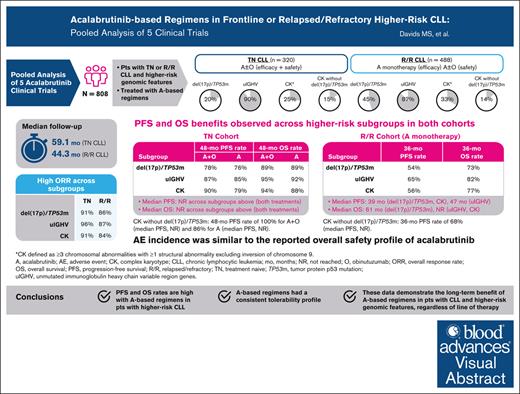
Key Points
Acalabrutinib-based regimens achieve long-term efficacy in patients with higher-risk CLL, across all lines of therapy.
Safety profile of acalabrutinib in patients with higher-risk CLL was similar to the overall safety profile of acalabrutinib.
Visual Abstract
Before targeted therapies, patients with higher-risk chronic lymphocytic leukemia (CLL), defined as del(17p) and/or TP53 mutation (TP53m), unmutated immunoglobulin heavy chain variable region genes (uIGHV), or complex karyotype (CK), had poorer prognosis with chemoimmunotherapy. Bruton tyrosine kinase inhibitors (BTKis) have demonstrated benefit in higher-risk patient populations with CLL in individual trials. To better understand the impact of the second-generation BTKi acalabrutinib, we pooled data from 5 prospective clinical studies of acalabrutinib as monotherapy or in combination with obinutuzumab (ACE-CL-001, ACE-CL-003, ELEVATE-TN, ELEVATE-RR, and ASCEND) in patients with higher-risk CLL in treatment-naive (TN) or relapsed/refractory (R/R) cohorts. A total of 808 patients were included (TN cohort, n = 320; R/R cohort, n = 488). Median follow-up was 59.1 months (TN cohort) and 44.3 months (R/R cohort); 51.3% and 26.8% of patients in the TN and R/R cohorts, respectively, remained on treatment at last follow-up. In the del(17p)/TP53m, uIGHV, and CK subgroups in the TN cohort, median progression-free survival (PFS) and median overall survival (OS) were not reached (NR). In the del(17p)/TP53m, uIGHV, and CK subgroups in the R/R cohort, median PFS was 38.6 months, 46.9 months, and 38.6 months, respectively, and median OS was 60.6 months, NR, and NR, respectively. The safety profile of acalabrutinib-based therapy in this population was consistent with the known safety profile of acalabrutinib in a broad CLL population. Our analysis demonstrates long-term benefit of acalabrutinib-based regimens in patients with higher-risk CLL, regardless of line of therapy.
Introduction
Chronic lymphocytic leukemia (CLL) is a heterogenous disease with a highly variable clinical course for which patients with higher-risk genomic aberrations, including del(17p13.1) (del(17p)) and/or TP53 mutation (TP53m), unmutated immunoglobulin heavy chain variable region genes (uIGHV), or complex karyotype (CK), have historically had inferior outcomes.1 Before the development of targeted therapies, patients with higher-risk genomic features had poorer prognosis when treated with standard chemoimmunotherapy.1 Additionally, fixed-duration treatment with venetoclax and an anti-CD20 antibody has shown shorter responses in patients with del(17p), uIGHV, and/or CK than in patients without those features.2-5 Targeted agents such as Bruton tyrosine kinase inhibitors (BTKis) administered as continuous therapy have demonstrated benefit in patients with higher-risk CLL and are preferred treatment options in patients with del(17p)/TP53m, uIGHV, and/or CK.6-12
Acalabrutinib is a second-generation, selective, covalent BTKi13 with demonstrated progression-free survival (PFS) and overall survival (OS) benefits in patients with treatment-naive (TN) and relapsed/refractory (R/R) CLL, including those with higher-risk genomic characteristics.6-10 Because the numbers of patients with higher-risk CLL in the individual studies of acalabrutinib are relatively small for most higher-risk features, there was a need to collate these data across trials to better understand treatment outcomes. Herein, we conducted a pooled analysis of 5 studies to evaluate the response and long-term efficacy and safety of acalabrutinib-based therapy in both the TN and R/R CLL settings in patients with higher-risk genomic features, including del(17p)/TP53m, uIGHV, and CK.
Methods
Pooled analysis
The data for this analysis were pooled from 5 clinical studies in patients with CLL with higher-risk genomic features (defined as del(17p)/TP53m, uIGHV, or CK [≥3 chromosomal abnormalities]; Table 1) or without any of the higher-risk features (lower-risk subgroup) treated with acalabrutinib monotherapy or acalabrutinib plus obinutuzumab. The number of patients with CK ≥5 abnormalities was too small to analyze and not included in this analysis. The analysis was conducted separately in patients who had previously untreated CLL (TN cohort) or patients who had received ≥1 prior therapy for CLL (R/R cohort). The TN CLL cohort included data from 3 clinical studies: ACE-CL-001 (TN cohort), ACE-CL-003 (TN cohort [ie, study cohort 2]), and ELEVATE-TN (ACE-CL-007). The R/R CLL cohort included data from 4 clinical studies: ACE-CL-001 (R/R cohort), ACE-CL-003 (R/R cohort [ie, study cohort 1]), ELEVATE-RR (ACE-CL-006), and ASCEND (ACE-CL-309). Detailed methods for each study were previously reported.6,8,9,14-16 Screening blood samples underwent centralized (except for CL-003) assessment of genomic aberrations with fluorescence in situ hybridization (FISH) and mutational analysis of the IGHV and cellular antigen TP53 genes by DNA sequencing; for CL-003, FISH, cytogenetics, and IGHV mutational analyses were performed in the Clinical Laboratory Improvement Amendments certified clinical pathology laboratories at The Ohio State University. FISH probes were used for cytogenetic profiling, and abnormalities in chromosomes 13q, 12, 11q, and 17p were assessed (Vysis CLL FISH Probe Kit; Abbott Molecular). IGHV mutations were assessed by standard Sanger sequencing using an assay sensitivity of 10% and a cutoff of 2%. TP53 mutations were analyzed by Sanger sequencing methods. CK was defined as having ≥3 chromosomal abnormalities with ≥1 structural abnormality, excluding inversion of chromosome 9. In all the included studies, acalabrutinib was given as 100 mg twice daily until progressive disease or unacceptable toxicity, with the exception of some patients in ACE-CL-001 and ACE-CL-003 who were initially treated at 200 mg once daily. Patients in ACE-CL-003 and patients in the acalabrutinib plus obinutuzumab arm of ELEVATE-TN also received 6 cycles of obinutuzumab (100 mg on day 1; 900 mg on day 2; and 1000 mg on days 8 and 15 of cycle 2; and 1000 mg on day 1 of cycles 3-7). Although some of these studies had treatment arms other than acalabrutinib monotherapy and acalabrutinib plus obinutuzumab, this analysis pooled data for acalabrutinib monotherapy and acalabrutinib plus obinutuzumab therapy, referred to as acalabrutinib-based treatment.
