Key Points
A concerning, progressive neurologic syndrome has emerged in patients with FA, with characteristic neuroradiological findings.
This neurodegenerative syndrome is distinct from malignancy and must be recognized by clinicians to avoid harmful, misguided treatment.
Visual Abstract
Fanconi anemia (FA) is a complex inherited bone marrow failure syndrome characterized by chromosomal instability and defective DNA repair, causing sensitivity to DNA interstrand crosslinking agents. Our understanding of the full adult phenotype of the disease continues to evolve, because most patients with FA died of marrow failure in the first decade of life before more recent advances in allogeneic hematopoietic cell transplantation. Herein, we report a previously undescribed, clinically concerning, progressive neurologic syndrome in patients with FA. Nine nonimmunosuppressed pediatric patients and young adults with FA presented with acute and chronic neurological signs and symptoms associated with distinct neuroradiological findings. Symptoms included, but were not limited to, limb weakness, papilledema, gait abnormalities, headaches, dysphagia, visual changes, and seizures. Brain imaging demonstrated a characteristic radiographic appearance of numerous cerebral and cerebellar lesions with associated calcifications and often a dominant ring-enhancing lesion. Tissue from the dominant brain lesions in 4 patients showed nonspecific atypical glial proliferation, and a small number of polyomavirus-infected microglial cells were identified by immunohistochemistry in 2 patients. Numerous interventions were pursued across this cohort, in general with no improvement. Overall, these patients demonstrated significant progressive neurologic decline. This cohort highlights the importance of recognizing FA neuroinflammatory syndrome, which is distinct from malignancy, and warrants careful ongoing evaluation by clinicians.
Introduction
Fanconi anemia (FA) is a genetic DNA repair disorder causing sensitivity to DNA crosslinking agents, associated with progressive bone marrow failure, small stature, congenital abnormalities, and predisposition to malignancy.1-7 Significant improvements in allogeneic hematopoietic cell transplantation (HCT) allow for most children with FA and marrow failure to survive.8,9 New manifestations of the FA phenotype are now becoming evident because a larger population of patients reach adulthood.2,10
Structural neurologic abnormalities have been previously reported in FA, including a small pituitary gland, Chiari I malformations, ectopic neurohypophysis, adenohypophysis hypoplasia, and platybasia.11-14 However, because these are typically present at birth and often not pathologic, neurologic imaging is not part of routine screening recommendations. Computed tomography is generally avoided due to increased sensitivity to radiation in patients with FA, and cranial imaging is typically only pursued in the setting of an acute, symptomatic presentation.
Our brain magnetic resonance imaging (MRI) in multiple patients with FA with new-onset neurologic symptoms have demonstrated previously undescribed neurologic lesions of uncertain etiology. The radiographic appearances are similar, with variability in clinical presentation depending on location of the lesions, and unlike any previously described neurologic lesions in FA. Our experience of this neurologic phenotype, FA neuroinflammatory syndrome (FANS), is described herein.
Methods
Patient population
Patients signed written informed consent to participate in the study, approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board. Demographics, clinical course, pathology results, and imaging were provided by the patients and families and/or abstracted from medical records.
Results
Clinical presentation
Nine patients with FA presented with new-onset neurologic changes, and demographics are outlined in Table 1. Of the 9 patients, 4 presented urgently to the emergency room, whereas the remaining 5 had a more indolent course, with either incidental findings or intermittent symptoms discovered at annual follow-up. The median age at presentation was 17 years (range, 11-24). Of the 9 patients, 8 had previously received an allogeneic HCT at a median of 9 years (range, 3-17) before symptom onset. Notably, 1 patient never received a transplant.
