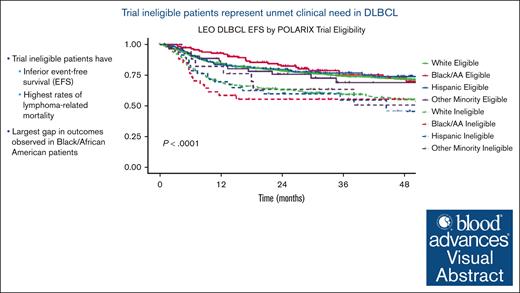
Key Points
Patients excluded from clinical trials of DLBCL are at a higher risk of dying from lymphoma when treated with standard-of-care therapy.
Minorities, in particular Black patients, are at a greater risk of being left behind on clinical trials of DLBCL.
Visual Abstract
Underrepresentation of racial and ethnic subgroups in cancer clinical trials remains a persistent challenge. Restrictive clinical trial eligibility criteria have been shown to exacerbate this problem. We previously identified that up to 24% of patients treated with standard immunochemotherapy would have been excluded from recent first-line trials in diffuse large B-cell lymphoma (DLBCL) based on 5 laboratory-based criteria. These ineligible patients had worse clinical outcomes and increased deaths related to lymphoma progression, suggesting the potential exclusion of patients who could have benefited most from the novel therapies being evaluated. Using data from the prospectively enrolled Lymphoma Epidemiology Outcomes cohort study, with demographics broadly similar to the US patients diagnosed with lymphoma, we evaluated the impact of laboratory eligibility criteria from recent first-line DLBCL trials across various racial and ethnic backgrounds. There were significant differences in the baseline laboratory values by race/ethnicity with Black/African American (AA) patients having the lowest mean hemoglobin and highest creatinine clearance. Based on recent clinical trial eligibility criteria, AA and Hispanic patients had higher rates of laboratory-based ineligibility than non-Hispanic White patients. The largest gap in the clinical outcomes between eligible and noneligible patients was noted within AA patients with an overall survival hazard ratio based on POLARIX clinical trial criteria of 4.09 (95% confidence interval, 1.83-9.14). A thoughtful approach to the utility of each criterion and cutoffs for eligibility needs to be evaluated in the context of its differential impact across various racial/ethnic groups.
Introduction
Therapeutic clinical trials are an essential component of providing care to cancer patients by enhancing discovery of new agents and providing access to precision medicine approaches. Representation in clinical trials is particularly important in the context of changing US demographics. Additionally, differences exist in disease biology, clinical presentations, and treatment tolerability based on race and ethnicity.1-3 Eligibility criteria are essential gatekeepers to prevent excessive toxicity from experimental treatments and to increase internal validity by creating a more homogeneous population to test the trial hypothesis.4 However, restrictive eligibility criteria can limit the generalizability of the trial data when the drugs are approved and used in populations underrepresented or not represented in the trials. Clinical trial eligibility criteria account for the reason for nonparticipation in cancer clinical trials in up to a quarter of patients.5-8 Furthermore, clinical trials have become more complex and may require a central review of pathology, molecular subtyping before enrollment, and an exhaustive trial enrollment process requiring special diagnostics that may delay enrollment to the point at which patients and physicians decide to proceed with standard therapy.
Diffuse large B-cell lymphoma (DLBCL) is the most common aggressive B-cell lymphoma in the United States.9 It is a clinically heterogenous disease with variable clinical presentations such as bulky disease, rapid tumor growth, or symptomatic disease. These high-risk patients in particular can have laboratory-based derangements as a manifestation of the disease itself. We previously identified that up to 24% of patients treated with standard immunochemotherapy (IC) would have been trial ineligible based on 5 laboratory-based criteria alone.10 Additionally, ineligible patients had worse clinical outcomes and increased deaths related to lymphoma progression, suggesting that the potential exclusion of patients who could have benefited most from novel therapies. According to the US Food and Drug Administration (FDA)’s 2018 Drug Trials Snapshots, a total of 5157 patients participated in oncology clinical trials that led to 17 new drug approvals. Of these, 68% were White, 5% were Asian, 4% were African American (AA), and 4% were Hispanic. These proportions sharply contrasted with the racial distribution of the general US population and US cancer population.11 This leads to significant limitations in applying data from the clinical trials pertaining to drug efficacy and safety/toxicity to the real-world population. The stakeholders from the American Society of Clinical Oncology, FDA, Friends of Cancer Research, and the Association of Community Cancer Centers have all published recommendations and commitment to increasing diversity, equity, and inclusion in clinical trials.12-16 Our prior study on the impact of trial eligibility in DLBCL was in a cohort of patients predominantly from the upper Midwest United States with limited racial and ethnic diversity.17 Therefore, we sought to confirm our findings in a larger, more diverse Lymphoma Epidemiology Outcomes (LEO) cohort and examine the differential impact of these laboratory-based criteria on trial exclusion based on race/ethnicity.
