Key Points
After CAR T-cell infusion, circulating PD-1+ CAR+ CD8+ T cells are highly associated with response to CAR T-cell therapy for NHL.
Spectral flow analysis reveals specific populations of stem-like and effector-like CAR T cells, which correlate with improved outcomes.
Visual Abstract
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized treatment for relapsed/refractory B-cell non-Hodgkin lymphoma (NHL). Robust biomarkers and a complete understanding of CAR T-cell function in the postinfusion phase remain limited. Here, we used a 37-color spectral flow cytometry panel to perform high dimensional single-cell analysis of postinfusion samples in 26 patients treated with CD28 costimulatory domain containing commercial CAR T cells for NHL and focused on computationally gated CD8+ CAR T cells. We found that the presence of postinfusion Programmed cell death protein 1 (PD-1)+ CD8+ CAR T cells at the day 14 time point highly correlated with the ability to achieve complete response (CR) by 6 months. Further analysis identified multiple subtypes of CD8+ PD-1+ CAR T cells, including PD-1+ T cell factor 1 (TCF1)+ stem-like CAR T cells and PD-1+ T-cell immunoglobulin and mucin-domain containing-3 (TIM3)+ effector-like CAR T cells that correlated with improved clinical outcomes such as response and progression-free survival. Additionally, we identified a subset of PD-1+ CD8+ CAR+ T cells with effector-like function that was increased in patients who achieved a CR and was associated with grade 3 or higher immune effector cell–associated neurotoxicity syndrome. Here, we identified robust biomarkers of response to CD28 CAR T cells and highlight the importance of PD-1 positivity in CD8+ CAR T cells after infusion in achieving CR.
Introduction
Chimeric antigen receptor (CAR) T-cells (CAR-Ts) directed against CD19 (CAR19) is standard of care (SOC) for patients with relapsed/refractory (R/R) B-cell non-Hodgkin lymphoma (NHL).1-8 Axicabtagene ciloleucel (axi-cel) and brexucabtagene autoleucel are commercially available CAR19 products containing a CD28 costimulatory domain (CD28 CAR19) used to treat R/R NHL. CD28 CAR19 products historically have resulted in complete response (CR) rates of 50% to 65% at 6 months, after which, disease progression is uncommon.4,8-11 However, a significant proportion of patients still experience CAR19 failure. Multiple studies have identified clinical risk factors for CAR-T failure including high disease burden, extranodal disease, poor performance status, and increased baseline inflammatory markers.12-14 However, patients with multiple clinical risk factors can still experience success with CAR19, and patients with low-risk disease still experience failure.
Prior studies focusing on commercial CAR T cells have described features that correlate with clinical outcomes. More naïve-like and memory-like CAR T cells present in leukapheresis products and CAR19 infusion products have been correlated with increased response rates whereas increased populations of exhausted CD8+ CAR T cells correlated with worse outcomes.13,15,16 Postinfusion peak expansion of CD28 CAR19 relative to tumor burden has been correlated with improved outcomes,13 however other studies reveal a weak or no association between CAR-T expansion and outcomes.17,18
Additionally, biomarkers to predict severe (grade ≥3) immune effector cell–associated neurotoxicity syndrome (ICANS) have been difficult to quantify for CD28 CAR19 products in NHL. Increased CAR-T expansion and higher baseline and peak inflammatory markers have been shown to correlate with severe ICANS in some studies but not in others.1,10,13,19 In acute lymphoblastic leukemia, higher pretreatment disease burden also correlates with ICANS, but less evidence of this has been found for NHL.20 A previous study has identified lower levels of CAR+ regulatory T cells regulatory T cells (Tregs) after infusion correlates with more severe ICANS18; however, no other features of potential direct mediators of ICANS (eg, CD8+ T cells) have been found. Thus, we sought to identify more robust predictive factors identifying patients at high risk of CAR19 failure and of developing high grade ICANS in order to improve management and the clinical outcomes of patients receiving CAR-T.
In this study, we performed high-dimensional flow cytometric analysis of postinfusion CD8+ CAR+ T-cell populations to identify features of patient’s CAR T cells associated with achieving CR by 6 months and who developed severe ICANS. High-dimensional analysis was performed with spectral flow cytometry, a next-generation platform using antibody–fluorochrome conjugates to detect a significantly increased number of surface and intracellular molecules.21 To account for variabilities in phenotype and functionality between CAR-T products with different constructs and costimulatory domains, we focused our analysis on commercial CD28 CAR19 products used for B-cell NHL. We found that the presence of postinfusion Programmed cell death protein 1 (PD-1)+ CD8+ CAR-Ts was highly associated with achievement of CR by 6 months. Further analysis identified multiple subtypes of PD-1+ CD8+ CAR-Ts that correlated with improved clinical outcomes, including PD-1+ T cell factor 1 (TCF1)+ stem-like CAR-T cells,22-26 and PD-1+ T-cell immunoglobulin and mucin-domain containing-3 (TIM3)+ effector-like CAR-T cells. Additionally, we identified a subset of CD8+ CAR+ T cells with effector-like function that was increased in patients who achieved a CR and had severe ICANS. Here, we identified robust biomarkers of response and toxicity to CD28 CAR-Ts and highlight the importance of PD-1 positivity in CD8+ CAR-Ts after infusion in achieving CR.
Methods
Patients
Patients who received SOC axi-cel or brexucabtagene autoleuce for R/R NHL at The Ohio State University (OSU) Comprehensive Cancer Center and who were consented to the Leukemia Tissue Bank protocol were included in this study. Inclusion criteria: patients with detectable CD19+ disease before CAR19 infusion. Exclusion criteria: patients who had ongoing partial response, which had not resolved to either CR or progressive disease (PD) at the time of final study evaluation. Patients who achieved stable disease as best response were included in the PD cohort.
Samples
Patients received CD28 CAR19 from February 2022 to March 2023 and had blood samples collected at time of SOC phlebotomy at, or near, day 14 after CAR T-cell infusion. The median day of sample collection after CAR-T infusion was 15.5 days for the CR cohort and 16.5 days for PD cohort. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh whole blood by density gradient centrifugation using Ficoll-Paque, and cryopreserved. Healthy donor PBMCs were purchased from STEMCELL Technologies.
Clinical data were obtained retrospectively from the electronic medical record under an institutional review board-approved protocol. Treatment response was assessed radiographically according to the Lugano criteria.27 Toxicity was evaluated by the American Society for Transplantation and Cellular Therapy consensus guidelines criteria for cytokine release syndrome and ICANS.28 Cutoffs for laboratory values being defined as within normal limits included: lactate dehydrogenase (LDH) of <190 U/L, ferritin of <322 ng/mL, and C-reactive protein (CRP) of <10 mg/L.
Spectral flow cytometry
The 37-color spectral flow cytometry panel is outlined in supplemental Table 1. Postinfusion PBMC samples were thawed and analyzed simultaneously along with healthy donor PBMs. Cryopreserved single-cell suspensions collected were thawed and washed with RPMI-1640 (Gibco). LIVE/DEAD fixable blue (Invitrogen) was applied to stain dead cells. Cells were washed twice with fluorescence-activated cell sorting (FACS) buffer and the surface molecule staining antibody cocktail including Fc block was applied for 45 minutes at 4°C. After incubation, cells were washed twice with FACS buffer, and the FOXP3/transcription factor staining buffer set (eBioscience) was applied overnight. Cells were washed twice in permeabilization buffer, and the intracellular staining antibody cocktail was added. After 2 hours of incubation at room temperature, cells were washed twice with FACS buffer; data were collected using the Aurora (Cytek) 5-laser spectral flow cytometry machine.