Patient demographics and clinical findings
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 27 | 24 | 20 | 21 | 29 | 20 | 18 | 27 | 17 |
| Age at FANS presentation, y | 20 | 19 | 13 | 11 | 22 | 17 | 17 | 24 | 16 |
| Sex | Female | Male | Male | Female | Female | Female | Male | Male | Male |
| FA complementation group | FANCL | FANCJ | FANCF | FANCD2 | FANCC | Unknown | Unknown | FANCD3 | Unknown |
| HCT, Y/N | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Age at HCT, y | 11 | N/A | 5 | 8 | 9 | 4 | 9 | 7 | 7 |
| Transplant type | MUD | N/A | MUD | MSD | MSCBT | MSD | MUCBT | MUD | MUD |
| Preparative regimen | Flu/Cy/ATG | N/A | Flu/Cy/ATG/TBI | Flu/Cy/ATG | Cy/TBI | Bu/Flu/ATG | Flu/Cy/ATG/TBI | Flu/Cy/ATG/TBI | Bu/Flu/Cy/ATG |
| GVHD prophylaxis | T-cell depletion; CsA, MP | N/A | T-cell depletion; CsA, MP | CsA | None | T-cell depletion; OKT3 | CsA, MP | T-cell depletion; CsA | T-cell depletion; CsA |
| Post-HCT complications | Skin GVHD, CKD, cholestasis, AVN | N/A | Ocular GVHD, hepatic dysfunction, and hypothyroidism | Hepatic dysfunction and hypothyroidism | Hepatic dysfunction, hypothyroidism, and feeding difficulties | Ocular GVHD and skin GVHD | Hepatic dysfunction, hypothyroidism, and hypertension | Skin GVHD and gastrointestinal GVHD | CKD, cholestasis, and feeding difficulties |
| Onset of FANS after HCT, y | 9 | N/A | 8 | 3 | 13 | 13 | 8 | 17 | 9 |
| Neurologic symptoms at presentation | Transient right sided numbness and slurred speech | Transient hemiplegia | Visual defect and slurred speech | Right hand weakness | Vertigo | Headache and neck pain | Headaches, blurry vision, and fatigue | Spastic paraparesis and progressive weakness | Papilledema (incidental finding) |
| Evolving neurologic symptoms | Unilateral hemianopsia, lower extremity hypertonia/hyperreflexia, and numbness episodes (arm, hip, and tongue) | Unilateral hemianopsia, hemiparesis, seizures, and intermittent explosive disorder | Cranial nerve palsies, paresthesias, seizures, ataxia, and depression | Intermittent hemiparesis, paraparesis, and left upper quadrantanopia | Vertigo, tics, tongue fasciculations, and myoclonic jerks | Upper and lower extremity paresthesias, leg pain, and dizziness | Seizures, ataxia, and less verbal | Headaches, dysphagia, dysarthria, incontinenc, altered mental status, facial droop, and hypophonia | Seizure |
| Brain MRI findings | Dominant ring-enhancing lesion with edema; multiple small, enhancing cerebral, and cerebellar lesions with associated calcifications | Dominant ring-enhancing lesion with edema; multiple small, enhancing cerebral, and cerebellar lesions with associated calcifications | Dominant ring-enhancing lesion with vasogenic edema; multiple small, enhancing cerebral, cerebellar, and brain stem lesions with calcifications | Dominant ring-enhancing lesion with edema; multiple small, enhancing cerebral and cerebellar lesions with associated calcifications | 2 frontal ring-enhancing lesions, with scattered multifocal enhancing foci throughout the cerebellum and cerebrum | Dominant ring-enhancing lesion; multiple small, enhancing cerebral, brain stem, and cerebellar lesions | Frontal lobe lesion with vasogenic edema, mass effect; scattered cerebral, cerebellar foci | Dominant enhancing frontal lobe lesion with vasogenic edema, mass effect; 2 brain stem lesions, 1 additional enhancing cerebral lesion | Dominant ring-enhancing lesion (bifrontal) with edema; multiple small, enhancing cerebral, and cerebellar lesions |
| Spinal MRI Findings | No | No | Focal lesion in the thoracic region | Three foci of abnormal enhancement in the spinal cord (thoracic) | No | Scattered enhancing foci within the cord, cervical, and lumbar | No | Enhancing foci in the cord at the cervical and thoracic regions | No |
| Brain tissue examined, Y/N | Y | Y | Y | N | N | N | Y | N | N |
| Duration of neurologic involvement, y | 7 | 5 | 7 | 10 | 10 | 4 | 2 | 2 | 1 |
| Cause of death | Renal failure | N/A | N/A | N/A | N/A | N/A | CNS nocardiosis | N/A | N/A |
| . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | Patient 7 . | Patient 8 . | Patient 9 . |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 27 | 24 | 20 | 21 | 29 | 20 | 18 | 27 | 17 |
