Key Points
Patients with MM receiving BsAbs or CAR T-cell therapies require frequent UHIs.
BsAb patients had more frequent unscheduled health care interactions than CAR T-cell therapy patients, largely due to more frequent URIs.
Visual Abstract
Outcomes for patients with relapsed/refractory multiple myeloma (R/RMM) have dramatically improved after the development and now growing utilization of B-cell maturation antigen–targeted chimeric antigen receptor (CAR) T-cell therapy and bispecific antibody (BsAb) therapy. However, health care utilization as a quality-of-life metric in these growing populations has not been thoroughly evaluated. We performed a retrospective cohort study evaluating the frequency and cause of unscheduled health care interactions (UHIs) among patients with R/RMM responding to B-cell maturation antigen–targeted BsAb and CAR T-cell therapies (N = 46). This included the analysis of remote UHIs including calls to physicians’ offices and messages sent through an online patient portal. Our results showed that nearly all patients with R/RMM (89%) receiving these therapies required a UHI during the first 125 days of treatment, with a mean of 3.7 UHIs per patient. Patients with R/RMM responding to BsAbs were significantly more likely to remotely contact their physicians’ offices (1.8-fold increase; P = .038) or visit an urgent care center (more than threefold increase; P = .012) than patients with R/RMM responding to CAR T-cell therapies. This was largely due to increased reports of mild upper respiratory tract infections in BsAb patients. Our results underscore the need to develop preemptive management strategies for commonly reported symptoms that patients with R/RMM experience while receiving CAR T-cell or BsAb therapies. This preemptive management may significantly reduce unnecessary health care utilization in this vulnerable patient population.
Introduction
T-cell redirecting therapies (TRTs) such as chimeric antigen receptor (CAR) T-cell and bispecific antibody (BsAb) therapies have profoundly expanded the treatment landscape for patients with relapsed/refractory multiple myeloma (R/RMM) over the last few years.1-8 With the commercial availability of these therapies and their high respective response rates, CAR T-cell therapies and BsAbs will be likely or expected options for patients with R/RMM.9-16 Because these therapies have only recently received approval from the US Food and Drug Administration, there are few studies evaluating quality-of-life (QoL) metrics in patients receiving them and no studies comparing metrics of any kind between these 2 classes of therapy.17,18 Additionally, therapy safety assessments have focused mainly on treatment-related adverse events reported in recent phase 2 and 3 clinical trials. This leaves a knowledge gap for understanding how these therapies affect patient reported outcomes outside of a clinical trial or outcomes not defined by common terminology criteria for adverse effects.
Health care utilization is not typically reported when evaluating risks associated with specific therapies and may be overlooked when evaluating therapy options. When health care utilization is considered, it is often limited to consideration of hospital admissions, treatment costs, and total inpatient time.19-21 Other relevant forms of health care utilization, including unplanned communications with physicians’ offices or office visits that do not result in inpatient admission, may not be considered, despite their meaningful impact on patient QoL. This is especially true at academic medical centers, where low acuity patient concerns managed entirely by nursing or advanced practice provider (APP) staff may be invisible to physician providers. TRTs purport to offer a long therapy-free interval (in the case of CAR T-cell therapies) or a period of infrequent office visits for therapy administration (in the case of BsAbs). However, health care utilization in patients with MM treated with commercial TRTs has not thoroughly been evaluated or compared.
In this study, we aimed to investigate the frequency and cause of unscheduled health care interactions (UHIs) among patients with heavily pretreated R/RMM receiving either commercial B-cell maturation antigen (BCMA)–targeted CAR T-cell or BsAb therapies. We also sought to quantify and characterize remote UHIs (rUHIs), which are often not considered when assessing health care utilization because they do not result in a face-to-face interaction with a health care provider.