High-dimensional flow cytometry data analysis
Flow cytometry data were uploaded to web-based software OMIQ (https://app.omiq.ai/). Live CD45+, CD8+, and CAR+ cells were gated, and uniform manifold approximation and projection (UMAP) dimension reduction and FlowSOM clustering analysis was performed. The proper number of clusters was determined using FlowSOM elbow metaclustering analysis; FlowSOM consensus metaclustering analysis was performed to cluster cells with unique features.29 Characteristics of each cluster were evaluated using individual marker expressions in UMAP space and validated using clustered heat map. For further validation, flow cytometry data were reevaluated using FlowJo (BD) by creating 2-dimensional (2D) plots and using concomitant statistical approaches. In order to quantify the amount of CAR+ CD8+ T cells present at the time of sample collection, live cell lymphocytes were gated followed by CD45+ CD3+ CD4− CD8+ CAR+ cells to quantify the percentage of CD8+ CAR+ T cells as a proportion of lymphocytes. This number was then multiplied by the clinically measured absolute lymphocyte count in order to obtain the absolute number of CD8+CAR+ T cells per μL of blood.
Statistical analyses
Two-sample t test was used for the analysis of the continuous variables, and Fisher exact test was used for the categorical variables. Normality assumption was checked before analysis, and the majority of the variables follow normal distribution. Wilcoxon rank-sum test was used to confirm our findings. The proportion of different cell types identified by flow cytometric analysis was compared using linear regression models that accounted for patient age, sex and tumor size; the models used the log2-transformed proportion as the response variable and the t-distribution as the reference distribution. Spearman correlation was used to analyze correlation between cell types and clinical variables, and a multivariate regression model controlling for clinical variables was used to associate cell types with response. Kaplan-Meier analysis was used for survival outcomes, and log-rank test was used to evaluate statistical significance. Thresholds for separation of patient cohorts based on cell types were selected based on the optimal response separation between the groups and denoted as “high” or “low,” as was performed in previously published analyses.18 Thus, P values should be interpreted with caution. SAS 9.4 and R were used for all the data analyses.
Patient samples were procured from OSU Comprehensive Cancer Center Leukemia Tissue Bank after informed consent and approval by OSU institution review board were obtained.
Results
Patient characteristics and outcomes with commercial CD28 CAR19
Twenty-six sequential patients treated with CD28 CAR19 between February 2022 and March 2023 met inclusion criteria and had day-14 PBMC samples analyzed simultaneously via spectral flow cytometry. Sixteen patients achieved CR by the 6-month time point and were included in the CR cohort. Ten patients had progression of disease within the first 6 months after CAR-T infusion and were included in the PD cohort. Table 1 describes patient and disease characteristics and all discrete patient data analyzed are included in supplemental File 2.
Patient and disease characteristics
| Characteristics . | Total . | CR, n = 16 (%) . | PD, n = 10 (%) . | P value∗ . |
|---|---|---|---|---|
| Age (y) at infusion | 55 ± 11 | 55 ± 9 | 54 ± 14 | .81 |
| Gender | ||||
| Female | 9 | 5 (31.25) | 4 (40.00) | .69 |
| Male | 21 | 11 (68.75) | 10 (60.00) | |
| Race/ethnicity | NA | |||
| Caucasian | 26 | 16 (100.00) | 10 (100.00) | |
| ECOG PS at baseline, n (%) | ||||
| 0 | 13 | 9 (56.25) | 4 (40.00) | .40 |
| 1 | 12 | 7 (43.75) | 5 (50.00) | |
| 2 | 1 | 0 (0) | 1 (10.00) | |
| Disease type, n (%) | ||||
| DLBCL | 16 | 9 (56.25) | 7 (70.00) | .54 |
| HGBCL | 3 | 1 (6.25) | 2 (20.00) | |
| PMBCL | 2 | 1 (6.25) | 1 (10.00) | |
| Richter transformation | 1 | 1 (6.25) | 0 (0.00) | |
| MCL | 1 | 1 (6.25) | 0 (0.00) | |
| FL | 3 | 3 (18.75) | 0 (0.00) | |
| Infusion product, n (%) | ||||
| Yescarta | 25 | 15 (93.75) | 10 (100.00) | 1 |
| Tecartus | 1 | 1 (6.25) | 0 (0) | |
| Primary refractory, n (%) | ||||
| No | 15 | 11 (73.33) | 4 (40.00) | .12 |
| Yes | 10 | 4(26.67) | 6 (60.00) | |
| MYC+FISH, n (%) | ||||
| No | 19 | 13 (86.67) | 6 (60.00) | .18 |
| Yes | 6 | 2 (13.33) | 4 (40.00) | |
| Double-hit FISH, n (%) | ||||
| No | 20 | 14 (93.33) | 6 (60.00) | .07 |
| Yes | 5 | 1 (6.67) | 4 (40.00) | |
| IPI at infusion (median) | 2 | 2 | 2 | 1 |
| IPI at infusion of ≥3, n (%) | ||||
| No | 19 | 13 (81.25) | 6 (60.00) | .37 |
| Yes | 7 | 3 (18.75) | 4 (40.00) | |
| Ann Arbor stage, n (%) | ||||
| I/II | 8 | 7 (33.75) | 1 (10.00) | .20 |
| III/IV | 18 | 9 (56.25) | 9 (90.00) | |
| Second-line or third-line or more CAR-T therapy, n (%) | ||||
| Second-line | 3 | 1 (6.25) | 2 (20.00) | .54 |
| Third-line or more | 23 | 15 (93.75) | 8 (80.00) | |
| Bridging therapy chemotherapy, n (%) | ||||
| No | 13 | 8 (50.00) | 5 (50.00) | 1 |
| Yes | 13 | 8 (50.00) | 5 (50.00) | |
| Bridging therapy, n (%) | ||||
| RT only | 1 | 0 (0) | 1 (16.67) | .43 |
| Systemic only | 13 | 8 (100.00) | 5 (83.33) | |
| Dexamethasone prophylaxis, n (%) | ||||
| No | 12 | 7 (43.75) | 5 (50.00) | 1 |
| Yes | 14 | 9 (56.25) | 5 (50.00) | |
| Characteristics | ||||
| Largest tumor diameter | ||||
| Mean, cm (range) | 5.77 (1.0-20.0) | 4.13 (1.1-10.0) | 8.41 (1.0-20.0) | .05 |
| Bulky, >5 cm, n (%) | ||||
| Yes | 10 | 4 (25.00) | 6 (60.00) | .11 |
| No | 16 | 12 (75.00) | 4 (40.00) | |
| Bulky, >7 cm, n (%) | ||||
| Yes | 7 | 2 (12.50) | 5 (50.00) | .07 |