Studies included in the pooled analysis of patients in the higher-risk subgroup treated with acalabrutinib-based regimens
| . | Study . | Study description . | Data cutoff date . | Number of patients treated with A-based regimens (N = 808) . | ||
|---|---|---|---|---|---|---|
| del(17p)/TP53m (n = 283) . | uIGHV (n = 712) . | CK∗ (n = 239) . | ||||
| TN CLL (n = 320) | ACE-CL-001 TN cohort NCT02029443 | Phase 1/2:A monotherapy in TN CLL/SLL | 15 July 2021 | 12 | 57 | 12 |
| ACE-CL-003 TN cohort (cohort 2) NCT02296918 | Phase 1b/2: A + O in TN CLL | 13 June 2021 | 4 | 9 | 8 | |
| ELEVATE-TN CL-007 NCT02475681 | Phase 3: A ± O vs O + Clb in TN CLL | 01 October 2021 | 48 | 221 | 59 | |
| Proportion of higher-risk genomic subgroup in TN cohort, n/N (%)† | 64/283 (23) | 287/712 (40) | 79/239 (33) | |||
| R/R CLL (n = 488) | ACE-CL-001 R/R cohort NCT02029443 | Phase 1/2:A monotherapy in R/R CLL/SLL | 15 July 2021 | 35 | 81 | 20 |
| ACE-CL-003 R/R cohort‡ (cohort 1) NCT02296918 | Phase 1b/2: A + O in R/R CLL | 13 June 2021 | 5 | 17 | 14 | |
| ELEVATE-RR CL-006 NCT02477696 | Phase 3:A monotherapy vs ibrutinib in R/R CLL | 15 September 2020 | 135 | 219 | 123 | |
| ASCEND CL-309 NCT02970318 | Phase 3:A monotherapy vs IdR/BR in R/R CLL | 03 September 2021 | 44 | 108 | 3 | |
| Proportion of higher-risk genomic subgroup in R/R cohort, n/N (%)† | 219/283 (77) | 425/712 (60) | 160/239 (67) | |||
| . | Study . | Study description . | Data cutoff date . | Number of patients treated with A-based regimens (N = 808) . | ||
|---|---|---|---|---|---|---|
| del(17p)/TP53m (n = 283) . | uIGHV (n = 712) . | CK∗ (n = 239) . | ||||
| TN CLL (n = 320) | ACE-CL-001 TN cohort NCT02029443 | Phase 1/2:A monotherapy in TN CLL/SLL | 15 July 2021 | 12 | 57 | 12 |
| ACE-CL-003 TN cohort (cohort 2) NCT02296918 | Phase 1b/2: A + O in TN CLL | 13 June 2021 | 4 | 9 | 8 | |
| ELEVATE-TN CL-007 NCT02475681 | Phase 3: A ± O vs O + Clb in TN CLL | 01 October 2021 | 48 | 221 | 59 | |
| Proportion of higher-risk genomic subgroup in TN cohort, n/N (%)† | 64/283 (23) | 287/712 (40) | 79/239 (33) | |||
| R/R CLL (n = 488) | ACE-CL-001 R/R cohort NCT02029443 | Phase 1/2:A monotherapy in R/R CLL/SLL | 15 July 2021 | 35 | 81 | 20 |
| ACE-CL-003 R/R cohort‡ (cohort 1) NCT02296918 | Phase 1b/2: A + O in R/R CLL | 13 June 2021 | 5 | 17 | 14 | |
| ELEVATE-RR CL-006 NCT02477696 | Phase 3:A monotherapy vs ibrutinib in R/R CLL | 15 September 2020 | 135 | 219 | 123 | |
| ASCEND CL-309 NCT02970318 | Phase 3:A monotherapy vs IdR/BR in R/R CLL | 03 September 2021 | 44 | 108 | 3 | |
| Proportion of higher-risk genomic subgroup in R/R cohort, n/N (%)† | 219/283 (77) | 425/712 (60) | 160/239 (67) | |||
A, acalabrutinib; BR, bendamustine plus rituximab; Clb, chlorambucil; IdR, idelalisib plus rituximab; O, obinutuzumab; SLL, small lymphocytic lymphoma.
CK was defined as having ≥3 chromosomal abnormalities with ≥1 structural abnormality excluding inversion of chromosome 9.
Genomic categories are not mutually exclusive.
Data in the R/R cohort of CL-003 were included only in demographics/baseline characteristics, study disposition, and safety analyses (Tables 2 and 3; supplemental Tables 1 and 2); CL-003 data were not included in the R/R efficacy analyses.
The institutional review board or independent ethics committees at each participating site approved each study protocol. Each study was conducted according to the principles of the Declaration of Helsinki and International Conference on Harmonization for Good Clinical Practice. All patients provided written informed consent.
Outcomes
PFS was defined as the time from the first dose of study drug (ACE-CL-001 and ACE-CL-003) or from randomization (ELEVATE-TN, ELEVATE-RR, and ASCEND) to documented disease progression, assessed based on the International Workshop on Chronic Lymphocytic Leukemia 2008 criteria,17 or death from any cause, whichever occurred first. OS was defined as the time from the first dose of study drug (ACE-CL-001 and ACE-CL-003) or from randomization (ELEVATE-TN, ELEVATE-RR, and ASCEND) to death due to any cause. Overall response rate (ORR) was defined as the proportion of patients who achieved a complete response (CR), CR with an incomplete blood count recovery, partial response (PR), or nodular PR; PR with lymphocytosis was not included in the ORR calculation. ORR was reported as the best response at any time over the course of the study for each patient. Safety was assessed based on treatment-emergent adverse events (TEAEs).
Statistical analysis
Analyses of patient demographics, baseline characteristics, and study disposition were reported by TN and R/R cohorts for all patients in the higher-risk subgroup and separately by TN and R/R cohorts for all patients in the lower-risk subgroup; safety data were reported only for patients in the higher-risk subgroup by TN and R/R cohorts.
In the higher-risk subgroup, efficacy analyses for the TN cohort reported data for acalabrutinib monotherapy, acalabrutinib plus obinutuzumab, and/or all acalabrutinib-based treatments combined, whereas efficacy analyses in the R/R cohort reported data for acalabrutinib monotherapy only. In the lower-risk subgroup, efficacy analyses reported data for acalabrutinib-based treatment combined in the TN cohort and for acalabrutinib monotherapy in the R/R cohort.
In the higher-risk subgroup, investigator-assessed response rates, investigator-assessed PFS, OS, and safety were reported for the TN CLL and R/R CLL cohorts. PFS and OS outcomes were estimated using the Kaplan-Meier method, and response rates were summarized with corresponding 95% confidence intervals (CIs) based on Wilson score.18 Additional analyses of investigator-assessed response rates, investigator-assessed PFS, and OS were conducted comparing data for the lower-risk subgroup vs the higher-risk subgroup in the TN and R/R cohorts. PFS and OS outcomes were compared between the risk subgroups using hazard ratios and 95% CIs, whereas response rates were summarized with corresponding 95% CIs based on Wilson score. The Statistical Analysis System Software, version 9.04.01, was used for data analysis.