With the background knowledge that this heavily pretreated population is already at significant risk for varied toxicities, especially serious infections, we want to create improved strategies for management and monitoring.22-26 The goal of our study was to quantify and compare health care utilization within these populations, while also identifying common causes of UHIs to identify targets for improved patient education and management to reduce unnecessary UHIs.27
Methods
Patient selection
We retrospectively analyzed UHIs for patients treated with commercial CAR T-cell and BsAb therapies at Memorial Sloan Kettering Cancer Center (MSKCC) via manual review of the electronic health record by individuals with nursing experience. Patients were included in the study if they received commercial BCMA-directed CAR T-cell (CAR T cohort) or BsAb (BsAb cohort) treatments and were “responders” to therapy. Responders were defined as patients who had at least 125 days of progression-free survival per International Myeloma Working Group criteria after therapy initiation for the BsAb cohort and after the day of cell infusion for the CAR T cohort.28 Patients were excluded if they were not exclusively managed by physicians at our center to avoid unaccounted for UHIs in our data set. Data collected included all unscheduled health care visits, including rUHIs, urgent care (UC) visits, emergency department (ED) visits, and hospital admissions. In this study, rUHIs were defined as all remote outpatient communications, including telephone calls to physicians’ offices and messages sent to physicians’ offices through MSKCC’s online patient portal that were not related to appointment logistics, medication refills, or review of laboratory results (supplemental Figure 1). UC visits were defined as unscheduled visits to UC centers that did not have hospital admitting capabilities. ED visits were defined as unscheduled visits to hospital EDs that did have admitting capabilities. UHIs were monitored for only the first 125 days after therapy initiation, and all included patients remained progression free for the entire 125-day observation period.
Patient management
Among patients in the BsAb cohort, all patients received at least the first 2 step-up doses of therapy in the inpatient setting. Scheduled follow-up visits for BsAb patients included weekly treatment visits during which patients were only seen by a registered nurse. Patients also had scheduled follow-up visits with a physician or APP every 4 weeks. The majority of patients in the CAR T cohort (20/23 [87%]) received cell infusion in the inpatient setting.29 After hospital discharge, CAR T patients were seen for follow-up office visits by a provider (APP or physician) at a provider-assessed frequency until acute therapy-related toxicities were deemed to be resolved and then every 4 weeks thereafter. Symptoms and patient questions addressed during scheduled visits were not considered UHIs.
Data analysis and statistical methods
The primary outcome measurement of our study was to compare the number of rUHIs between patients in the CAR T cohort and BsAb cohort during the observation period. Secondary outcomes included comparing the number of UC and ED visits between the 2 cohorts during the observation period. Exploratory outcomes included comparing the total number of inpatient days for each patient cohort during the observation period, as well as categorizing the causes of UHIs in these cohorts. This study was approved by the MSKCC institutional review board (IRB 23-070 and 14-276) and conducted in accordance with the Declaration of Helsinki and the Belmont report. Statistical comparisons of the number of events in each patient cohort was performed using the Mann-Whitney U test.30 Outlier analysis was performed only for the primary outcome measurement and was done using the robust regression and outliter removal (ROUT) method (Q = 0.2%).31
Results
Patient cohort
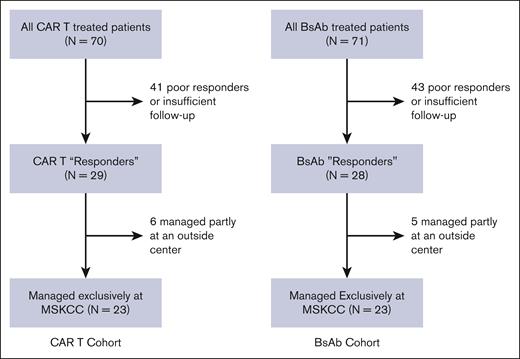
Between 25 October 2021 and our data cutoff of 3 October 2023, we identified 70 patients with MM treated with commercial CAR T-cell therapy and 71 patients treated with commercial BsAb therapy at our center. Among these patients, 29 CAR T patients and 28 BsAb patients were identified as responders to therapy. Among the cohort of responders, 6 CAR T patients and 5 BsAb patients were not exclusively managed at our center and were excluded from the analysis to avoid unaccounted for UHIs. This yielded a cohort consisting of 23 CAR T patients and 23 BsAb patients meeting our inclusion criteria (Figure 1).
CONSORT diagram. Recruited patients included patients treated with commercial CAR T-cell and BsAb products, with a response to therapy, who were managed exclusively at our single center, MSKCC.