| Age at FANS presentation, y | 20 | 19 | 13 | 11 | 22 | 17 | 17 | 24 | 16 |
| Sex | Female | Male | Male | Female | Female | Female | Male | Male | Male |
| FA complementation group | FANCL | FANCJ | FANCF | FANCD2 | FANCC | Unknown | Unknown | FANCD3 | Unknown |
| HCT, Y/N | Y | N | Y | Y | Y | Y | Y | Y | Y |
| Age at HCT, y | 11 | N/A | 5 | 8 | 9 | 4 | 9 | 7 | 7 |
| Transplant type | MUD | N/A | MUD | MSD | MSCBT | MSD | MUCBT | MUD | MUD |
| Preparative regimen | Flu/Cy/ATG | N/A | Flu/Cy/ATG/TBI | Flu/Cy/ATG | Cy/TBI | Bu/Flu/ATG | Flu/Cy/ATG/TBI | Flu/Cy/ATG/TBI | Bu/Flu/Cy/ATG |
| GVHD prophylaxis | T-cell depletion; CsA, MP | N/A | T-cell depletion; CsA, MP | CsA | None | T-cell depletion; OKT3 | CsA, MP | T-cell depletion; CsA | T-cell depletion; CsA |
| Post-HCT complications | Skin GVHD, CKD, cholestasis, AVN | N/A | Ocular GVHD, hepatic dysfunction, and hypothyroidism | Hepatic dysfunction and hypothyroidism | Hepatic dysfunction, hypothyroidism, and feeding difficulties | Ocular GVHD and skin GVHD | Hepatic dysfunction, hypothyroidism, and hypertension | Skin GVHD and gastrointestinal GVHD | CKD, cholestasis, and feeding difficulties |
| Onset of FANS after HCT, y | 9 | N/A | 8 | 3 | 13 | 13 | 8 | 17 | 9 |
| Neurologic symptoms at presentation | Transient right sided numbness and slurred speech | Transient hemiplegia | Visual defect and slurred speech | Right hand weakness | Vertigo | Headache and neck pain | Headaches, blurry vision, and fatigue | Spastic paraparesis and progressive weakness | Papilledema (incidental finding) |
| Evolving neurologic symptoms | Unilateral hemianopsia, lower extremity hypertonia/hyperreflexia, and numbness episodes (arm, hip, and tongue) | Unilateral hemianopsia, hemiparesis, seizures, and intermittent explosive disorder | Cranial nerve palsies, paresthesias, seizures, ataxia, and depression | Intermittent hemiparesis, paraparesis, and left upper quadrantanopia | Vertigo, tics, tongue fasciculations, and myoclonic jerks | Upper and lower extremity paresthesias, leg pain, and dizziness | Seizures, ataxia, and less verbal | Headaches, dysphagia, dysarthria, incontinenc, altered mental status, facial droop, and hypophonia | Seizure |
| Brain MRI findings | Dominant ring-enhancing lesion with edema; multiple small, enhancing cerebral, and cerebellar lesions with associated calcifications | Dominant ring-enhancing lesion with edema; multiple small, enhancing cerebral, and cerebellar lesions with associated calcifications | Dominant ring-enhancing lesion with vasogenic edema; multiple small, enhancing cerebral, cerebellar, and brain stem lesions with calcifications | Dominant ring-enhancing lesion with edema; multiple small, enhancing cerebral and cerebellar lesions with associated calcifications | 2 frontal ring-enhancing lesions, with scattered multifocal enhancing foci throughout the cerebellum and cerebrum | Dominant ring-enhancing lesion; multiple small, enhancing cerebral, brain stem, and cerebellar lesions | Frontal lobe lesion with vasogenic edema, mass effect; scattered cerebral, cerebellar foci | Dominant enhancing frontal lobe lesion with vasogenic edema, mass effect; 2 brain stem lesions, 1 additional enhancing cerebral lesion | Dominant ring-enhancing lesion (bifrontal) with edema; multiple small, enhancing cerebral, and cerebellar lesions |
| Spinal MRI Findings | No | No | Focal lesion in the thoracic region | Three foci of abnormal enhancement in the spinal cord (thoracic) | No | Scattered enhancing foci within the cord, cervical, and lumbar | No | Enhancing foci in the cord at the cervical and thoracic regions | No |
| Brain tissue examined, Y/N | Y | Y | Y | N | N | N | Y | N | N |
| Duration of neurologic involvement, y | 7 | 5 | 7 | 10 | 10 | 4 | 2 | 2 | 1 |
| Cause of death | Renal failure | N/A | N/A | N/A | N/A | N/A | CNS nocardiosis | N/A | N/A |
ATG, antithymocyte globulin; AVN, avascular necrosis; Bu, busulfan; CKD, chronic kidney disease; CsA, cyclosporine; Cy, cyclophosphamide; Flu, fludarabine; MP, methylprednisolone; MSCBT, matched sibling cord blood transplant; MSD, matched sibling donor; MUCBT, matched unrelated cord blood transplant; MUD, matched unrelated donor; N/A, not applicable; OKT3, muromonab-CD3; Y/N, yes/no.
