Key Points
In this R/R population with high unmet need, patients responding to tabelecleucel demonstrated a clinically meaningful survival benefit.
Tabelecleucel has a favorable safety profile and shows durable clinical benefit in R/R EBV+ PTLD after HCT or SOT.
Visual Abstract
Patients with Epstein-Barr virus (EBV)–positive posttransplant lymphoproliferative disease (EBV+ PTLD) in whom initial treatment fails have few options and historically low median overall survival (OS) of 0.7 months after allogeneic hematopoietic cell transplant (HCT) and 4.1 months after solid organ transplant (SOT). Tabelecleucel is an off-the-shelf, allogeneic EBV-specific cytotoxic T-lymphocyte immunotherapy for EBV+ PTLD. Previous single-center experience showed responses in patients with EBV+ PTLD after HCT or SOT. We now report outcomes from a multicenter expanded access protocol in HCT (n = 14) and SOT (n = 12) recipients treated with tabelecleucel for EBV+ PTLD that was relapsed/refractory (R/R) to rituximab with/without chemotherapy. The investigator-assessed objective response rate was 65.4% overall (including 38.5% with a complete and 26.9% with a partial response), 50.0% in HCT, and 83.3% in SOT. The estimated 1- and 2-year OS rates were both 70.0% (95% confidence interval [CI], 46.5-84.7) overall, both 61.5% (95% CI, 30.8-81.8) in HCT, and both 81.5% (95% CI, 43.5-95.1) in SOT (median follow-up: 8.2, 2.8, and 22.5 months, respectively). Patients responding to tabelecleucel had higher 1- and 2-year OS rates (94.1%) than nonresponders (0%). Treatment was well tolerated, with no reports of tumor flare, cytokine release syndrome, or rejection of marrow and SOT. Results demonstrate clinically meaningful outcomes across a broad population treated with tabelecleucel, indicating a potentially transformative and accessible treatment advance for R/R EBV+ PTLD after HCT or SOT. This trial was registered at www.ClinicalTrials.gov as #NCT02822495.
Introduction
Epstein-Barr virus (EBV), an oncogenic herpes virus that initially infects B cells and epithelial cells,1,2 typically persists in a latent state after primary infection.2-4 Over 90% of the population is EBV seropositive, with latency maintained by a normal immune system.2,3,5,6 However, in the setting of immune system dysregulation, infection can result in several types of EBV-positive (EBV+) lymphoid proliferations.7 One such complication is EBV+ posttransplant lymphoproliferative disease (PTLD), an ultrarare, aggressive, and potentially deadly hematologic malignancy.8-10 Patients undergoing either allogeneic hematopoietic cell transplant (HCT) or solid organ transplant (SOT) are at risk of developing EBV+ PTLD.6,8,11-14
Risk factors for developing EBV+ PTLD after HCT include conditioning regimen (particularly antithymocyte globulin use), T-cell depletion of the stem cell graft, the intensity and duration of immunosuppression, EBV serostatus mismatch (particularly EBV seronegativity in the SOT recipient and EBV seropositivity in the donor at the time of transplantation), and unrelated or HLA-mismatched donor and graft type.10,11,15,16 Risk factors after SOT include the type of organ(s) transplanted (recipients of multiorgan and intestinal transplants have the highest incidence of PTLD, and kidney transplant recipients the lowest11,17), degree and duration of immunosuppressive therapy, EBV serostatus mismatch, and age of the recipient.8,11,18 EBV+ PTLD most commonly occurs within the first year after allogeneic HCT,11,12,14 whereas the timing of PTLD diagnoses after SOT is bimodal, with a first peak occurring during the first year after transplantation and the second peak 5 to 10 years (or more) after transplantation.11,19,20 Allograft survival in patients with PTLD compromises graft rejection rates ranging from 9% to 67%, depending on the type of organ transplanted.13,21-23 Prognostic factors associated with poor outcomes for HCT and SOT recipients with PTLD include older age at PTLD diagnosis, bone marrow involvement, and failure to achieve a response to initial therapy.13 Age, Eastern Cooperative Oncology Group (ECOG) performance status, and elevated baseline lactate dehydrogenase are also used in a PTLD-specific prognostic index to stratify patients into high-, intermediate-, and low- risk categories for survival.24
