TO THE EDITOR:
Acquired pure red cell aplasia (PRCA) is a rare hematological disorder that results from failure of erythropoiesis1,2 and can be distinguished from other bone marrow failure disorders by reticulocytopenia, normal granulopoiesis, and megakaryopoiesis.3,4 PRCA is often idiopathic and likely due to cytotoxic T-cell–mediated destruction of early erythroid precursors. Indeed, it can be associated with conditions such as T-cell large granular lymphocytic leukemia, B-cell dyscrasia, thymoma, immunodeficiency, and infections.5-8
Thrombocytosis refers to an abnormal elevation in platelet count of >450 000 × 109/L. This condition can be related to a primary process, usually associated with a myeloproliferative neoplasm known as essential thrombocythemia, but it more commonly presents as an epiphenomenon of other causes that include iron deficiency, chronic inflammatory conditions, and asplenia.9,10 Thrombocytosis has not been associated with PRCA. Herein, we report a cohort of patients with PRCA and thrombocytosis that have not been defined in the literature.
A retrospective analysis was conducted on patient records diagnosed with PRCA at the University of Texas Southwestern and Cleveland Clinic Foundation between 2000 and 2022. This study was approved by the institutional review board of the University of Texas Southwestern Medical Center and Cleveland Clinic Foundation. The primary objective was to investigate the presence of thrombocytosis and/or megakaryocyte changes in these PRCA cases. Clinical, laboratory, and molecular data were abstracted, adhering to the guidelines established by the Declaration of Helsinki and the respective participating institutions.
Among a total of 90 patients, comprehensive analysis of 27 cases were identified as having acquired PRCA with thrombocytosis and/or megakaryocyte changes noted on bone marrow biopsies (Table 1) examined. To assess treatment efficacy, hemoglobin and platelet count before and after resolution of PRCA were compared. The Shapiro-Wilk test was used to assess normality. For normally distributed data, a paired t test was performed. For nonnormally distributed data, a Wilcoxon matched-pair signed-rank test was performed. For all relevant comparisons, a P value <.05 was used to set statistical significance.
Cohort of patients with PRCA
| . | Changes in megakaryocyte only . | Changes in thrombocytosis only . | Changes in both . | Changes in megakaryocyte and/or thrombocytosis . | Neither (PRCA in the absence of thrombocytosis or megakaryocyte changes) . | Total . |
|---|---|---|---|---|---|---|
| Number of patients | 16 | 4 | 7 | 27 | 63 | 90 |
| Mean age at diagnosis, y | 57.6 ± 8.3 (33.8-82) | 68 ± 16.1 (45.3-84.3) | 53.5 ± 11.2 (28-73.9) | 58.1 ± 6.2 (28-84.3) | 56.7 ± 5.1 (0-85) | 57.1 ± 4 (0-85) |