Methods
Study population
Patients were enrolled within 6 months of diagnosis in the LEO cohort study (ClinicalTrials.gov identifier: NCT02736357) at 1 of 8 institutions: Mayo Clinic (Rochester, MN), MD Anderson (Houston, TX), University of Miami (Miami, FL), Emory University (Atlanta, GA), University of Iowa (Iowa City, IA), Washington University (St. Louis, MO), Weill Cornell Medicine (New York, NY), and University of Rochester (Rochester, NY), and prospectively followed-up.18 Baseline clinical data were abstracted using a standard protocol. Central pathology review was performed by an expert hematopathologist at each LEO center. Patients were managed by the treating physician (either at 1 of 8 academic centers or locally) and contacted prospectively and systematically every 6 months for the first 3 years, and then annually thereafter. Events (new treatments, progression, and death) were validated by medical record review. Patients included in this analysis were enrolled in LEO from 1 July 2015 to 31 May 2020. All patients provided informed consent to enroll in the LEO cohort study. Use of the LEO data for this study was approved by the Mayo Clinic institutional review board.
This analysis included adult patients aged ≥18 years with a diagnosis of DLBCL who initiated first-line treatment with anthracycline plus CD20 antibody–based IC. The exclusion criteria were: Burkitt lymphoma, Burkitt-like intensive therapy (eg, cyclophosphamide, vincristine, doxorubicin, and methotrexate and cyclophosphamide, vincristine, doxorubicin and dexamethasone), lack of information regarding race/ethnicity, and/or missing values for ≥3 of 5 laboratory-based criteria. Creatinine clearance was calculated per Cockcroft-Gault with/without race adjustment as per the protocol from the POLARIX clinical trial.19 Race and ethnicity were self-reported by the patients at the time of LEO enrollment and were categorized as follows: Hispanic (any race), non-Hispanic Black or AA, non-Hispanic White (NHW), and all other race/ethnicities (ie, non-White race and non-Hispanic).
Organ-function laboratory values at the time of diagnosis were abstracted from the medical record as part of standard LEO data collection. Baseline laboratory-based eligibility criteria parameters were identified from recent phase 3 first-line DLBCL clinical trials, as previously described. These included hemoglobin, absolute neutrophil count, platelet count, creatinine clearance, and bilirubin. The cutoff values for different laboratory parameters reported in the respective clinical trials’ protocol (POLARIX, ENGINE, PHOENIX, ROBUST, ECOG 1412, REMoDL-B, GOYA, and CALGB 50303) were identified (supplement Table 1).19-26
Statistical methods
The percentage of patients excluded based on clinical trial criteria was determined for each laboratory value individually as well as across all laboratory parameters. The percentage exclusion across trial was then compared between various race/ethnicity groups. Event-free survival (EFS) was defined as the time from diagnosis to relapse, progression, retreatment (second-line therapy), or death from any cause. Overall survival (OS) was defined as the time from diagnosis until death from any cause. EFS was reported at 24 months (EFS24), as previously described.27 Kaplan-Meier curves and Cox models were used to compare EFS and OS outcomes between eligible and ineligible patients. Logistic regression was used to compare EFS24 between eligible and ineligible patients. Causes of death were evaluated using a competing risk approach. An interactive tool was developed in R-Shiny to allow users to estimate the percentage of patients who would be excluded by changing organ function cutoffs and race/ethnicity. All analyses were performed using R version 3.6.2, R-Shiny, and SAS version 9.4M5.1.
Results
Baseline characteristics
A total of 7746 patients were enrolled in the LEO cohort between July 2015 and December 2020; 2748 had DLBCL or other aggressive B-cell lymphoma, and 2353 patients initiated first-line IC. Of these, 2185 patients had ≥3 of 5 laboratory values available at the time of diagnosis (Figure 1). Approximately 79% of the cohort was treated at 1 of 8 US academic centers and the remaining at referring sites. The baseline characteristics of the total cohort (2185 patients) and race/ethnicities are shown in Table 1. The median age at diagnosis for the entire cohort was 63 years (interquartile range [IQR], 52-72), with males accounting for 57% of patients. The median time from diagnosis to treatment was 21 days (IQR, 12-33). A total of 9% of patients were treated on various first-line clinical trials available at the time of presentation. The median follow-up of the cohort was 37 months; 420 patients (19%) died during the follow-up period, and 73% achieved EFS24.