Historical treatment options for EBV+ PTLD after HCT and SOT include reduction in immunosuppression, surgery, radiation therapy, rituximab (anti-CD20 monoclonal antibody therapy), and chemotherapy.11,25 However, reduction in immunosuppression can lead to graft-versus-host disease (GVHD) and rejection of transplanted organs,11,12,14,26-28 and toxicity associated with standard lymphoma-directed chemotherapy in transplant recipients11,25,29,30 can result in high treatment-related mortality, especially in patients who are immunocompromised (7%-11%).31-33 Additionally, organ impairment associated with prior HCT or SOT may complicate efforts to fully and effectively treat subsequent PTLD, especially early after HCT when reduced organ function because of medications or infection is common.12,24,34 Median overall survival (OS) in patients with EBV+ PTLD after HCT relapsed or refractory to rituximab with/without chemotherapy is 0.7 months,35 whereas reported median OS in SOT recipients with EBV+ PTLD relapsed or refractory to rituximab plus chemotherapy is 4.1 months.36 Thus, there is clearly an unmet need for effective and well-tolerated therapies in this disease.
Adoptive immunotherapy with EBV-specific cytotoxic T lymphocytes (EBV-CTLs) is a promising approach for EBV+ PTLD after HCT or SOT,11,14,26,37 and is included in the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) as potential second-line therapy.26,38 Adoptive transfer of autologous37,39 and HCT donor–derived EBV-CTLs11,26,37,39 has resulted in complete (CR) and partial responses (PR),37,39,40 but EBV-CTLs cannot be generated from all HCT donors14 or SOT recipients,37 and the weeks to months required for in vitro generation limits the potential for timely administration.12,26,37,40 Furthermore, access to EBV-specific T-cell therapy has previously been limited to a few specialized cell-therapy centers able to manufacture these cell products.14,26,41 Early studies using readily available banks of allogeneic EBV-CTLs have addressed some of these limitations and shown safety and efficacy.42-45
Tabelecleucel is an off-the-shelf, allogeneic, EBV-specific T-cell immunotherapy consisting of EBV-specific CTLs generated using Good Manufacturing Practice and derived from EBV-seropositive donors that targets and eliminates EBV-expressing cells in an HLA-restricted manner. Specifically, tabelecleucel targets the type 3 EBV latency profile commonly expressed in EBV+ PTLD,2,46 making the disease a good candidate for treatment with tabelecleucel.2,43 Tabelecleucel is manufactured, extensively characterized, and stored in advance of patient need, allowing for rapid delivery from inventory for treatment, in contrast to generating autologous or donor-derived EBV-CTLs once a patient has been identified to require therapy.26,47 We have previously shown in a single-center analysis that treatment with tabelecleucel is well tolerated and results in high objective response rates (ORRs; 68% and 54%), durable responses, and promising survival (2-year OS rates of 57% and 54%) in patients with rituximab-refractory EBV+ PTLD after HCT and SOT, respectively.37 With the recent European Union marketing authorization, tabelecleucel is the first and, currently, only allogeneic T-cell therapy to receive an approval in relapsed/refractory (R/R) EBV+ PTLD. Here, we report the efficacy and safety of tabelecleucel for patients with EBV+ PTLD after HCT or SOT in a multicenter expanded access protocol, EBV-CTL-201 (ClinicalTrials.gov identifier: NCT02822495).