| Patients with BM megakaryocyte changes | ||||||
| Dysplasia | 1 | 0 | 0 | 1 | 0 | 1 |
| Hyperlobation | 3 | 0 | 1 | 4 | 0 | 4 |
| Hyperplasia | 13 | 0 | 7 | 20 | 0 | 20 |
| Total with any changes | 16 | 0 | 7 | 23 | 0 | 23 |
| Mean hemoglobin at diagnosis, g/dL | 7.6 ± 0.9 (3.5-9.6) | 6.4 ± 1.3 (4.5-7.4) | 8.3 ± 1.6 (5.1-12.4) | 7.6 ± 0.7 (3.5-12.4) | 7.7 ± 0.6 (2.6-11.7) | 7.6 ± 0.5 (2.6-12.4) |
| Mean platelet count, × 109/L | 232.7 ± 70.3 (9-440) | 550.8 ± 96.6 (476-695) | 564.7 ± 59.2 (472-693) | 389 ± 80.4 (9-695) | 254.2 ± 26.7 (45-432) | 299.1 ± 35.2 (9-695) |
| Mean ferritin at diagnosis, μg/L | 2309.4 ± 1750.2 (648.4-6579) | 2254.9 ± 2350.7 (434.1-5505) | 705 ± 310.1 (315-1 569) | 1 635.9 ± 861.5 (315-6 579) | 2 021 ± 583.6 (50.1-7 597) | 1 899.8 ± 481 (50.1-7 597) |
| Mean WBC at diagnosis, 1 000 WBC per μL | 8.1 ± 2.7 (3.9-18.8) | 8.3 ± 0.7 (7.8-9.4) | 8 ± 1.8 (3.3-10.3) | 8.1 ± 1.5 (3.3-18.8) | 5.6 ± 0.7 (2-12.6) | 6.4 ± 0.7 (2-18.8) |
| Female (% of total) | 6 (37.5%) | 2 (50%) | 6 (85.7%) | 14 (51.9%) | 29 (46.0%) | 43 (47.8%) |
| Mean BM cellularity, % | 55.3 ± 10.9 (25-90) | 53.8 ± 26.9 (40-95) | 73.6 ± 8.2 (50-80) | 60 ± 8.2 (25-95) | 39.6 ± 5.4 (5-90) | 1 973.2 ± 745.8 (46.6-11 560) |
| Next generation sequencing findings | • STAT3 p.S614RC 8 • IDH1, SETBP1 | • SF3B1 p.K666N 18.21% • BCOR p.1252_1253del 9% | • c.490G>T p.G164C 43.2%, c.1292C>T p.P431L 41.7%, c.6460G>A p.D2154N 41.4%, c.3974A>G p.K1325R 40.6%, c.588_589insACCCGC p.P196_P197insTR 13.0% • NF-kappaB2, JAK2, TYK 2 • c.490G>T p.G164C 43.2%, c.1292C>T p.P431L 41.7%, c.6460G>A p.D2154N 41.4%, c.3974A>G p.K1325R 40.6%, c.588_589insACCCGC p.P196_P197insTR 13.0% | • SPTB, SPTA1, EPB42 • TET2 p.N275lfs 4.3% • RPS19, HFE C282Y, H63D • PB, ASXL1, U2AF1 • ASXL1, JAK2, STAT3, U2AF1 VUS • STAT3 p.D661Y VAF 12.7% • ASXL1 p.K912Q c.2734A>C VAF 50.3% and PTPN11 p.K131R c.392A>G 51.1% VAF N6471 mutation in STAT3 gene | ||
| Mean absolute reticulocyte count at diagnosis, reticulocytes per μL | 2.924 ± 3.162 (0.01-10) | 0.013 ± 0.004 (0.011-0.015) | 3.685 ± 3.322 (0.007-8.9) | 2.84 ± 1.987 (0.007-10) | 1.45 ± 0.884 (0-7.1) | 1.894 ± 0.881 (0-10) |
| Mean reticulocyte percent at diagnosis, % | 0.684 ± 0.647 (0.3-2) | 0.543 ± 0.283 (0.3-0.8) | 0.263 ± 0.084 (0.04-0.4) | 0.459 ± 0.231 (0.04-2) | 0.5 ± 0.191 (0.1-2.1) | 0.487 ± 0.149 (0.04-2.1) |
| Mean erythropoietin level, mU/mL | 748.6 ± 532.1 (46.6-1646) | 2012 (n = 1) | 2 553.9 ± 3 290 (72.4-10 815) | 1 679 ±1 542.6 (46.6-10 815) | 2 105 ± 845.3 (91-11 560) | 1 973.2 ± 745.8 (46.6-11 560) |
| Parvovirus B19 | 0 | 2 | 1 | 3 | 3 | 6 |
| Chronic lymphocytic leukemia | 0 | 1 | 0 | 1 | 1 | 2 |
| Large granular lymphocyte leukemia | 4 | 2 | 4 | 10 | 15 | 25 |
