Visual Abstract
TO THE EDITOR:
Vaso-occlusive pain episodes (VOE) are the clinical hallmarks of sickle cell disease (SCD) and a leading cause of morbidity and mortality.1 Therapies targeting the underlying mechanisms of pain are lacking, which renders opioid analgesics the current standard of care. Multiple controlled trials in both the United States and Sub-Saharan Africa support the safety and efficacy of arginine therapy in children with SCD-VOE.2-6 Arginine is an obligate substrate for the production of nitric oxide (NO), a potent vasodilator that is low in SCD-VOE and contributes to vaso-occlusive complications.3,7 Mechanistically, arginine supplementation increases NO metabolites (NOx),8,9 improves mitochondrial function, and decreases oxidative stress.10 Clinically, it improves cardiopulmonary function,4 decreases pain, and has opioid-sparing effects in children with SCD.2,3
Arginine is also a precursor of kyotorphin, an endogenous opioid-like analgesic first described in 1979 in Kyoto, Japan.11 It is produced from its amino acids precursors L-arginine and L-tyrosine by the action of the enzyme kyotorphin synthetase.12,13 Kyotorphin exerts its analgesic effects indirectly by inducing met-enkephalin and β-endorphin, which bind to μ- and/or δ-opioid receptors.11,13 Oral administration of arginine (1 g/kg) to wild-type mice increased kyotorphin levels in the midbrain and medulla, where the sites of morphine analgesia are located.12 Subcutaneous administration of arginine inhibited carrageenin-induced hyperalgesia in rat and mouse models, an effect that was reversed by naloxone (a δ-opioid inhibitor).14 In addition, intracerebroventricular administration of arginine produced antinociception in intact mice after the mechano-nociceptive and thermo-nociceptive tests.14,15 Furthermore, clinical studies have shown that persistent analgesia is reversible by naloxone in patients with chronic pain treated with IV arginine.16,17 Although kyotorphin has not been previously evaluated in SCD, these studies suggest a functional link between kyotorphin, arginine, and met-enkephalin/β-endorphin in suppressing pain. Prior kyotorphin studies have focused on chronic pain; however, no studies to date have explored the arginine-kyotorphin relationship in acute pain.
SCD-VOE represents an acute pain model characterized by arginine deficiency.7 Hemolysis plays a key role in arginine dysregulation;7,18 release of erythrocyte-arginase, an arginine-metabolizing enzyme that competes with NO synthase for its obligate substrate L-arginine, hydrolyzes arginine to form ornithine and urea, while diverting away from NO production.18,19 Low levels of kyotorphin-precursor tyrosine have also been reported in SCD during VOE.20 However, the relationship between arginine bioavailability and kyotorphin levels in SCD and pain is unknown. Our objective was to evaluate the impact of IV arginine therapy on plasma arginine, NOx, and kyotorphin concentrations in children hospitalized with SCD-VOE.
We conducted a single-center, institutional review board-approved, prospective, randomized, open-label pharmacokinetics (pK)/pharmacodynamics study of IV arginine at a Children’s Hospital in Atlanta, GA (Arginine Therapy for the Treatment of Pain in Children with Sickle Cell Disease; ClinicalTrials.gov identifier #NCT02447874 under IND#66,943) to assess the impact of arginine therapy on plasma arginine and NOx concentrations over time. Kyotorphin assessment was a post hoc analysis. Patients with SCD (homozygous sickle hemoglobin [Hb-SS] or hemoglobin sickle beta zero thalassemia [Hb-Sβ0-thalassemia]) aged 7 to 21 years hospitalized for VOE requiring parenteral opioids were eligible. Written informed consent, and assent when appropriate, were obtained from all participants. Exclusion criteria included hemoglobin <5 gm/dL, hepatic/renal dysfunction, acute stroke, allergy to arginine, pregnancy, emergency department discharge, hospital discharge within the past 7 days, or previous enrollment into the study.