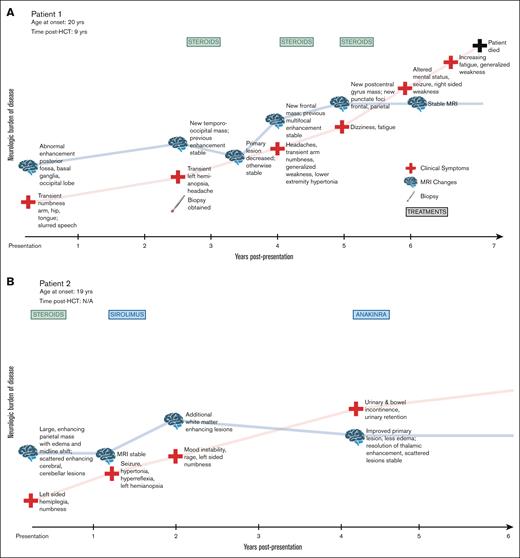
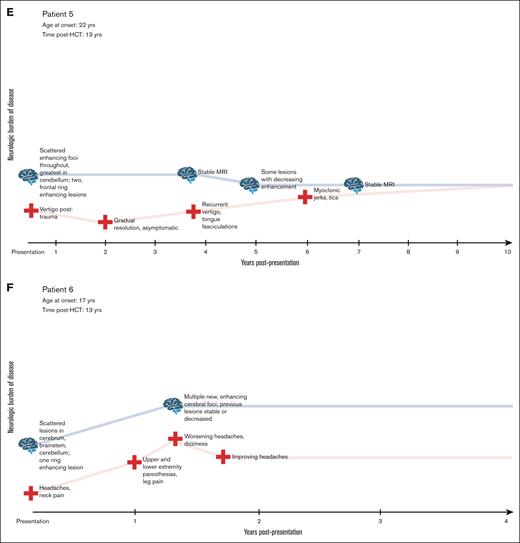
Signs and symptoms at presentation included visual abnormalities, headache, paresthesias, stroke-like symptoms, weakness, and fatigue. As the process evolved in patients, we noted seizures, ataxia, pain, spasticity, and worsening neurologic function. Clinical course for each patient is shown in Figure 1. At the time of reporting, the median follow-up for this cohort is 5 years (range, 1-10). All 9 patients showed progressive neurologic decline. Two patients died in the setting of worsening neurologic symptoms, after 10 years and 2 years of decline, respectively.
Clinical timecourses of patients with FANS, showing progressive neurologic decline. (A-I) Visual timeline of the clinical course of each patient is depicted, including evolution of symptoms, neurologic burden of disease, MRI findings, and treatments administered.
Clinical timecourses of patients with FANS, showing progressive neurologic decline. (A-I) Visual timeline of the clinical course of each patient is depicted, including evolution of symptoms, neurologic burden of disease, MRI findings, and treatments administered.