CONSORT diagram. Recruited patients included patients treated with commercial CAR T-cell and BsAb products, with a response to therapy, who were managed exclusively at our single center, MSKCC.
When analyzing patient characteristics within this cohort, the BsAb cohort had a higher percentage of patients aged ≥75 years than the CAR T cohort (30% vs 13%). BsAb patients were more heavily pretreated (43% vs 17% penta-drug refractory) but less likely to have a high-risk cytogenetic profile (22% vs 48%) than CAR T patients. The majority of patients in the total cohort had received a prior autologous stem cell transplant (80%) and had an Eastern Cooperative Oncology Group score ≥1 (67%; Table 1).
Baseline characteristics: characteristics of patients meeting inclusion criteria
| Characteristic . | Total (N = 46) . | CAR T (n = 23) . | BsAb (n = 23) . |
|---|---|---|---|
| Age, median (range) | 62 (41-88) | 65 (41-78) | 67 (51-88) |
| Age ≥75, n (%) | 10 (22) | 3 (13) | 7 (30) |
| ISS, n (%) | |||
| I | 16 (35) | 8 (35) | 8 (35) |
| II | 14 (30) | 7 (30) | 7 (30) |
| III | 8 (17) | 5 (22) | 3 (13) |
| Unknown | 8 (17) | 3 (13) | 5 (22) |
| High-risk Cytogenetics, n (%)∗ | 16 (35) | 11 (48) | 5 (22) |
| ECOG ≥1, n (%) | 31 (67) | 16 (70) | 15 (65) |
| Extramedullary disease, n (%) | 12 (26) | 6 (26) | 6 (26) |
| Prior autologous stem cell transplantation, n (%) | 37 (80) | 21 (91) | 16 (70) |
| Prior Allogeneic stem cell transplantation, n (%) | 5 (11) | 4 (17) | 1 (4) |
| Triple class refractory, n (%) | 36 (78) | 16 (70) | 20 (87) |
| Penta-drug refractory, n (%) | 14 (30) | 4 (17) | 10 (43) |
| Prior BCMA-directed therapy, n (%) | 14 (30) | 6 (26) | 8 (35) |
| Characteristic . | Total (N = 46) . | CAR T (n = 23) . | BsAb (n = 23) . |
|---|---|---|---|
| Age, median (range) | 62 (41-88) | 65 (41-78) | 67 (51-88) |
| Age ≥75, n (%) | 10 (22) | 3 (13) | 7 (30) |
| ISS, n (%) | |||
| I | 16 (35) | 8 (35) | 8 (35) |
| II | 14 (30) | 7 (30) | 7 (30) |
| III | 8 (17) | 5 (22) | 3 (13) |
| Unknown | 8 (17) | 3 (13) | 5 (22) |
| High-risk Cytogenetics, n (%)∗ | 16 (35) | 11 (48) | 5 (22) |
| ECOG ≥1, n (%) | 31 (67) | 16 (70) | 15 (65) |
| Extramedullary disease, n (%) | 12 (26) | 6 (26) | 6 (26) |
| Prior autologous stem cell transplantation, n (%) | 37 (80) | 21 (91) | 16 (70) |
| Prior Allogeneic stem cell transplantation, n (%) | 5 (11) | 4 (17) | 1 (4) |
| Triple class refractory, n (%) | 36 (78) | 16 (70) | 20 (87) |
| Penta-drug refractory, n (%) | 14 (30) | 4 (17) | 10 (43) |
| Prior BCMA-directed therapy, n (%) | 14 (30) | 6 (26) | 8 (35) |
ISS, international staging system.
High-risk cytogenetics defined as either t(4;14), t(14;16), del (17p), or +1q.
Within this population, there were no differences between the length of initial hospital admissions for treatment initiation between BsAb and CAR T patients (median, 7 vs 8 days; P = .18). We also compared the number of scheduled health care visits 29 days after CAR T-cell infusion and 29 days after the first step-up dose for BsAb patients. Scheduled health care visits included any planned appointment in which a patient was seen by either a physician, an APP, or a registered nurse. There were no significant differences in the total number of scheduled health care interactions between day 29 and the end of the 125-day observation period between BsAb and CAR T patients (mean scheduled visits, 11.7 vs 9.4; P = .14).
