Key Points
Recipient CH found before alloBMT transplantation predicts posttransplant mortality.
Recipient CH is an important independent predictor of NRM after alloBMT.
Visual Abstract
Allogeneic blood and marrow transplantation (alloBMT) is increasingly being used in older patients with blood cancer. Aging is associated with an increasing incidence of clonal hematopoiesis (CH). Although the effects of donor CH on alloBMT has been reported, the impact of recipient CH on alloBMT outcomes is unknown. In this retrospective study, alloBMT recipients age 60 and older with lymphoid malignancies were included. Among 97 consecutive patients who received alloBMT between 2017 and 2022, CH was detected in 60 (62%; 95% confidence interval [CI], 51-72). CH was found in 45% (95% CI, 28-64) of patients aged 60 to 64, 64% (95% CI, 44-81) of patients aged 65% to 69%, and 73% (95% CI, 59-87) in those above 70. Pretransplant CH was associated with worse survival after alloBMT: 3-year overall survival (OS) was 78% (95% CI, 65-94) for patients without CH vs 47% (95% CI, 35-63) for those with CH, (unadjusted HR, 3.1; [95% CI, 1.4-6.8; P < .001]). Nonrelapse mortality (NRM) was higher in patients with CH; cumulative incidence of NRM at 1-year was 11% (95% CI, 1-22) vs 35% (95% CI, 23-48), (HR, 3.4; [95% CI, 1.4-8.5], P = .009]). Among CH patients, worse OS and NRM was associated with CH burden and number of mutations. Recipient CH had no effect on relapse. In conclusion, older patients with CH experience worse outcomes after alloBMT, almost exclusively attributable to increased NRM. CH is a strong, independent predictor of outcomes. Novel strategies to ameliorate the adverse impacts of patient CH on transplant outcomes are being evaluated.
Introduction
The median age of patients diagnosed with most hematologic malignancies is 65 years or older.1 Refinements over the years to allogeneic blood and marrow transplantation (alloBMT), including the use of nonmyeloablative/reduced intensity conditioning regimens and newer graft-versus-host disease (GVHD) prophylaxis platforms, allow patients at least up to age 80 to undergo the procedure.2 Despite progress, transplant-related toxicities are higher in older patients,3 and traditional pretransplant risk assessment tools such as the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI)4 offer limited predictive accuracy for outcomes in this age group.5 This limitation can be attributed to the development of the HCT-CI in younger patients who received myeloablative conditioning regimens. It suggests that common measures of organ dysfunction alone cannot account for excess mortality in older patients.
Clonal hematopoiesis (CH) is a common age–related condition characterized by clonal expansion of hematopoietic stem cells that usually carry 1 or more somatic gene mutations associated with myeloid malignancies.6 The incidence of CH increases steadily with age and is especially prevalent after age 60.
CH is a precursor to myeloid malignancies including myelodysplastic syndrome and acute myeloid leukemia.7-9 However, many studies have shown that CH exerts most of its adverse effects through worse outcomes in a variety of chronic diseases. CH has been associated with an increased incidence or severity of cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and gout among other diseases.10-17 A shared characteristic among these conditions is there frequent association with age and inflammation, both of which are also linked to CH.
CH is prevalent in patients with cancer, and can be caused by cancer treatments.18 In this population CH has been associated with survival outcomes in older patients undergoing cancer treatment,19,20 and with worse survival after autologous BMT for lymphoma21-24 but not multiple myeloma.25 Although CH has been associated with an increased risk of therapy-related myeloid neoplasms (tMN) in autologous BMT recipients, the adverse outcomes extend beyond therapy-related myeloid neoplasms.
In the setting of alloBMT, the impact of donor CH on outcomes has been studied: donor CH was associated with a higher incidence of GVHD and lower relapse rates after tacrolimus/methotrexate- but not high dose posttransplant cyclopohsphamide (PTCy)–based GVHD prophylaxis.26-29 In animal models, clonal donor-derived lymphocytes promote inflammation that causes acute GVHD.30 CH in the donor also has been associated with a higher risk of donor-derived leukemia.26,31
Recipient CH has been studied in the context of molecular measurable residual disease, and was not associated with an increased risk of relapse.32 Chronic inflammation is associated with frailty in aging individuals,33-35 and frail older patients are known to have worse outcomes after alloBMT despite HCT-CI scores similar to nonfrail recipients.36-41 Animal studies implicate older host antigen presenting cells in GVHD, suggesting that components of the aging host immune system are important to transplant outcomes.42 CH has been associated with numerous age-related conditions and adverse outcomes in the general population, and may be a surrogate marker for fraility. Its role in recipient fitness and alloBMT outcome has not been studied, thus we investigated the effect of pretransplant recipient CH on alloBMT outcomes.