Imaging
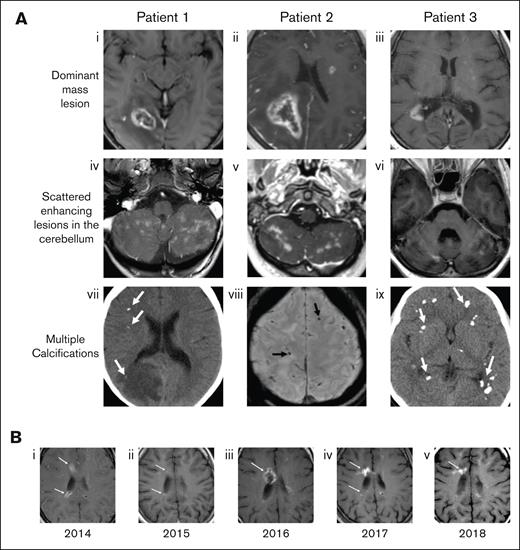
MRI was performed in all patients at the time of presentation. Defining features of the radiographic appearance are shown in Figure 2 and include a dominant, ring-enhancing lesion together with small, scattered enhancing foci, and calcifications throughout the cerebellum. The parietal lobe was the most frequent location of the dominant lesion. Significant vasogenic edema with accompanying midline shift as well as areas of diffusion restriction were common. Four patients also had enhancing lesions in the spinal cord. Repeat imaging throughout the clinical course of each patient demonstrated the waxing and waning nature of the lesions, with increases and decreases in enhancement over time. Patients demonstrated declining neurological function, both motor and cognitive, over a period of years.
Characteristic radiographic findings of FANS and their evolution over time. (A) Representative MRI findings shown for 3 patients with FA in our cohort. (Ai-iii) Axial postcontrast T1-weighted MRIs show focal ring-enhancing mass lesions. (Aiv-vi) Contrast-enhanced images through the cerebellum show multiple enhancing lesions. (Avii,ix) Noncontrast computed tomography and (Aviii) susceptibility weighted MR images demonstrate multiple calcifications in each case (arrows). (B) Axial T1-weighted MR images from patient 3 over a 5-year span, demonstrating the waxing and waning of lesions. (Bi) In 2014, enhancing lesions are seen on the anterior and posterior margin of the right lateral ventricle (arrows), both of which markedly regress a year later (Bii, arrows). (Biii) In 2016, the anterior lesion recrudesces (arrow), then decreases again over the next 2 years, with some waxing and waning of the posterior lesion (Biv-v, arrows).
Characteristic radiographic findings of FANS and their evolution over time. (A) Representative MRI findings shown for 3 patients with FA in our cohort. (Ai-iii) Axial postcontrast T1-weighted MRIs show focal ring-enhancing mass lesions. (Aiv-vi) Contrast-enhanced images through the cerebellum show multiple enhancing lesions. (Avii,ix) Noncontrast computed tomography and (Aviii) susceptibility weighted MR images demonstrate multiple calcifications in each case (arrows). (B) Axial T1-weighted MR images from patient 3 over a 5-year span, demonstrating the waxing and waning of lesions. (Bi) In 2014, enhancing lesions are seen on the anterior and posterior margin of the right lateral ventricle (arrows), both of which markedly regress a year later (Bii, arrows). (Biii) In 2016, the anterior lesion recrudesces (arrow), then decreases again over the next 2 years, with some waxing and waning of the posterior lesion (Biv-v, arrows).
Histology and molecular analyses
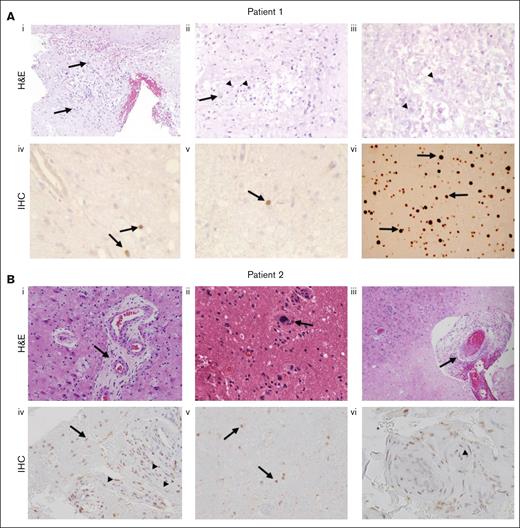
Three patients underwent biopsy of their dominant brain lesion, and a fourth patient’s lesion was assessed on autopsy. Pathology for 2 biopsied patients (patient 1 and patient 2) with available tissue is shown in Figure 3. In patient 1 (Figure 3Ai-iii), we observed a nonspecific necrotic-inflammatory process with reactive glial proliferation and macrophages, necrotic zones (some with specks of mineralization), and vasculature with plump reactive endothelium and focal hyalinization but no neoplastic changes. Staining for tumor markers, bacteria, acid-fast bacteria, fungi, and Toxoplasma, as well as in situ hybridization for herpes virus, adenovirus, cytomegalovirus, and Epstein-Barr virus were negative. In patient 2 (Figure 3Bi-iii), biopsy of the dominant parietal lesion showed necrosis, axonal spheroids, gliosis, abundant macrophages, and thick-walled hyalinized microvessels, some with luminal occlusion. Staining for tumor markers, bacteria, acid-fast bacteria, fungi, and Toxoplasma, as well as in