Methods
Tabelecleucel manufacturing
Peripheral blood mononuclear cells were collected from healthy, EBV-seropositive donors. Donor B cells were infected with EBV yielding EBV transformed B-lymphoblastoid cell lines (EBV-BLCLs), capable of presenting a broad range of EBV antigens. After lethal irradiation, EBV-BLCLs were then cocultured with T cells from the same donor to stimulate initially a polyclonal T-cell population. T cells were restimulated with the same EBV-BLCL approximately weekly. After a minimum 17 days of culture, the resulting enriched EBV-CTLs were characterized for EBV-specific cytotoxicity, alloreactivity, and for phenotype by flow cytometry. EBV-specific cytotoxicity of the EBV-CTLs was tested against a panel of EBV-BLCL targets sharing single HLA alleles with the donor T cells to confirm ≥1 HLA restrictions of EBV-specific cytotoxicity, information used in subsequent lot selection for a specific patient. Alloreactivity was similarly tested against HLA-mismatched EBV-BLCLs or phytohemagglutinin-stimulated T-cell blasts. Product quality was also confirmed by sterility, cell viability, and purity testing (ie, percent CD3+). EBV-CTLs meeting EBV-specific cytotoxicity and alloreactivity thresholds and other release criteria were harvested, aliquoted into single-use vials, cryopreserved, further tested to complete batch-release criteria, and stored for off-the-shelf use, resulting in a tabelecleucel product inventory of HLA-characterized lots that span a variety of haplotypes to provide broad population coverage.
Tabelecleucel lot selection
Selecting tabelecleucel for each patient was based on clinical information, including, but not limited to, cytomegalovirus status, weight, high-resolution HLA, as well as availability of a lot with an HLA restriction recognizing at least 1 HLA allele expressed by the patient’s disease cell of origin (as clinically determined by the investigator). Per protocol, patients were only permitted to enter screening once appropriate product lots meeting study requirements were identified. Our library is intended to cover 90% of the patient population. Among appropriately HLA-restricted lots, a minimum of 2 HLA alleles had to match the patient’s HLA using high resolution typing. This minimum was selected based on previously published data37 that found no correlation between response and an increase in the number of matching HLA alleles beyond 2.
Study design and patients
EBV-CTL-201 (ClinicalTrials.gov identifier: NCT02822495) was a multicenter, open-label, single-arm, expanded access protocol of tabelecleucel in patients with EBV+ diseases for whom there were no appropriate alternative therapies and who were not eligible to enroll in other clinical studies involving tabelecleucel. Although labeled an expanded access protocol, EBV-CTL-201 included periodic data collection for efficacy and safety analyses. Patients with diverse EBV+ diseases were enrolled, including EBV+ PTLD after HCT or SOT, EBV-associated lymphoproliferative disorders (LPDs) associated with primary or acquired immunodeficiency, EBV-associated LPD not associated with immunodeficiency, EBV+ nasopharyngeal carcinoma, leiomyosarcoma, other EBV+ solid tumors, and EBV viremia. The efficacy and safety analyses presented here solely involve outcomes in patients with EBV+ PTLD enrolled at 10 US sites. Key inclusion criteria included a lack of approved alternative therapies and ECOG performance status score of ≤4; key exclusion criteria included an ongoing need for methotrexate or extracorporeal photopheresis and corticosteroid therapy of >0.5 mg/kg daily. Refer to supplemental Table 1 for additional information on patient eligibility.
Details of excluded immunosuppressants and required washout periods before study entry can be found in supplemental Table 1. Per treatment guidelines, concomitant immunosuppressants were expected to have been minimized to the lowest level considered clinically acceptable at study enrollment. The dose of any ongoing immunosuppressants was at the discretion of the treating physicians based on the immunologic risk of a given patient.
The study was conducted in accordance with the protocol, International Council for Harmonization, Good Clinical Practice standards, and the Declaration of Helsinki. The protocol and amendments were approved by the relevant institutional review boards (IRBs) or ethics committees of participating institutions. All patients provided written informed consent.