Methods
This study was conducted with institutional review board approval and according to the declaration of Helsinki.
Clinical care
Sequential patients aged 60 years and above who underwent first alloBMT between 1 March 2017 through 21 October 2022 for lymphoid malignancies at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Hospital (JHH) were studied. Patients undergoing alloBMT for myeloid malignancies were excluded, as the distinction between the underlying CH and residual disease is often challenging. All patients received conditioning consisting of fludarabine, cyclophosphamide, and either 200 cGy or 400 cGy total body irradiation and PTCy–based GVHD prophylaxis as previously described.43-45 Cytokine release syndrome (CRS) was graded according to American Society for Transplantation and Cellular Therapy criteria.46
Genetic sequencing
Stored samples of DNA previously isolated for clinical chimerism determination were submitted for sequencing. CH was determined by targeted error–corrected next-generation sequencing restricted to 48 genes commonly mutated in CH47 (supplemental Table 1). The average, unique molecular identifier–corrected read depth allowed for reliable detection of mutant alleles with variant allele frequency (VAF) ≥1%.
Outcome definitions
Graft failure was defined as failure to reach an absolute neutorophil count >500/uL by day 56 or T-cell chimerism <5% donor in the absence of relapse at any point. Progressive disease (PD) or relapse was diagnosed clinically and confirmed upon record-review based on radiological and pathological studies; of note, persistence of measurable residual disease or administration of planned or study maintenance medication were not tallied as progression. Nonrelapse mortality (NRM) was defined as death in the absence of progression or relapse. While estimating the cumulative incidence of NRM, relapse was considered a competing event; while estimating the cumulative incidence of relapse, NRM was considered a competing event. Acute GVHD was graded according to Keystone Criteria48; competing risks for GVHD were graft failure and death in the absence of GVHD. Time-to-event outcomes were measured from allograft infusion. Overall survival (OS) was measured from date of allograft infusion to date of death from any cause, or censored at the last follow-up date in patients who were alive.
Statistical methods
Proportions were reported with 95% exact confidence intervals (CIs). OS was estimated using the Kaplan-Meier method, and the group difference was assessed using the log-rank test. For time-to-event outcomes considering competing risks, the group differences in the corresponding cumulative incidence function were assessed using Gray's k-sample method.49 Initially, a comparison of survival was made between groups with and without pretransplant CH. Since there was a significant association between CH and OS, patients with CH were further divided based on VAF levels and the number of mutations to examine the associations with the amount of CH on the respective outcomes. The cut-point for dichotomizing VAF was determined by visualizing age-adjusted log relative hazards of OS within patients who are CH-positive, using a restricted cubic spline function of VAF (supplemental Figure 1). Three knots of VAF were placed at the 55th, 75th, and 95th quantiles (corresponding to VAF values of 3.4, 5.1, and 37.7) due to a right-skewed distribution of VAF in these older patients and a lower limit of positivity of 1%. The relative risk for each 10% increase in VAF level within patients who are CH-positive was further examined. To assess the group differences while adjusting for covariates, the Cox proportional hazards model was applied for OS and progression free survival (PFS), and Fine-Gray’s subdistribution hazard models were applied for other time-to-event outcomes accounting for competing risks.50 The adjusted covariates included the patient's age at BMT (as a linear continuous variable), the donor’s age (as a linear continuous variable), HCT-CI (categorized as 0, 1-2, or 3+) and lines of cytotoxic therapy (categorized as 3+ vs 0-2). These variables were chosen a priori based on a reported association with mortality after alloBMT or CH incidence. Analyses were compiled in R 4.2.2 environment (R Foundation for Statistical Computing, Vienna, Austria).
This study was approved by the institutional review board at JHH.