Patient characteristics by race/ethnicity
| Characteristic . | Total N = 2185 . | NHW n = 1666 . | Black/AA (non-Hispanic) n = 155 . | Hispanic (any) n = 288 . | Other minority n = 76 . | P value . |
|---|---|---|---|---|---|---|
| Age (y) at diagnosis, median (IQR) | 63 (42-72.5) | 65 (55-73) | 51 (39-62) | 56 (41-65.5) | 63 (42-72.5) | <.0001 |
| Male, (%) | 1237 (56.6%) | 959 (57.6%) | 80 (51.6%) | 160 (55.6%) | 38 (50.0%) | .30 |
| ECOG PS ≥2 (%) | 333 (16.2%) | 269 (17.1%) | 19 (13.1%) | 34 (12.5%) | 11 (16.9%) | .19 |
| Ann Arbor stage, III-IV (%) | 1331 (63.9%) | 1008 (63.4%) | 108 (73.5%) | 171 (62.6%) | 44 (60.3%) | .085 |
| Extranodal sites > 1 (%) | 568 (26.6%) | 414 (25.4%) | 48 (31.8%) | 88 (31.0%) | 18 (24.3%) | .1099 |
| Elevated LDH (%) | 1143 (56.4%) | 849 (54.8%) | 87 (61.7%) | 161 (59.9%) | 46 (66.7%) | .059 |
| IPI | .020 | |||||
| 0-2 | 1353 (62%) | 1020 (61%) | 101 (65%) | 183 (64%) | 49 (64%) | |
| 3-5 | 832 (38%) | 646 (39%) | 54 (35%) | 105 (36%) | 27 (36%) | |
| DTI in days, median (IQR) | 21 (12-33) | 21 (12-32) | 24 (13-39) | 21 (13-33) | 17 (9-33) | .027 |
| B-symptoms (%) | 699 (32.0%) | 508 (30.5%) | 68 (43.9%) | 107 (37.2%) | 16 (21.1%) | .0007 |
| Bone marrow involvement (%) | 276 (15.9%) | 217 (16.5%) | 26 (20.8%) | 26 (10.9%) | 7 (11.7%) | .14 |
| 1L Treatment received | <.0001 | |||||
| R-CHOP | 1402 (64.2%) | 1111 (66.7%) | 86 (55.5%) | 162 (56.3%) | 43 (56.6%) | |
| R-EPOCH | 576 (26.4%) | 385 (23.1%) | 56 (36.1%) | 107 (37.2%) | 28 (36.8%) | |
| Clinical trial | 189 (8.6%) | 159 (9.5%) | 11 (7.1%) | 14 (4.9%) | 5 (6.6%) | |
| Other IC | 18 (0.8%) | 11 (0.7%) | 2 (1.3%) | 5 (1.7%) | 0 (0.0%) |
| Characteristic . | Total N = 2185 . | NHW n = 1666 . | Black/AA (non-Hispanic) n = 155 . | Hispanic (any) n = 288 . | Other minority n = 76 . | P value . |
|---|---|---|---|---|---|---|
| Age (y) at diagnosis, median (IQR) | 63 (42-72.5) | 65 (55-73) | 51 (39-62) | 56 (41-65.5) | 63 (42-72.5) | <.0001 |
| Male, (%) | 1237 (56.6%) | 959 (57.6%) | 80 (51.6%) | 160 (55.6%) | 38 (50.0%) | .30 |
| ECOG PS ≥2 (%) | 333 (16.2%) | 269 (17.1%) | 19 (13.1%) | 34 (12.5%) | 11 (16.9%) | .19 |
| Ann Arbor stage, III-IV (%) | 1331 (63.9%) | 1008 (63.4%) | 108 (73.5%) | 171 (62.6%) | 44 (60.3%) | .085 |
| Extranodal sites > 1 (%) | 568 (26.6%) | 414 (25.4%) | 48 (31.8%) | 88 (31.0%) | 18 (24.3%) | .1099 |
| Elevated LDH (%) | 1143 (56.4%) | 849 (54.8%) | 87 (61.7%) | 161 (59.9%) | 46 (66.7%) | .059 |
| IPI | .020 | |||||
| 0-2 | 1353 (62%) | 1020 (61%) | 101 (65%) | 183 (64%) | 49 (64%) | |
| 3-5 | 832 (38%) | 646 (39%) | 54 (35%) | 105 (36%) | 27 (36%) | |
| DTI in days, median (IQR) | 21 (12-33) | 21 (12-32) | 24 (13-39) | 21 (13-33) | 17 (9-33) | .027 |
| B-symptoms (%) | 699 (32.0%) | 508 (30.5%) | 68 (43.9%) | 107 (37.2%) | 16 (21.1%) | .0007 |
| Bone marrow involvement (%) | 276 (15.9%) | 217 (16.5%) | 26 (20.8%) | 26 (10.9%) | 7 (11.7%) | .14 |
| 1L Treatment received | <.0001 | |||||
| R-CHOP | 1402 (64.2%) | 1111 (66.7%) | 86 (55.5%) | 162 (56.3%) | 43 (56.6%) | |
| R-EPOCH | 576 (26.4%) | 385 (23.1%) | 56 (36.1%) | 107 (37.2%) | 28 (36.8%) | |
| Clinical trial | 189 (8.6%) | 159 (9.5%) | 11 (7.1%) | 14 (4.9%) | 5 (6.6%) | |
| Other IC | 18 (0.8%) | 11 (0.7%) | 2 (1.3%) | 5 (1.7%) | 0 (0.0%) |
ECOG PS, Eastern Cooperative Oncology Group performance status; DTI, diagnosis to treatment interval; 1L, first line; LDH, lactate dehydrogenase; IPI, international prognostic index; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; R-EPOCH, rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide and prednisone.