Treatments and assessments
Patients were treated with 1.6 × 106 to 2.0 × 106 cells per kg per dose of intravenous tabelecleucel on days 1, 8, and 15, without any prior lymphodepleting therapy, followed by an observation period to complete a 35-day cycle. Evaluation by imaging occurred between day 28 and 35 of each cycle. Response was assessed by investigators using Lugano Response Criteria with Lymphoma Response to Immunomodulatory Therapy Criteria modification48 read at the treating center. Per study protocol, radiographic assessments were performed at screening before cycle 1, during the precycle assessment for each subsequent cycle, and at 180 days after last dose for patients whose best response was CR, PR, or stable disease. Treatment continued until maximal response, unacceptable toxicity, patient or investigator decision, or withdrawal of consent. Maximal response was defined as 3 consecutive cycles with PR or 2 consecutive cycles with CR outcomes. Patients were switched to tabelecleucel with a different HLA restriction (restriction switch) if their PTLD progressed or they were assessed as having stable disease for 2 consecutive cycles. A maximum of 4 different tabelecleucel lots was allowed.
The primary efficacy end point was investigator-assessed ORR, defined as the proportion of patients who achieved best overall response of CR or PR. Key secondary end points included OS, time to response, duration of response, progression-free survival, durable response rate, and time to progression; only end points with adequate response assessment follow-up are presented. Definitions for all secondary efficacy end points are shown in supplemental Table 2. Safety end points included frequency of treatment-emergent adverse events (TEAEs), TEAEs of special interest (GVHD, transmission of infectious diseases [TIDs], infusion-related reactions [IRRs], and cytokine release syndrome [CRS]), and incidence of graft rejection or loss.
Analysis
All patients with PTLD who received ≥1 dose of tabelecleucel were included in the safety and efficacy analyses presented. ORR and 95% confidence interval (CI) were estimated by using the exact binomial method. The Kaplan-Meier method was used to estimate OS; 95% CI was provided using log-log transformation method. Patients without a death event were censored on the last-known-alive date. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities, version 23.0 to system organ classes and preferred terms and were graded using National Cancer Institute’s Common Terminology Criteria for Adverse Events version, 4.03 as assessed by investigator. Related AEs were defined as those AEs reported as “possibly related” or “related” by investigator. Acute GVHD was graded using the Center for International Blood and Marrow Transplant Research consensus grading system.49 All AEs were reported as per IRB/ethics committee requirements.
TEAEs included any events that occurred after the initiation of the first dose of tabelecleucel through 30 days after the last dose of tabelecleucel, or any event already present before the first dose of tabelecleucel that worsened in either intensity or frequency after exposure to tabelecleucel through 30 days after the last dose, or any AE with date of onset on or after the first dose of tabelecleucel that was deemed treatment-related by the investigator. TEAEs of special interest were systematically captured.
Enrollment period for the study was July 2016 through December 2018. Per study protocol, patients were followed-up for at least 30 days from last infusion and up to 24 months. The study completion date was 8 September 2020 (last patient, last visit); the overall study duration was 4.2 years from enrollment of the first patient.
Results
Patient baseline characteristics
Twenty-six patients who developed EBV+ PTLD after HCT (n = 14) or SOT (n = 12) and whose disease failed to respond to, or recurred after, rituximab with/without chemotherapy were enrolled and received treatment with tabelecleucel (Figure 1). Patient characteristics and duration of tabelecleucel exposure are shown in Table 1. Pediatric (aged <16 years; 23.1%) and adult (76.9%) patients were included. Median age was 36.0 years (range, 2-74). The most reported EBV+ PTLD histology was diffuse large B-cell lymphoma (46.2%), followed by EBV+ PTLD not otherwise specified (23.1%) and polymorphic EBV+ PTLD (11.3%). Median number of lines of prior therapy was 1.0 (range, 1-3); most patients (96%) received prior treatment with rituximab. Most patients (58%) in the SOT cohort and 7% of patients in the HCT cohort had also received chemotherapy. Across both HCT and SOT cohorts, median time from transplant to diagnosis of EBV+ PTLD was 5.1 months (range, 1.4-275.9). Median time from initial EBV+ PTLD diagnosis to first administration of tabelecleucel was 2.3 months (range, 0.2-67.6).
Study disposition. ∗Removed from study by sponsor because of concurrent cytotoxic T-lymphocyte treatment with different agent for cytomegalovirus disease (n = 1); primary disease relapse (n = 1); patient noncompliance with follow-up appointments (n = 1); patient exiting study 5 months after treatment discontinuation because of start of subsequent therapy (n = 1); patient enrolling on different protocol (n = 1); and physician decision (n = 1).