Results
Patient and CH characteristics
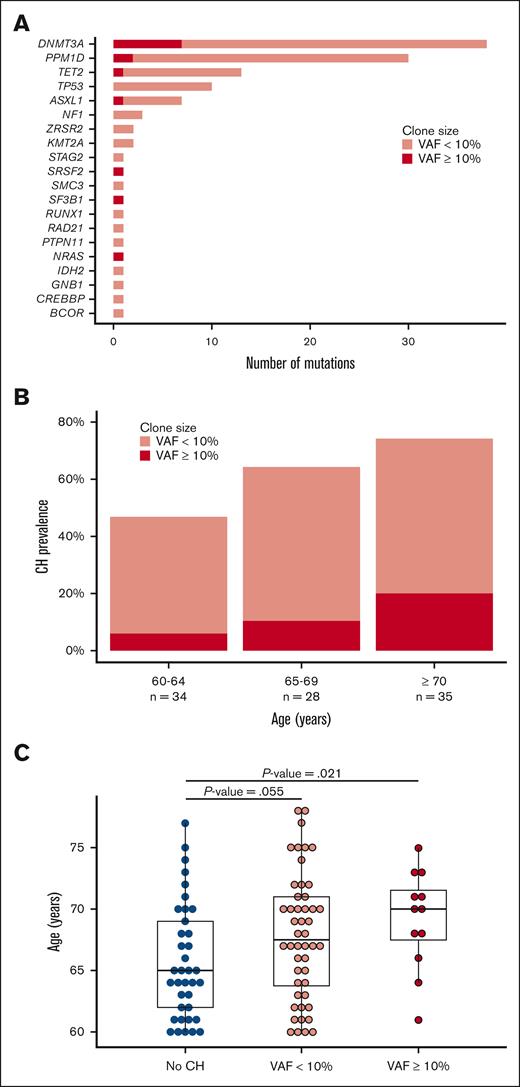
The samples from 97 consecutive patients aged 60 or above met the technical requirements for the sequencing analysis. CH was found in 60 out of 97 (62%; 95% CI, 51-72) patients, involving 117 total variants (supplemental Table 2). The median age of patients with pre-alloBMT CH was 68 years, compared with 65 years in those without CH (P = .01). Otherwise, demographic, disease and BMT characteristics were well balanced between the CH positive and CH-negative cohorts (Table 1). DNMT3A, TET2, and ASXL1 mutations were common (Figure 1A); the prevalence of PPM1D variants (21/97, 22%) and TP53 variants (7/97, 7%) in our study was higher than that of the general population,47 but consistent with that seen following cytotoxic chemotherapy.19 The prevalence of CH varied by age: CH was present in 45% (95% CI, 28-64) of patients aged 60 to 64 years, 64% (95% CI, 44-81) of patients aged 65 to 69 years, and 75% (95% CI, 58-88) of those 70 years and above (Figure 1B). CH clone size also positively correlated with patient age (Figure 1B-C). Patients received a median of 2 lines of cytotoxic chemotherapy (range, 0-4) prior to transplant, and median values were not different between the groups (Table 1). The vast majority of patients received only 2 lines (initial therapy and salvage before BMT); only 2 patients received 4 distinct cycles of cytotoxic chemotherapy, both of whom had CH.
Clinical and transplant characteristics of sequential patients aged 60 and above, separated into 3 groups by size of CH clone
| Characteristic . | All (n = 97) . | No CH (n = 37) . | CH positive VAF 1%-10% (n = 48) . | CH positive VAF | P value . |
|---|---|---|---|---|---|
| Median age (range) | 67 (60-78) | 65 (60-77) | 68 (60-78) | 70 (61-75) | .04 |
| Male sex | 62 (64%) | 24 (65%) | 31 (65%) | 7 (58%) | .9 |
| Graft source | .7 | ||||
| PB | 26 (28%) | 8 (22%) | 15 (31%) | 3 (25%) | |
| BM | 71 (72%) | 29 (78%) | 33 (69%) | 9 (75%) | |
| Match | .6 | ||||
| Haploidentical | 85 (88%) | 30 (81%) | 44 (92%) | 11 (92%) | |
| Full (inc 9/10) | 5 (5%) | 3 (8%) | 2 (4%) | 0 | |
| mMUD | 7 (7%) | 4 (11%) | 2 (4%) | 1 (8%) | |
| HCT-CI, med (range) | 1 (0-7) | 1 (0-6) | 1 (0-7) | 1 (0-4) | .9 |
| 0 | 27 (28%) | 12 (32%) | 13 (27%) | 2 (17%) | .9 |
| 1 to 2 | 48 (49%) | 17 (46%) | 24 (50%) | 7 (58%) | |
| 3+ | 22 (23%) | 8 (22%) | 11 (23%) | 3 (25%) | |
| Disease | .56 | ||||
| T ALL | 2 | 0 | 2 | 0 | |
| Ph+ B ALL | 6 | 2 | 4 | 0 | |
| Ph- ALL | 3 | 1 | 1 | 1 | |