Retrospective analyses were conducted to evaluate the association of higher-risk genomic features including del(17p)/TP53m, uIGHV, and CK with PFS and OS in both the TN and R/R cohorts. First, a univariable analysis was performed with each mutation or comutation to assess statistical significance and the predictive ability of each genetic status. Then, a multivariable analysis including all genetic mutations/comutations was performed using backward selection method to determine the final model. The univariable and multivariable analyses were performed using Cox proportional hazards model, and the predictive ability of the models was assessed via concordance index (C-index).
Results
Patients
A total of 808 patients with higher-risk CLL (TN cohort, n = 320; R/R cohort, n = 488) were included in the pooled analysis (Table 2). In the TN cohort (n = 320), 64 patients (20%) had del(17p)/TP53m, 287 (90%) had uIGHV, 79 (25%) had CK overall, and 49 (15%) had CK without del(17p)/TP53m. In the R/R cohort (n = 488), 219 patients (45%) had del(17p)/TP53m, 425 (87%) had uIGHV, 160 (33%) had CK overall, and 69 (14%) had CK without del(17p)/TP53m. In the higher-risk subgroup, baseline genetic status was generally similar between the TN and R/R cohorts, with the exception of relatively lower proportions of patients with del(17p)/TP53m and CK overall in the TN cohort than the R/R cohort, as expected. Baseline characteristics were generally similar between the higher-risk and lower-risk subgroups in both cohorts, although in the R/R cohort, the median number of prior therapies was higher in the higher-risk subgroup vs the lower-risk subgroup (2 vs 1; supplemental Table 1).
Patient demographics and baseline characteristics among patients with higher-risk CLL
| Characteristic . | TN CLL (n = 320) . | R/R CLL (n = 488)∗ . | Total (N = 808) . |
|---|---|---|---|
| Age, median (range), y | 68 (34-88) | 66 (32-89) | 67 (32-89) |
| Male, n (%) | 206 (64) | 346 (71) | 552 (68) |
| Number of prior therapies, median (range) | 0 (0-1)† | 2 (1-10) | 1 (0-10) |
| ECOG PS, n (%) | |||
| 0-1 | 301 (94) | 451 (92) | 752 (93) |
| ≥2 | 19 (6) | 37 (8) | 56 (7) |
| Genetic status‡, n (%) | |||
| del(17p) and/or TP53m | 64 (20) | 219 (45) | 283 (35) |
| del(17p)/TP53m alone§ | 11 (3) | 35 (7) | 46 (6) |
| del(17p)/TP53m and uIGHV | 23 (7) | 93 (19) | 116 (14) |
| del(17p)/TP53m and CK | 9 (3) | 14 (3) | 23 (3) |
| del(17p)/TP53m with uIGHV and CK | 21 (7) | 77 (16) | 98 (12) |
| del(17p)/TP53m with either uIGHV or CK | 53 (17) | 184 (38) | 237 (29) |
| uIGHV | 287 (90) | 425 (87) | 712 (88) |
| uIGHV alone‖ | 207 (65) | 200 (41) | 407 (50) |
| uIGHV and CK | 36 (11) | 55 (11) | 91 (11) |
| uIGHV and del(17p)/TP53m | 23 (7) | 93 (19) | 116 (14) |
| uIGHV with CK and del(17p)/TP53m | 21 (7) | 77 (16) | 98 (12) |
| uIGHV with either CK or del(17p)/TP53m | 80 (25) | 225 (46) | 305 (38) |
| CK (≥3 chromosomal abnormalities)¶ | 79 (25) | 160 (33) | 239 (30) |
| CK alone§ | 13 (4) | 14 (3) | 27 (3) |
| CK and uIGHV | 36 (11) | 55 (11) | 91 (11) |
| CK and del(17p)/TP53m | 9 (3) | 14 (3) | 23 (3) |
| CK with uIGHV and del(17p)/TP53m | 21 (7) | 77 (16) | 98 (12) |
| CK with either uIGHV or del(17p)/TP53m | 66 (21) | 146 (30) | 212 (26) |
| CK without del(17p) and/or TP53m | 49 (15) | 69 (14) | 118 (15) |
| Characteristic . | TN CLL (n = 320) . | R/R CLL (n = 488)∗ . | Total (N = 808) . |
|---|---|---|---|
| Age, median (range), y | 68 (34-88) | 66 (32-89) | 67 (32-89) |
| Male, n (%) | 206 (64) | 346 (71) | 552 (68) |
| Number of prior therapies, median (range) | 0 (0-1)† | 2 (1-10) | 1 (0-10) |
| ECOG PS, n (%) | |||
| 0-1 | 301 (94) | 451 (92) | 752 (93) |
| ≥2 | 19 (6) | 37 (8) | 56 (7) |
| Genetic status‡, n (%) | |||
| del(17p) and/or TP53m | 64 (20) | 219 (45) | 283 (35) |
| del(17p)/TP53m alone§ | 11 (3) | 35 (7) | 46 (6) |
| del(17p)/TP53m and uIGHV | 23 (7) | 93 (19) | 116 (14) |
| del(17p)/TP53m and CK | 9 (3) | 14 (3) | 23 (3) |
| del(17p)/TP53m with uIGHV and CK | 21 (7) | 77 (16) | 98 (12) |
| del(17p)/TP53m with either uIGHV or CK | 53 (17) | 184 (38) | 237 (29) |
| uIGHV | 287 (90) | 425 (87) | 712 (88) |
| uIGHV alone‖ | 207 (65) | 200 (41) | 407 (50) |
| uIGHV and CK | 36 (11) | 55 (11) | 91 (11) |
| uIGHV and del(17p)/TP53m | 23 (7) | 93 (19) | 116 (14) |
| uIGHV with CK and del(17p)/TP53m | 21 (7) | 77 (16) | 98 (12) |
| uIGHV with either CK or del(17p)/TP53m | 80 (25) | 225 (46) | 305 (38) |
| CK (≥3 chromosomal abnormalities)¶ | 79 (25) | 160 (33) | 239 (30) |
| CK alone§ | 13 (4) | 14 (3) | 27 (3) |
| CK and uIGHV | 36 (11) | 55 (11) | 91 (11) |
| CK and del(17p)/TP53m | 9 (3) | 14 (3) | 23 (3) |
| CK with uIGHV and del(17p)/TP53m | 21 (7) | 77 (16) | 98 (12) |
| CK with either uIGHV or del(17p)/TP53m | 66 (21) | 146 (30) | 212 (26) |
| CK without del(17p) and/or TP53m | 49 (15) | 69 (14) | 118 (15) |
ECOG PS, Eastern Cooperative Oncology Group performance status.
R/R data set includes acalabrutinib plus obinutuzumab data from the CL-003 study in addition to the acalabrutinib monotherapy data from the CL-001, ELEVATE-R/R, and ASCEND studies.
One patient in the CL-001 study received an interrupted prior course of treatment.
Genomic categories are not mutually exclusive.
Includes patients with mIGHV, which is not considered an unfavorable genetic feature, and excludes the other high-risk genetic features.
Includes patients with uIGHV without del(17p)/TP53m or CK.
CK was defined as having ≥3 chromosomal abnormalities with ≥1 structural abnormality excluding inversion of chromosome 9.
For the efficacy analyses comparing data for the higher-risk vs lower-risk subgroups, a total of 475 patients were included in the TN cohort (higher-risk subgroup, n = 320; lower-risk subgroup, n = 155), and 554 patients (those treated with acalabrutinib monotherapy only) were included in the R/R cohort (higher-risk subgroup, n = 468; lower-risk subgroup, n = 86).