situ hybridization for herpes virus, adenovirus, cytomegalovirus, and Epstein-Barr virus were negative. Biopsy tissue from patient 3 was unavailable for images (biopsy not shown), but pathology review of the dominant lesion similarly revealed a macrophage-rich inflammatory lesion with areas of necrosis. Staining for tumor markers, bacteria, acid-fast bacteria, and fungi was negative. Postmortem examination of the brain of a fourth patient (patient 7, not shown) demonstrated a chronic, multifocal, necrotizing encephalopathy predominantly of the white matter, along with an acute bacterial infection causing purulent abscesses that varied in age from acute to 2 weeks. Microscopic examination of the areas of chronic leukoencephalopathy shared features with the biopsies of patients 1 to 3 (above), with coagulative necrosis, reactive glial proliferation with macrophage infiltration, and axonal spheroids.
Brain biopsies from 2 patients with FANS. (A) H&E and immunohistochemical IHC stains of the brain biopsy of patient 1; (Ai) demonstrates a nonspecific necrotic-inflammatory reactive process with reactive astrocytes (arrows) and macrophages (arrowheads), 100×; (Aii) 200×; (Aiii) necrosis with minute flecks of mineralization (arrowheads). (Aiv-v) IHC staining of the brain biopsy of patient 1 with an antibody against SV40 T antigen that cross-reacts with JCV T antigen, demonstrating positive staining of scattered parenchymal cells (arrows). (vi) IHC staining of a positive control brain biopsy from a patient with progressive multifocal leukoencephalopathy (PML), showing more widespread staining of cells (arrows). (B) H&E and IHC stains of the brain biopsy of patient 2; (Bi-ii) abundant reactive appearing endothelium within many of the vessels, and necrosis, axonal spheroids, gliosis, abundant macrophages, and thick-walled hyalinized microvessels (arrows); (Biii) luminal occlusion (arrows); (Biv-v) IHC staining of the brain biopsy of patient 2 with an antibody against SV40 T antigen, demonstrating positive staining of scattered cells in the parenchyma (arrows) and the walls of blood vessels (arrowheads); and (Bvi) endothelial cells (arrowheads), more widespread than seen in patient 1. H&E, hematoxylin and eosin; IHC, immunohistochemical. JCV, JC virus. PML, progressive multifocal leukoencephalopathy. SV40, polyoma simian virus 40.
Brain biopsies from 2 patients with FANS. (A) H&E and immunohistochemical IHC stains of the brain biopsy of patient 1; (Ai) demonstrates a nonspecific necrotic-inflammatory reactive process with reactive astrocytes (arrows) and macrophages (arrowheads), 100×; (Aii) 200×; (Aiii) necrosis with minute flecks of mineralization (arrowheads). (Aiv-v) IHC staining of the brain biopsy of patient 1 with an antibody against SV40 T antigen that cross-reacts with JCV T antigen, demonstrating positive staining of scattered parenchymal cells (arrows). (vi) IHC staining of a positive control brain biopsy from a patient with progressive multifocal leukoencephalopathy (PML), showing more widespread staining of cells (arrows). (B) H&E and IHC stains of the brain biopsy of patient 2; (Bi-ii) abundant reactive appearing endothelium within many of the vessels, and necrosis, axonal spheroids, gliosis, abundant macrophages, and thick-walled hyalinized microvessels (arrows); (Biii) luminal occlusion (arrows); (Biv-v) IHC staining of the brain biopsy of patient 2 with an antibody against SV40 T antigen, demonstrating positive staining of scattered cells in the parenchyma (arrows) and the walls of blood vessels (arrowheads); and (Bvi) endothelial cells (arrowheads), more widespread than seen in patient 1. H&E, hematoxylin and eosin; IHC, immunohistochemical. JCV, JC virus. PML, progressive multifocal leukoencephalopathy. SV40, polyoma simian virus 40.