Study disposition. ∗Removed from study by sponsor because of concurrent cytotoxic T-lymphocyte treatment with different agent for cytomegalovirus disease (n = 1); primary disease relapse (n = 1); patient noncompliance with follow-up appointments (n = 1); patient exiting study 5 months after treatment discontinuation because of start of subsequent therapy (n = 1); patient enrolling on different protocol (n = 1); and physician decision (n = 1).
Baseline characteristics of patients, and extent of tabelecleucel exposure
| Characteristics . | HCT (n = 14) . | SOT (n = 12) . | Total (N = 26) . |
|---|---|---|---|
| Median age, y (range) | 46.0 (2-74) | 27.5 (7-66) | 36.0 (2-74) |
| Age category, n (%) | |||
| <16 y | 2 (14.3) | 4 (33.3) | 6 (23.1) |
| ≥16 y | 12 (85.7) | 8 (66.7) | 20 (76.9) |
| Male, n (%) | 7 (50.0) | 6 (50.0) | 13 (50.0) |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 1 (7.1) | 2 (16.7) | 3 (11.5) |
| Not Hispanic/Latino | 11 (78.6) | 8 (66.7) | 19 (17.3) |
| Not given | 2 (14.3) | 2 (16.7) | 4 (15.4) |
| Race, n (%) | |||
| Caucasian | 10 (71.4) | 8 (66.7) | 18 (69.2) |
| Black | 1 (7.1) | 0 | 1 (3.8) |
| Asian | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| Other/unknowns | 1 (7.1) | 3 (25.0) | 4 (15.4) |
| Median ECOG PS score (range)∗ | 1.5 (0-4) | 1.0 (0-3) | 1.0 (0-4) |
| Median Lansky score (range)† | 55.0 (50-60) | 80.0 (20-90) | 65.0 (20-90) |
| Disease risk parameters, n (%)∗ | |||
| Age of ≥60 y | 2 (16.7) | 1 (12.5) | 3 (15.0) |
| ECOG PS score of ≥2 | 6 (50.0) | 3 (37.5) | 9 (45.0) |
| Elevated serum LDH | 7 (58.3) | 4 (50.0) | 11 (55.0) |
| Risk score∗,‡ | |||
| High | 3 (25.0) | 2 (25.0) | 5 (25.0) |
| Intermediate | 8 (66.7) | 4 (50.0) | 12 (60.0) |
| Low | 1 (8.3) | 2 (25.0) | 3 (15.0) |
| Disease morphology/histology, n (%)§ | |||
| Diffuse large B-cell lymphoma | 4 (28.6) | 8 (66.7) | 12 (46.2) |
| PTLD NOS | 6 (42.9) | 0 | 6 (23.1) |
| Polymorphic PTLD | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| Hodgkin lymphoma | 0 | 1 (8.3) | 1 (3.8) |
| Infectious mononucleosis–like PTLD | 0 | 1 (8.3) | 1 (3.8) |
| Lymphoproliferative disorder NOS | 1 (7.1) | 0 | 1 (3.8) |
| Monomorphic B-cell PTLD | 0 | 1 (8.3) | 1 (3.8) |
| Transplanted organ, n (%) | |||
| Kidney | N/A | 6 (50.0) | N/A |
| Heart | N/A | 2 (16.7) | N/A |
| Lung | N/A | 2 (16.7) | N/A |
| Intestine | N/A | 2 (16.7) | N/A |
| Median time | |||
| Median time from transplant to diagnosis of EBV+ PTLD, mo (range) | 4.4 (1.4-198.4) | 7.2 (2.1-275.9) | 5.1 (1.4-275.9) |
| Median time from transplant to first dose of tabelecleucel, mo (range) | 6.4 (2.3-202.2) | 20.5 (2.3-281.3) | 9.3 (2.3-281.3) |
| Median time from initial EBV-related disease diagnosis to first tabelecleucel dose, mo (range) | 1.4 (0.2-8.2) | 5.0 (0.2-67.6) | 2.3 (0.2-67.6) |
