TO THE EDITOR:
Mature plasmacytoid dendritic cell (pDC) expansions have been described in the blood, lymph node, and bone marrow in various myeloid neoplasms ([MNs]; chronic myelomonocytic leukemia [CMML], myelodysplastic syndrome [MDS], myeloproliferative neoplasm, and acute myeloid leukemia [AML]).1-5 Blood pDCs in pDC-AML are clonally related to blast cells.6,7 The cutaneous infiltration by pDCs and/or indeterminate dendritic cells (iDCs) has been reported in patients with CMML, and the term pDC dermatosis has recently been coined to describe this pathological finding.8,9 It can be differentiated from CMML skin tumors, blastic pDC neoplasm, and blastic iDC neoplasm based on morphology and phenotype.8 Although pDC infiltrate in bone marrow seems to be associated with poor prognosis in CMML,10 the significance and clinical findings of cutaneous pDC expansion in CMML/MDS/AML has not yet been well documented. Here, we report 14 cases of pDC dermatosis manifesting as papulo-nodular itchy dermatosis associated with MN and study its clonal relationship to underlying MN.
We included all patients followed at Hôpital Saint-Louis, Paris, France, between January 2010 and April 2022 according to the following criteria: (1) nonblastic pDC (with or without iDC) cutaneous infiltrate; (2) skin eruption; and (3) diagnosis of MN (AML, MDS, or CMML) according to the 2022 International Consensus Classification of MNs.11 Mature pDCs were defined as CD123+ TCF4+ CD2AP+ CD1a− S100− CD207− CD163− granzyme B–positive/negative CD56− MPO−, and mature iDCs were defined as CD1a+ S100+/− CD207− CD163− CD123− TCF4− CD2AP− CD56− MPO−. The percentage of pDCs, iDCs, and CD163+ myeloid cells over total nucleated cells in skin sample was evaluated independently by 3 pathologists. We used next-generation sequencing (NGS) to screen at least 30 genes recurrently mutated in MN on DNA extracted from skin lesion formalin-fixed paraffin-embedded tissue sections, bone marrow aspirate, or peripheral blood from patients. MN prognosis was evaluated using the Molecular International Prognostic Scoring System for MDS and the molecular CMML-specific prognostic scoring system for CMML.2,12,13
Clinico-histological characteristics and NGS analysis of the 14 patients are summarized in Table 1. Patients were mainly male (sex ratio, 3.6; 11 males and 3 females). Median age at first skin lesion appearance and at MN diagnosis was 78 and 79 years, respectively. Diagnosis of pDC dermatosis occurred before the diagnosis of MN in 4 cases, at the same time in 7, and during follow-up in 3. Patients had diffuse pruritus (n = 12 [86%]) with fixed or recurrent flares of papules (n = 12 [86%]) and nodules (n = 5), mainly on the trunk (n = 6; Figure 1A-D). Two patients had lesions limited to the face. No clinical difference was seen according to the presence of iDCs. Associated MNs were CMML (n = 10), MDS (n = 2), and AML (n = 1), whereas 1 patient had clonal cytopenia of undetermined significance rapidly evolving to AML. No increase in pDCs was reported in flow cytometry of bone marrow aspirate or blood at MN diagnosis.