| T-PLL | 2 | 1 | 1 | 0 | |
| T-cell lymphoma | 11 | 5 | 4 | 2 | |
| CLL | 3 | 0 | 1 | 2 | |
| B-cell lymphoma∗ | 64 | 25 | 32 | 7 | |
| HL | 6 | 3 | 3 | 0 | |
| Disease risk index | .6 | ||||
| Low | 28 | 11 | 14 | 3 | |
| Intermediate | 65 | 25 | 32 | 8 | |
| High/very high | 4 | 0 | 2 | 1 | |
| Lines of cytotoxic chemotherapy | .65 | ||||
| 0 | 5 (5%) | 2 (5%) | 2 (4%) | 1 (8%) | |
| 1 | 25 (26%) | 8 (22%) | 14 (29%) | 3 (25%) | |
| 2 | 49 (51%) | 23 (62%) | 20 (42%) | 6 (50%) | |
| 3 | 16 (16%) | 4 (11%) | 10 (21%) | 2 (17%) | |
| 4 | 2 (2%) | 0 | 2 (4%) | 0 | |
| Donor age, median (range) | 31 (14-54) | 29 (16-50) | 32 (14-49) | 37 (26-54) | .11 |
| Characteristic . | All (n = 97) . | No CH (n = 37) . | CH positive VAF 1%-10% (n = 48) . | CH positive VAF | P value . |
|---|---|---|---|---|---|
| Median age (range) | 67 (60-78) | 65 (60-77) | 68 (60-78) | 70 (61-75) | .04 |
| Male sex | 62 (64%) | 24 (65%) | 31 (65%) | 7 (58%) | .9 |
| Graft source | .7 | ||||
| PB | 26 (28%) | 8 (22%) | 15 (31%) | 3 (25%) | |
| BM | 71 (72%) | 29 (78%) | 33 (69%) | 9 (75%) | |
| Match | .6 | ||||
| Haploidentical | 85 (88%) | 30 (81%) | 44 (92%) | 11 (92%) | |
| Full (inc 9/10) | 5 (5%) | 3 (8%) | 2 (4%) | 0 | |
| mMUD | 7 (7%) | 4 (11%) | 2 (4%) | 1 (8%) | |
| HCT-CI, med (range) | 1 (0-7) | 1 (0-6) | 1 (0-7) | 1 (0-4) | .9 |
| 0 | 27 (28%) | 12 (32%) | 13 (27%) | 2 (17%) | .9 |
| 1 to 2 | 48 (49%) | 17 (46%) | 24 (50%) | 7 (58%) | |
| 3+ | 22 (23%) | 8 (22%) | 11 (23%) | 3 (25%) | |
| Disease | .56 | ||||
| T ALL | 2 | 0 | 2 | 0 | |
| Ph+ B ALL | 6 | 2 | 4 | 0 | |
| Ph- ALL | 3 | 1 | 1 | 1 | |
| T-PLL | 2 | 1 | 1 | 0 | |
| T-cell lymphoma | 11 | 5 | 4 | 2 | |
| CLL | 3 | 0 | 1 | 2 | |
| B-cell lymphoma∗ | 64 | 25 | 32 | 7 | |
| HL | 6 | 3 | 3 | 0 | |
| Disease risk index | .6 | ||||
| Low | 28 | 11 | 14 | 3 | |
| Intermediate | 65 | 25 | 32 | 8 | |
| High/very high | 4 | 0 | 2 | 1 | |
| Lines of cytotoxic chemotherapy | .65 | ||||
| 0 | 5 (5%) | 2 (5%) | 2 (4%) | 1 (8%) | |
| 1 | 25 (26%) | 8 (22%) | 14 (29%) | 3 (25%) | |
| 2 | 49 (51%) | 23 (62%) | 20 (42%) | 6 (50%) | |
| 3 | 16 (16%) | 4 (11%) | 10 (21%) | 2 (17%) | |
| 4 | 2 (2%) | 0 | 2 (4%) | 0 | |
| Donor age, median (range) | 31 (14-54) | 29 (16-50) | 32 (14-49) | 37 (26-54) | .11 |
ALL, acute lymphoblastic leukemia; BM, bone marrow; HL, Hodgkin lymphoma; inc, including; mMUD, mismatched unrelated donor; NHL, non-Hodgkin lymphoma.
Includes 1 patient with multiple myeloma (small CH) and 1 patient with plasmablastic lymphoma (no CH).