| No | 19 | 14 (87.5) | 5 (50.00) | |
| Bulky, >10 cm, n (%) | ||||
| Yes | 6 | 2 (12.5) | 4 (40.00) | .16 |
| No | 20 | 14 (87.5) | 6 (60.00) | |
| SUVmax(mean ± SD) | 18.65 ± 10.94 | 14.97 ± 8.34 | 23.90 ± 12.64 | .10 |
| Disease response on preinfusion imaging, n (%) | ||||
| PD | 13 | 9 (56.25) | 4 (40.00) | .83 |
| Partial response | 8 | 4 (25.00) | 4 (40.00) | |
| Stable disease | 1 | 1 (6.25) | 0 (0.00) | |
| Stable disease/mixed | 2 | 1 (6.25) | 1 (10.00) | |
| N/A | 2 | 1 (6.25) | 1 (10.00) | |
| Circulating disease prior, n (%) | ||||
| Yes | 3 | 2 (13.33) | 1 (11.11) | 1 |
| No | 21 | 13 (86.67) | 8 (88.89) | |
| Extranodal disease, number of sites, n (%) | ||||
| 0 | 11 | 8 (50.00) | 3 (30.00) | .64 |
| 1 | 11 | 6 (37.50) | 5 (50.00) | |
| 2 | 3 | 2 (12.50) | 1 (10.00) | |
| 3 | 1 | 0 (0.00) | 1 (10.00) | |
| LDH at cell infusion, (mean ± STD), U/L | 288 ± 487 | 189 ± 32 | 445 ± 78 | .20 |
| LDH >190 U/L (ULN), n (%) | ||||
| No | 11 | 7 (43.75) | 4 (40.00) | 1 |
| Yes | 15 | 9 (56.25) | 6 (60.00) | |
| Ferritin at cell infusion, (mean ± SD), ng/mL | 456 ± 394 | 314 ± 202 | 684 ± 519 | .02 |
| Ferritin of >WNL, n (%) | ||||
| No | 16 | 13 (81.25) | 3 (30.00) | .02 |
| Yes | 10 | 3 (18.75) | 7 (70.00) | |
| CRP at cell infusion, (mean ± SD), mg/L | 30 ± 44 | 19 ± 27 | 48 ± 60 | .10 |
| CRP of >WNL, n (%) | ||||
| Yes | 10 | 3 (18.75) | 7 (70.00) | .02 |
| No | 16 | 13 (81.25) | 3 (30.00) |
| Characteristics . | Total . | CR, n = 16 (%) . | PD, n = 10 (%) . | P value∗ . |
|---|---|---|---|---|
| Age (y) at infusion | 55 ± 11 | 55 ± 9 | 54 ± 14 | .81 |
| Gender | ||||
| Female | 9 | 5 (31.25) | 4 (40.00) | .69 |
| Male | 21 | 11 (68.75) | 10 (60.00) | |
| Race/ethnicity | NA | |||
| Caucasian | 26 | 16 (100.00) | 10 (100.00) | |
| ECOG PS at baseline, n (%) | ||||
| 0 | 13 | 9 (56.25) | 4 (40.00) | .40 |
| 1 | 12 | 7 (43.75) | 5 (50.00) | |
| 2 | 1 | 0 (0) | 1 (10.00) | |
| Disease type, n (%) | ||||
| DLBCL | 16 | 9 (56.25) | 7 (70.00) | .54 |
| HGBCL | 3 | 1 (6.25) | 2 (20.00) | |
| PMBCL | 2 | 1 (6.25) | 1 (10.00) | |
| Richter transformation | 1 | 1 (6.25) | 0 (0.00) | |
| MCL | 1 | 1 (6.25) | 0 (0.00) | |
| FL | 3 | 3 (18.75) | 0 (0.00) | |
| Infusion product, n (%) | ||||
| Yescarta | 25 | 15 (93.75) | 10 (100.00) | 1 |
| Tecartus | 1 | 1 (6.25) | 0 (0) | |
| Primary refractory, n (%) | ||||
| No | 15 | 11 (73.33) | 4 (40.00) | .12 |
| Yes | 10 | 4(26.67) | 6 (60.00) | |
| MYC+FISH, n (%) | ||||
| No | 19 | 13 (86.67) | 6 (60.00) | .18 |
| Yes | 6 | 2 (13.33) | 4 (40.00) | |
| Double-hit FISH, n (%) | ||||
| No | 20 | 14 (93.33) | 6 (60.00) | .07 |
| Yes | 5 | 1 (6.67) | 4 (40.00) | |
| IPI at infusion (median) | 2 | 2 | 2 | 1 |
| IPI at infusion of ≥3, n (%) | ||||
| No | 19 | 13 (81.25) | 6 (60.00) | .37 |
| Yes | 7 | 3 (18.75) | 4 (40.00) | |
| Ann Arbor stage, n (%) | ||||
| I/II | 8 | 7 (33.75) | 1 (10.00) | .20 |
| III/IV | 18 | 9 (56.25) | 9 (90.00) | |
| Second-line or third-line or more CAR-T therapy, n (%) | ||||
| Second-line | 3 | 1 (6.25) | 2 (20.00) | .54 |
| Third-line or more | 23 | 15 (93.75) | 8 (80.00) | |
| Bridging therapy chemotherapy, n (%) | ||||
| No | 13 | 8 (50.00) | 5 (50.00) | 1 |
| Yes | 13 | 8 (50.00) | 5 (50.00) | |
| Bridging therapy, n (%) | ||||
| RT only | 1 | 0 (0) | 1 (16.67) | .43 |
| Systemic only | 13 | 8 (100.00) | 5 (83.33) | |
| Dexamethasone prophylaxis, n (%) | ||||
| No | 12 | 7 (43.75) | 5 (50.00) | 1 |
| Yes | 14 | 9 (56.25) | 5 (50.00) | |
| Characteristics | ||||
| Largest tumor diameter | ||||
| Mean, cm (range) | 5.77 (1.0-20.0) | 4.13 (1.1-10.0) | 8.41 (1.0-20.0) | .05 |
| Bulky, >5 cm, n (%) | ||||
| Yes | 10 | 4 (25.00) | 6 (60.00) | .11 |
| No | 16 | 12 (75.00) | 4 (40.00) | |
| Bulky, >7 cm, n (%) | ||||
| Yes | 7 | 2 (12.50) | 5 (50.00) | .07 |
| No | 19 | 14 (87.5) | 5 (50.00) | |
| Bulky, >10 cm, n (%) | ||||
| Yes | 6 | 2 (12.5) | 4 (40.00) | .16 |
| No | 20 | 14 (87.5) | 6 (60.00) | |
| SUVmax(mean ± SD) | 18.65 ± 10.94 | 14.97 ± 8.34 | 23.90 ± 12.64 | .10 |
| Disease response on preinfusion imaging, n (%) | ||||
| PD | 13 | 9 (56.25) | 4 (40.00) | .83 |
| Partial response | 8 | 4 (25.00) | 4 (40.00) | |
| Stable disease | 1 | 1 (6.25) | 0 (0.00) | |
| Stable disease/mixed | 2 | 1 (6.25) | 1 (10.00) | |
| N/A | 2 | 1 (6.25) | 1 (10.00) | |
| Circulating disease prior, n (%) | ||||
| Yes | 3 | 2 (13.33) | 1 (11.11) | 1 |
| No | 21 | 13 (86.67) | 8 (88.89) | |
| Extranodal disease, number of sites, n (%) | ||||
| 0 | 11 | 8 (50.00) | 3 (30.00) | .64 |
| 1 | 11 | 6 (37.50) | 5 (50.00) | |
| 2 | 3 | 2 (12.50) | 1 (10.00) | |
| 3 | 1 | 0 (0.00) | 1 (10.00) | |
| LDH at cell infusion, (mean ± STD), U/L | 288 ± 487 | 189 ± 32 | 445 ± 78 | .20 |
| LDH >190 U/L (ULN), n (%) | ||||
| No | 11 | 7 (43.75) | 4 (40.00) | 1 |
| Yes | 15 | 9 (56.25) | 6 (60.00) | |
| Ferritin at cell infusion, (mean ± SD), ng/mL | 456 ± 394 | 314 ± 202 | 684 ± 519 | .02 |
| Ferritin of >WNL, n (%) | ||||
| No | 16 | 13 (81.25) | 3 (30.00) | .02 |
| Yes | 10 | 3 (18.75) | 7 (70.00) | |
| CRP at cell infusion, (mean ± SD), mg/L | 30 ± 44 | 19 ± 27 | 48 ± 60 | .10 |
| CRP of >WNL, n (%) | ||||
| Yes | 10 | 3 (18.75) | 7 (70.00) | .02 |
| No | 16 | 13 (81.25) | 3 (30.00) |
DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; FL, follicular lymphoma; HGBCL, high grade B-cell lymphoma; IPI, International Prognostic Index; MCL, mantle cell lymphoma; N/A, not applicable; RT, radiation therapy; PMBCL, primary mediastinal B-cell lymphoma; SD, standard deviation; SUV, standard update value; ULN, upper limit of normal (based on institutional parameters); WLN, within normal limits.
P value ≤.05 considered as significant.