In the higher-risk subgroup, the median study follow-up duration was 59.1 months in the TN cohort and 44.3 months in the R/R cohort (Table 3). The proportion of patients who remained on treatment in the TN and R/R cohorts was 51.3% and 26.8%, respectively. Among the patients in the TN cohort, the most common reason for treatment discontinuation was TEAEs (13.8%); 8.1% of patients discontinued due to disease progression. Among the patients in the R/R cohort, the most common reason for treatment discontinuation was disease progression (30.5%); 16.6% of patients discontinued due to TEAEs. In the lower-risk subgroup, the median study follow-up duration was 61.0 months in the TN cohort and 48.6 months in the R/R cohort, and the most common reason for treatment discontinuation after study termination by sponsor was TEAEs (14.8%) in the TN cohort and TEAEs (19.6%) and disease progression (19.6%) in the R/R cohort (supplemental Table 2).
Patient disposition among patients with higher-risk CLL
| Parameter . | TN CLL (n = 320) . | R/R CLL (n = 488)∗ . | Total (N = 808) . |
|---|---|---|---|
| Follow-up, median (range), mo | 59.1 (1-82) | 44.3 (0-88) | 49.1 (0-88) |
| Patients remaining on treatment, n (%) | 164 (51) | 131 (27) | 295 (37) |
| Reasons for treatment discontinuation, n (%) | |||
| Progressive disease | 26 (8) | 149 (31) | 175 (22) |
| Adverse event | 44 (14) | 81 (17) | 125 (15) |
| Study terminated by sponsor | 42 (13) | 74 (15) | 116 (14) |
| Death | 11 (3) | 18 (4) | 29 (4) |
| Lost to follow-up | 2 (0.6) | 1 (0.2) | 3 (0.4) |
| Richter transformation | 1 (0.3) | 2 (0.4) | 3 (0.4) |
| Other† | 30 (9) | 32 (7) | 62 (8) |
| Parameter . | TN CLL (n = 320) . | R/R CLL (n = 488)∗ . | Total (N = 808) . |
|---|---|---|---|
| Follow-up, median (range), mo | 59.1 (1-82) | 44.3 (0-88) | 49.1 (0-88) |
| Patients remaining on treatment, n (%) | 164 (51) | 131 (27) | 295 (37) |
| Reasons for treatment discontinuation, n (%) | |||
| Progressive disease | 26 (8) | 149 (31) | 175 (22) |
| Adverse event | 44 (14) | 81 (17) | 125 (15) |
| Study terminated by sponsor | 42 (13) | 74 (15) | 116 (14) |
| Death | 11 (3) | 18 (4) | 29 (4) |
| Lost to follow-up | 2 (0.6) | 1 (0.2) | 3 (0.4) |
| Richter transformation | 1 (0.3) | 2 (0.4) | 3 (0.4) |
| Other† | 30 (9) | 32 (7) | 62 (8) |
R/R data set includes acalabrutinib plus obinutuzumab data from the CL-003 study in addition to the acalabrutinib monotherapy data from the CL-001, ELEVATE-R/R, and ASCEND studies.
Other includes physician decision (TN, n = 11; R/R, n = 15), withdrawal of consent (TN, n = 5; R/R, n = 11), pregnancy (TN, n = 1; R/R, n = 0), and other (TN, n = 13; R/R, n = 6).
Efficacy
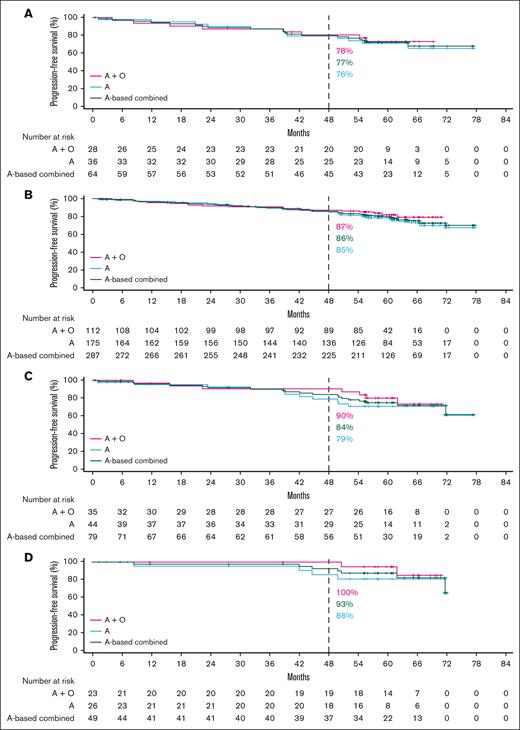
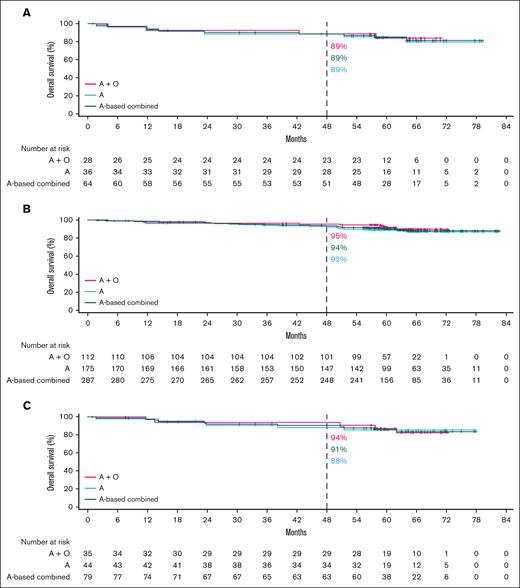
In the TN cohort, ORR data were generally similar in the overall pooled higher-risk and lower-risk subgroups (95.0% vs 92.3%, respectively; supplemental Figure 1A). Favorable PFS outcomes were observed overall with acalabrutinib monotherapy and acalabrutinib plus obinutuzumab across higher-risk subgroups (Figure 1A-D), with no statistically significant difference in PFS between lower-risk vs higher-risk patients (supplemental Figure 2A). There was also no statistically significant difference in PFS between lower-risk patients vs patients with uIGHV, CK, or CK without del(17p)/TP53m (supplemental Figure 2B); however, PFS was significantly shorter in patients with del(17p)/TP53m vs lower-risk patients. Favorable OS outcomes were also observed overall with acalabrutinib monotherapy and acalabrutinib plus obinutuzumab across higher-risk subgroups (Figure 2A-C). Similar to the PFS outcomes, no statistically significant difference in OS was observed between the lower-risk patients vs higher-risk patients or specifically those with del(17p)/TP53m (supplemental Figure 3A-B).
PFS in TN CLL by higher-risk genomic feature. Shown are acalabrutinib-based regimens for (A) del(17p)/TP53m, (B) uIGHV, (C) CK overall, and (D) CK without del(17p)/TP53m. A, acalabrutinib; O, obinutuzumab.
PFS in TN CLL by higher-risk genomic feature. Shown are acalabrutinib-based regimens for (A) del(17p)/TP53m, (B) uIGHV, (C) CK overall, and (D) CK without del(17p)/TP53m. A, acalabrutinib; O, obinutuzumab.