Prevalence and characteristics of CH according to age of alloBMT recipient. (A) Distribution of CH mutations in the cohort. (B) Prevalence of CH at different ages. (C) Distribution of CH proportion according to patient age.
Prevalence and characteristics of CH according to age of alloBMT recipient. (A) Distribution of CH mutations in the cohort. (B) Prevalence of CH at different ages. (C) Distribution of CH proportion according to patient age.
Survival
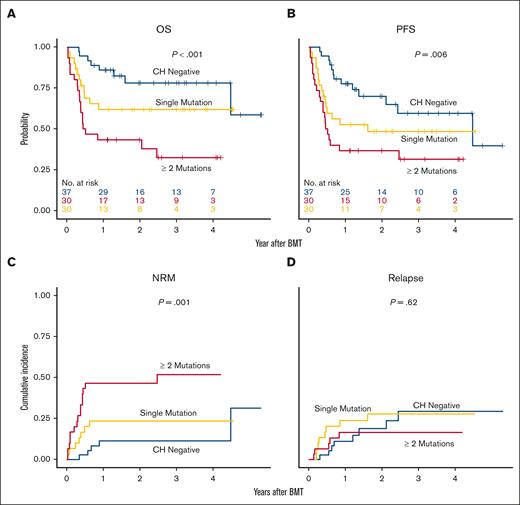
The median follow-up for the entire cohort was estimated to be 32 months after alloBMT using a reverse Kaplan-Meier approach. The presence of pretransplant CH was associated with a significantly shorter survival after alloBMT: 3-year OS 78% (95% CI, 65-94) without CH vs 47% (95% CI, 35-63) with CH (unadjusted hazard ratio [HR], 3.1; 95% CI, 1.4-6.8; P < .001; Figure 2A). PFS was also worse in those patients with CH (Figure 2B), with a 3-year PFS of 39% (95% CI, 28-55) vs 60% (95% CI, 43-82) in CH-negative patients. The risk of NRM was significantly higher in recipients with CH (cumulative incidence of 1-year NRM was 11% [95% CI, 1-22] vs 35% [95% CI, 23-48], unadjusted subdistribution HR [SDHR], 3.4; [95% CI, 1.4-8.5], P = .009; Figure 2C). Three patients experienced graft failure, 1 with CH and 2 without. CH was not associated with the risk of relapse (Figure 2D).
Clinical outcomes after alloBMT are associated with VAF pretransplant CH. Kaplan-Meier curves of (A) OS and (B) PFS; cumulative incidence of (C) NRM and (D) relapse by CH-negative or CH-positive. Estimates in CH positive group were further stratified by ([VAF] 1%-10% and ≥10%).
Clinical outcomes after alloBMT are associated with VAF pretransplant CH. Kaplan-Meier curves of (A) OS and (B) PFS; cumulative incidence of (C) NRM and (D) relapse by CH-negative or CH-positive. Estimates in CH positive group were further stratified by ([VAF] 1%-10% and ≥10%).
In addition to CH, older recipient age was also significantly associated with OS and NRM in univariate analysis (supplemental Table 3). Donor age was also associated with NRM and with relapse (supplemental Table 3). HCT-CI was not associated with OS (compared with HCT-CI of 0, HR, 1.34; [95% CI, 0.61-2.95; P = .46] for HCT-CI 1 to 2, and for 3+ HR, 1.35; [95% CI, 0.53-3.40; P = .53]) or with NRM (compared with HCT-CI of 0; HR, 1.18 [95% CI, 0.48-2.90; P = .40] for 1 to 2, and for 3+ HR, 0.99 [95% CI, 0.59-7.61; P = .25]).
Clone size had a strong effect on survival. The 3-year OS was 78% (65%-94%) for patients without CH, 50% (37%-68%; HR, 2.74; [95% CI, 1.22-6.16]) for those with a VAF between 1% and 10%, and 33% (15%-74%; HR, 5.08; [95% CI, 1.9-13.59]) in those with a VAF ≥10% (Figure 2A). The 1-year cumulative incidence of NRM was 11% (1%-22%) for those without CH, 27% (SDHR, 2.57; [95% CI, 0.98-6.76, P = .056]) in patients with VAF 1% to 10%, and 67% (HR, 7.97; [95% CI, 2.68-23.68]) with VAF ≥10% (Figure 2B). Although the numbers are small (12), 0 out of 7 matched sibling and unrelated patients without CH died within 1 year of alloBMT compared with 2 out of 5 with CH. An analysis of the number of distinct CH mutations on outcomes showed similar results, with worse outcomes in patients with more than 1 CH mutation with regard to OS, PFS, and NRM (Figures 3A-C), but no difference in relapse (Figure 3D).