Clinico-pathologic, genetic, and therapeutic features of 14 patients with MN and with pDC dermatosis
| Patient,Sex,age . | Clinical presentation . | Pruritus . | Myeloid neoplasm (ICC 2022) and time of diagnosis related to pDC dermatosis . | Pronostic scoring (IPSS-M or CPSS-mol) . | Skin histology (% of cell type in the sample∗); total percentage of pDC+iDC in the sample∗ . | Percentage of CD163+ myeloid cells in the skin sample . | Targeted sequencing of skin biopsy (gene, VAF %) . | Targeted sequencing of bone marrow or blood (gene, VAF%) / cytogenetic . | Hematological Treatment and response . | Skin-directed treatment and response . | Death, follow-up time after cutaneous sign, after diagnosis of myeloid neoplasm and cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1, F, 46 | Papules, face, trunk | No | AML with myelodysplasia-related gene mutations concurrent | — | Dermal infiltrate of pDC (30), iDC (1); 31% | 5 | RUNX1 x 2 (26,23) BCORL1 (23) FLT3-ITD (duplication) | RUNX1 x2 (45,43) BCORL1 (41) FLT3-ITD (duplication) /normal | Allo-HCT (CR) | Allo-HCT (CR) | No, 3 y and 3 y |
| #2, M, 74 | Papules, legs | yes | MDS, NOS with multilineage dysplasia 4 y later | Low –0.81 | Dermal infiltrate of pDC (10), iDC (15); 25% | 10 | BRCC3 (16) TET2 (10) SRSF2 (9) RUNX1 (9) | TET2 (38) SRSF2 (43) RUNX1 (44) BRCC3 (NA) /normal | NA | NA | No, 4 y and 4.4 y |
| #3, M, 65 | Papules, head, trunk, arms, legs | yes | CMML 1 y before | Intermediate 1 | Dermal infiltrate of pDC (20), iDC (10); 30% | 15 | IDH2 (19) BRCC3 (18) SRSF2 (10) NF1 (8) CBL (6) | CBL (73) IDH2 (43) SRSF2 (35) NF1 (4) CBL (73) /normal | Azacitidine and Allo-HCT (CR) | Azacitidine and Allo-HCT (CR) | No, 6 y and 7 y |
| #4, M, 63 | Papules, trunk | yes | CMML 3 mo later | Intermediate 2 | Dermal infiltrate of pDC (25),iDC (10); 35% | 10 | TET2 x2 (23, 19) 20CTCF (1930) NRAS (1019) ASXL101 (17)20 | TET2 x2 (53, 22) NRAS (53) SRSF2 (40) ASXL1 (35) /normal | Allo-HCT (CR) | Allo-HCT (CR) | No, 2.25 y 2 y |
| #5, M, 84 | papules, trunk, arms, legs | yes | CMML Concurrent | Intermediate 1 | Dermal infiltrate of pDC (35); 35% | 20 | JAK2 (3106) TET2 5 (27) SRSF2 10(22) | JAK2 (70) TET2 (44) SRSF2 (43) /normal | NA | NA | NA |
| #6, M, 84 | Papules, nodules, Head | yes | CMML 1 y later | Intermediate 2 | Dermal infiltrate of pDC (45), iDC (2); 47% | 30 | TET2 x3 (55,20,17) ASXL1 (31) NRAS (23) SRSF2 (21) | TET2 x3 (88,4,4) SRSF2 (44) ASXL1 (42) NRAS (24) /46,XY,add(11)(p11)/46,XY | No treatment, surveillance | High potent topical corticosteroids and tacrolimus (PR) Prednisone 1 mg/kg per day (CR) | Yes, 4 y, 3 y, sepsis from lung infection |