Patient and disease characteristics were, for the most part, well balanced between the 2 cohorts and were similar to those treated in real-world studies of axi-cel.9,11 Baseline ferritin and CRP were significantly increased in the PD cohort compared with the CR cohort when analyzed as categorical variables. There were no other significant differences in clinical characteristics between the CR and PD cohorts. Toxicity, including cytokine release syndrome and ICANS, was not significantly different between the 2 cohorts (supplemental Table 2). Progression-free survival (PFS) and overall survival for the entire 26 patient cohort (supplemental Figure 1) were similar to real-world studies with axi-cel.9,11 Overall, our patient cohort was found to be comparable with the majority of real-world patients treated with CD28 CAR19.
Differentially abundant postinfusion CD8+ CAR+ T-cell clusters in CR and PD cohorts
We developed a 37-color CD8+ CAR-T–specific spectral flow cytometry panel to identify features of postinfusion CD8+ CAR+ T cells that may correlate with clinical outcomes. We targeted day-14 samples for the analysis to ensure all patients had reached maximum expansion, because it has been shown that the range of peak expansion for CD28 CAR19 products is 5 to 14 days.17,30
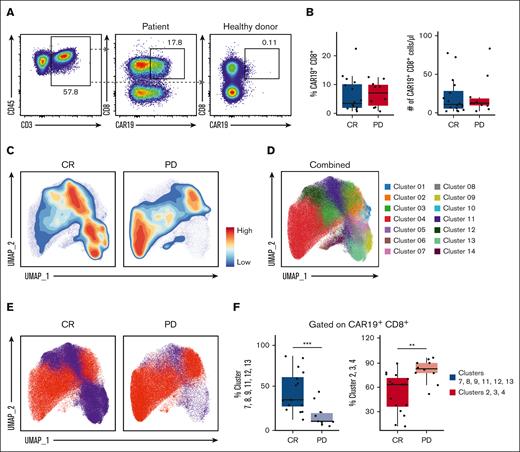
We first looked at the quantity of CD8+ CAR+ T cells (gating strategy shown in supplemental Figure 2). Similar to previous reports,18 we observed no differences in the quantity of circulating CAR+ T cells between CR and PD cohorts. This was measured as the absolute number of CD8+ CAR+ T cells (Figure 1A) and as the percent of CD3+ (supplemental Figure 3) and CD8+ CAR+ T cells in circulation (Figure 1B). However, visualization of cells through contour plots revealed areas of the UMAP in which CD8+ CAR+ T-cell events were more prevalent in the CR vs PD cohort (Figure 1C). Single-cell clustering analysis resulted in 14 clusters, which were mapped back to the CD8+ CAR+ T-cell UMAP space (Figure 1D). Clusters 7, 8, 9, 11, 12, and 13 were found to be more abundant in the CR cohort, whereas clusters 2, 3, and 4 were more abundant in the PD cohort (Figure 1E). When combined and analyzed via linear regression model, the abundance of clusters 7, 8, 9, 11, 12, and 13 was significantly higher in the CR than the PD cohort, whereas the abundance of clusters 2, 3, and 4 was significantly lower in the CR than the PD cohort (Figure 1F). These results suggest that significant qualitative differences are present within CD8+ CAR+ T cells between CR and PD cohorts.
Differentially abundant CD8+ CAR+ T-cell clusters in CR and PD cohorts. PBMCs from the day-14 post-CAR-T time point were analyzed via spectral flow cytometry. (A) Representative 2-dimensional flow cytometry plot showing gating strategy for CAR19+ CD8+ CAR-Ts. Healthy donor samples were used as negative controls. Percentages of CD3+ T cells as well as CAR19+ CD8+ T cells are indicated. (B) Box plot of CAR19+ CD8+ cells as a percent of live CD45+ CD3+ lymphocytes in CR vs PD cohorts (left); absolute number of CAR19+ CD8+ lymphocytes in CR vs PD cohorts (right). (C) Contour UMAP plots of CAR19+ CD8+ T cells in CR vs PD cohorts. (D) UMAP dot plot of CAR19+ CD8+ T cells from the total cohort (n = 26). (E) UMAP of CAR19+ CD8+ T cells in CR vs PD cohorts with clusters increased in CR colored blue (clusters 7, 8, 9, 11, 12, and 13) and clusters increased in PD colored red (clusters 2, 3, and 4). (F) Box plots showing combined clusters percentages of CAR19+ CD8+ cells. Combined clusters 7, 8, 9, 11, 12, and 13 in CR vs PD (left). Combined clusters 2, 3, and 4 in CR vs PD (right). Box plots in panels B and F show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis; ∗P < .05, ∗∗P < .01; ∗∗∗P < .001.
Differentially abundant CD8+ CAR+ T-cell clusters in CR and PD cohorts. PBMCs from the day-14 post-CAR-T time point were analyzed via spectral flow cytometry. (A) Representative 2-dimensional flow cytometry plot showing gating strategy for CAR19+ CD8+ CAR-Ts. Healthy donor samples were used as negative controls. Percentages of CD3+ T cells as well as CAR19+ CD8+ T cells are indicated. (B) Box plot of CAR19+ CD8+ cells as a percent of live CD45+ CD3+ lymphocytes in CR vs PD cohorts (left); absolute number of CAR19+ CD8+ lymphocytes in CR vs PD cohorts (right). (C) Contour UMAP plots of CAR19+ CD8+ T cells in CR vs PD cohorts. (D) UMAP dot plot of CAR19+ CD8+ T cells from the total cohort (n = 26). (E) UMAP of CAR19+ CD8+ T cells in CR vs PD cohorts with clusters increased in CR colored blue (clusters 7, 8, 9, 11, 12, and 13) and clusters increased in PD colored red (clusters 2, 3, and 4). (F) Box plots showing combined clusters percentages of CAR19+ CD8+ cells. Combined clusters 7, 8, 9, 11, 12, and 13 in CR vs PD (left). Combined clusters 2, 3, and 4 in CR vs PD (right). Box plots in panels B and F show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis; ∗P < .05, ∗∗P < .01; ∗∗∗P < .001.
PD-1+ CD8+ CAR+ T-cell clusters are increased in the CR cohort
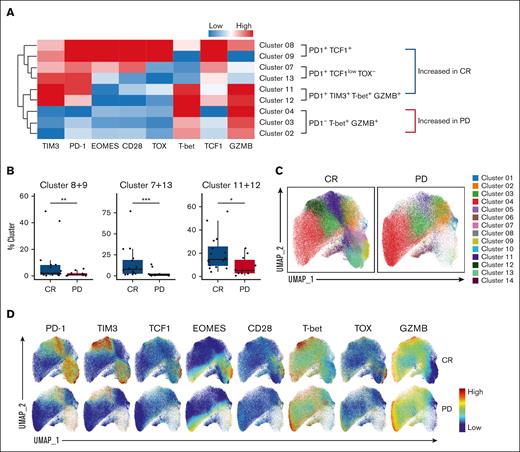
Next, we examined key markers of these differentially abundant clusters in CR vs PD cohorts. Characteristics of each cluster and individual marker expression were first evaluated by a clustered heat map (Figure 2A). We first noted that all cell clusters that were increased in the CR group were PD-1+, whereas clusters that were increased in the PD group were PD-1−. Clusters 8 and 9, increased in the CR cohort, were PD-1+ TCF1+ T-box expressed in T cells (T-bet)−, which is consistent with the phenotype of stem-like T cells.22-26 These clusters also had high expression of other stem-like T-cell markers such as CD28 and Eomesodermin (EOMES).22-26 Clusters 11 and 12, increased in the CR group, were PD-1+ TIM3+, and expressed high levels of effector T-cell markers including Granzyme B (GZMB) and T-bet. Overall, these PD-1+ TIM3+ T-bet+ GZMB+ cells had an expression pattern similar to previously described CD8+ effector-like transitory T cells.22-26,31 Clusters 7 and 13, also increased in the CR cohort, were PD-1+, TCF1low, and Thymocyte selection-associated high mobility group box protein (TOX)− with a diverse expression of EOMES and CD28 and appeared to be in transition from stem-like to effector-like transitory cells. On the other hand, clusters 2, 3 and 4, increased in the PD group, were T-bet+ GZMB+, but PD-1-. This is in contrast with the PD-1 positivity present on T-bet+ and GZMB+ cells found in clusters 11 and 12 which were increased in the CR cohort. Statistical analysis revealed that the CR cohort had a significantly increased population of each PD-1+ CD8+ CAR-T subgroup (Figure 2B): PD-1+ TCF1+ (clusters 8 and 9), PD-1+ TCF1low TOX− (clusters 7 and 13) and PD-1+ TIM3+ T-bet+ GZMB+ (clusters 11 and 12). The PD-1− clusters 2, 3, and 4, as shown in Figure 1, were noted to be significantly increased in the PD cohort. Comparison of UMAP dot plots depicting cellular dynamics and marker expression plots overlaid in UMAP space were used to confirm these findings (Figure 2C-D). Expression of all markers on all clusters is shown in supplemental Figure 4A. Collectively, our data showed that at day 14 after CAR-T infusion, PD-1+ CAR-T groups including PD-1+TCF1+ stem-like T cells and PD-1+ TIM3+ effector-like T cells were increased in patients who achieved CR by 6 months.