Clinical outcomes after alloBMT are associated with number of CH mutations with variant allele frequencies >1%. Kaplan-Meier curves of (A) OS and (B) PFS; cumulative incidence of (C) NRM and (D) relapse by CH-negative and number of mutations (single mutation, 2 or more mutations).
Clinical outcomes after alloBMT are associated with number of CH mutations with variant allele frequencies >1%. Kaplan-Meier curves of (A) OS and (B) PFS; cumulative incidence of (C) NRM and (D) relapse by CH-negative and number of mutations (single mutation, 2 or more mutations).
In a multivariable analysis that included recipient age, donor age, HCT-CI, and number of prior lines of therapy (3+ vs 0-2), CH remained the only significant predictor of NRM (Table 2) and it was also significantly associated with OS and PFS, but not relapse. Recipient age was also associated with OS and PFS, while donor age was associated with relapse. Number of lines of therapy was associated with relapse but not NRM or OS, and HCT-CI was not prognostic of any outcome.
Association between CH and survival outcomes in multivariable analyses
| Variables . | OS . | PFS . | NRM . | Relapse . | ||||
|---|---|---|---|---|---|---|---|---|
| Adj HR (95% CI) . | P value . | Adj HR (95% CI) . | P value . | Adj SDHR (95% CI) . | P value . | Adj SDHR (95% CI) . | P value . | |
| CH negative | Ref | Ref | Ref | Ref | ||||
| CH positive | 2.47 (1.11-5.52) | .03 | 1.98 (1.02-3.82) | .04 | 3.04 (1.18-7.80) | .02 | 0.95 (0.37-2.45) | .92 |
| Recipient age at BMT (per 10 y) | 2.95 (1.44-6.08) | .003 | 2.24 (1.20-4.18) | .01 | 1.77 (0.80-3.92) | .16 | 1.59 (0.60-4.23) | .36 |
| Donor’s age (per 10 y) | 0.99 (0.69-1.42) | .94 | 0.83 (0.60-1.16) | .29 | 1.38 (0.89-2.13) | .15 | 0.43 (0.24-0.79) | .007 |
| HCT-CI | ||||||||
| 0 | Ref | Ref | Ref | Ref | ||||
| 1-2 | 1.23 (0.55-2.75) | .62 | 1.49 (0.73-3.05) | .28 | 0.92 (0.3-2.37) | .86 | 1.88 (0.61-5.80) | .27 |
| 3+ | 1.19 (0.46-3.08) | .71 | 1.50 (0.65-3.47) | .34 | 0.76 (0.26-2.22) | .61 | 2.94 (0.94-9.20) | .06 |
| No. of prior cytotoxic therapies | ||||||||
| 0 to 2 | Ref | Ref | Ref | Ref | ||||
| 3+ | 1.39 (0.64-3.02) | .41 | 1.92 (0.97-3.79) | .06 | 0.71 (0.25-2.03) | .53 | 4.53 (1.75-11.70) | .002 |
| Variables . | OS . | PFS . | NRM . | Relapse . | ||||
|---|---|---|---|---|---|---|---|---|
| Adj HR (95% CI) . | P value . | Adj HR (95% CI) . | P value . | Adj SDHR (95% CI) . | P value . | Adj SDHR (95% CI) . | P value . | |
| CH negative | Ref | Ref | Ref | Ref | ||||
| CH positive | 2.47 (1.11-5.52) | .03 | 1.98 (1.02-3.82) | .04 | 3.04 (1.18-7.80) | .02 | 0.95 (0.37-2.45) | .92 |
| Recipient age at BMT (per 10 y) | 2.95 (1.44-6.08) | .003 | 2.24 (1.20-4.18) | .01 | 1.77 (0.80-3.92) | .16 | 1.59 (0.60-4.23) | .36 |
| Donor’s age (per 10 y) | 0.99 (0.69-1.42) | .94 | 0.83 (0.60-1.16) | .29 | 1.38 (0.89-2.13) | .15 | 0.43 (0.24-0.79) | .007 |
| HCT-CI | ||||||||
| 0 | Ref | Ref | Ref | Ref | ||||
| 1-2 | 1.23 (0.55-2.75) | .62 | 1.49 (0.73-3.05) | .28 | 0.92 (0.3-2.37) | .86 | 1.88 (0.61-5.80) | .27 |
| 3+ | 1.19 (0.46-3.08) | .71 | 1.50 (0.65-3.47) | .34 | 0.76 (0.26-2.22) | .61 | 2.94 (0.94-9.20) | .06 |
| No. of prior cytotoxic therapies | ||||||||
| 0 to 2 | Ref | Ref | Ref | Ref | ||||
| 3+ | 1.39 (0.64-3.02) | .41 | 1.92 (0.97-3.79) | .06 | 0.71 (0.25-2.03) | .53 | 4.53 (1.75-11.70) | .002 |
The analysis included patient age, donor age, number of lines of cytotoxic chemotherapy, and HCT-CI score.