| #7, F, 86 | Papules | no | CMML, 8 mo before | Intermediate 2 | Dermal infiltrate of pDC (30), iDC (1); 31% | 10 | KRAS(22), SF3B1 (21) SMC3 (17) RUNX1 (17) FLT3-ITD | KRAS (43) SF3B1 (42) SMC3 (42) RUNX1 (13) FLT3-ITD /normal | Sorafenib 200 mg twice a day and decitabine (NR) | High potent topical corticosteroids (NR) Prednisone 1m/kg per day (CR) | Yes, 1.1 y, 6 mo, AML |
| #8, M, 89 | Papules | Yes | CMML, concurrent | Intermediate 1 | Dermal infiltrate of pDC (10), iDC (30); 40% | 10 | ASXL1 (17) ETV6 (19) BRAF(17) CUX1 (15) SRSF2 (11) TET2 (11) | ASXL1 (41), ETV6 (38), BRAF (37) CUX1 (35) SRSF2 (32) TET2 (16) /normal (BLOOD) | Hydroxycarbamide (NR) | high potent topical corticosteroids (NR) | Yes, 3 y, 3 y AML |
| #9, M, 80 | Papules | yes | MDS, NOS with multilineage dysplasia, concurrent | Low –1.41 | Dermal infiltrate of pDC (10), iDC (10); 20% | 20 | ASXL2 (11) CBL (9) CHEK2 (9) TET2 (15) BRCC3 (15) TP53 (2) | TET2 (40) BRCC3 (40) ASXL2 (25) CBL (16) CHEK2 (15) /normal | No treatment | High potent topical corticosteroids (PR) Prednisone 0.1mg/kg per day (CR) | No, 7 y, 7 y |
| #10, M, 64 | Papules | yes | CMML concurrent | Intermediate 2 | Dermal infiltrate of pDC (20), iDC (5); 25% | 10 | ZRSR2 (56) TET2 (9) ASLX1 (4) | TET2x2 (35, 42) ASXL1 (32) ZRSR2 (3) PTPN11 (2) /normal | NA | High potent topical corticosteroids (PR) | No, 2.5 y 2.5 y |
| #11, M, 87 | Papules, face | yes | CMML concurrent | NA | Dermal infiltrate of pDC (5), iDC (80); 85% | 5 | TET2 (73) ZRSR2 (19) KRAS (17) ASXL1 (7) | TET2 (86) ZRSR2 (19) ASXL1 (9) /normal (BLOOD) | Transfusion, no treatment | No treatment | No, 3 mo, 3 mo |
| #12, F, 80 | Papules,and nodules Trunk | yes | CCUS 6 mo later | NA | Dermal infiltrate of pDC (40); 40% | 10 | RUNX1 (25) SF3B1 (22) WT1 (16) KRAS (9) | NA | no | Anti-H1 (NR) | Yes, 1.1 y, 6 mo AML |
| #13, M, 76 | Papules, Nodules, Trunk, legs, arms | yes | CMML concurrent | Intermediate 2 | Dermal infiltrate of pDC (15), iDC (10); 25% | 10 | ARID2 (24) RIT1 (24) ASXL1 (24) SRSF2 (19) SMC3 (8) | ARID2 (NA) RIT1 (NA) ASXL1 (NA) /normal | EPO for anemia (no progression) | Anti H1 (NR) High potent topical corticosteroids (NR) Prednisone 0.5mg/kg per day (CR) | No, 2 y, 2 y |
| # 14, M, 60 | Papules, head and neck | no | CMML 3 y before | Intermediate 2 | Dermal infiltrate of pDC (20), iDC (15); 35% | 15 | SH2B3 (22) NF1 (21) SRSF2 (16) IDH2 (11) | SH2B3 (36) NF1x3 (50, 33 & 2), SRSF2 (42) IDH2 (46) KRAS (7) MPL (5) /normal | Hydroxycarbamide (NR) | — | No, 4 y, 3 y |