PD-1+ CD8+ CAR+ T-cell clusters are increased in the CR cohort. (A) Clustered heat map showing key marker expressions on differentially abundant CAR+ CD8+ clusters. Clusters 8 and 9: PD-1+ TCF1+. Clusters 7 and 13: PD-1+ TCF1low TOX−. Clusters 11 and 12: PD-1+ TIM3+ T-bet+ GZMB+. Clusters 2, 3, and 4: PD-1− T-bet+ GZMB+. Color scale was determined by median normalization of each individual marker with blue representing low expression, white representing median expression, and red representing high expression. (B) Box plots of clusters in CR vs PD cohorts. Cluster abundance was reported as a percentage of CAR+ CD8+ T cells. (C) UMAP of CAR+CD8+ T cells in CR vs PD cohorts, colored by cluster. (D) Expression plots of phenotypical and functional markers present on CAR+ CD8+ T cells. Expression of markers on individual cells were overlaid onto the UMAP space in CR (top) vs PD (bottom) cohorts. Box plots show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis; ∗P < .05, ∗∗P < .01; ∗∗∗P < .001.
PD-1+ CD8+ CAR+ T-cell clusters are increased in the CR cohort. (A) Clustered heat map showing key marker expressions on differentially abundant CAR+ CD8+ clusters. Clusters 8 and 9: PD-1+ TCF1+. Clusters 7 and 13: PD-1+ TCF1low TOX−. Clusters 11 and 12: PD-1+ TIM3+ T-bet+ GZMB+. Clusters 2, 3, and 4: PD-1− T-bet+ GZMB+. Color scale was determined by median normalization of each individual marker with blue representing low expression, white representing median expression, and red representing high expression. (B) Box plots of clusters in CR vs PD cohorts. Cluster abundance was reported as a percentage of CAR+ CD8+ T cells. (C) UMAP of CAR+CD8+ T cells in CR vs PD cohorts, colored by cluster. (D) Expression plots of phenotypical and functional markers present on CAR+ CD8+ T cells. Expression of markers on individual cells were overlaid onto the UMAP space in CR (top) vs PD (bottom) cohorts. Box plots show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis; ∗P < .05, ∗∗P < .01; ∗∗∗P < .001.
Identification of key CD8+ CAR+ T-cell types that correlate with improved clinical outcomes
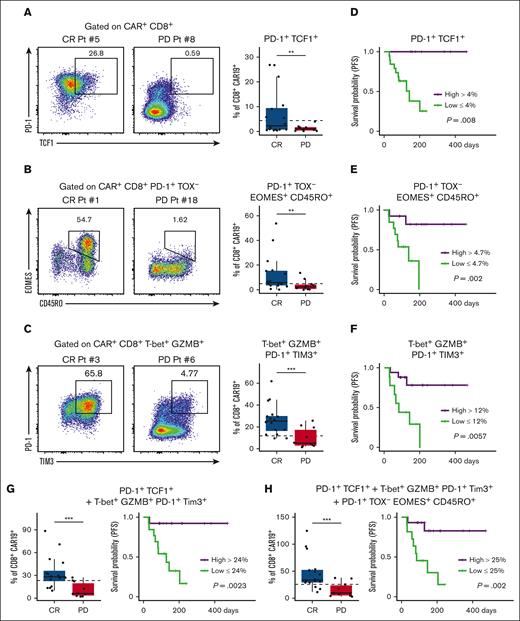
To further delineate the importance of PD-1+ CD8+ CAR-T clusters in patients who achieved CR, we performed a separate analysis of spectral flow data via 2D plotting and correlated this with clinical outcomes (Figure 3; supplemental Figure 5). Similar to dimensional reduction, 2D plot analysis again clearly identified a PD-1+ TCF1+ population of CD8+ CAR-Ts that was more prevalent in patients who achieved a CR at 6 months (Figure 3A). A population of PD-1+ TOX− EOMES+ CD45RO+ cells was also found to be increased in the CR cohort (Figure 3B). Additionally, the population of PD-1+ TIM3+ T-bet+ GZMB+ Toxlow effector-like CD8+ CAR-Ts was also found to be more abundant in patients with CR (Figure 3C). In the PD cohort, a PD-1− T-bet+ GZMB+ CD45RA+ population of cells was noted to be increased (supplemental Figure 6). This population of cells appeared to share characteristics of T-cell effector memory CD45RA re-expressors,32,33 although PD-1 was notably absent in the majority of T-cell effector memory CD45RA re-expressors in the PD group.
Key postinfusion PD-1+ CAR+ CD8+ T-cell populations correlate with clinical outcomes. 2D flow plot analysis was performed on spectral flow cytometry data from day-14 post-CAR-T samples. (A) Representative 2D flow cytometry plots showing individual patient PD-1 and TCF1 expression in CAR+ CD8+ T cells and corresponding box plot quantification in CR vs PD cohorts. (B) Representative 2D flow cytometry plots showing individual patient EOMES and CD45RO expression in CAR+ CD8+ PD-1+ TOX− T cells and corresponding box plot quantification in CR vs PD cohorts. (C) Representative 2D flow cytometry plots showing individual patient PD-1 and TIM3 expression in CAR+ CD8+ T-bet+ GZMB+ T cells and corresponding box plot quantification in CR vs PD cohorts. (D, E, F) Kaplan-Meier (KM) analysis was used to generate PFS curves stratified by high vs low percent of CD8+ CAR-T populations. (D) KM analysis of PFS for patients with high (>4%) or low (<4%) percent of this cell type; high group, n = 6; low group, n = 20. (E) KM analysis of PFS for patients with high (>4.7%) or low (<4.7%) percent of this cell type; high group, n = 13; low group, n = 13. (F) KM analysis of PFS for patients with high (>12%) or low (<12%) percent of this cell type; high group, n = 17; low group, n = 9. (G) Box plot of the combination of PD-1+ TCF1+ cells and PD-1+ TIM3+ T-bet+ GZMB+ cells in CR vs PD (left). KM analysis of PFS for patients with high (>24%) or low (<24%) percent of this combination of cell types (right); high group, n = 13; low group, n = 13. (H) Box plot of the combination of PD-1+ TCF1+ cells, PD-1+ TOX− EOMES+ CD45RO+ cells, and PD-1+ TIM3+ T-bet+ GZMB+ cells in CR vs PD (left). KM analysis of PFS for patients with high (>25%) or low (<25%) percent of this combination of cell types (right); high group, n = 15; low group, n = 11. For all KM curves, the x-axis was time in days from date of CAR-T infusion. Dotted lines on box plots indicate separation lines between high and low percentages of CAR-Ts in each population and were selected based on optimal response separation between cohorts. Because clinical outcomes were known during patient stratification, P values need to be interpreted with caution. Box plots in panels A, B, and C show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis (cell type % changes) and log-rank tests (PFS); ∗P < .05, ∗∗P < .01; ∗∗∗P < .001. Pt, patient.