| . | Changes in megakaryocyte only . | Changes in thrombocytosis only . | Changes in both . | Changes in megakaryocyte and/or thrombocytosis . | Neither (PRCA in the absence of thrombocytosis or megakaryocyte changes) . | Total . |
|---|---|---|---|---|---|---|
| Number of patients | 16 | 4 | 7 | 27 | 63 | 90 |
| Mean age at diagnosis, y | 57.6 ± 8.3 (33.8-82) | 68 ± 16.1 (45.3-84.3) | 53.5 ± 11.2 (28-73.9) | 58.1 ± 6.2 (28-84.3) | 56.7 ± 5.1 (0-85) | 57.1 ± 4 (0-85) |
| Patients with BM megakaryocyte changes | ||||||
| Dysplasia | 1 | 0 | 0 | 1 | 0 | 1 |
| Hyperlobation | 3 | 0 | 1 | 4 | 0 | 4 |
| Hyperplasia | 13 | 0 | 7 | 20 | 0 | 20 |
| Total with any changes | 16 | 0 | 7 | 23 | 0 | 23 |
| Mean hemoglobin at diagnosis, g/dL | 7.6 ± 0.9 (3.5-9.6) | 6.4 ± 1.3 (4.5-7.4) | 8.3 ± 1.6 (5.1-12.4) | 7.6 ± 0.7 (3.5-12.4) | 7.7 ± 0.6 (2.6-11.7) | 7.6 ± 0.5 (2.6-12.4) |
| Mean platelet count, × 109/L | 232.7 ± 70.3 (9-440) | 550.8 ± 96.6 (476-695) | 564.7 ± 59.2 (472-693) | 389 ± 80.4 (9-695) | 254.2 ± 26.7 (45-432) | 299.1 ± 35.2 (9-695) |
| Mean ferritin at diagnosis, μg/L | 2309.4 ± 1750.2 (648.4-6579) | 2254.9 ± 2350.7 (434.1-5505) | 705 ± 310.1 (315-1 569) | 1 635.9 ± 861.5 (315-6 579) | 2 021 ± 583.6 (50.1-7 597) | 1 899.8 ± 481 (50.1-7 597) |
| Mean WBC at diagnosis, 1 000 WBC per μL | 8.1 ± 2.7 (3.9-18.8) | 8.3 ± 0.7 (7.8-9.4) | 8 ± 1.8 (3.3-10.3) | 8.1 ± 1.5 (3.3-18.8) | 5.6 ± 0.7 (2-12.6) | 6.4 ± 0.7 (2-18.8) |
| Female (% of total) | 6 (37.5%) | 2 (50%) | 6 (85.7%) | 14 (51.9%) | 29 (46.0%) | 43 (47.8%) |
| Mean BM cellularity, % | 55.3 ± 10.9 (25-90) | 53.8 ± 26.9 (40-95) | 73.6 ± 8.2 (50-80) | 60 ± 8.2 (25-95) | 39.6 ± 5.4 (5-90) | 1 973.2 ± 745.8 (46.6-11 560) |
| Next generation sequencing findings | • STAT3 p.S614RC 8 • IDH1, SETBP1 | • SF3B1 p.K666N 18.21% • BCOR p.1252_1253del 9% | • c.490G>T p.G164C 43.2%, c.1292C>T p.P431L 41.7%, c.6460G>A p.D2154N 41.4%, c.3974A>G p.K1325R 40.6%, c.588_589insACCCGC p.P196_P197insTR 13.0% • NF-kappaB2, JAK2, TYK 2 • c.490G>T p.G164C 43.2%, c.1292C>T p.P431L 41.7%, c.6460G>A p.D2154N 41.4%, c.3974A>G p.K1325R 40.6%, c.588_589insACCCGC p.P196_P197insTR 13.0% | • SPTB, SPTA1, EPB42 • TET2 p.N275lfs 4.3% • RPS19, HFE C282Y, H63D • PB, ASXL1, U2AF1 • ASXL1, JAK2, STAT3, U2AF1 VUS • STAT3 p.D661Y VAF 12.7% • ASXL1 p.K912Q c.2734A>C VAF 50.3% and PTPN11 p.K131R c.392A>G 51.1% VAF N6471 mutation in STAT3 gene | ||
| Mean absolute reticulocyte count at diagnosis, reticulocytes per μL | 2.924 ± 3.162 (0.01-10) | 0.013 ± 0.004 (0.011-0.015) | 3.685 ± 3.322 (0.007-8.9) | 2.84 ± 1.987 (0.007-10) | 1.45 ± 0.884 (0-7.1) | 1.894 ± 0.881 (0-10) |
| Mean reticulocyte percent at diagnosis, % | 0.684 ± 0.647 (0.3-2) | 0.543 ± 0.283 (0.3-0.8) | 0.263 ± 0.084 (0.04-0.4) | 0.459 ± 0.231 (0.04-2) | 0.5 ± 0.191 (0.1-2.1) | 0.487 ± 0.149 (0.04-2.1) |