OS in TN CLL by higher-risk genomic feature. Shown are acalabrutinib-based regimens for del(17p)/TP53m (A), uIGHV (B), or CK (C). A, acalabrutinib; O, obinutuzumab.
OS in TN CLL by higher-risk genomic feature. Shown are acalabrutinib-based regimens for del(17p)/TP53m (A), uIGHV (B), or CK (C). A, acalabrutinib; O, obinutuzumab.
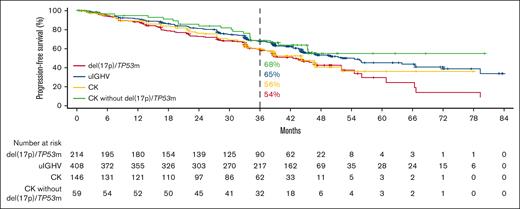
In the R/R cohort, ORR data were also similar in the overall pooled higher-risk and lower-risk subgroups (87.2% vs 84.9%, respectively; supplemental Figure 1B). Favorable PFS outcomes were observed with acalabrutinib monotherapy across higher-risk subgroups (Figure 3), whereas PFS was significantly shorter in the higher-risk vs lower-risk patients (supplemental Figure 2C). PFS was also significantly shorter in patients with del(17p)/TP53m, uIGHV, or CK vs lower-risk patients; however, there was no significant difference in PFS between lower-risk patients vs patients with CK without del(17p)/TP53m (supplemental Figure 2D). Favorable OS outcomes were also observed across higher-risk subgroups (Figure 4). However, OS was significantly shorter in higher-risk patients and in patients with del(17p)/TP53m vs lower-risk patients (supplemental Figure 3C-D).
PFS in R/R CLL with acalabrutinib monotherapy for del(17p)/TP53m, uIGHV, CK overall, and CK without del(17p)/TP53m.
PFS in R/R CLL with acalabrutinib monotherapy for del(17p)/TP53m, uIGHV, CK overall, and CK without del(17p)/TP53m.
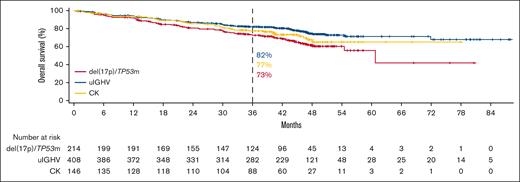
OS in R/R CLL with acalabrutinib monotherapy for del(17p)/TP53m, uIGHV, or CK.
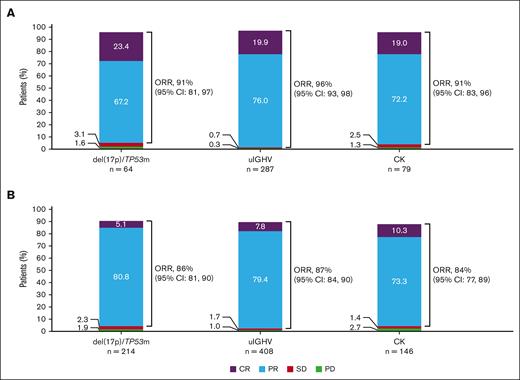
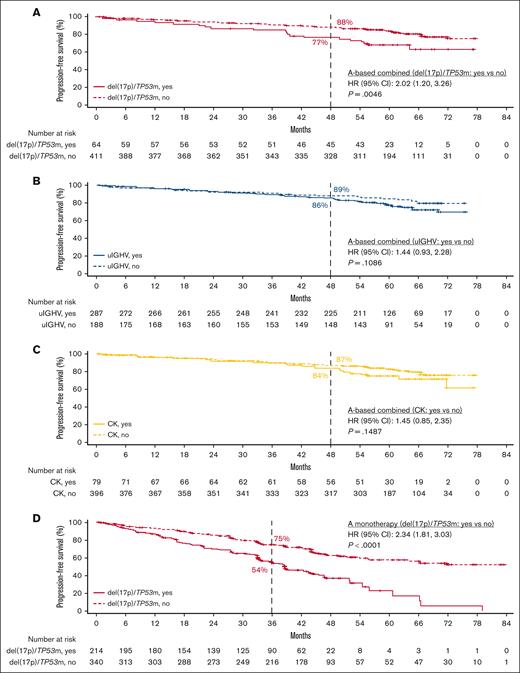
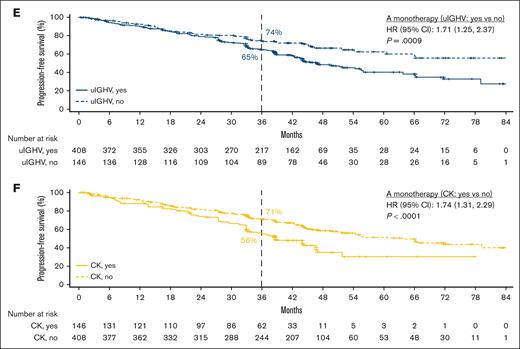
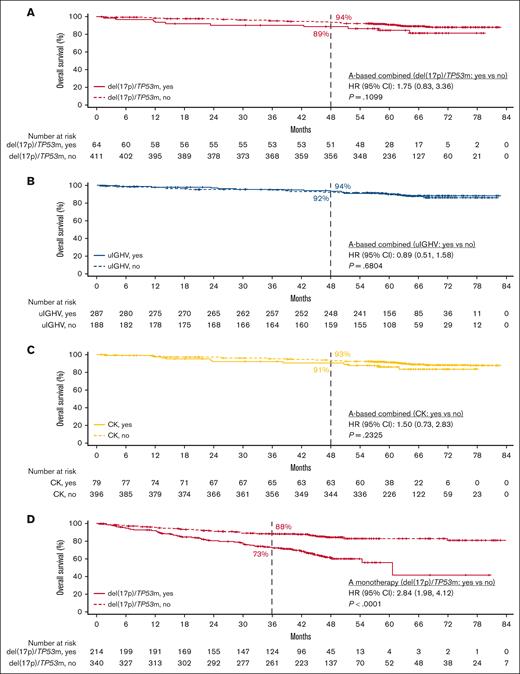
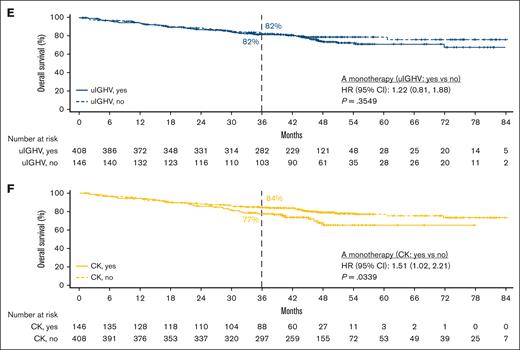
In patients with del(17p)/TP53m, the ORR was 90.6% (CR, 23.4%) in the TN cohort and 86.0% (CR, 5.1%) in the R/R cohort (Figure 5). In the TN cohort, the median PFS for patients with del(17p)/TP53m was not reached (NR), and the 48-month PFS rate was 76.9%. In the R/R cohort, the median PFS for patients with del(17p)/TP53m was 38.6 months, and the 36-month PFS rate was 54.4%. PFS was significantly shorter in patients with del(17p)/TP53m vs patients without del(17p)/TP53m in both the TN and R/R cohorts (Figure 6A, D, respectively). In the TN cohort, the median OS for patients with del(17p)/TP53m was NR, and the 48-month OS rate was 88.6%. In the R/R cohort, the median OS for patients with del(17p)/TP53m was 60.6 months, and the 36-month OS rate was 72.5%. No statistically significant difference in OS was observed in patients with vs without del(17p)/TP53m in the TN cohort (Figure 7A); however, in the R/R cohort, OS was significantly shorter in patients with del(17p)/TP53m vs patients without del(17p)/TP53m (Figure 7D).