The research pharmacist performed blocked randomization using lists prepared by the biostatistician to randomize the patients into 1 of 3 IV arginine dosing arms: (1) 100 mg/kg every 8 hours (standard dose, n = 4); (2) loading dose (200 mg/kg) followed by standard dose (n = 5); or (3) loading dose (200 mg/kg) followed by continuous infusion (300 mg/kg per day) (n = 4). Arginine was administered over 30 minutes per the manufacturer’s recommendations (R-Gene10; Pfizer). Blood was obtained at 6 time points: preinfusion (time 0) and at 1, 1.5, 2, 4, and 8 hours after the initiation of the first arginine infusion, and then at ∼8 AM daily until discharge or for 7 days, whichever came first. Plasma arginine, kyotorphin, and NOx levels were measured, through previously described methods.7,18 pK/pharmacodynamics analyses were performed, including determination of arginine maximum concentration (Cmax), time to reach Cmax (Tmax), area under the curve (AUC; calculated using the trapezoid rule), rate of clearance, and half-life. Numeric pain scores were extracted from the electronic medical records. The daily highest/worst, lowest, and mean pain scores were assessed for correlations with the peak kyotorphin concentration, arginine Cmax, change in arginine concentration from baseline to discharge (μM), and peak NOx. Mean ± standard deviation, paired t tests, and Pearson correlation analyses between groups were performed where appropriate, using Prism-v9.5.1.
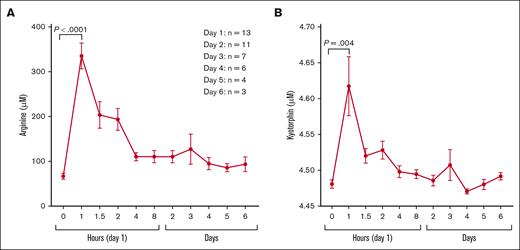
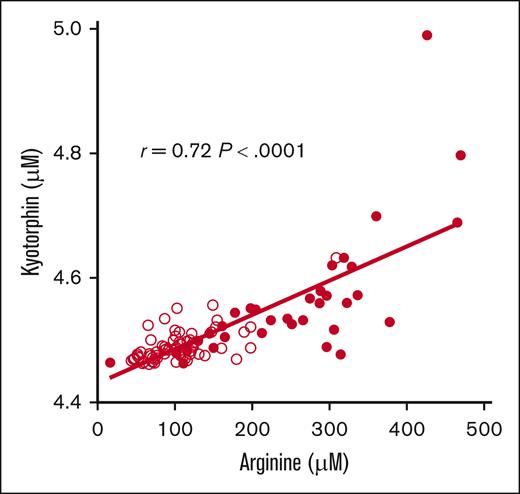
Sixteen patients were consented and 13 patients were randomized. Three patients were excluded: 1 with elevated creatinine and 2 with emergency department discharge. Participant demographics, clinical characteristics, and laboratory values at initial presentation (predose) are summarized in supplemental Table 1. Although no statistically significant differences between randomized study arms were identified, participants randomized to the standard dose arm (100 mg/kg IV every 8 hours) trended to be younger in age and had clinically relevant lower hemoglobin levels and blood biomarkers, suggesting an increased hemolytic rate that could impact arginine bioavailability. Plasma arginine and kyotorphin levels (Figure 1) were significantly higher after arginine infusion, peaking at 1 hour, with no significant differences in the peak concentrations across the study arms. pK parameters are summarized in supplemental Table 2. The mean plasma arginine peak for all participants was 331.6 ± 95.4 μM, 30 minutes after infusion completion (Tmax). All participant except 1 achieved peak plasma arginine levels above the Km (100-150 μM) of the cationic amino acid transporter-1 after arginine infusion. The AUC was the highest in the loading dose + continuous infusion arm. Kyotorphin levels were strongly correlated with plasma arginine concentration (r = 0.72; P < .0001; Figure 2). Arginine and kyotorphin levels over time, broken down by study arm, are illustrated in supplemental Figure 1. Plasma NOx also significantly increased from predose to Tmax (within 1-2 hours; mean absolute change 12.1 ± 16.2 μM; P = .02; supplemental Figure 2), returning to baseline by 8 hours. Although NOx increased primarily in those receiving an arginine-loading dose, no correlation was found between arginine and NOx concentration, arginine Cmax and peak NOx levels, or the mean change/percent change in NOx. No significant changes in the kyotorphin-precursor tyrosine were observed (supplemental Figure 3). Significant inverse correlations were identified between daily pain scores and changes in plasma arginine concentration (μM) from baseline to discharge and peak kyotorphin levels on day 1 when the arginine-loading dose arms were combined (supplemental Table 3). Onalo et al also reported a significant difference in the worst pain scores after oral arginine vs placebo.2 Nonsignificant inverse correlations between daily pain scores and day 1 peak kyotorphin, arginine Cmax, and peak NOx levels for all participants were also noted when standard dose was included (data not shown).