Key postinfusion PD-1+ CAR+ CD8+ T-cell populations correlate with clinical outcomes. 2D flow plot analysis was performed on spectral flow cytometry data from day-14 post-CAR-T samples. (A) Representative 2D flow cytometry plots showing individual patient PD-1 and TCF1 expression in CAR+ CD8+ T cells and corresponding box plot quantification in CR vs PD cohorts. (B) Representative 2D flow cytometry plots showing individual patient EOMES and CD45RO expression in CAR+ CD8+ PD-1+ TOX− T cells and corresponding box plot quantification in CR vs PD cohorts. (C) Representative 2D flow cytometry plots showing individual patient PD-1 and TIM3 expression in CAR+ CD8+ T-bet+ GZMB+ T cells and corresponding box plot quantification in CR vs PD cohorts. (D, E, F) Kaplan-Meier (KM) analysis was used to generate PFS curves stratified by high vs low percent of CD8+ CAR-T populations. (D) KM analysis of PFS for patients with high (>4%) or low (<4%) percent of this cell type; high group, n = 6; low group, n = 20. (E) KM analysis of PFS for patients with high (>4.7%) or low (<4.7%) percent of this cell type; high group, n = 13; low group, n = 13. (F) KM analysis of PFS for patients with high (>12%) or low (<12%) percent of this cell type; high group, n = 17; low group, n = 9. (G) Box plot of the combination of PD-1+ TCF1+ cells and PD-1+ TIM3+ T-bet+ GZMB+ cells in CR vs PD (left). KM analysis of PFS for patients with high (>24%) or low (<24%) percent of this combination of cell types (right); high group, n = 13; low group, n = 13. (H) Box plot of the combination of PD-1+ TCF1+ cells, PD-1+ TOX− EOMES+ CD45RO+ cells, and PD-1+ TIM3+ T-bet+ GZMB+ cells in CR vs PD (left). KM analysis of PFS for patients with high (>25%) or low (<25%) percent of this combination of cell types (right); high group, n = 15; low group, n = 11. For all KM curves, the x-axis was time in days from date of CAR-T infusion. Dotted lines on box plots indicate separation lines between high and low percentages of CAR-Ts in each population and were selected based on optimal response separation between cohorts. Because clinical outcomes were known during patient stratification, P values need to be interpreted with caution. Box plots in panels A, B, and C show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis (cell type % changes) and log-rank tests (PFS); ∗P < .05, ∗∗P < .01; ∗∗∗P < .001. Pt, patient.
Next, we divided patients into cohorts based on high or low percentage of each PD-1+ CD8+ CAR-T population and assessed PFS using Kaplan-Meier analysis. Three populations were analyzed: PD-1+ TCF1+, PD-1+ TOX− EOMES+ CD45RO+, and PD-1+ TIM3+ T-bet+ GZMB+ CD8+ CAR-T. We found that patients who had higher percentages of each individual population alone had significantly improved PFS (Figure 3D-F). We also found that the combined abundance of groupings of these cell types was significantly higher in the CR cohort (Figure 3G-H), and higher levels of these combinations of cell types correlated with increased PFS, as assessed by Kaplan-Meier analysis (Figure 3G-H). To investigate whether tumor burden or baseline inflammation correlated with the presence of the PD-1+, PD-1+ TCF1+, or PD-1+ TIM3+ T-bet+ GZMB+ CD8+ CAR-Ts, we performed Spearman correlation and multivariate regression analysis. We found no direct correlation between cell types and tumor diameter and baseline LDH. In the multivariate model (controlling for LDH, CRP, ferritin, and largest tumor diameter) we found that PD-1+ CD8+ CAR-Ts (although not other cell types) correlated with both patient response and tumor diameter but not baseline LDH, ferritin, or CRP. When controlling for tumor burden, a higher quantity PD-1+ CD8+ CAR-T cells correlated with increased chance for CR. Taken together, these results provide a basis for quantifying easily identifiable postinfusion CD8+ CAR-T types by 2D flow and establish associations with these cell types to improved PFS for patients treated with CD28 CAR19 for NHL.
PD-1 expression on postinfusion CD8+ CAR-Ts correlates with improved clinical outcomes
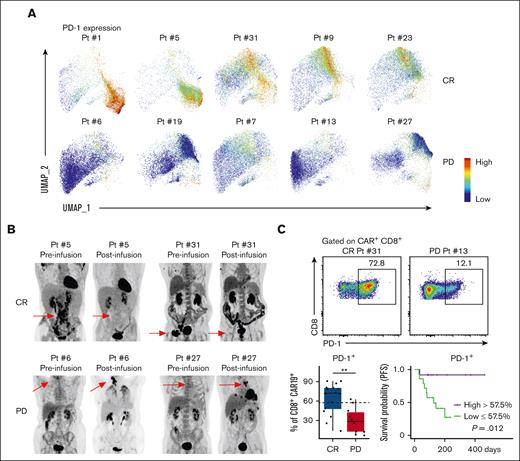
Given PD-1 expression was present on all key clusters and cell types that correlated with improved clinical outcomes, we analyzed whether PD-1 expression alone correlated with better clinical outcomes. Individual patient PD-1 expression plots revealed increased expression of PD-1 on CD8+ CAR+ T cells of patients in the CR cohort (Figure 4A). Preinfusion and postinfusion 18F-fluorodeoxyglucose–positron emission tomography scans (Figure 4B) revealed complete responses achieved by patients with the presence of high PD-1 expression (patient number 5 and patient number 31). In comparison, positron emission tomography scans revealed progressive disease in patients with low PD-1 expression (patient number 6 and patient number 27). Statistical analysis of PD-1 expression on CD8+ CAR+ T cells revealed that PD-1+ CD8+ CAR+ T cells were significantly increased in CR vs PD (Figure 4C). Additionally, patients with a higher percentage of PD-1+ CD8+ CAR-Ts had increased PFS, as assessed by Kaplan-Meier analysis (Figure 4C). Analysis of PD-1 expression as a continuous variable revealed that for each 1% increase in PD-1+ expression on CAR+ CD8+ cells at day 14, the odds of achieving a CR increased by 5%. A 5% increase in PD-1+ CD8+ CAR-Ts resulted in a 28% increase in the odds of CR, and a 20% increase resulted in a 260% increase in the odds of achieving a CR. Overall, these analyses reveal that increased PD-1 expression on postinfusion CD8+ CAR-Ts at day 14 correlates with improvement in clinical outcomes.
PD-1 expression on postinfusion CD8+ CAR-Ts correlates with improved clinical outcomes. (A) Individual patient PD-1 expression on CAR+ CD8+ T cells was plotted in the UMAP space; CR (top) vs PD (bottom). (B) Individual patient pre- and post-CAR-T infusion 18F-fluorodeoxyglucose–positron emission tomography (PET) scans. Preinfusion PETs were performed within 30 days of CAR-T infusion; red arrows point to site of preinfusion lymphoma lesions. Postinfusion PETs were performed 30 to 60 days after CAR-T infusion; red arrows point to areas of resolution or progression of lymphoma. (C) Representative 2D flow cytometry plot showing PD-1 expression in CR vs PD cohorts (top). Box plot showing percentages of PD-1+ CAR+ CD8+ T cells in CR vs PD cohorts (bottom left). KM analysis of PFS for patients with high (>57.5%) or low (<57.5%) percent of PD-1+ CD8+ CAR-Ts (bottom right); high group, n = 12; low group, n = 14. x-axis is time in days from date of CAR-T infusion. Dotted lines on box plots indicate separation lines between high and low percentages of CAR-Ts and were selected based on optimal response separation between cohorts. Because clinical outcomes were known during patient stratification, P values should be interpreted with caution. Box plots show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis (cell type % changes) and log-rank test (PFS); ∗P < .05, ∗∗P < .01; ∗∗∗P < .001. Pt, Patient.