ORR in TN and R/R CLL. Shown are ORR by higher-risk genomic feature in the TN CLL cohort (acalabrutinib-based regimens) (A) and R/R CLL cohort (acalabrutinib monotherapy) (B). CR includes CRi; PR includes nPR. CRi, CR with incomplete blood count recovery; nPR, nodular PR; PD, progressive disease; SD, stable disease.
ORR in TN and R/R CLL. Shown are ORR by higher-risk genomic feature in the TN CLL cohort (acalabrutinib-based regimens) (A) and R/R CLL cohort (acalabrutinib monotherapy) (B). CR includes CRi; PR includes nPR. CRi, CR with incomplete blood count recovery; nPR, nodular PR; PD, progressive disease; SD, stable disease.
PFS in TN and R/R CLL by higher-risk vs lower-risk genomic feature. Shown are PFS in TN CLL with acalabrutinib-based regimens for del(17p)/TP53m vs no del(17p)/TP53m (A), uIGHV vs mIGHV (B), and CK vs no CK (C) and PFS in R/R CLL with acalabrutinib monotherapy for del(17p)/TP53m vs no del(17p)/TP53m (D), uIGHV vs mIGHV (E), and CK vs no CK (F).
PFS in TN and R/R CLL by higher-risk vs lower-risk genomic feature. Shown are PFS in TN CLL with acalabrutinib-based regimens for del(17p)/TP53m vs no del(17p)/TP53m (A), uIGHV vs mIGHV (B), and CK vs no CK (C) and PFS in R/R CLL with acalabrutinib monotherapy for del(17p)/TP53m vs no del(17p)/TP53m (D), uIGHV vs mIGHV (E), and CK vs no CK (F).
OS in TN and R/R CLL by higher-risk vs lower-risk genomic feature. Shown are OS in TN CLL with acalabrutinib-based regimens for del(17p)/TP53m vs no del(17p)/TP53m (A), uIGHV vs mIGHV (B), and CK vs no CK (C) and OS in R/R CLL with acalabrutinib monotherapy for del(17p)/TP53m vs no del(17p)/TP53m (D), uIGHV vs mIGHV (E), and CK vs no CK (F).
OS in TN and R/R CLL by higher-risk vs lower-risk genomic feature. Shown are OS in TN CLL with acalabrutinib-based regimens for del(17p)/TP53m vs no del(17p)/TP53m (A), uIGHV vs mIGHV (B), and CK vs no CK (C) and OS in R/R CLL with acalabrutinib monotherapy for del(17p)/TP53m vs no del(17p)/TP53m (D), uIGHV vs mIGHV (E), and CK vs no CK (F).
In patients with uIGHV, ORR was 95.8% (CR, 19.9%) in the TN cohort and 87.3% (CR, 7.8%) in the R/R cohort (Figure 5). In the TN cohort, the median PFS for patients with uIGHV was NR, and the 48-month PFS rate was 85.6%. In the R/R cohort, the median PFS for patients with uIGHV was 46.9 months, and the 36-month PFS rate was 64.6%. No statistically significant difference in PFS was observed in patients with uIGHV vs mutated IGHV (mIGHV) in the TN cohort (Figure 6B); however, in the R/R cohort, PFS was significantly shorter in patients with uIGHV vs patients with mIGHV (Figure 6E). In the TN cohort, the median OS for patients with uIGHV was NR, and the 48-month OS rate was 93.5%. In the R/R cohort, the median OS for patients with uIGHV was NR, and the 36-month OS rate was 82.0%. No statistically significant difference in OS was observed in patients with uIGHV vs mIGHV in the TN cohort and R/R cohort (Figure 7B,E).
In patients with CK, ORR was 91.1% (CR, 19.0%) in the TN cohort and 83.6% (CR, 10.3%) in the R/R cohort (Figure 5). In the TN cohort, the median PFS for patients with CK overall was NR, and the 48-month PFS rate was 84.1%. Examining data in the subset of patients with CK without del(17p)/TP53m, the 48-month PFS rate was 92.7% (Figure 1D). In the R/R cohort, the median PFS for patients with CK overall was 38.6 months, and the 36-month PFS rate was 55.7%, whereas in the subset of patients with CK without del(17p)/TP53m, the 36-month PFS rate was 68.4%. No statistically significant difference in PFS was observed in patients with vs without CK in the TN cohort (Figure 6C); however, in the R/R cohort, PFS was significantly shorter in patients with CK vs patients without CK (Figure 6F). In the TN cohort, the median OS for patients with CK overall was NR, and the 48-month OS rate was 90.6%. In the R/R cohort, the median OS for patients with CK overall was NR, and the 36-month OS rate was 77.4%. No statistically significant difference in OS was observed in patients with vs without CK in the TN cohort (Figure 7C); however, in the R/R cohort, OS was significantly shorter in patients with CK vs patients without CK (Figure 7F).
A multivariable analysis of TN patients demonstrated that the presence of all 3 genomic features (del(17p)/TP53m, uIGHV, and CK combined) was significantly associated with shorter PFS (supplemental Table 3), whereas none of the genomic features were significantly associated with shorter OS (supplemental Table 4). Furthermore, multivariable analysis of patients with R/R disease showed that the presence of both del(17p)/TP53m and uIGHV as well as all 3 genomic features (del(17p)/TP53m, uIGHV, and CK) was significantly associated with shorter PFS. Similarly, the presence of del(17p)/TP53m alone, both del(17p)/TP53m and uIGHV, or all 3 genomic features (del(17p)/TP53m, uIGHV, and CK) was significantly associated with shorter OS in patients with R/R disease.
Patients with higher-risk CLL in both the TN and R/R cohorts received a median of 1 subsequent line of therapy (supplemental Table 5). The most common subsequent therapy in the TN cohort was chemotherapy- and/or immunotherapy-based treatments (4.7%) followed by targeted therapies (2.8%), and it was targeted therapies (14.3%) followed by chemotherapy- and/or immunotherapy-based treatments (7.2%) in the R/R cohort.