There were significant differences in clinical presentation and management by race and ethnicity within the LEO cohort. In comparison with NHW patients, AA patients and Hispanic patients who enrolled in the LEO were much younger, with a median age of 51 years (IQR, 39-62) for AA patients and 56 years (IQR, 41-65) for Hispanic patients compared with 65 years (IQR, 55-73) in NHW. AA (44%) and Hispanic (37%) patients with DLBCL presented with significantly higher rates of B symptoms than NHW patients (30%). NHW patients (10%) were also more likely to receive first-line therapy on a clinical trial compared with AA (7%) and Hispanic (5%) patients.
Impact of laboratory-based criteria on trial exclusion based on race/ethnicity
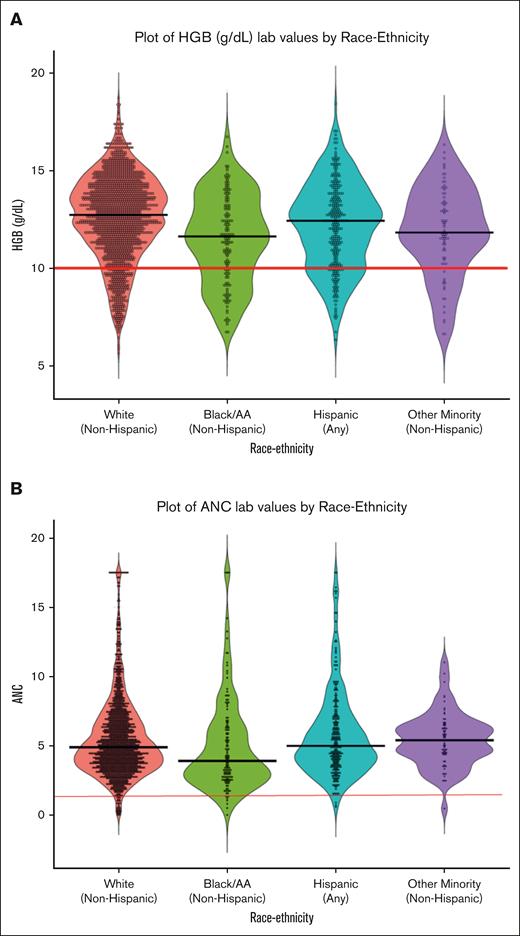
We observed significant differences in the distributions of laboratory-based criteria by race/ethnicity (Table 2). NHW and Hispanic patients with DLBCL had the highest median levels of hemoglobin in the LEO cohort, with significantly lower hemoglobin levels observed in AA and other non-White minority patients; a 10-gm/dL cutoff for hemoglobin as used in the ENGINE trial, would exclude 28% of AA LEO patients with DLBCL compared with only 13% of NHW patients (Figure 2A). There was also a significant difference in neutrophil counts by race/ethnicity, with AA patients having the lowest neutrophil counts. However, a cutoff of 1.0 × 109/L as used in the POLARIX trial would have excluded very few patients across all race and ethnicity groups (Figure 2B). The race/ethnicities with the highest distributions of creatinine clearance were AA and Hispanic patients (supplemental Figure 1), which were also the race/ethnicities with the youngest age distributions.