Impact of IV arginine therapy on plasma. (A) Arginine and (B) kyotorphin concentrations (μM) over 8 hours and daily. Both plasma arginine and kyotorphin levels peaked within 30 minutes of completion of IV arginine infusion delivered over 30 minutes. Pooled data from the 3 dosing arms are represented, as there was no significant difference in the peak concentration across the study arms. For the 8-hour pK study, plasma arginine concentration troughs by 4 hours but remains significantly above baseline through 8 hours (P = .01) and day 2 (P = .01). Plasma kyotorphin levels were significantly elevated between 1 to 2 hours (P = .004), before dropping toward baseline. Morning blood draws occurred at ∼8 AM daily, >6 hours from the last arginine infusion, representing a trough in plasma arginine and kyotorphin levels. The participants available for daily blood analysis varied based on the clinical resolution of their vaso-occlusive pain and discharge day; there were 13 individuals analyzed on day 1, 11 individuals on day 2, 7 individuals on day 3, 6 individuals on day 4, 4 individuals on day 5, and 3 individuals on day 6.
Impact of IV arginine therapy on plasma. (A) Arginine and (B) kyotorphin concentrations (μM) over 8 hours and daily. Both plasma arginine and kyotorphin levels peaked within 30 minutes of completion of IV arginine infusion delivered over 30 minutes. Pooled data from the 3 dosing arms are represented, as there was no significant difference in the peak concentration across the study arms. For the 8-hour pK study, plasma arginine concentration troughs by 4 hours but remains significantly above baseline through 8 hours (P = .01) and day 2 (P = .01). Plasma kyotorphin levels were significantly elevated between 1 to 2 hours (P = .004), before dropping toward baseline. Morning blood draws occurred at ∼8 AM daily, >6 hours from the last arginine infusion, representing a trough in plasma arginine and kyotorphin levels. The participants available for daily blood analysis varied based on the clinical resolution of their vaso-occlusive pain and discharge day; there were 13 individuals analyzed on day 1, 11 individuals on day 2, 7 individuals on day 3, 6 individuals on day 4, 4 individuals on day 5, and 3 individuals on day 6.
Pearson correlation between plasma arginine and kyotorphin levels (μM) for all available time point values. A strong correlation exists between plasma arginine and plasma kyotorphin concentrations (r = 0.72; P < .0001). When an outlier time point with a peak kyotorphin level of 5.0 μM was excluded from the analysis, the correlation was even stronger (r = 0.77; P < .0001). Filled circles represent data at 1, 1.5, and 2 hours after the initiation of arginine infusion, reflective of the significant acute increase in plasma kyotorphin levels. Unfilled circles represent time 0 (predose), 4 and 8 hours after initiation of arginine infusion, and daily values for patients remaining in the hospital.