| Baseline CNS PTLD involvement, n (%)‖ | 1 (7.1) | 1 (8.3) | 2 (7.7) |
| Baseline extranodal PTLD (including bone marrow), n (%)¶ | 1 (7.1) | 3 (25.0) | 4 (15.4) |
| Prior rituximab therapy, n (%)# | 14 (100) | 11 (91.7) | 25 (96.2) |
| Prior chemotherapy, n (%) | 1 (7.1) | 7 (58.3) | 8 (30.8) |
| Median number of lines of prior systemic therapies (range) | 1.0 (1-3) | 1.5 (1-3) | 1.0 (1-3) |
| Use of immunosuppressive medications at start of tabelecleucel, n (%) | 1 (7.1) | 11 (91.7) | 12 (46.2) |
| Treatment on trial | |||
| Median of average cells administered per dose (×106 cells per kg) (range) | 1.98 (1.6-2.0) | 1.98 (1.6-2.0) | 1.98 (1.6-2.0) |
| Median duration of tabelecleucel treatment, mo (range) | 1.3 (0.03-3.1) | 2.5 (1.2-10.4) | 1.8 (0.03-10.4) |
| Median no. of tabelecleucel doses received (range) | 4.0 (1-9) | 7.0 (4-27) | 6.0 (1-27) |
| Median no. of tabelecleucel cycles received (range) | 2.0 (1-4) | 2.5 (2-9) | 2.0 (1-9) |
| Reason for treatment discontinuation | |||
| Death | 3 (21.4) | 1 (8.3) | 4 (15.4) |
| Disease progression∗∗ | 3 (21.4) | 1 (8.3) | 4 (15.4) |
| AEs other than disease progression†† | 0 | 0 | 0 |
| Required subsequent EBV therapy‡‡ | 2 (14.3) | 1 (8.1) | 3 (11.5) |
| Received maximum available tabelecleucel cell products | 1 (7.1) | 1 (8.3) | 2 (7.7) |
| Physician decision | 1 (7.1) | 1 (8.3) | 2 (7.7) |
| Patient preference | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| Other§§ | 1 (7.1) | 0 | 1 (3.8) |
| No. of tabelecleucel lots received, n (%) | |||
| 1 | 14 (100) | 8 (66.7) | 22 (84.6) |
| 2 | 0 | 3 (25.0) | 3 (11.5) |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 1 (8.3) | 1 (3.8) |
| Characteristics . | HCT (n = 14) . | SOT (n = 12) . | Total (N = 26) . |
|---|---|---|---|
| Median age, y (range) | 46.0 (2-74) | 27.5 (7-66) | 36.0 (2-74) |
| Age category, n (%) | |||
| <16 y | 2 (14.3) | 4 (33.3) | 6 (23.1) |
| ≥16 y | 12 (85.7) | 8 (66.7) | 20 (76.9) |
| Male, n (%) | 7 (50.0) | 6 (50.0) | 13 (50.0) |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 1 (7.1) | 2 (16.7) | 3 (11.5) |
| Not Hispanic/Latino | 11 (78.6) | 8 (66.7) | 19 (17.3) |
| Not given | 2 (14.3) | 2 (16.7) | 4 (15.4) |
| Race, n (%) | |||
| Caucasian | 10 (71.4) | 8 (66.7) | 18 (69.2) |
| Black | 1 (7.1) | 0 | 1 (3.8) |
| Asian | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| Other/unknowns | 1 (7.1) | 3 (25.0) | 4 (15.4) |
| Median ECOG PS score (range)∗ | 1.5 (0-4) | 1.0 (0-3) | 1.0 (0-4) |
| Median Lansky score (range)† | 55.0 (50-60) | 80.0 (20-90) | 65.0 (20-90) |
| Disease risk parameters, n (%)∗ | |||
| Age of ≥60 y | 2 (16.7) | 1 (12.5) | 3 (15.0) |
| ECOG PS score of ≥2 | 6 (50.0) | 3 (37.5) | 9 (45.0) |
| Elevated serum LDH | 7 (58.3) | 4 (50.0) | 11 (55.0) |
| Risk score∗,‡ | |||
| High | 3 (25.0) | 2 (25.0) | 5 (25.0) |
| Intermediate | 8 (66.7) | 4 (50.0) | 12 (60.0) |