Immunohistochemical stains for the polyoma simian virus 40 (SV40) large T antigen, an antibody commonly used to identify polyoma virus, on tissues from patients 1 and 2 revealed scattered nuclei of microglial-appearing cells staining positively, more frequent in the brain biopsy of patient 2 than patient 1. In addition, nuclei of mesenchymal-appearing cells in the enlarged wall of microvessels of patient 2 also stained positive (Figure 3). In situ hybridization for BK virus RNA was negative. T-cell subsets in our patients revealed normal CD4 and CD8 T-cell counts, and patients were HIV seronegative.
Management
Due to the novelty of this clinical presentation in FA and the absence of clinical experience, numerous interventions were tried in this cohort aimed at improving neurologic symptoms or slowing decline. Steroids were the most common intervention, given to 7 patients to reduce edema around dominant lesions. Although edema was reduced, the overall course of the illness did not seem changed. Patients also received IV immunoglobulin, cyclosporin, rituximab, sirolimus, mycophenolate, and plasmapheresis at various times throughout their illness, with no striking response to any therapy.
Discussion
Herein, we detail a previously undescribed chronic neuroinflammatory condition in pediatric patients and young adults with FANS. Patients presented with both focal acute and chronic neurological deficits, including motor impairment, sensory disruption, and visual field abnormalities. Across our cohort of 9 patients, the clinical course was as long as 10 years at the time of reporting, with progressively worsening neurologic symptoms accompanied by waxing and waning enhancement of radiological abnormalities and appearance of additional lesions over time. Importantly, overall neurological function declined over time, significantly reducing the quality of life.
In several cases, MRI at presentation had features consistent with an aggressive brain tumor. The dominant lesion was frequently characterized by ring-enhancement, areas of diffusion restriction, and significant vasogenic edema, in some cases with midline shift. A diagnosis of malignant glioma was considered in multiple patients upon initial review of their imaging. Awareness of this neuroinflammatory condition in patients with FA among hematologists is important to avoid misguided cancer-directed treatment.
We noted polyomavirus-infected microglial-appearing cells in 2 tissue biopsies and polyomavirus-infected mesenchymal-appearing cells in 1 of those.
Our patients are not immunosuppressed but have brain cells with increased susceptibility to viral infection and/or proliferation due to FA. Increased in vitro susceptibility of FA pathway–deficient cells to polyomavirus infection has been described previously.15-17 Moreover, evidence of increased quiescent polyoma infection has been reported in persons with FA, and polyoma positivity has been reported in tumors found in patients with FA.18,19 This situation is distinct from progressive multifocal leukoencephalopathy (PML), which occurs in the setting of immunosuppression, and is characterized by lytic infection of glial cell by JC virus (JCV), causing demyelinating lesions, and typically progresses rapidly.20,21 It is uncertain whether JCV is the virus responsible for SV40 staining in the tissue biopsies of 2 of our patients, and if so, whether JCV represents a trigger for this encephalitic process or an unrelated, concurrent colonization in a vulnerable population. However, given the susceptibility to JCV in patients with FA, we feel this warrants careful ongoing consideration.
Our therapeutic approach was primarily geared toward symptomatic management, with high-dose steroids commonly used in the acute setting to alleviate intracranial swelling and vasogenic edema. Although this often improved the appearance of edema on MRI, the benefit was temporary and at times independent of persistent clinical symptoms. In the absence of clinical experience or a clear etiology for this entity, some patients received immunomodulatory treatments directed at potential autoimmune or proinflammatory processes that were thought to be driving the decline. None of these agents led to striking clinical benefit, and in general, the process of gradual neurological decline continued regardless of these interventions.