Unscheduled health care interaction frequency
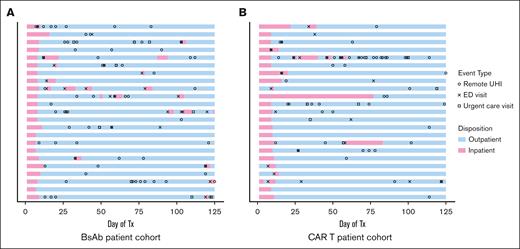
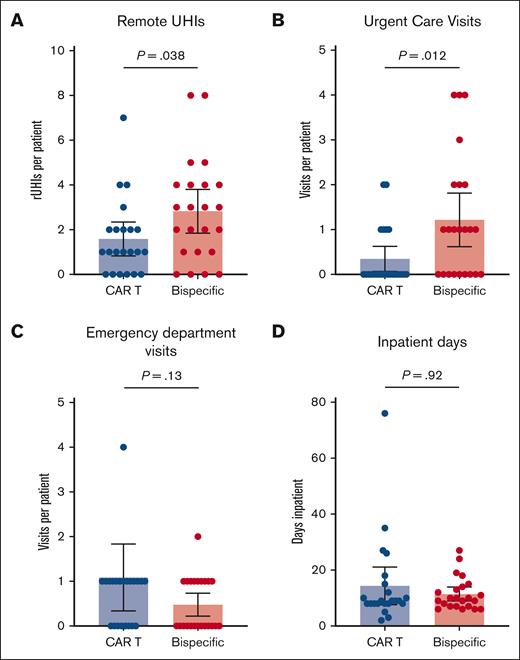
All patients required frequent UHIs, with 89% of patients having at least 1 UHI during the observation period and with an average of 3.7 UHIs per patient among the total cohort (Figure 2). The number of rUHIs, including phone calls and messages sent via the online patient portal, was significantly higher in the BsAb cohort than the CAR T cohort (mean rUHIs per patient, 2.8 vs 1.6; P = .038; Figure 3A). Only 1 outlier was identified on outlier analysis, which was identified as a patient calling the physician's office 19 times during the observation period for the same instance of gastrointestinal (GI)-related symptoms, and was excluded from statistical analysis.
UHIs. Swimmer plot describing patient disposition and the incidence of UHIs among patients included in study cohorts over the 125-day observation period. Symbols indicate UHIs and colors indicate patient disposition for BsAb patients (A) and CAR T patients (B). Tx, treatment.
UHIs. Swimmer plot describing patient disposition and the incidence of UHIs among patients included in study cohorts over the 125-day observation period. Symbols indicate UHIs and colors indicate patient disposition for BsAb patients (A) and CAR T patients (B). Tx, treatment.
Relative UHI frequency. (A) BsAb patients had more frequent rUHIs during the observation period than CAR T patients. (B) BsAb patients more frequently visited UC center offices during the observation period than CAR T patients. There were no statistically significant differences between the number of ED visits (C) or total inpatient days (D) during the observation period between the 2 cohorts.
Relative UHI frequency. (A) BsAb patients had more frequent rUHIs during the observation period than CAR T patients. (B) BsAb patients more frequently visited UC center offices during the observation period than CAR T patients. There were no statistically significant differences between the number of ED visits (C) or total inpatient days (D) during the observation period between the 2 cohorts.
The frequency of UC visits was also significantly higher in the BsAb cohort than the CAR T cohort (mean UC visits per patient, 1.2 vs 0.35; P = .012; Figure 3B). There were no statistically significant differences between the BsAb cohort and the CAR T cohort in terms of the number of ED visits (mean visits per patient, 0.50 vs 1.1; P = .13; Figure 3C) or in the total number of inpatient days (mean days per patient, 11.4 vs 14.4; P = .92; Figure 3D) during the observation period.