| Low | 1 (8.3) | 2 (25.0) | 3 (15.0) |
| Disease morphology/histology, n (%)§ | |||
| Diffuse large B-cell lymphoma | 4 (28.6) | 8 (66.7) | 12 (46.2) |
| PTLD NOS | 6 (42.9) | 0 | 6 (23.1) |
| Polymorphic PTLD | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| Hodgkin lymphoma | 0 | 1 (8.3) | 1 (3.8) |
| Infectious mononucleosis–like PTLD | 0 | 1 (8.3) | 1 (3.8) |
| Lymphoproliferative disorder NOS | 1 (7.1) | 0 | 1 (3.8) |
| Monomorphic B-cell PTLD | 0 | 1 (8.3) | 1 (3.8) |
| Transplanted organ, n (%) | |||
| Kidney | N/A | 6 (50.0) | N/A |
| Heart | N/A | 2 (16.7) | N/A |
| Lung | N/A | 2 (16.7) | N/A |
| Intestine | N/A | 2 (16.7) | N/A |
| Median time | |||
| Median time from transplant to diagnosis of EBV+ PTLD, mo (range) | 4.4 (1.4-198.4) | 7.2 (2.1-275.9) | 5.1 (1.4-275.9) |
| Median time from transplant to first dose of tabelecleucel, mo (range) | 6.4 (2.3-202.2) | 20.5 (2.3-281.3) | 9.3 (2.3-281.3) |
| Median time from initial EBV-related disease diagnosis to first tabelecleucel dose, mo (range) | 1.4 (0.2-8.2) | 5.0 (0.2-67.6) | 2.3 (0.2-67.6) |
| Baseline CNS PTLD involvement, n (%)‖ | 1 (7.1) | 1 (8.3) | 2 (7.7) |
| Baseline extranodal PTLD (including bone marrow), n (%)¶ | 1 (7.1) | 3 (25.0) | 4 (15.4) |
| Prior rituximab therapy, n (%)# | 14 (100) | 11 (91.7) | 25 (96.2) |
| Prior chemotherapy, n (%) | 1 (7.1) | 7 (58.3) | 8 (30.8) |
| Median number of lines of prior systemic therapies (range) | 1.0 (1-3) | 1.5 (1-3) | 1.0 (1-3) |
| Use of immunosuppressive medications at start of tabelecleucel, n (%) | 1 (7.1) | 11 (91.7) | 12 (46.2) |
| Treatment on trial | |||
| Median of average cells administered per dose (×106 cells per kg) (range) | 1.98 (1.6-2.0) | 1.98 (1.6-2.0) | 1.98 (1.6-2.0) |
| Median duration of tabelecleucel treatment, mo (range) | 1.3 (0.03-3.1) | 2.5 (1.2-10.4) | 1.8 (0.03-10.4) |
| Median no. of tabelecleucel doses received (range) | 4.0 (1-9) | 7.0 (4-27) | 6.0 (1-27) |
| Median no. of tabelecleucel cycles received (range) | 2.0 (1-4) | 2.5 (2-9) | 2.0 (1-9) |
| Reason for treatment discontinuation | |||
| Death | 3 (21.4) | 1 (8.3) | 4 (15.4) |
| Disease progression∗∗ | 3 (21.4) | 1 (8.3) | 4 (15.4) |
| AEs other than disease progression†† | 0 | 0 | 0 |
| Required subsequent EBV therapy‡‡ | 2 (14.3) | 1 (8.1) | 3 (11.5) |
| Received maximum available tabelecleucel cell products | 1 (7.1) | 1 (8.3) | 2 (7.7) |
| Physician decision | 1 (7.1) | 1 (8.3) | 2 (7.7) |
| Patient preference | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| Other§§ | 1 (7.1) | 0 | 1 (3.8) |
| No. of tabelecleucel lots received, n (%) | |||
| 1 | 14 (100) | 8 (66.7) | 22 (84.6) |
| 2 | 0 | 3 (25.0) | 3 (11.5) |
| 3 | 0 | 0 | 0 |
| 4 | 0 | 1 (8.3) | 1 (3.8) |
LDH, lactate dehydrogenase; N/A, not applicable; NOS, not otherwise specified; PS, performance status.