Trial eligibility laboratory values by race/ethnicity
| Laboratory values (mean, SD) . | Total N = 2185 . | NHW n = 1666 . | Black/AA (non-Hispanic) n = 155 . | Hispanic (any) n = 288 . | Other minority n = 76 . | P value . |
|---|---|---|---|---|---|---|
| ANC (×109/L) | 5.2 (2.3) | 5.3 (2.3) | 4.7 (2.6) | 5.5 (2.4) | 5.4 (1.9) | .0005 |
| PLT (×109/L) | 267 (104) | 261.4 (101) | 283 (115) | 285 (111) | 278 (104) | .0037 |
| Hb (g/dL) | 12.4 (2.2) | 12.5 (2.2) | 11.5 (2.3) | 12.2 (2.2) | 11.9 (2.3) | <.0001 |
| Bilirubin (mg/dL) | 0.6 (0.6) | 0.6 (0.6) | 0.6 (0.4) | 0.6 (0.7) | 0.6 (0.6) | .0023 |
| Creatinine clearance (mL/min) | 100 (45) | 98 (44) | 111 (49) | 109 (45) | 97 (50) | <.0001 |
| Laboratory values (mean, SD) . | Total N = 2185 . | NHW n = 1666 . | Black/AA (non-Hispanic) n = 155 . | Hispanic (any) n = 288 . | Other minority n = 76 . | P value . |
|---|---|---|---|---|---|---|
| ANC (×109/L) | 5.2 (2.3) | 5.3 (2.3) | 4.7 (2.6) | 5.5 (2.4) | 5.4 (1.9) | .0005 |
| PLT (×109/L) | 267 (104) | 261.4 (101) | 283 (115) | 285 (111) | 278 (104) | .0037 |
| Hb (g/dL) | 12.4 (2.2) | 12.5 (2.2) | 11.5 (2.3) | 12.2 (2.2) | 11.9 (2.3) | <.0001 |
| Bilirubin (mg/dL) | 0.6 (0.6) | 0.6 (0.6) | 0.6 (0.4) | 0.6 (0.7) | 0.6 (0.6) | .0023 |
| Creatinine clearance (mL/min) | 100 (45) | 98 (44) | 111 (49) | 109 (45) | 97 (50) | <.0001 |
ANC, absolute neutrophil count; Hb, hemoglobin; PLT, platelet count; SD, standard deviation.
Baseline laboratory values based on race/ethnicity. Violin plots showing distribution of baseline hemoglobin (A) and absolute neutrophil count (B) in the LEO cohort among various racial/ethnic subgroups. Cutoff values (red solid line) show differential impact among the subgroups for hemoglobin (HGB; 10 g/dL) and absolute neutrophil count (ANC; 1.0 × 109) cutoffs.
Baseline laboratory values based on race/ethnicity. Violin plots showing distribution of baseline hemoglobin (A) and absolute neutrophil count (B) in the LEO cohort among various racial/ethnic subgroups. Cutoff values (red solid line) show differential impact among the subgroups for hemoglobin (HGB; 10 g/dL) and absolute neutrophil count (ANC; 1.0 × 109) cutoffs.
When the laboratory-based cutoffs were applied, between 9% and 26% of the LEO cohort patients were considered ineligible across the different trials (Table 3), with the REMoDL-B trial being the least restrictive, and the ENGINE trial being the most restrictive. Notably, as the trials got more restrictive, the impact was greater on minorities compared with NHW patients (Table 3). There was a significantly higher ineligibility of the AA (37%), Hispanic (29%), and other non-Hispanic minority (30%) patients when compared with NHW patients (24%) in the LEO cohort based on the ENGINE trial’s laboratory-based criteria. Similar findings were noted for the GOYA and POLARIX trials. An interactive tool to further evaluate the impact of potential cutoffs on eligibility using study data from the LEO cohort is publically available at www.rtools.mayo.edu/leo_dlbcl_left_behind/.