Pearson correlation between plasma arginine and kyotorphin levels (μM) for all available time point values. A strong correlation exists between plasma arginine and plasma kyotorphin concentrations (r = 0.72; P < .0001). When an outlier time point with a peak kyotorphin level of 5.0 μM was excluded from the analysis, the correlation was even stronger (r = 0.77; P < .0001). Filled circles represent data at 1, 1.5, and 2 hours after the initiation of arginine infusion, reflective of the significant acute increase in plasma kyotorphin levels. Unfilled circles represent time 0 (predose), 4 and 8 hours after initiation of arginine infusion, and daily values for patients remaining in the hospital.
To our knowledge, this is the first report of an acute increase in the plasma concentration of the opioid-like analgesic kyotorphin in patients with SCD-VOE after IV arginine infusion. Kyotorphin concentrations remained elevated for 2 hours before returning to the predose baseline level by 4 hours, strongly correlating with arginine concentration. Low arginine bioavailability is associated with SCD mortality and morbidity,18 including acute pain severity.2,3,7 Multiple phase-2 trials support the safety and efficacy of arginine therapy in children with SCD-VOE,2-6 whereas marked analgesia has been reported in patients with non-SCD with various forms of pain 30 to 40 minutes after IV arginine compared with placebo, with a dose-dependent effect that lasted 6 to 24 hours.16,17 As an obligate substrate for NO production, arginine’s mechanism of action is unknown but is thought to be related in part to NO production. However, arginine is likely to be the rate-limiting amino acid for kyotorphin production,11,13 potentially contributing to the efficacy of arginine therapy for pain reduction.2,3,16 In particular, the opioid-sparing effect of arginine supplementation is not fully understood in SCD; induction of an endogenous opioid-like dipeptide, such as kyotorphin, represents a potential mechanism of analgesia that would decrease opioid utilization during VOE. Although our study was limited by its small sample size, lack of a control arm, and single-center enrollment, it is a pK study meant to identify the dose-dependent effects of arginine therapy and potential mechanisms of action, leading to a larger controlled trial in SCD-VOE.21
This study demonstrated that IV arginine rapidly increased plasma arginine concentration 2 to 5 times above baseline at presentation for VOE, reaching a maximum concentration within 1 hour of infusion initiation, regardless of the study-dose administered. Although there was intersubject variability in the peak arginine levels achieved, the loading dose, which was double the standard dose, interestingly, did not result in a significantly higher Cmax. However, previous studies have demonstrated a dose-dependent impact of arginine on NOx production,9 mitochondrial function, and oxidative stress.10 In addition, we previously demonstrated that a significantly lower peak arginine concentration was achieved in children with SCD at the onset of acute VOE compared with levels achieved with the same arginine dose given at steady state.9 Although greater renal excretion or elevated metabolism of arginine are potential explanations not evaluated in this study, this observation may potentially reflect higher arginine intracellular transport in the loading dose arms, ultimately leading to changes in pharmacodynamic outcomes, such as mitochondrial function and oxidative stress, which favor the utilization of higher doses.10 Kyotorphin levels strongly correlated with arginine concentration and rapidly peaked within 1 hour of arginine infusion initiation. Although there were no significant changes in plasma tyrosine concentration after arginine infusion, we also found no significant difference in peak kyotorphin levels between our loading (200 mg/kg) and the standard dose (100 mg/kg). However, because the AUC was the greatest in the loading/continuous infusion group, a larger sample size might reveal a dose-dependent response. It is also possible that the administration of higher doses of IV arginine may have a greater impact on kyotorphin production and, ultimately, pain relief. Given the excellent safety profile of arginine6 and practices utilizing up to 500 mg/kg for urea cycle disorders and hyperammonemia,22 mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS),23 and growth hormone stimulation testing,6 studies evaluating higher doses for SCD-VOE are indicated to potentially maximize pain management, particularly in the acute care setting.