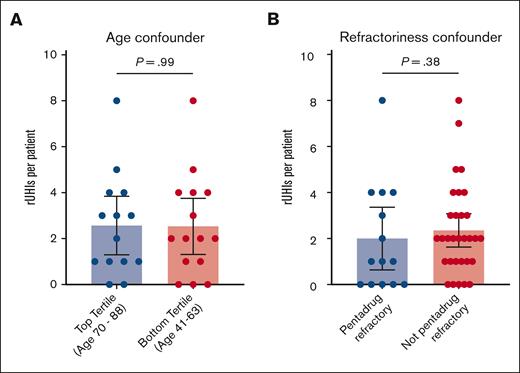
Data were then stratified to identify possible confounders not related to therapy modality. There were no statistically significant differences between the number of rUHIs between top and bottom tertiles by age (mean rUHIs per patient, 2.3 vs 2.5; P = .99; Figure 4A) or between patients with and without pentarefractory disease (mean rUHIs per patient, 2.0 vs 2.3; P = .38; Figure 4B) when looking at the combined cohort.
Accounting for confounders. When stratifying rUHI frequency by top vs bottom age tertiles (A) or prior therapy refractoriness (B), no significant differences in rUHI frequency are observed.
Accounting for confounders. When stratifying rUHI frequency by top vs bottom age tertiles (A) or prior therapy refractoriness (B), no significant differences in rUHI frequency are observed.
Reasons for unscheduled health care interactions
rUHIs in the BsAb cohort were more frequently concerning upper respiratory tract infection (URI) symptoms than in the CAR T cohort (33% vs 24%). Complaints leading to UC visits were also more frequently concerning URI symptoms in the BsAb cohort than in the CAR T cohort (62% vs 25%). Cytokine release syndrome (CRS)–related symptoms more frequently contributed to ED visits and inpatient hospitalizations in the CAR T cohort than in the BsAb cohort (ED, 16% vs 4%; Hospitalizations, 31% vs 5%).
Patients responding to CAR T-cell therapy most frequently visited an ED for GI symptoms (28%). Other reasons patients in this group visited an ED were fever/infection (non-URI; 20%), CRS (16%), and URI symptoms (12%). Patients responding to BsAb therapy visited EDs for fever/infection (non-URI; 24%), URI (16%), GI symptoms (8%), CRS (4%), disease-related pain (4%), and volume overload (4%). Forty percent of ED visits for patients in the BsAb group and 15% of visits for patients in the CAR T group were for “other” symptoms and were likely unrelated to their treatment. The overwhelming majority of UC and ED visits did not lead to hospital admissions.
The most common causes for hospital admissions for patients receiving CAR T-cell therapy were fever/infection (non-URI; 31%) and CRS (31%). Patients receiving BsAb therapy were admitted most frequently for fever/infection (non-URI; 23%) and URIs (9%). A summary of common complaints leading to UHIs is shown in Table 2.
Reasons for UHIs: the most common symptoms leading to each category of UHI are shown
. | rUHIs . | UC visit . | ED . | Hospital admission . | ||||
|---|---|---|---|---|---|---|---|---|
| CAR T, % . | BsAb, % . | CAR T, % . | BsAb, % . | CAR T, % . | BsAb, % . | CAR T, % . | BsAb, % . | |
| URI | 24 | 33 | 25 | 62 | 12 | 16 | 0 | 9 |
| Fever/infection (non-URI) | 9 | 0 | 13 | 8 | 20 | 24 | 31 | 23 |
| GI symptoms | 28 | 19 | 38 | 0 | 28 | 8 | 8 | 5 |
| CRS | 6 | 0 | 0 | 8 | 16 | 4 | 31 | 5 |
| Disease-related pain | 2 | 15 | 0 | 8 | 0 | 4 | 0 | 5 |
| Volume overload | 6 | 9 | 0 | 0 | 0 | 4 | 0 | 0 |
| Injection site reaction | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 26 | 16 | 25 | 15 | 20 | 40 | 31 | 52 |
. | rUHIs . | UC visit . | ED . | Hospital admission . | ||||
|---|---|---|---|---|---|---|---|---|
| CAR T, % . | BsAb, % . | CAR T, % . | BsAb, % . | CAR T, % . | BsAb, % . | CAR T, % . | BsAb, % . | |
| URI | 24 | 33 | 25 | 62 | 12 | 16 | 0 | 9 |
| Fever/infection (non-URI) | 9 | 0 | 13 | 8 | 20 | 24 | 31 | 23 |
| GI symptoms | 28 | 19 | 38 | 0 | 28 | 8 | 8 | 5 |
| CRS | 6 | 0 | 0 | 8 | 16 | 4 | 31 | 5 |
| Disease-related pain | 2 | 15 | 0 | 8 | 0 | 4 | 0 | 5 |
| Volume overload | 6 | 9 | 0 | 0 | 0 | 4 | 0 | 0 |
| Injection site reaction | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 26 | 16 | 25 | 15 | 20 | 40 | 31 | 52 |
URI symptoms caused a plurality of rUHIs and UC visits in the BsAb population. Common causes of ED visits and hospital admissions in both cohorts included CRS and non-URI infections.