| Mean erythropoietin level, mU/mL | 748.6 ± 532.1 (46.6-1646) | 2012 (n = 1) | 2 553.9 ± 3 290 (72.4-10 815) | 1 679 ±1 542.6 (46.6-10 815) | 2 105 ± 845.3 (91-11 560) | 1 973.2 ± 745.8 (46.6-11 560) |
| Parvovirus B19 | 0 | 2 | 1 | 3 | 3 | 6 |
| Chronic lymphocytic leukemia | 0 | 1 | 0 | 1 | 1 | 2 |
| Large granular lymphocyte leukemia | 4 | 2 | 4 | 10 | 15 | 25 |
BM, bone marrow; WBC, white blood cell.
Subset of 7 patients who had resolution of transfusion dependency
| Variable . | Mean before resolution ± CI . | Mean after resolution ± CI . | P value . |
|---|---|---|---|
| Platelet count, × 109/L | 576.3 ± 71.8 | 431.6 ± 78.6 | .0120 |
| Hemoglobin, g/dL | 8.5 ± 1.4 | 11.3 ± 1.3 | .0225 |
| Variable . | Mean before resolution ± CI . | Mean after resolution ± CI . | P value . |
|---|---|---|---|
| Platelet count, × 109/L | 576.3 ± 71.8 | 431.6 ± 78.6 | .0120 |
| Hemoglobin, g/dL | 8.5 ± 1.4 | 11.3 ± 1.3 | .0225 |
CI, confidence interval.
In a cohort of 90 patients with PRCA, we identified 27 individuals with concurrent thrombocytosis and/or megakaryocyte changes (Table 1).
Of the 27 patients, the mean hemoglobin count at diagnosis was 7.6 ± 0.7 g/dL, and the mean platelet count at diagnosis was 389 000 ± 80 400 × 109/L. In total, 51.9% were female. A total of 20 demonstrated megakaryocyte hyperplasia, 4 demonstrated hyperlobation, and 1 displayed megakaryocyte dysplasia. Three patients tested positive for parvovirus on polymerase chain reaction, 1 patient had chronic lymphocytic leukemia, and 10 patients had large granular lymphocytic leukemia.
To delve deeper into the potential mechanisms linking PRCA and thrombocytosis, we then examined the clinical variables in patients exhibiting thrombocytosis (n = 7) following the clinical response of their PRCA (Table 2). This was defined as the achievement of a hemoglobin level >9 g/dL with red blood cell transfusion independence. We excluded 4 patients because of unresolved hemoglobin levels or insufficient chart information. Bone marrow biopsies at the time of resolution were not available for any of the examined patients because this procedure is generally not clinically indicated at this time point.
Among the subset of 7 patients with PRCA resolution, the mean hemoglobin count at diagnosis was 8.5 ± 1.4 g/dL, and the mean platelet count was 576 300 ± 71 800 × 109/L. After the resolution of PRCA, the mean hemoglobin count increased to 11.3 ± 1.3 g/dL, and the mean platelet count decreased to 431 600 ± 78 600 × 109/L. Statistical analysis revealed significant differences in mean hemoglobin and mean platelet count before and after resolution, with P values of .0225 and .0120, respectively. Interestingly, of the 7 patients, 2 patients still exhibited thrombocytosis even after resolution of their PRCA.