Evaluating other potential etiologies of this process, the role of HCT as a predisposing or exacerbating factor must be considered. Although 1 of the 9 patients never received a bone marrow transplant, half of the patients who underwent transplantation received total body irradiation as part of their preparative regimen. Similarly, calcineurin inhibitors and cytotoxic agents were ubiquitous. Despite this, post-HCT neurologic complications including posterior reversible encephalopathy syndrome, thrombotic microangiopathy, cerebrovascular disease, metabolic encephalopathy, or demyelinating disease were not observed in any of our patients. Likewise, we considered central nervous system (CNS) involvement of graft-versus-host disease (GVHD; CNS-GVHD). Heterogenous in presentation and often nonspecific, CNS-GVHD can include stroke-like symptoms, lacunar syndromes, encephalopathy, and declining neurologic function.22 However, only 1 patient had active (ocular) GVHD at the time of presentation with neurologic symptoms, and biopsies did not demonstrate findings consistent with the diagnosis. We did note that tissue from patients in our cohort showed abnormal vasculature with plump reactive endothelium, focal hyalinization, and coagulative necrosis, suggesting vascular involvement. Aspects of the phenotype we describe overlap with a class of interferonopathies that cause leukodystrophies, namely retinal vasculopathy and cerebral leukodystrophy and Aicardi-Goutières syndrome.23-25 These processes have a wide spectrum of clinical evolution and encompass severe, small vessel vasculopathies, manifesting as visual field abnormalities along with hemiparesis, weakness, hemianopsia, and aphasia.24,25 Multiple genes have been associated with these conditions, most notably TREX1 (3' repair exonuclease 1), which functions to remove excess single- and double-stranded DNA to prevent overactivation of the immune system. When mutated, an autoimmune, neuroinflammatory vasculopathy results, not unlike the clinical, radiographic, and pathologic features we observed in our patients with FA. There is, similarly, overlap in the clinical presentation of the patients in our cohort with some other DNA repair disorders that exhibit neurodegeneration, including ataxia telangiectasia and xeroderma pigmentosa. The similarities in phenotype, coupled with an underlying mechanism rooted in defective DNA replication/repair as it relates to neurodegeneration,26,27 might lead to further comparisons toward a better understanding of FA phenotypes described here.
The emergence of numerous cases of this condition raised concern that perhaps there were undiagnosed FANS cases in other patients with FA that we have cared for. Systematic review was performed on all available brain imaging from patients with FA at Cincinnati Children’s Hospital Medical Center and the University of Minnesota (data not shown). Comprehensive review was performed for 34 patients, including evaluation of 132 brain MRI and 90 head computed tomography scans. This cohort of patients had a median age of 11 years (range, 1-31), with a wide range of reasons for pursuing imaging, including but not limited to infectious concerns, tumor screening, vascular concerns, ventriculoperitoneal (VP) shunt evaluation, and posterior reversible encephalopathy syndrome. This internal review did not reveal any asymptomatic patients with abnormal imaging nor incidental findings consistent with FANS. This suggests that routine screening for FANS with intracranial imaging, in the absence of symptoms, is unlikely to be beneficial.
In this report, we highlight a previously undescribed aspect of the FA phenotype, FANS. The neurologic symptoms present in pediatric patients and young adults and appear to be independent of bone marrow transplant, because 1 patient in our cohort did not receive transplant. Radiographic appearances can be mistaken for aggressive CNS malignancy, so recognition of this distinct, insidious process is paramount to avoid unnecessary exposure to detrimental chemotherapy and/or radiation treatment in this vulnerable patient population. As our clinical awareness around this neurodegenerative process in FA improves, we hope to gain a greater understanding of its natural course and importantly, effective therapy.
Acknowledgments
The authors are indebted to the children and families who participated in this study for their invaluable contribution to this research. The authors are grateful for the regulatory assistance of Erica Miller.
Visual abstract created with BioRender.com.
Authorship
Contribution: A.L.B. and S.M.D. designed the research; A.L.B., B.J., A.S., C.F., and S.M.D. performed the research; A.L.B., J.E.W., A.S., and S.M.D. examined the patients; B.J. reviewed radiological studies; C.F. performed the pathology review; A.L.B., J.E.W., B.J., S.W., A.S., C.F., and S.M.D. analyzed the data; and all authors contributed to writing the manuscript and reviewed and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Allison L. Bartlett, Bone Marrow Transplantation and Immune Deficiency, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, MLC 11027, Cincinnati, OH 45229; email: allison.bartlett@cchmc.org.
References
Author notes
Study data will be shared in the form of scientific publication. This publication will be immediately available to the research community. Original data are available on request from the corresponding author, Allison L. Bartlett (allison.bartlett@cchmc.org).