Among the 3 CAR T cohort patients who received infusion as outpatients, all 3 visited the ED and required subsequent hospital admission within the first 2 weeks after infusion. Two were admitted for grade 1 CRS symptoms. One was admitted with URI symptoms and was discharged the following day.
Discussion
In this retrospective analysis, we report that the incidence of UHIs was frequent among both the CAR T-cell therapy and BsAb cohorts during a 125-day observation period among responders to each therapy. Importantly, unlike other studies evaluating health care utilization in patients with hematologic malignancies, this analysis included remote forms of health care utilization that did not involve in-person interaction with physicians. The frequency of rUHIs in our MM patient cohorts receiving BsAbs or CAR T-cell therapies underscores the need to better account for this form of health care utilization.
Despite this analysis being restricted only to patients exhibiting a response to therapy, nearly all patients had a UHI at some point during the first 125 days of therapy (Figure 2). The overwhelming majority of UHIs did not ultimately lead to inpatient admissions, highlighting the need to establish preemptive management plans for common symptoms associated with therapy.
There was a significantly higher incidence of rUHIs and UC visits in the BsAb cohort than the CAR T cohort (Figure 3). However, the number of ED visits and total inpatient days were not significantly different between the 2 cohorts, and there were no significant differences in the number of scheduled health care interactions between the 2 groups. Many of the rUHIs and UC visits in the BsAb cohort were due to URI symptoms, which is consistent with the increased incidence of infections observed in the frequently hypogammaglobulinemic BsAb patient population.32-35 Because URIs represent mild infections that are rarely associated with significant morbidity and mortality, our data indicate that BsAb patients in particular would benefit from additional education on distinguishing URIs from more severe infections in the outpatient setting. If executed, this may significantly reduce both in-person and remote health care utilization among a heavily pretreated R/RMM population, for whom QoL and avoidance of health care settings is critical. Pretreatment counseling for CAR T-cell therapy–treated patients is often more comprehensive than for BsAb patients, given the many logistical concerns associated with therapy. More thorough patient education may partially contribute to differences in UHI frequency in these populations. This, therefore, emphasizes the value of similarly thorough patient education for BsAb patients to reduce UHI frequency.
It should be noted that, because our patient cohort was limited to patients receiving BCMA-directed therapies, side effects specific to G protein–coupled receptor, class C, group 5, member D (GPRC5D)–targeted therapies are not accounted for. These therapies, particularly talquetamab, a GPRC5D-directed BsAb, are associated with “on-target, off-tumor” side effects that may result in a unique adverse event profile not examined in this study. Additionally, GPRC5D-directed therapies have been shown, in some reports, to carry a lower risk of infectious complications, which may affect UHI and rUHI frequency.5,36
Overall, our study identifies common causes of remote and in-person UHIs in a vulnerable patient population and offers a framework for a future quality improvement study to reduce health care utilization in patients with R/RMM.
Acknowledgment
Portions of the graphical abstract were made using BioRender.com.