Next, the mean erythropoietin (EPO) levels in patients with thrombocytosis were examined. Of the 7 patients who had EPO levels drawn at the time of diagnosis, all had levels well over the upper limit of normal (26 mU/mL), 2476.5 ± 2784.7 mU/mL (range, 72.4-10 815).
In this study, we provide a detailed characterization of a cohort of patients with PRCA exhibiting notable alterations in megakaryocytes. Our cohort was heterogenous and the etiology of each patient’s PRCA was different. Therefore, there may not be 1 mechanism by which PRCA is linked to thrombocytosis. However, we theorize that there are 2 potential mechanisms that may explain the phenomenon in some cases.
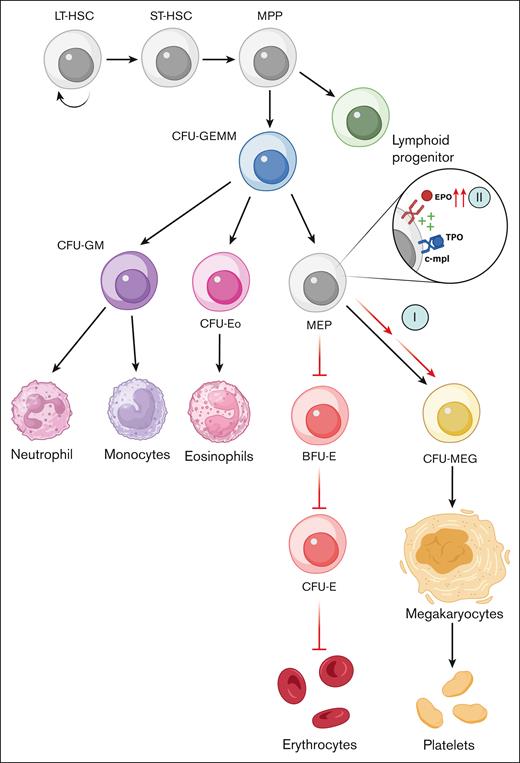
We first hypothesized that the scarcity of erythroid production may redirect hematopoietic precursors toward the megakaryocytic lineage. The process of hematopoiesis, encompassing both erythroid and megakaryocyte lineages arising from bipotential megakaryocyte-erythrocyte progenitor (MEP), is well-documented.11-15 We suggest that the interruption of erythroid differentiation at a critical stage may funnel differentiation toward the megakaryocytic lineage (Figure 1, I). Our findings lend support to this hypothesis, as the initial thrombocytosis observed before treatment resolves upon PRCA resolution with treatment.
Proposed mechanism for PRCA-related thrombocytosis. (I) Proposed mechanism I: decreased erythropoiesis directs differentiation toward the megakaryocyte lineage. (II) Proposed mechanism II: increased EPO due to PRCA leads to synergistic signaling between EPO and TPO at bipotential MEP, stimulating TPO receptors and resulting in increased megakaryocyte formation. BFU, burst-forming unit; CFU, colony-forming unit; E, erythrocyte; Eo, eosinophil; GEMM, granulocyte-erythrocyte-monocyte-megakaryocyte; GM, granulocyte-monocyte; LT-HSC, long-term hematopoietic stem cell; MEG, megakaryocyte; MPP, multipotent progenitor, ST-HSC, short-term hematopoeitic stem cell. Figure created with BioRender.com.
Proposed mechanism for PRCA-related thrombocytosis. (I) Proposed mechanism I: decreased erythropoiesis directs differentiation toward the megakaryocyte lineage. (II) Proposed mechanism II: increased EPO due to PRCA leads to synergistic signaling between EPO and TPO at bipotential MEP, stimulating TPO receptors and resulting in increased megakaryocyte formation. BFU, burst-forming unit; CFU, colony-forming unit; E, erythrocyte; Eo, eosinophil; GEMM, granulocyte-erythrocyte-monocyte-megakaryocyte; GM, granulocyte-monocyte; LT-HSC, long-term hematopoietic stem cell; MEG, megakaryocyte; MPP, multipotent progenitor, ST-HSC, short-term hematopoeitic stem cell. Figure created with BioRender.com.