Safety
Among the overall population of patients with higher-risk CLL (N = 808), the duration of treatment exposure was 59.3 months in the TN cohort and 39.1 months in the R/R cohort (supplemental Table 6). Of the 808 patients, 568 (70.3%) experienced at least 1 grade ≥3 TEAE; the most common grade ≥3 TEAEs were neutropenia (19.3%), pneumonia (9.5%), anemia (8.4%), thrombocytopenia (6.1%), and hypertension (5.4%). TEAEs of any grade that led to treatment discontinuation were reported in 16.5% of patients, most commonly pneumonia and thrombocytopenia, which occurred in 7 (0.9%) and 5 patients (0.6%), respectively (supplemental Table 7). The most common events of clinical interest were infections (any grade, 78.3%; grade ≥3, 28.5%) and neutropenia (any grade, 23.9%; grade ≥3, 22.3%), with incidences of any-grade atrial fibrillation/flutter of 7.4% (grade ≥3, 2.6%) and any-grade hemorrhage of 45.7% (grade ≥3, 4.2%; supplemental Table 8). The safety profile of acalabrutinib-based treatment in the lower-risk subgroup (data not shown) was similar to that seen in the higher-risk subgroup in both the TN and R/R cohorts.
Deaths were reported in 34 patients (10.6%) in the TN cohort and in 114 patients (23.4%) in the R/R cohort, most commonly due to adverse events in both cohorts (n = 19 [5.9%] and n = 57 [11.7%], respectively; supplemental Table 9). The most common cause of death due to adverse event per system organ class was infections and infestations in both cohorts (7 [2.2%] and 28 [5.7%], respectively); the most common infection and infestation event was COVID-19 (n = 3 [0.9%]) in the TN cohort and pneumonia (n = 8 [1.6%]) in the R/R cohort (supplemental Table 10). Disease progression was the cause of death in 3 patients (0.9%) in the TN cohort and in 33 patients (6.8%) in the R/R cohort; only 1 of these patients (in the R/R cohort) was on active treatment at the time of death. Death due to Richter transformation was uncommon in both cohorts (TN cohort, n = 1 [0.3%]; R/R cohort, n = 6 [1.2%]).
Discussion
In this pooled analysis of clinical trial data in 808 patients with CLL and higher-risk genomic features, PFS and OS rates were high with acalabrutinib-based regimens across higher-risk genomic features in both the TN and R/R cohorts at a median follow-up of ∼5 and 4 years, respectively. Although significantly shorter PFS was only observed in patients with del(17p)/TP53m vs without del(17p)/TP53m in the TN cohort, findings differed in the R/R cohort in which significantly shorter PFS was observed in patients with del(17p)/TP53m vs without del(17p)/TP53m, with uIGHV vs mIGHV, and with CK vs without CK. This is further supported by the multivariable analysis in which the presence of both del(17p)/TP53m and uIGHV or all 3 genomic features (del(17p)/TP53m, uIGHV, and CK) was significantly associated with shorter PFS in the R/R cohort. Although no statistically significant OS difference was observed in patients with vs without each individual genomic feature in the TN cohort, significantly shorter OS was observed in patients with del(17p)/TP53m vs without del(17p)/TP53m, and with CK vs without CK in the R/R cohort. This is further supported by the multivariable analysis in which the presence of del(17p)/TP53m alone, both del(17p)/TP53m and uIGHV, or all 3 genomic features (del(17p)/TP53m, uIGHV, and CK) was significantly associated with shorter OS in the R/R cohort. Therefore, in the R/R cohort, del(17p)/TP53m, regardless of other comutations, was predictive of OS. This finding, however, was not evident in the TN cohort, indicating that the impact of first-line treatment with acalabrutinib was comparable regardless of higher-risk genomic features. Of note, interpretation of findings from the multivariable analysis is limited due to small sample sizes in some subgroups. The safety profile of acalabrutinib in patients with higher-risk CLL was consistent with the known safety profile of the drug. Although these patients had higher-risk genomic features, discontinuation rates due to Richter transformation were low in both the TN (0.3%) and R/R cohorts (0.4%). At the time of the analysis, more than half of the patients in the TN cohort remained on treatment, with up to 82 months of follow-up in the patient on treatment for the longest duration.
In the TN cohort, in the subgroup of 64 patients with del(17p)/TP53m treated with acalabrutinib-based therapy, the ORR was 91%, the 24-month PFS and OS rates were 87% and 90%, respectively, and the 48-month PFS and OS rates were 77% and 89%, respectively, at a median follow-up of 59.1 months. These findings are similar to those reported in a pooled analysis of ibrutinib-based treatments in 89 patients with TN CLL and TP53 aberrations, for which the ORR was 93%, and the 48-month PFS and OS rates were 79% and 88%, respectively, at a median follow-up of 49.8 months.19 Outcomes reported from the cohort of 110 patients with TN CLL and del(17p) treated with zanubrutinib monotherapy in arm C of the SEQUOIA study also appear to be similar, with estimated 42-month PFS and OS rates of 79% and 90%, respectively, reported at a median follow-up of 47.9 months.20-22 In contrast to our findings, a long-term follow-up analysis of the Alliance for Clinical Trials in Oncology A041202 study at a median follow-up of 55 months showed no significant difference in PFS between ibrutinib-treated patients with vs without TP53 abnormalities.23 However, this could be due to differences in sample size.
In the R/R cohort (median of 2 prior lines of therapy) subgroup of 214 patients with del(17p)/TP53m treated with acalabrutinib monotherapy, the ORR was 86%, the 24-month PFS and OS rates were 70% and 81%, respectively, and the 36-month PFS and OS rates were 54% and 73%, respectively, at a median follow-up of 44.3 months in the overall R/R cohort. By comparison, at a shorter median follow-up of 29.6 months in the head-to-head phase 3 ALPINE study of zanubrutinib vs ibrutinib in patients with R/R CLL (median of 1 prior line of therapy), patients with del(17p) and/or TP53m had an ORR of 85% and 24-month PFS rate of 78% with zanubrutinib (n = 75), compared with 71% and 56%, respectively, with ibrutinib (n = 75).24 Although these analyses all evaluated the efficacy of BTKis in higher-risk CLL, crosstrial comparisons are limited by differences in trial design, time period of study recruitment, heterogeneity in patient populations, and study follow-up duration. Notably, most of the patients in the R/R cohort in this analysis were from the ELEVATE-RR study,8 which included a more heavily pretreated population than patients from the ALPINE study.24 The impact of follow-up duration is most important because very few patients with CLL progress before 2 years across all relevant covalent BTKi trials.
The safety profile of acalabrutinib in this analysis was similar to the reported overall safety profile of acalabrutinib, with a relatively low incidence of grade ≥3 hypertension (5.4%), any-grade atrial fibrillation/flutter (7.4%), and grade ≥3 hemorrhage (4.2%) events observed with long-term follow-up. Discontinuation rates due to TEAEs remained low in both the TN (14%) and R/R (17%) cohorts, at a median treatment exposure of 59.3 months and 39.1 months, respectively.
With the emergence of BTKis, patients with TN and R/R CLL have several highly effective and well-tolerated treatment options available. Venetoclax plus obinutuzumab is also a highly effective treatment approach in TN CLL. Of note, higher-risk patients with uIGHV and/or TP53-aberrant CLL treated with the time-limited venetoclax plus obinutuzumab combination have shorter PFS than patients without higher-risk CLL.2,3 In contrast, our analysis has demonstrated that acalabrutinib-based regimens in TN CLL are comparably efficacious in patients with or without higher-risk CLL. The possibility of retreatment with venetoclax plus obinutuzumab for patients who relapse after initially achieving remission with the regimen is a theoretically appealing idea. However, there are currently limited prospective data to understand how effective such a retreatment strategy might be; ongoing studies will hopefully help to address this data gap (eg, NCT04895436). Until such data are available, continuous BTKi-based strategies appear to be the most evidence-based approach for patients with higher-risk CLL.