Similar to previous reports,7,8,24 arginine supplementation in this cohort significantly increased plasma NOx levels, supporting the role of vasodilation as an additional potential mechanism of action during SCD-VOE. Finally, hemolysis depletes tetrahydrobiopterin (BH4),25 an essential cofactor for both tyrosine synthesis and NO production from arginine. BH4 converts phenylalanine to tyrosine and is also a cofactor for NO synthase in the production of NO from arginine. Because BH4 is unstable, it becomes nonenzymatically oxidized to dihydrobiopterin under oxidative stress,25 disrupting both metabolic pathways and compromising tyrosine synthesis and NO production. In malaria, BH4 is oxidized to dihydrobiopterin, which contributes to endothelial dysfunction.25 Particularly relevant to SCD, BH4 activity warrants further study as it could disrupt both metabolic pathways, compromising tyrosine synthesis, and NO production, adversely impacting kyotorphin production, and potentially contributing to pain.
Our findings highlight a novel mechanism of action of arginine therapy in SCD-VOE, which requires further research. Although a phase-3 randomized controlled trial of IV arginine for children and young adults with SCD-VOE is currently underway,21 our kyotorphin-related observation has significant implications for the potential use of arginine as an opioid-sparing therapy for pain syndromes beyond SCD.
Acknowledgments: This study was supported in part by grants from the National Institutes of Health (NIH; National Heart, Lung, and Bood Institute [NHLBI; grant R34HL122557] and National Center for Complementary and Integrative Health [grant K24AT009893; C.R.M.], NIH-NHLBI [grants 1K23HL140142 and 1K23HL140142-03S1] [N.B.]), the Doris Duke Charitable Foundation COVID-19 Fund to Retain Clinical Scientists-PeRSEVERE Program at Emory University School of Medicine, and the Georgia Clinical and Translational Science Alliance (award UL1-TR002378; N.B.). This study used the Emory Pediatric Biomarker Core facility.
Contribution: C.R.M. designed the research question, wrote the study protocol, obtained funding, obtained informed consent or assent, analyzed and interpreted the data, and wrote the manuscript; R.K. and D.H. analyzed and interpreted the data and wrote the manuscript; L.A.B. assisted with study protocol development, supervised sample processing, assisted with interpretation of data, and critically reviewed the manuscript; F.H. processed and analyzed biological samples, assisted with interpretation of the data, and critically reviewed the manuscript; H.W. assisted with the pharmacokinetic/pharmacodynamics data analysis and final critical review of the manuscript; C.A.R., D.R.A., and N.B. assisted with the interpretation of the data and critically reviewed the manuscript; and C.D. assisted with study protocol design, patient enrollment and consent, interpretation of the data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: C.R.M. is the inventor or coinventor of several University of California San Francisco-Benioff Children’s Hospital Oakland patents, that include nutritional supplements; is an inventor of the Emory University School of Medicine patent applications for nutritional supplements for autism/apraxia, coronaviruses, and pain; serves as a consultant for Roche and CSL Behring; sits on the scientific advisory board of TRILITY; acts as an editor for the sickle cell disease-fever and sickle cell disease-pain web-based reference for UpToDate; and is the founder and executive director for Food as Medicine Therapeutics, Limited Liability Company. C.D. has received research support from Pfizer. D.R.A. received research funding from Pfizer/Global Blood Therapeutics and Disc Medicine. The remaining authors declare no competing financial interests.
Correspondence: Claudia R. Morris, Department of Pediatrics and Emergency Medicine, Emory University School of Medicine, 1760 Haygood Dr NE, W458, Atlanta, GA 30322; email: claudia.r.morris@emory.edu.
References
Author notes
R.K. and D.H. contributed equally to this study.
R.K. and D.H. are joint first authors.
Deidentified participant data are available on reasonable request from the corresponding author, Claudia R. Morris (claudia.r.morris@emory.edu).
The full-text version of this article contains a data supplement.