A similar phenomenon has been reported in the context of iron-deficient anemia leading to secondary thrombocytosis, although the precise underlying mechanism remains elusive.16 One proposed mechanism suggests that once the hematopoietic growth factors and cytokines required for erythrocyte development become available, differentiation of the MEP cell veers away from the megakaryocyte lineage and reverts toward the erythroid lineage. This skewing may be attributed to heightened MKL1 expression;14 transcription factors determining the megakaryocyte lineage;17 or an increase in thrombopoietin (TPO), stem cell factor, stromal-derived factor 1, or cytokines known to exert thrombopoietic effects.17 In addition, 1 study found that low iron in the bone marrow environment can bias MEP differentiation toward the megakaryocyte lineage via a reduction in extracellular signal-regulated kinase signaling.18 It is plausible that 1 or more of these mechanisms may account for the PRCA-related thrombocytosis we have observed, although further research is warranted for a comprehensive understanding.
In our cohort, many patients for whom EPO levels were measured at the time of PRCA diagnosis exhibited levels well above the normal range. Therefore, we hypothesized that EPO may play a role in the development of thrombocytosis (Figure 1, II). In addition, EPO signaling has been shown to have a synergistic effect with TPO on thrombopoiesis.19 A study involving TPO-knockout mice demonstrated that EPO exerts a direct and TPO-independent influence on late-stage thrombopoiesis, resulting in the increased production of large platelets.20 Furthermore, several human studies in healthy volunteers, uremic patients, and patients with chronic liver disease have reported significant short-term increases in platelet counts after EPO injections.21-24 Thus, it is conceivable that in certain patients with PRCA, synergistic signaling between EPO and TPO may be occurring upstream at the level of the bipotential MEP cell, leading to increased megakaryocyte production and thrombocytosis.
The implications of thrombocytosis and megakaryocyte alterations in the context of PRCA remain enigmatic, necessitating comprehensive data sets to unravel their role in the pathophysiology of the disease and their potential as prognostic markers for treatment response. It is important to acknowledge the limitations of this study, including a modest sample size secondary to the rarity of the disorder and the absence of posttreatment bone marrow biopsy results, which would have provided visual confirmation of the resolved megakaryocyte abnormalities.
Our findings in aggregate suggest that perhaps the definition of PRCA be expanded to include those with megakaryote changes or thrombocytosis. Further research is warranted to establish a definitive correlation and assess whether the course and response of PRCA to standard immunosuppressive treatment differ in cases with and without thrombocytosis. Future investigations should also encompass an examination of megakaryocyte hyperplasia and other abnormalities, such as hyper and hypolobation, associated with PRCA.
Contribution: J.A., R.G.W., and T.B. generated and conceived the study design; J.A., R.G.W., G.R., M.M., Y.O., W.C., and T.B. contributed to the table and manuscript preparation; C.G., H.A., and J.P.M. reviewed the clinical data, participated in patient selection, and helped with manuscript writing; J.A., R.G.W., G.R., M.M., Y.O., H.A., M.O., W.C., C.G., J.P.M., and T.B. reviewed the clinical data and contributed to manuscript writing; and all authors participated in data interpretation, provided critical review of the final manuscript and submission, and have read and agreed to the published version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taha Bat, Division of Hematology-Oncology, UT Southwestern Medical Center, Dallas, TX, 5323 Harry Hines Blvd, Dallas, TX 75390-9255; email: a.bat@utsouthwestern.edu.
References
Author notes
J.A. and R.G.W. contributed equally to this study.
All data are presented in the manuscript. Additional information is available on request from the corresponding author, Taha Bat (taha.bat@utsouthwestern.edu).