PD-1 expression on postinfusion CD8+ CAR-Ts correlates with improved clinical outcomes. (A) Individual patient PD-1 expression on CAR+ CD8+ T cells was plotted in the UMAP space; CR (top) vs PD (bottom). (B) Individual patient pre- and post-CAR-T infusion 18F-fluorodeoxyglucose–positron emission tomography (PET) scans. Preinfusion PETs were performed within 30 days of CAR-T infusion; red arrows point to site of preinfusion lymphoma lesions. Postinfusion PETs were performed 30 to 60 days after CAR-T infusion; red arrows point to areas of resolution or progression of lymphoma. (C) Representative 2D flow cytometry plot showing PD-1 expression in CR vs PD cohorts (top). Box plot showing percentages of PD-1+ CAR+ CD8+ T cells in CR vs PD cohorts (bottom left). KM analysis of PFS for patients with high (>57.5%) or low (<57.5%) percent of PD-1+ CD8+ CAR-Ts (bottom right); high group, n = 12; low group, n = 14. x-axis is time in days from date of CAR-T infusion. Dotted lines on box plots indicate separation lines between high and low percentages of CAR-Ts and were selected based on optimal response separation between cohorts. Because clinical outcomes were known during patient stratification, P values should be interpreted with caution. Box plots show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis (cell type % changes) and log-rank test (PFS); ∗P < .05, ∗∗P < .01; ∗∗∗P < .001. Pt, Patient.
Increased PD-1+ CD8+ CAR+ effector-like cells correlate with severe ICANS in the CR cohort
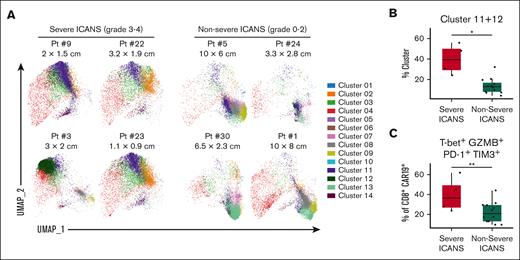
We also evaluated toxicity to investigate for factors associated with severe (grade ≥3) ICANS. The rate of any grade ICANS for the total cohort was 50%, and the rate of severe ICANS was 23% (supplemental Table 2), similar to that of real-world studies.9,11 We then analyzed for features present on CD8+ CAR-Ts that may have correlated with severe ICANS. Six patients had severe ICANS whereas 20 patients had no or nonsevere ICANS (grade 0-2). Of 6 patients with severe ICANS, 4 had CR and 2 had PD. Using individual patient UMAPs, we found that clusters 11 and 12 were enriched in patients who achieved CR who had severe ICANS compared with those without severe ICANS (Figure 5A). These clusters were not enriched in the 2 PD patients with severe ICANS (supplemental Figure 7). We thus continued our analysis with a focus on patient with CR, and ICANS. Patient and disease characteristics in patient with CR were analyzed and no clinical characteristics were found to correlate with the presence of severe ICANS (supplemental Table 3). Further analysis of baseline and peak (within 30 days after CAR-Ts) inflammatory markers (LDH, ferritin, and CRP) as well as expansion of CAR-Ts at the time of sample collection revealed no correlation with severe ICANS (supplemental Figure 8). However, linear regression analysis confirmed that clusters 11 and 12, which were previously characterized as PD-1+TIM3+T-bet+GZMB+ effector-like CD8+ CAR-Ts (Figure 2A), were significantly increased in patients with severe ICANS (Figure 5B). Clusters 7 and 13 were significantly increased in patients with no/non-severe ICANS whereas other clusters did not correlate with ICANS (supplemental Figure 9). Subsequently, 2D flow analysis confirmed that PD-1+TIM3+T-bet+GZMB+ effector-like CD8+ CAR-Ts were increased in the severe ICANS cohort (Figure 5C). With this analysis, we identified a CD8+ effector-like CAR-T population present at the day 14 time point that correlated with severe ICANS in patients who achieved a CR.
Higher quantities of PD1+ TIM3+ effector-like CD8+ CAR-Ts correlate with severe ICANS in CR. Patients who achieved CR (n = 16) were separated into a severe ICANS (grade 3-4) cohort (n = 4) and a no/nonsevere ICANS (grade 0-2) cohort (n = 12). (A) Day-14 postinfusion PBMCs were analyzed by spectral flow cytometry and dimensional reduction. Individual patient UMAPs of CAR+ CD8+ T-cell clusters in ICANS (left) vs no/nonsevere ICANS cohorts (right) are shown, colored by cluster. Largest cross sectional tumor diameter before CAR-T infusion is listed underneath patient identifying numbers. Box plots in panels B and C compare characteristics in severe ICANS vs no/nonsevere ICANS cohorts. (B) Combined clusters 11 and 12, reported as a percent of CAR+ CD8+ T cells. (C) PD-1+ TIM3+ T-bet+ GZMB+ cells quantified by 2D flow analysis, reported as a percentage of CAR+ CD8+ cells. Box plots show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis; ∗P < .05, ∗∗P < .01; ∗∗∗P < .001. Pt, Patient.
Higher quantities of PD1+ TIM3+ effector-like CD8+ CAR-Ts correlate with severe ICANS in CR. Patients who achieved CR (n = 16) were separated into a severe ICANS (grade 3-4) cohort (n = 4) and a no/nonsevere ICANS (grade 0-2) cohort (n = 12). (A) Day-14 postinfusion PBMCs were analyzed by spectral flow cytometry and dimensional reduction. Individual patient UMAPs of CAR+ CD8+ T-cell clusters in ICANS (left) vs no/nonsevere ICANS cohorts (right) are shown, colored by cluster. Largest cross sectional tumor diameter before CAR-T infusion is listed underneath patient identifying numbers. Box plots in panels B and C compare characteristics in severe ICANS vs no/nonsevere ICANS cohorts. (B) Combined clusters 11 and 12, reported as a percent of CAR+ CD8+ T cells. (C) PD-1+ TIM3+ T-bet+ GZMB+ cells quantified by 2D flow analysis, reported as a percentage of CAR+ CD8+ cells. Box plots show quartiles with bands at the median; whiskers indicate 1.5 interquartile range; all observations overlaid as dots. P values are from linear regression analysis; ∗P < .05, ∗∗P < .01; ∗∗∗P < .001. Pt, Patient.
Discussion
In this study, we found that postinfusion PD-1+CD8+CAR-Ts were critical for patients to achieve CR by 6 months with CD28 CAR19 for NHL. Deeper analysis identified subtypes of PD-1+CD8+ CAR-Ts that correlated with improved clinical outcomes, including PD-1+TCF1+ stem-like CAR-Ts and PD-1+ TIM3+ effector-like CAR-Ts. Furthermore, we identified a subset of CD8+ CAR+ T cells with effector-like function that was increased in the CR cohort in patients who had severe ICANS.