Laboratory-based trial eligibility by race/ethnicity
| Trial . | Total (N = 2185) . | NHW (n = 1666) . | Black/AA (non-Hispanic) (n = 155) . | Hispanic (any) (n = 288) . | Other minority (non-Hispanic) (n = 76) . | P value . |
|---|---|---|---|---|---|---|
| REMoDL-B, n (%) | .51 | |||||
| Ineligible | 194 (8.9%) | 144 (8.6%) | 16 (10.3%) | 24 (8.3%) | 10 (13.2%) | |
| Eligible | 1991 (91.1%) | 1522 (91.4%) | 139 (89.7%) | 264 (91.7%) | 66 (86.8%) | |
| ROBUST, n (%) | .20 | |||||
| Ineligible | 218 (10.0%) | 161 (9.7%) | 22 (14.2%) | 25 (8.7%) | 10 (13.2%) | |
| Eligible | 1967 (90.0%) | 1505 (90.3%) | 133 (85.8%) | 263 (91.3%) | 66 (86.8%) | |
| ECOG 1412, n (%) | .30 | |||||
| Ineligible | 237 (10.8%) | 177 (10.6%) | 23 (14.8%) | 27 (9.4%) | 10 (13.2%) | |
| Eligible | 1948 (89.2%) | 1489 (89.4%) | 132 (85.2%) | 261 (90.6%) | 66 (86.8%) | |
| PHOENIX, n (%) | .52 | |||||
| Ineligible | 261 (11.9%) | 199 (11.9%) | 17 (11.0%) | 32 (11.1%) | 13 (17.1%) | |
| Eligible | 1924 (88.1%) | 1467 (88.1%) | 138 (89.0%) | 256 (88.9%) | 63 (82.9%) | |
| CALGB 50303, n (%) | .50 | |||||
| Ineligible | 361 (16.5%) | 280 (16.8%) | 22 (14.2%) | 43 (14.9%) | 16 (21.1%) | |
| Eligible | 1824 (83.5%) | 1386 (83.2%) | 133 (85.8%) | 245 (85.1%) | 60 (78.9%) | |
| POLARIX, n (%) | .12 | |||||
| Ineligible | 362 (16.6%) | 263 (15.8%) | 34 (21.9%) | 48 (16.7%) | 17 (22.4%) | |
| Eligible | 1823 (83.4%) | 1403 (84.2%) | 121 (78.1%) | 240 (83.3%) | 59 (77.6%) | |
| GOYA, n (%) | .022 | |||||
| Ineligible | 374 (17.1%) | 270 (16.2%) | 39 (25.2%) | 48 (16.7%) | 17 (22.4%) | |
| Eligible | 1811 (82.9%) | 1396 (83.8%) | 116 (74.8%) | 240 (83.3%) | 59 (77.6%) | |
| ENGINE, n (%) | .0028 | |||||
| Ineligible | 573 (26.2%) | 409 (24.5%) | 58 (37.4%) | 83 (28.8%) | 23 (30.3%) | |
| Eligible | 1612 (73.8%) | 1257 (75.5%) | 97 (62.6%) | 205 (71.2%) | 53 (69.7%) |
| Trial . | Total (N = 2185) . | NHW (n = 1666) . | Black/AA (non-Hispanic) (n = 155) . | Hispanic (any) (n = 288) . | Other minority (non-Hispanic) (n = 76) . | P value . |
|---|---|---|---|---|---|---|
| REMoDL-B, n (%) | .51 | |||||
| Ineligible | 194 (8.9%) | 144 (8.6%) | 16 (10.3%) | 24 (8.3%) | 10 (13.2%) | |
| Eligible | 1991 (91.1%) | 1522 (91.4%) | 139 (89.7%) | 264 (91.7%) | 66 (86.8%) | |
| ROBUST, n (%) | .20 | |||||
| Ineligible | 218 (10.0%) | 161 (9.7%) | 22 (14.2%) | 25 (8.7%) | 10 (13.2%) | |
| Eligible | 1967 (90.0%) | 1505 (90.3%) | 133 (85.8%) | 263 (91.3%) | 66 (86.8%) | |
| ECOG 1412, n (%) | .30 | |||||
| Ineligible | 237 (10.8%) | 177 (10.6%) | 23 (14.8%) | 27 (9.4%) | 10 (13.2%) | |
| Eligible | 1948 (89.2%) | 1489 (89.4%) | 132 (85.2%) | 261 (90.6%) | 66 (86.8%) | |
| PHOENIX, n (%) | .52 | |||||
| Ineligible | 261 (11.9%) | 199 (11.9%) | 17 (11.0%) | 32 (11.1%) | 13 (17.1%) | |
| Eligible | 1924 (88.1%) | 1467 (88.1%) | 138 (89.0%) | 256 (88.9%) | 63 (82.9%) | |
| CALGB 50303, n (%) | .50 | |||||
| Ineligible | 361 (16.5%) | 280 (16.8%) | 22 (14.2%) | 43 (14.9%) | 16 (21.1%) | |
| Eligible | 1824 (83.5%) | 1386 (83.2%) | 133 (85.8%) | 245 (85.1%) | 60 (78.9%) | |
| POLARIX, n (%) | .12 | |||||
| Ineligible | 362 (16.6%) | 263 (15.8%) | 34 (21.9%) | 48 (16.7%) | 17 (22.4%) | |
| Eligible | 1823 (83.4%) | 1403 (84.2%) | 121 (78.1%) | 240 (83.3%) | 59 (77.6%) | |
| GOYA, n (%) | .022 | |||||
| Ineligible | 374 (17.1%) | 270 (16.2%) | 39 (25.2%) | 48 (16.7%) | 17 (22.4%) | |
| Eligible | 1811 (82.9%) | 1396 (83.8%) | 116 (74.8%) | 240 (83.3%) | 59 (77.6%) | |
| ENGINE, n (%) | .0028 | |||||
| Ineligible | 573 (26.2%) | 409 (24.5%) | 58 (37.4%) | 83 (28.8%) | 23 (30.3%) | |
| Eligible | 1612 (73.8%) | 1257 (75.5%) | 97 (62.6%) | 205 (71.2%) | 53 (69.7%) |
Impact of trial exclusion on outcomes based on race/ethnicity
To confirm our prior results, we compared clinical outcomes and cause of death in the LEO cohort based on eligibility and race/ethnicity. Patients with DLBCL enrolled in LEO who did not meet trial eligibility based on the 5 laboratory criteria had significantly inferior EFS and OS. When applying laboratory-based cutoffs from the recent POLARIX trial, EFS24 was 79% (95% confidence interval [CI], 77-81) in trial-eligible patients compared with 62% (95% CI, 57-68) in trial-ineligible patients (P < .001; supplemental Figure 4). Additionally, patients that were trial ineligible had a significantly increased risk of dying from progressive lymphoma, with no increase in therapy-related deaths. Five-year OS was 80% (95% CI, 78-83) vs 55% (95% CI, 48-62) with a risk of death from progressive disease at 5 years of 20% (95% CI, 16-25) vs 8% (95% CI, 7-9) for trial eligible and ineligible, respectively (supplemental Figure 5). This observation was consistent across the various trial eligibility criteria examined (data not shown).