Some limitations to this retrospective analysis include differences in trial design and pooling data from nonrandomized, single-arm studies, which may introduce selection bias. Despite these limitations, the pooling of 5 clinical studies in both TN and R/R CLL allowed for data to be collated from all patients with higher-risk CLL to better understand the impact of acalabrutinib-based treatment. CK defined as ≥3 chromosomal abnormalities (with ≥1 structural abnormality excluding inversion of chromosome 9) did not appear to be prognostic in patients with TN CLL in this analysis, as previously suggested in the era of chemoimmunotherapy25; the possibility that poorer outcomes would be seen in patients with CK defined as ≥5 abnormalities cannot be excluded, but the number of patients was too small to perform this analysis.
Overall, our results demonstrate the long-term benefit of acalabrutinib-based regimens in patients with CLL and higher-risk genomic features, regardless of line of therapy, with no new safety signals identified in the analysis. Our data continue to support the approach of continuous acalabrutinib as a highly effective and well-tolerated option for treating a broad population of patients with CLL, particularly those with higher-risk genetic features. However, treatment optimization in patients with del(17p)/TP53m is still an urgent unmet need, particularly in the R/R setting.
Acknowledgments
The authors thank the patients who participated in these trials and their families for their support. The authors also thank all the study staff at each site and among the companies supporting this work who are not recognized as authors in this article.
This analysis was sponsored by AstraZeneca. M.S.D. is a scholar in Clinical Research of the Leukemia & Lymphoma Society and is supported by a National Institutes of Health award (1R01CA266298-01A1). Medical writing support was provided by Sarah Huh of Peloton Advantage, LLC (Parsippany, NJ), an OPEN Health company, and funded by AstraZeneca. The authors directed development of the manuscript and are fully responsible for all content and editorial decisions.
Authorship
Contribution: M.S.D., P.M., M.S., and J.C.B. designed the study; M.S.D., J.P.S., P.G., J.A.W., T.A.E., W.J., T.S., and J.C.B. were study investigators; M.S.D., J.P.S., P.G., J.A.W., T.A.E., W.J., T.S., and J.C.B. provided patients or study materials; M.S.D., J.P.S., P.G., J.A.W., T.A.E., W.J., T.S., J.C.B., and U.E. collected and assembled the data; M.S.D., P.M., M.S., A.B., and U.E. analyzed the data; M.S.D., T.A.E., P.M., U.E., and J.C.B. interpreted the data; M.S.D., T.A.E., P.M., and J.C.B. were involved in manuscript preparation; and all authors participated in the critical review and revision of the manuscript and provided approval of the manuscript for submission.
Conflict-of-interest disclosure: M.S.D. reports consultancy fees from AbbVie, Adaptive Biotechnologies, Ascentage Pharma, AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), Eli Lilly, Genentech, Genmab, Janssen, Merck, MingSight Pharmaceuticals, Secura Bio, Takeda, TG Therapeutics, and Zentalis; research funding from AbbVie, Ascentage Pharma, AstraZeneca, Genentech, MEI Pharma, Novartis, Surface Oncology, TG Therapeutics, and Verastem; and honoraria from Research To Practice. J.P.S. reports employment with The US Oncology Network; research funding from Pharmacyclics, Genentech, Celgene, Acerta Pharma, AstraZeneca, Gilead Sciences, Seattle Genetics, TG Therapeutics, Merck, Takeda, BeiGene, and Lilly; and consulting/advisory roles in Pharmacyclics, Celgene, TG Therapeutics, Genentech, AbbVie, Acerta Pharma, AstraZeneca, BeiGene, Pfizer, BMS, and VelosBio. P.G. reports research funding from AbbVie, Gilead, Janssen, Novartis, and Sunesis; honoraria from AbbVie, AstraZeneca, ArQule/Merck Sharp and Dohme, BeiGene, Celgene/Juno/BMS, Gilead, Janssen, Lilly/Loxo, Adaptive, and Roche; and consulting/advisory roles in AbbVie, AstraZeneca, ArQule/Merck Sharp and Dohme, BeiGene, Celgene/Juno/BMS, Gilead, Janssen, Lilly/Loxo, Adaptive, and Roche. J.A.W. reports consulting/advisory roles in Pharmacyclics, Janssen, AstraZeneca, BeiGene, Loxo, Newave Pharmaceutical, and Genentech, and research funding from Janssen, Karyopharm, MorphoSys, AbbVie, and Schrodinger. T.A.E. reports honoraria from Roche, Gilead, Kite, Janssen, AbbVie, AstraZeneca, Loxo Oncology, BeiGene, Incyte, Secura Bio, and Autolus; travel fees from Roche, Gilead, AbbVie, and AstraZeneca; research support from Gilead, AstraZeneca, and BeiGene; and trial steering committee membership in Loxo Oncology. W.J. reports research funding from GlaxoSmithKline, Acerta, AstraZeneca, BeiGene, Nordic Nanovector, Incyte, Debiopharm, Incyte, Genentech, Janssen, Loxo, MEI Pharma, MorphoSys, Takeda, and TG Therapeutics, and consulting/advisory roles in MEI Pharma, Debiopharm, Loxo, Takeda, AstraZeneca, and BeiGene. T.S. reports research funding from Ascentage, AstraZeneca, BeiGene, BMS, Juno Therapeutics, Kite, Oncternal, Pharmacyclics, and TG Therapeutics; membership on an entity’s board of directors or advisory committees in AstraZeneca, BeiGene, BMS, Celgene, Kite, and AbbVie; and speakers bureau fees from AstraZeneca, BeiGene, and BMS. P.M., M.S., A.B., and U.E. report stock and employment with AstraZeneca. J.C.B. reports stock and other ownership interests with Vincerx Pharma; honoraria from Pharmacyclics, AstraZeneca, Novartis, Syndax, and Trillium Therapeutics; consulting/advisory roles in Acerta Pharma, Janssen, Kura Oncology, Novartis, Syndax, and AstraZeneca; research funding from Acerta Pharma, Pharmacyclics, and Zencor; patents, royalties, and other intellectual property with The Ohio State University patents; and travel, accommodations, and expenses from Gilead Sciences, Janssen, Novartis, Pharmacyclics, and TG Therapeutics.
Correspondence: Matthew S. Davids, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: matthew_davids@dfci.harvard.edu.
References
Author notes
Presented in part at the European Hematology Association Annual Meeting, Vienna, Austria, 9 to 12 June 2022, and virtually; and at the American Society of Hematology Annual Meeting, New Orleans, LA, 10 to 13 December 2022, and virtually.
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details at https://vivli.org/ourmember/astrazeneca/. Sequencing data were not deposited into a public database because (1) sequencing was not the primary outcome of the research and (2) the sequencing analyses were conducted as part of the included clinical trials that were previously published (PubMed IDs: 33786588 and 31876911 [ACE-CL-001], 31915195 [ACE-CL-003], 32305093 [ELEVATE-TN], 34310172 [ELEVATE-RR], and 32459600 [ASCEND]).
The full-text version of this article contains a data supplement.