PD-1+TCF1+ stem-like T cells are characterized by high proliferative capacity and the capacity for self-renewal. In models of chronic infection and cancer, they are responsible for providing a pool of functional CD8+ T cells that mediate disease control, and, with immune checkpoint inhibitor therapy, the presence and frequency of stem-like T cells correlates with improved clinical outcomes.22-26 To our knowledge, to date, the presence of PD-1+TCF1+ stem-like CD8+ CAR-T cells and their correlation to CAR-T response has not been described. Here, we clearly show that a higher frequency of stem-like CD8+CAR-Ts correlated with better clinical outcomes with CD28 CAR19 for NHL. We also identified a PD-1+TIM3+GZMB+ T-bet+ Toxlow population of effector-like CD8+ CAR-Ts, which correlated with improved outcomes. These effector-like CD8+ cells, as progeny of stem-like T cells, have been found to be highly proliferative and also mediate disease control.34-36
Our study also highlights the importance of PD-1 expression on CD8+ CAR+ T cells after infusion. PD-1 is often described as a marker of T-cell exhaustion; however, this is only when combined with expression of other coinhibitory receptors (eg, TIGIT, CTLA4, and TIM3). PD-1 first and foremost is a marker of T-cell activation.37 When antigen-specific T cells encounter antigen and are adequately activated, PD-1 is upregulated.38-40 Thus, one might expect PD-1 upregulation to be ubiquitous on CAR-Ts after infusion, given the abundant expression of CD19 on B cells in circulation and lymphoma cells. However, we, along with others looking at day +7, have shown negative, low, and high PD-1 expression on CAR-Ts after infusion.17,18 Poor PD-1 upregulation by day 14 may denote suboptimal activation caused by intrinsic T-cell dysfunction or other influences (ie, systemic or tumor-related immunosuppression). Here, we found that neither tumor burden nor LDH correlated with less PD-1+ expression, and when controlling for LDH or tumor burden, PD-1+ expression on CD8+ CAR+ T cells still associated with improved response. This suggests that the lack of PD1+ upregulation may not be affected by systemic inflammation or tumor burden but may be a T-cell–intrinsic phenomenon directly related to a patient’s apheresis product or final CAR-T infusion product.
Furthermore, we found that higher PD-1 expression on postinfusion CD8+ CAR-Ts at day 14 correlated with improved longer-term outcomes. Prior studies have described the role of PD-1 in protecting T-cell longevity, proliferation, and optimal memory formation in stem-like T-cell models of cancer, chronic infection, and immune checkpoint inhibitor therapy.22-26 A PD-1 knockout on CD8+ T cells can lead to increased expansion but also more rapid development of T-cell dysfunction and exhaustion, and loss of memory formation.41 Therefore, it may be that low levels of PD-1 after infusion result in a reduced ability for CAR-Ts to maintain and mediate ongoing response; further studies are ongoing to investigate this.
Previous studies have shown that increased tumor burden, expansion, and peak inflammation, as well as lower levels CAR+ Tregs after infusion correlate with more severe ICANS.1,18,20,42 Here, we found that a subset of postinfusion CD8+CAR+ T cells with the effector-like phenotype (PD-1+TIM3+GZMB+T-bet+) was increased in patients in the CR cohort who had severe ICANS, suggesting that these effector-like CD8+ CAR-Ts may potentially play a role in the development of severe ICANS. Of note, this specific population of effector-like T cells was not prevalent in patients with PD, likely because the majority of effector-like cells in patients with PD lacked both PD-1 and TIM3. Further analysis with larger numbers of patients with PD with severe ICANS and further studies investigating the interplay between CAR+ Tregs, CAR-T expansion, inflammation, and effector-like CD8+ CAR-Ts are required.
Some limitations of our study include its correlative, retrospective, and descriptive nature. With regard to the Kaplan-Meier curve analysis for PFS, given there is no known biologically relevant value for the newly described cell types, we had no predetermined way to specify cutoffs for each value. Thus, cutoffs were determined post hoc, and P values and PFS based on these values should be interpreted with caution. We also did not report on CD4+ CAR-T analyses. Although there were some differences noted, our panel was focused on CD8+ CAR-T phenotype and function, and further studies with a focus on CD4+ CAR-Ts are under way.
With this study, we highlight the importance of PD-1 positivity in CD8+ CAR-Ts after infusion, describe specific populations of postinfusion CAR-Ts (including stem-like and effector-like CD8+ CAR-Ts), and investigate how CD8+ CAR-T phenotype and function may play a role in outcomes to CAR-T therapy. These results allow us to identify robust biomarkers of clinical response and toxicity to CD28 CAR19 and help further elucidate patterns of CAR-T population dynamics in the post-CAR-T infusion setting.
Acknowledgments
This work was funded in part by a Conquer Cancer Endowed Young Investigator Award (N.D.). N.D. was also in part supported by National Cancer Institute (NCI) training grant (T32 CA090223). This study was supported by grants from the NCI (R01 CA193167 [Y.Y.]; R01 CA260858 [Y.Y. and X.H.]; R01 CA262069 [Z.L.]; and P01 CA278732 [Z.L.]), and a grant from National Heart, Lung, and Blood Institute (R01 HL151195 [Y.Y. and X.H.]);. This work was also supported by the The Ohio State University-Leukemia Tissue Bank Shared Resource (NCI P30CA016058).
Authorship
Contribution: N.D. conceptualized the project, designed, and performed the experiments, collected and analyzed the data, and wrote the manuscript; N.-J.S. performed experiments, collected and analyzed the data, and edited the manuscript; X.Z., H.J., J.M., and D.C. performed statistical analyses; C.P, Y.W., K.R., R.M.B, C.S., D.W., W.K.C., W.H., L.A., and M.P.R. assisted in performing research experiments, and edited the manuscript; E.B., N.E., T.J.V., A.S.K., D.A.B., Y.S., A.S., J.C.R., W.H., B.C., R.A.B., K.M., L.A., S.V., M.d.L., J.B., and S.J. assisted with participant recruitment, and edited the manuscript; Z.L. participated in discussion and interpretation of data, and edited the manuscript; X.H. participated in designing various parts of the study, and in discussion and interpretation of the results, and edited the manuscript; Y.Y. assisted with designing the experiments, analyzed the data, and wrote the manuscript; and Z.L., X.H. and Y.Y. supervised the work.
Conflict-of-interest disclosures: T.J.V receives research funding from MorphoSys, Incyte, Genmab, AbbVie, Recordati, Viracta Therapeutics, and AstraZeneca; and has consulted for Novartis and Recordati. A.S.K. receives research funding from AstraZeneca and BeiGene; and consults for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), Kite, a Gilead Company, Janssen, and Loxo@Lilly. D.A.B. receives research funding from Novartis, Nurix Therapeutics, Kite, a Gilead Company, and Incyte; and has consulted for Novartis, Nurix Therapeutics, ADC Therapeutics, and Kite, a Gilead Company. Y.S. has received research funding from BMS, Celgene, TG Therapeutics, BeiGene, AbbVie, and Genmab. J.C.R receives research funding from Merck, Corvus Pharmaceuticals, and Kymera Therapeutics; and consults for Acrotech Biopharma, and Kyowa Kirin. W.H. receives research funding from Incyte. B.C. received research funding from Genentech, Acerta, Millenium, and BMS. S.J. received research funding from Kite, a Gilead Company and BMS; and serves on advisory boards for Kite, a Gilead Company, BMS, Caribou Biosciences, and CRISPR Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Yiping Yang, Division of Hematology, The Ohio State University, 508 Biomedical Research Tower, 460 West 12th Ave, Columbus, OH 43210; email: yiping.yang2@osumc.edu; Xiaopei Huang, Division of Hematology, The Ohio State University, 512 Biomedical Research Tower, 460 West 12th Ave, Columbus, OH 43210; email: xiaopei.huang@osumc.edu; and Zihai Li, Pelotonia Institute for Immuno-Oncology, The Ohio State University Comprehensive Cancer Center, 580 Biomedical Research Tower, 460 West 12th Ave, Columbus, OH 43210; email: zihai.li@osumc.edu.
References
Author notes
N.D. and N.-J.S. contributed equally to this study.
For original data please contact the author, Nathan Denlinger (Nathan.denlinger@osumc.edu).
The full-text version of this article contains a data supplement.