| Patient,Sex,age . | Clinical presentation . | Pruritus . | Myeloid neoplasm (ICC 2022) and time of diagnosis related to pDC dermatosis . | Pronostic scoring (IPSS-M or CPSS-mol) . | Skin histology (% of cell type in the sample∗); total percentage of pDC+iDC in the sample∗ . | Percentage of CD163+ myeloid cells in the skin sample . | Targeted sequencing of skin biopsy (gene, VAF %) . | Targeted sequencing of bone marrow or blood (gene, VAF%) / cytogenetic . | Hematological Treatment and response . | Skin-directed treatment and response . | Death, follow-up time after cutaneous sign, after diagnosis of myeloid neoplasm and cause of death . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1, F, 46 | Papules, face, trunk | No | AML with myelodysplasia-related gene mutations concurrent | — | Dermal infiltrate of pDC (30), iDC (1); 31% | 5 | RUNX1 x 2 (26,23) BCORL1 (23) FLT3-ITD (duplication) | RUNX1 x2 (45,43) BCORL1 (41) FLT3-ITD (duplication) /normal | Allo-HCT (CR) | Allo-HCT (CR) | No, 3 y and 3 y |
| #2, M, 74 | Papules, legs | yes | MDS, NOS with multilineage dysplasia 4 y later | Low –0.81 | Dermal infiltrate of pDC (10), iDC (15); 25% | 10 | BRCC3 (16) TET2 (10) SRSF2 (9) RUNX1 (9) | TET2 (38) SRSF2 (43) RUNX1 (44) BRCC3 (NA) /normal | NA | NA | No, 4 y and 4.4 y |
| #3, M, 65 | Papules, head, trunk, arms, legs | yes | CMML 1 y before | Intermediate 1 | Dermal infiltrate of pDC (20), iDC (10); 30% | 15 | IDH2 (19) BRCC3 (18) SRSF2 (10) NF1 (8) CBL (6) | CBL (73) IDH2 (43) SRSF2 (35) NF1 (4) CBL (73) /normal | Azacitidine and Allo-HCT (CR) | Azacitidine and Allo-HCT (CR) | No, 6 y and 7 y |
| #4, M, 63 | Papules, trunk | yes | CMML 3 mo later | Intermediate 2 | Dermal infiltrate of pDC (25),iDC (10); 35% | 10 | TET2 x2 (23, 19) 20CTCF (1930) NRAS (1019) ASXL101 (17)20 | TET2 x2 (53, 22) NRAS (53) SRSF2 (40) ASXL1 (35) /normal | Allo-HCT (CR) | Allo-HCT (CR) | No, 2.25 y 2 y |
| #5, M, 84 | papules, trunk, arms, legs | yes | CMML Concurrent | Intermediate 1 | Dermal infiltrate of pDC (35); 35% | 20 | JAK2 (3106) TET2 5 (27) SRSF2 10(22) | JAK2 (70) TET2 (44) SRSF2 (43) /normal | NA | NA | NA |
| #6, M, 84 | Papules, nodules, Head | yes | CMML 1 y later | Intermediate 2 | Dermal infiltrate of pDC (45), iDC (2); 47% | 30 | TET2 x3 (55,20,17) ASXL1 (31) NRAS (23) SRSF2 (21) | TET2 x3 (88,4,4) SRSF2 (44) ASXL1 (42) NRAS (24) /46,XY,add(11)(p11)/46,XY | No treatment, surveillance | High potent topical corticosteroids and tacrolimus (PR) Prednisone 1 mg/kg per day (CR) | Yes, 4 y, 3 y, sepsis from lung infection |
| #7, F, 86 | Papules | no | CMML, 8 mo before | Intermediate 2 | Dermal infiltrate of pDC (30), iDC (1); 31% | 10 | KRAS(22), SF3B1 (21) SMC3 (17) RUNX1 (17) FLT3-ITD | KRAS (43) SF3B1 (42) SMC3 (42) RUNX1 (13) FLT3-ITD /normal | Sorafenib 200 mg twice a day and decitabine (NR) | High potent topical corticosteroids (NR) Prednisone 1m/kg per day (CR) | Yes, 1.1 y, 6 mo, AML |