The discrepancy in outcomes in the LEO cohort was most notable in AA patients. When eligibility cutoffs from the POLARIX trial were applied, AA patients had the most disparate outcomes between eligible and ineligible patients (Figure 3). This effect remained significant after adjusting for international prognostic index, with AA trial ineligible patients having significantly inferior EFS (hazard ratio, 2.56; 95% CI, 1.35-4.85) and OS (hazard ratio, 4.09; 95% CI, 1.83-9.14) compared with AA trial eligible patients.
Kaplan-Meier curves for EFS and OS in the LEO cohort based on trial eligibility (POLARIX) among various racial/ethnic subgroups.
Kaplan-Meier curves for EFS and OS in the LEO cohort based on trial eligibility (POLARIX) among various racial/ethnic subgroups.
Discussion
This study confirms our previous findings of the impact of laboratory-based eligibility criteria in patients with newly diagnosed DLBCL and extends these results to a much more diverse population. Patients ineligible for trials because of 5 laboratory-based criteria had worse clinical outcomes as well as increased risk of dying from progressive lymphoma. Furthermore, these laboratory-based eligibility criteria led to a disproportionately higher exclusion of Hispanic, AA, and other minority patients than NHW patients. To our knowledge, these data have not been previously reported in first-line DLBCL and will help in future clinical trial design. We also confirmed the previously reported findings of AA patients presenting with DLBCL at a much younger age and with more adverse/high-risk disease than NHW patients, which may be responsible for worse laboratory-based criteria.28 This suggests an even greater unmet need for such patients who could potentially benefit from novel treatments in clinical trials than standard-of-care IC.
In the last few decades clinical trials have become increasingly more restrictive. Loh et al analyzed 42 phase 3 clinical trials in patients with DLBCL receiving first-line treatment and reported that the total number of criteria per study increased from 14.5 between 1993 and 2005, to 23 between 2014 and 2020.8 Furthermore, in the same study when these criteria were applied to a cohort of patients with newly diagnosed DLBCL from an institutional database, the percent of patients ineligible also increased from 32% to 53% between these time periods. Although these ineligibility numbers are higher than our current report, the ineligibility in our study is only based on 5 laboratory-based criteria. The percentage of patients with DLBCL ineligible from the LEO cohort is similar to our previous report as well as a recently reported Danish nationwide cohort study (18%-29% exclusion).29
Many efforts are currently underway to modernize clinical trial eligibility criteria.12,15,30-33 Laboratory-based criteria are easily modifiable in trial design. However, the progress remains slow because of a paucity of data regarding toxicities related to investigational drugs in the early phase trials for patients with organ dysfunction because they are typically excluded, causing further regulatory issues. Determination of laboratory-based eligibility criteria can be subjective and may not necessarily be related to the mechanism of action of the investigational agent. The differential impact of various laboratory-based criteria on patients with DLBCL based on race/ethnicity has not been reported previously. Although it can be hypothesized that some differences in laboratory values exist because of race/ethnicity, such as AA patients having higher proportion of benign ethnic neutropenia; the absolute neutrophil count threshold used in current trial eligibility did not show a substantial impact on eligibility. In contrast, hemoglobin eligibility cutoffs were >10 g/dL in the ENGINE trial and >9 g/dL in the POLARIX trial whereas not present in half of the trials examined. This high threshold for hemoglobin contributed to a 37% exclusion of AA patients in the LEO cohort based on the ENGINE criteria compared with 24% in NHW patients. This is notable as AA patients were the youngest and had the highest kidney function distributions across the race/ethnicity groups. In addition, the largest gap in the clinical outcomes was noted for the AA trial eligible and trial ineligible patients. This suggests a true unmet need in a population that could benefit the most from trial participation and novel therapeutics. Similar findings have been reported in a recent report from the FDA in multiple myeloma trials.34 Sixteen myeloma trials over a 14-year (2006-2019) period were evaluated for specific trial eligibility criteria as a potential barrier to enrollment of underrepresented racial and ethnic subgroups. Ineligibility rates were highest among AA patients (24%) compared with those for White patients (17%).