Authorship
Contribution: A.J.H. and R.S.F. conceived the study and designed the project; A.J.H., I.C., A.X.W., I.S.H., M.H., S.M., C.T., N.K., A.M.L., H.H., U.A.S., K.H.M., S.R., H.J.L., M.S., G.L.S., O.B.L., D.J.C., S.G., S.Z.U., and R.S.F. collected clinical data and recruited patients; A.J.H., I.C., and R.S.F. analyzed the data; A.J.H., I.C., and R.S.F. wrote the manuscript; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: A.J.H. reports honoraria from Janssen. M.H. reports research funding from Amgen, Daiichi Sankyo, and GlaxoSmithKline, and has received honoraria for consultancy from/participated in advisory boards for Curio Science LLC, Intellisphere LLC, Bristol Myers Squibb (BMS), and GlaxoSmithKline. S.M. received consulting fees from Evicore, Optum, Bio Ascend, Janssen Oncology, BMS, AbbVie, HMP Education, and Legend Biotech, and received honoraria from OncLive, Physician Education Resource, MJH Life Sciences, and Plexus Communications. Memorial Sloan Kettering Cancer Center receives research funding from the NCI, Janssen Oncology, BMS, Allogene Therapeutics, Fate Therapeutics, Caribou Therapeutics, and Takeda Oncology for conducting research. C.T. reports research funding from Janssen and Takeda; personal fees from Physician Educations Resource and MJH Life Sciences; and has participated in advisory boards for Janssen and Sanofi, outside of the submitted work. N.K. reports research funding through Amgen, Janssen, Epizyme, and AbbVie; consults for CCO, OncLive, and Intellisphere Remedy Health; and participated in advisory boards for Janssen and MedImmune. A.M.L. reports grants from Novartis, during the conduct of the study; grants from BMS; personal fees from Trillium Therapeutics; grants, personal fees, and nonfinancial support from Pfizer; grants and personal fees from Janssen, outside the submitted work; and also has a patent US20150037346A1 with royalties paid. H.H. reports grants from Celgene, Takeda, and Janssen, outside the submitted work. U.A.S. reports grants from National Institutes of Health/National Cancer Institute Cancer Center (Support Grant P30 CA008748), MSK Paul Calabresi Career Development Award for Clinical Oncology K12CA184746, Paula and Rodger Riney Foundation, Parker Institute for Cancer Immunotherapy at MSK, HealthTree Foundation, International Myeloma Society, American Society of Hematology, and Allen Foundation Inc, as well as nonfinancial support from American Society of Hematology Clinical Research Training Institute and TREC Training Workshop R25CA203650 (PI: Melinda Irwin); reports other research support from Celgene/BMS and Janssen; and personal fees from ACCC, MashUp MD, Janssen Biotech, Sanofi, BMS, MJH LifeSciences, Intellisphere, Phillips Gilmore Oncology Communications, i3 Health, and RedMedEd. K.H.M. reports grant support from ASH, MMRF, and IMS. H.J.L. has served as a paid consultant for Takeda, Genzyme, Janssen, Karyopharm, Pfizer, Celgene, and Caelum Biosciences, and has received research support from Takeda. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc, and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc, Omeros Corporation, and Amgen, Inc; served on ad hoc advisory boards for Kite, a Gilead company; and received honoraria from i3 Health, Medscape, and CancerNetwork for CME-related activity, and IDEOlogy. G.L.S. reports research funding from Janssen, Amgen, BMS, and Beyond Spring, and serves on the data safety monitoring board for ArcellX; research funding to the institution from Janssen, Amgen, BMS, Beyond Spring, and GPCR; and is on the DSMB for ArcellX. O.B.L. reports serving on advisory board for MorphoSys, Kite, Daiichi Sankyo Inc, and Incyte, and has served as a paid consultant for Incyte. D.J.C. receives research funding from Genentech. S.G. reports personal fees and advisory role (scientific advisory board) from Actinium, Celgene, BMS, Sanofi, Amgen, Pfizer, GlaxoSmithKline, Jazz, Janssen, Omeros, Takeda, and Kite, outside the submitted work. S.Z.U. reports grants and personal fees from AbbVie, 404 Amgen, BMS, Celgene, GlaxoSmithKline, Janssen, Merck, MundiPharma, Oncopeptides, 405 Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Ross S. Firestone, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: firestor@mskcc.org; and Saad Z. Usmani, Department of Medicine, Myeloma Service, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; email: usmanis@mskcc.org.
References
Author notes
A.J.H. and I.C. contributed equally to this study.
Data are available on request from the corresponding authors, Saad Z. Usmani (usmanis@mskcc.org) or Ross S. Firestone (firestor@mskcc.org).
The full-text version of this article contains a data supplement.