| #8, M, 89 | Papules | Yes | CMML, concurrent | Intermediate 1 | Dermal infiltrate of pDC (10), iDC (30); 40% | 10 | ASXL1 (17) ETV6 (19) BRAF(17) CUX1 (15) SRSF2 (11) TET2 (11) | ASXL1 (41), ETV6 (38), BRAF (37) CUX1 (35) SRSF2 (32) TET2 (16) /normal (BLOOD) | Hydroxycarbamide (NR) | high potent topical corticosteroids (NR) | Yes, 3 y, 3 y AML |
| #9, M, 80 | Papules | yes | MDS, NOS with multilineage dysplasia, concurrent | Low –1.41 | Dermal infiltrate of pDC (10), iDC (10); 20% | 20 | ASXL2 (11) CBL (9) CHEK2 (9) TET2 (15) BRCC3 (15) TP53 (2) | TET2 (40) BRCC3 (40) ASXL2 (25) CBL (16) CHEK2 (15) /normal | No treatment | High potent topical corticosteroids (PR) Prednisone 0.1mg/kg per day (CR) | No, 7 y, 7 y |
| #10, M, 64 | Papules | yes | CMML concurrent | Intermediate 2 | Dermal infiltrate of pDC (20), iDC (5); 25% | 10 | ZRSR2 (56) TET2 (9) ASLX1 (4) | TET2x2 (35, 42) ASXL1 (32) ZRSR2 (3) PTPN11 (2) /normal | NA | High potent topical corticosteroids (PR) | No, 2.5 y 2.5 y |
| #11, M, 87 | Papules, face | yes | CMML concurrent | NA | Dermal infiltrate of pDC (5), iDC (80); 85% | 5 | TET2 (73) ZRSR2 (19) KRAS (17) ASXL1 (7) | TET2 (86) ZRSR2 (19) ASXL1 (9) /normal (BLOOD) | Transfusion, no treatment | No treatment | No, 3 mo, 3 mo |
| #12, F, 80 | Papules,and nodules Trunk | yes | CCUS 6 mo later | NA | Dermal infiltrate of pDC (40); 40% | 10 | RUNX1 (25) SF3B1 (22) WT1 (16) KRAS (9) | NA | no | Anti-H1 (NR) | Yes, 1.1 y, 6 mo AML |
| #13, M, 76 | Papules, Nodules, Trunk, legs, arms | yes | CMML concurrent | Intermediate 2 | Dermal infiltrate of pDC (15), iDC (10); 25% | 10 | ARID2 (24) RIT1 (24) ASXL1 (24) SRSF2 (19) SMC3 (8) | ARID2 (NA) RIT1 (NA) ASXL1 (NA) /normal | EPO for anemia (no progression) | Anti H1 (NR) High potent topical corticosteroids (NR) Prednisone 0.5mg/kg per day (CR) | No, 2 y, 2 y |
| # 14, M, 60 | Papules, head and neck | no | CMML 3 y before | Intermediate 2 | Dermal infiltrate of pDC (20), iDC (15); 35% | 15 | SH2B3 (22) NF1 (21) SRSF2 (16) IDH2 (11) | SH2B3 (36) NF1x3 (50, 33 & 2), SRSF2 (42) IDH2 (46) KRAS (7) MPL (5) /normal | Hydroxycarbamide (NR) | — | No, 4 y, 3 y |
CCUS, clonal cytopenia of undetermined significance; CPSS-mol, molecular CMML-specific prognostic scoring system; CR, complete response; ICC, International Consensus Classification; IPSS-M, International Prognostic Scoring System; MDN, myelodysplastic neoplasm; NA, not available; NR, no response; PR, partial response; VAF, variant allele frequency.
Percentages of cells in the skin sample correspond to the following: the number of CD123+ TCF4+ CD2AP+ cells on immunohistochemistry out of total nucleated cells of the sample for pDCs; the number of CD1a+ S100+/− CD207− cells on immunohistochemistry out of total nucleated cells of the sample for iDCs; the sum of pDC percentage and iDC percentage for total percentage of pDC+iDC in the sample.