Several barriers such as lack of access, financial disadvantage, mistrust in the health system, low health literacy, limited access to transportation, increased comorbidity burden, and others have been reported as reasons for low minority accrual on clinical trials. Unger et al reported that more than half of patients if offered clinical trial were willing to participate, with no differences in the participation rates for Black vs White patients.35 The minority patients in the LEO cohort represent a patient population that has access to large academic center and is willing to participate in research, as evidenced by providing informed consent for the LEO cohort study. Exclusion of such high-risk population despite a younger age at presentation based on eligibility criteria requires a thorough reevaluation of these criteria in the context of race/ethnicity.
The strengths of the study include a large well studied prospective patient cohort enrolled at 8 US academic medical centers that is representative of patients considered for clinical trials. Limitations include lack of standardized timing of laboratory evaluations before initiation of treatment across centers and potential changes in these parameters between diagnosis and treatment initiation. This study specifically focused on 5 laboratory-based criteria for newly diagnosed DLBCL only, so the impact of other criteria and in other disease settings is limited. However, the study was specifically designed to evaluate these criteria because they are objective and are easily modifiable once their impact is identified. The patients in LEO cohort self-report their racial/ethnicity status, and those with overlaps were first identified based on Hispanic ethnicity and then segregated based on race. Lastly, data regarding chemotherapy dosing and modifications were unavailable for this study and, hence, the effect of differences in the chemotherapy dose intensity on outcomes between the groups cannot be identified.
In conclusion, laboratory-based eligibility criteria have a substantial impact on clinical trial enrollment, study design, and generalizability of its findings. The trial exclusion based on these laboratory criteria also disproportionately affects AA, Hispanic, and other non-White minority groups compared with the NHW population. Exclusion of patients, especially those belonging to minority groups that are willing participants in research and have access to trials because of eligibility criteria, requires a strategic approach and close evaluation of relevance of each criterion for improvement of trial designs. Future studies focused on modification of early phase studies to include patients with organ dysfunction in separate arms or with provisions for additional support and monitoring are required to bypass some regulatory barriers for large phase 3 trials and broadening of eligibility criteria. Optimization strategies aimed at reversing organ dysfunction before trial enrollment need further evaluation to identify a cohort of these high-risk DLBCL cases that can be safely brought back in clinical trials without additional toxicity burden.
Acknowledgments
This work was supported by grants from the National Cancer Institute: Lymphoma Epidemiology of Outcomes (U01 CA195568) and the University of Iowa/Mayo Clinic Lymphoma SPORE (P50 CA97274).
Authorship
Contribution: A.K., T.E.W., G.S.N., and M.J.M. conceptualized and designed the study; A.K., R.M., L.J.N., P.M.R., U.F., J.T.R., T.J.M., S.M.R., I.S.L., B.S.K., P.M., J.R.C., C.R.F., G.S.N., and M.J.M. collected and/or assembled data; A.K., R.M., S.M.R., and M.J.M. performed statistical analysis; and all authors wrote and reviewed the manuscript.
Conflict-of-interest disclosure: L.J.N. has received honorarium for participation in advisory boards/consulting from AbbVie, ADC Therapeutics, Atara Biotherapeutics, Bristol Myers Squibb (BMS)/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Janssen, Incyte, MorphoSys, Novartis, and Takeda, and research support from BMS/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda. J.R.C., unrelated to this analysis, has received research funding to Mayo from BMS, Genmab, and NanoString, and compensation to Mayo for participation on a scientific advisory board from BMS. M.J.M. received research funding from BMS, Genetech/Roche, GenMab, and reports advisory and board/consulting role with BMS, AstraZeneca, and Adaptive Biotechnologies. The remaining authors declare no competing financial interests.
Correspondence: Matthew J. Maurer, Department of Quantitative Health Sciences, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; email: maurer.matthew@mayo.edu.
References
Author notes
G.S.N. and M.J.M. contributed equally to this study.
Presented in abstract form at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 12 December 2022.
These data are not publicly available. Data sharing policies and the process to request Lymphoma Epidemiology Outcomes data that support the findings of this study can be found on the Lymphoma Epidemiology Outcomes cohort website (https://leocohort.org/).
The full-text version of this article contains a data supplement.