Adj, adjustment with patient age, donor age, HCT-CI, and lines of cytotoxic chemotherapy; NOM, number of mutations; Ref, reference.
Causes of death
PD was the most common cause of death in CH-negative patients (8%). In CH-positive patients, PD (13%), GVHD (12%), CRS (5%), sepsis (7%) and multiorgan failure (5%) were the most common causes of death. CH was present in all patients who died prior to day 100 (n = 10) (supplemental Table 4).
CRS and GVHD
The incidence of grade 2 to 4 acute GVHD was not significantly different between the cohorts (supplemental Figure 2A). Grade 3 to 4 GVHD incidence did appear to be higher as the level of CH increased, although the relationship was not statistically significant: the 1-year cumulative incidence of grade 3 to 4 GVHD was 5% (95% CI, 0-13) in patients without CH, 10% (SDHR, 2.0 [95% CI, 0.39-10.2]; P = .4) in those with 1% to 10% VAF, and 25% (95% CI, 0-52; SDHR, 4.99; [95% CI, 0.86-28.88]; P = .07) in those with >10% VAF (supplemental Figure 2B). In patients with CH, 8 of 19 (42%) cases of acute GVHD were grade 3 to 4 compared with 2 of 15 (13%) without CH (P = .13). Eight patients died with acute GVHD, 7 of whom (88%) had CH. Chronic GVHD incidence was low in all 3 groups: 5 of 37 patients without CH, 5 of 48 with 1% to 10% VAFs, and 0 of 13 with >10% VAF.
CRS is an early complication of mismatched alloBMT, especially in older recipients and those receiving peripheral blood grafts.51 Of the 26 patients who received peripheral blood grafts, CRS occurred in 19. The incidence was 50% (4/8 [95% CI, 16-84], with no cases of severe CRS) in patients without CH, and 83% (15/18 [95% CI, 59-96]) in patients with CH, including 4 cases of severe CRS (P = .08).
Discussion
Older patients with aggressive blood cancers are increasingly treated with alloBMT. In this study, recipient CH predicted significantly increased morbidity and mortality despite subsequent engraftment of donor hematopoiesis. Indeed, CH appeared to account for most of the excess risk attributable to age in this cohort of older patients after alloBMT. The size and number of clones both appeared to mediate outcomes, which was consistent with a dose-response relationship.
The prevalence of CH in this cohort of alloBMT recipients was high (62%) and might reflect exposure to DNA damaging agents such as chemotherapy and radiation, proliferative stress, chronic inflammation,52,53 or an aged bone marrow microenvironment.54 Both PPM1D55,56 and TP5357,58 mutations can emerge after chemotherapy, which may be due to CH clones having a selective advantage in repopulating bone marrow exposed to chemotherapy.59
It is unclear as to why CH is associated with increased toxicity after alloBMT. CH may simply be a marker of frailty or a biomarker of aging.60,61 We also explored the possibility that CH may be a surrogate for other known or predicted markers of mortality such as patient age, disease status (using the disease risk index), or amount of prior therapy. However, there was no difference in lines of therapy between patients with and without CH, and a multivariable analysis that included prior number of therapies showed that CH remained the only predictor of NRM.
Perhaps more likely, CH exerts its effects by enhancing inflammatory responses. Most studies on CH and alloBMT have focused on engrafting donor CH,14-19 and some studies reported the association between donor CH and GVHD. However, host tissue–resident macrophages persist for prolonged periods after alloBMT.42,62 CH-derived macrophages secrete proinflammatory cytokines such as interleukin-6,10 which could result in an exuberant inflammatory response resulting in CRS and GVHD. Strategies that could ameliorate the negative effects of CH on outcomes if they are related to inflammation include JAK inhibitors, potent anti-inflammatory agents that have been studied in the early posttransplant period.63 Studies of the JAK inhibitor itaciinib in older alloBMT recipients are ongoing.