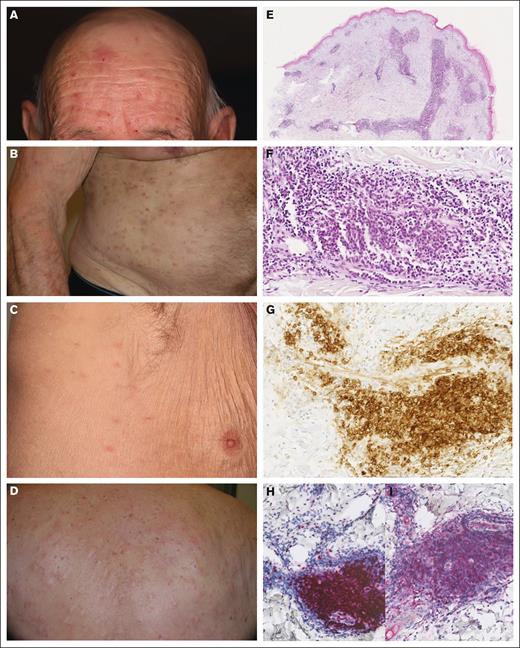
Clinico-pathological features of mature pDC dermatosis. (A-D) Papulo-nodular skin eruption in patients with pDC dermatosis. (E-F) Dense perivascular dermal infiltrate without blast cells (case 12; HES; original magnifications ×40 and ×330). (G) CD123 expression in mature pDCs (case 12; original magnification ×330). (H) TCF4 expression in mature pDCs (case 12; original magnification ×200). (I) CD2AP expression in mature pDCs (case 12; original magnification ×200). HES, haematoxylin, eosin, saffron.
Clinico-pathological features of mature pDC dermatosis. (A-D) Papulo-nodular skin eruption in patients with pDC dermatosis. (E-F) Dense perivascular dermal infiltrate without blast cells (case 12; HES; original magnifications ×40 and ×330). (G) CD123 expression in mature pDCs (case 12; original magnification ×330). (H) TCF4 expression in mature pDCs (case 12; original magnification ×200). (I) CD2AP expression in mature pDCs (case 12; original magnification ×200). HES, haematoxylin, eosin, saffron.
Histologically, the pDC and iDC skin infiltrates were perivascular or diffuse in the dermis (Figure 1E-H), with variable density (Table 1), always intermixed with CD3+ T cells and rarely with neutrophils (n = 3) or eosinophils (n = 4). Myeloid CD163+ cells were less abundant than pDC and iDC populations, no blast cells were present in the skin, and Ki67 was ≤20% in all skin infiltrates.
Paired NGS of skin and bone marrow (or blood) samples were available for 13 patients. The same gene mutations were detected in both tissues in all patients, with small differences in clone sizes and detection of smaller clones in skin, blood, or bone marrow. Pathogenic mutations of TET2, SRSF2, ASXL1, and RUNX1 were the most frequent in skin biopsies (57%, 50%, 42%, and 28% respectively). Mutations of RAS pathway genes (KRAS, NRAS, CBL, and NF1) were detected in 8 cases (57%). Maximum variant allele frequency of mutated genes in the skin was positively correlated with pDC plus iDC percentage in the skin sample (r = 0.72, Pearson correlation) but not with CD163+ myeloid cells (r = 0.06).
Six patients were treated with high potent topical corticosteroids (3 partial response [skin lesions disappearance up to 30%] and 3 no response [NR]). Prednisone (0.1-1 mg/kg per day) was consistently efficient in 4 patients (4 complete response; median duration of response, 15 months) with relapse after treatment discontinuation. MN-directed therapies did not show efficacy both on the dermatosis and MNs. Allogeneic hematopoietic stem cell transplantation was consistently efficient on skin lesions (complete response, 3/3).
Median follow-up was 36 months (range, 3-84), and 4 patients of 14 died (29%). Three patients with CMML (n = 2) or clonal cytopenia of undetermined significance (n = 1) died of progression to AML (time from skin symptoms and CMML diagnosis to death, 13 and 6 months, respectively), and 1 patient with CMML died of sepsis.
Herein, we describe the features of pDC dermatosis in MNs and demonstrate the clonal relationship between skin infiltrate and underlying MN. Among myeloid cells in skin infiltrate, pDCs and iDCs were the predominant populations and positively correlated to gene mutation variant allele frequency. Further work using single-cell or flow-sorted cell sequencing approaches, mutational signatures, and phylogenomic analyses on fresh tissue samples will be necessary to precisely assess the mutational landscape of pDCs and iDCs in this context, because we cannot exclude that other myeloid cells may also carry mutations detected in the skin sample.
All our patients had a chronical, itchy, popular, or nodular skin eruption occurring mostly during the course of an MN and sometimes before its diagnosis. As in previous reports, pDC dermatosis was mostly associated with CMML.8,9 Differential diagnosis included prurigo nodularis, but lesions mostly involved the trunk and face and less predominantly the limbs; the dermal infiltrate was usually too dense and/or deep; and phenotyping showed abundant mature pDC and iDC infiltrate. Myelodysplasia cutis was ruled out by the absence of nonblastic histiocytoid MPO+ cells in the infiltrate and the absence of plaques and annularity of skin lesions.14 Leukemia cutis in AML and blastic pDC neoplasm typically presents as nonitchy erythemato-violaceous nodules, with infiltrate made of blast cells.
As reported in myelodysplasia cutis,14,15 pDC dermatosis can predate MN diagnosis. Physicians should be aware of this entity and may perform hematological workup (blood and/or bone marrow) including NGS and blood monocyte subset phenotyping16 when this type of skin infiltrate is found. Nodal and bone marrow pDC proliferation in CMML have been associated with poor prognosis, with high risk of AML transformation.4,10 The prognosis of pDC dermatosis in CMML is less clear. A previous report of 16 patients with CMML and mature pDC dermatosis suggested a better prognosis than that of patients with other CMML-related skin infiltrates.8 In a more recent study, 4 of 6 patients with pDC dermatosis died of infection or hematological progression.9 In our series, 4 of 14 patients died after 36 months of follow-up. Thus, more data are needed to clarify the prognosis of pDC dermatosis.
The mechanism of skin pDC expansion in CMML and other MNs is unclear. Clonal expansion of skin pDCs could derive from a common mutated myeloid progenitor or from the differentiation of circulating tumor cells. TET2 mutation, found in 50% of our skin samples, is known to promote IRF7 expression, a transcription factor responsible for pDC proliferation.17 Similarly, RUNX1 mutation found in 28% of our cases is known to upregulate pDC transcriptional programs in pDC-AML.6 Another interesting hypothesis is the involvement of UV radiation in the skin in shaping the mutation landscape of cells that may recirculate, as described in blastic pDC neoplasm.18 UV radiation or various other local stimuli may participate in dendritic cell transdifferentiation, as described in juvenile CMML.19
Overall, clonal mature pDC dermatosis is characterized by (1) a chronic itchy skin eruption with papules or nodules, (2) a frequent occurrence at the time of underlying MN diagnosis, (3) a high association with CMML, (4) shared mutational profile in skin sample and MN, (5) efficacy of oral corticosteroids and allogeneic hematopoietic stem cell transplantation, and (6) an uncertain risk of AML progression.
This noninterventional study was performed in accordance with the Declaration of Helsinki. Patients gave written informed consent for the publication of their clinical pictures and for the inclusion of their tissue sample in the Lymphoteq tissue bank.
Contribution: T.M., A.O., M.D.V.P., and M.B. designed the research; T.M., A.O., L.L., E.C., W.K., L.A., P.F., M.S., J.D., M.J., F.C., E.C., O.C., R.I., M.W., N.M., J.M.B., A.D.M., N.D., T.M.H., and J.D.B. provided data; T.M., A.O., L.L., E.C., W.K., R.I., N.D., and M.B. analyzed data; T.M., A.O., and M.B. wrote the manuscript; and all authors revised the manuscript and gave final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maxime Battistella, Pathology Department, Hôpital Saint-Louis AP-HP, 1 Ave Claude Vellefaux, 75010, Paris, France; email: maxime.battistella@aphp.fr.
References
Author notes
Sequencing data are available upon request from the corresponding author, Maxime Battistella (maxime.battistella@aphp.fr).