Limitations of this study include the relatively small number of patients from a single center. All the patients received PTCy, and there was a preponderance of haploidentical donors. The study also excluded patients with myeloid diseases like myelodysplastic syndrome and acute myeloid leukemia to distinguish clearly between preexisting CH and detectable residual myeloid disease. In the patients with lymphoid malignancy studied here, the distinction is clearer. We chose to include only patients over 60 because CH prevalence and NRM are both more common in older patients; CH may contribute to NRM in younger patients and this question should be addressed in future studies. Fewer than one-third of our patients received all of their cancer care at JHH, a rate which is common for referral transplant centers but made it impossible to determine the precise contribution of each component of therapy to CH incidence or clinical outcomes; lines of cytotoxic chemotherapy is only an approximation of chemotherapy exposure.
In this study of older alloBMT patients with lymphoid malignancies, recipient CH was a strong, independent predictor of survival. Since chronologic age alone is no longer an absolute contraindication to alloBMT, improved predictive tools are important to care for the growing population of healthy older patients with aggressive blood cancer. Detection of CH may add important prognostic information to current risk stratification paradigms. Results from a large, prospective, recently completed study of risk stratification for older alloBMT recipients using several geriatric assessment tools (Bone Marrow Translant Clinical Trials Network 1704 Composite Health Risk Assessment Model: [CHARM; NCT03992352]) should aid further in risk prediction and identify groups who might benefit from prospective, interventional trials.
In conclusion, pretransplant CH predicts NRM and survival after alloBMT in older patients. These findings should be confirmed in an independent cohort. Further studies in older patients with myeloid malignancies also should be pursued, because these patients represent nearly 80% of alloBMT indications. Targeting CH-mediated inflammation in the early alloBMT period may represent a novel strategy leading to improved outcomes.
Acknowledgments
The authors thank Lisa Haley for preparing the samples, Inpatient/Outpatient Ambulatory Transplant Clinic), and blood or marrow transplantation staff; patients, their families, and caregivers.
The study was supported by grants from the National Cancer Institute (P01 CA225618-01A1 and 2P30CA006973-54), National Institute on Aging (UH3AG056933), and National Heart, Lung, and Blood Institute (R01HL156144).
Authorship
Contribution: P.H.I., R.J.J., and L.P.G. designed the research; P.H.I., S.P., Y.M.A., and L.P.G. performed the research; P.H.I., S.P., H.-L.T., R.V., R.J.J., and L.P.G. analyzed data; and all authors wrote the manuscript.
Conflict-of-intreset disclosure: The authors declare no competing financial interests.
Correspondence: Philip H. Imus, Oncology, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital, CRB1 Room 2M10, 1650 Orleans St, Baltimore, MD 21231; email: pimus1@jhmi.edu.
References
Author notes
Presented in abstract form at the 2023 Annual Tandem Meetings of the American Society of Transplantation ond Cellular Therapy/Center for International Blood and Marrow Transplant Research, 16 February 2023, Orlando, FL.
Data are available on request from the corresponding author, Philip Imus (pimus1@jhmi.edu). The accession number is PRJNA1096370 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1096370).
The full-text version of this article contains a data supplement.


![Clinical outcomes after alloBMT are associated with VAF pretransplant CH. Kaplan-Meier curves of (A) OS and (B) PFS; cumulative incidence of (C) NRM and (D) relapse by CH-negative or CH-positive. Estimates in CH positive group were further stratified by ([VAF] 1%-10% and ≥10%).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/14/10.1182_bloodadvances.2023011761/1/m_blooda_adv-2023-011761-gr2.jpeg?Expires=1770002867&Signature=kkkJrNUDly5bYr-5qQptYNVjOR5xIhMBpDF~176sa7QNt30dYld2UPf7J7m1adc~vIzLh7Nm9muc6YHMheo1FKVxdG4p6Ldsy7SW1V1gAhrMUt1mhU2~LHuw37mYi1xIkMRCk7~xQ215JyUalCdH6lp964vVkeLdczAiOguHj5BZWdlpKGLzTcKzmfvGOZd52LsXN2rl4WF0qPt6IY0NK4-32tLBr~UBiSr6DYpkaZEzxMdLbYskfkDcC-fzSpw~6KY4diQkJgHrShkRArov~5CJ5lPgAYuRi9upxSF51j3cCjU~M5vbh9zzsxg310zSGA9t7LufbXYd7pf2rJ0pcA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)