For patients aged >16 years.
For patients aged ≤16 years.
Scored using PTLD–adapted prognostic index. Per Choquet et al,24 high-risk patients had ≥2 of the following: age ≥60 years, ECOG PS score of ≥2, and/or elevated LDH at, or before, first dose of tabelecleucel.
Disease morphology/histology was collected for 25 of 26 patients.
Baseline CNS disease was not officially evaluated by imaging because of low clinical suspicion in 21 of 26 patients (10/12 of SOT and 11/14 of HCT).
Baseline extranodal disease was missing in 1 patient and not evaluable in 2 patients.
Administered as a monotherapy; however, patients may have received other prior treatments for PTLD.
Includes 1 patient with primary disease progression.
All AEs that led to treatment discontinuation were unrelated to tabelecleucel.
Subsequent EBV therapies included immunotherapy, chemotherapy, or radiotherapy.
Initiation of non-protocol CTL treatment for cytomegalovirus disease.
Adult patients with EBV+ PTLD after HCT or SOT were classified into high, intermediate, and low risk according to the PTLD prognostic index.24 Of these patients, 25% were high risk based on ≥2 of the following: age ≥60 years, ECOG performance status score of ≥2 (assessable in patients aged >16 years), and/or elevated baseline lactate dehydrogenase (Table 1). An additional 60% of patients were intermediate risk.
Patients received a median dose (averaged across up to 3 doses administered per cycle) of 1.98 × 106 cells per dose (range, 1.6 × 106 to 2.0 × 106 cells per dose), for a median 2 cycles (range, 1-9 cycles) and a median 6 doses (range, 1-27 doses) of tabelecleucel. Median treatment duration was 1.8 months across cohorts (range, 0.03-10.4 months); most (85%) patients did not require restriction switch. Common reasons for discontinuing treatment were death (15%), starting other EBV+ PTLD therapy (12%), and AEs (12%; Table 1).
Efficacy
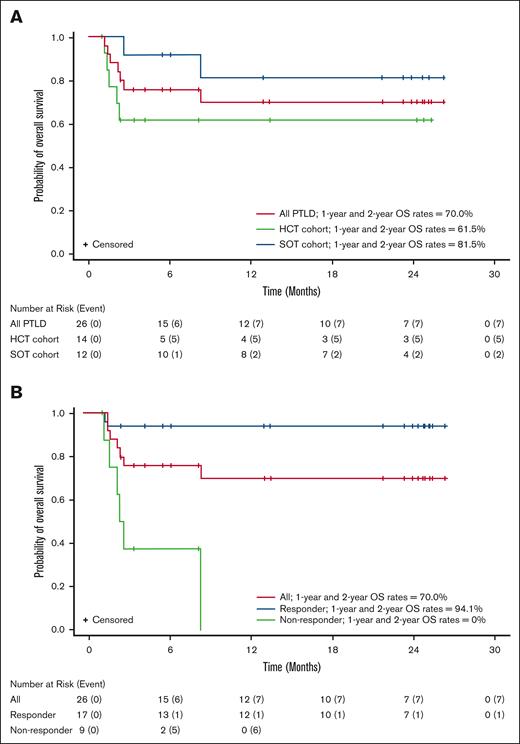
Responses to tabelecleucel were observed in 17 of 26 (65.4%) patients overall, 7 of 14 (50.0%) patients in the HCT cohort, and 10 of 12 (83.3%) patients in the SOT cohort, with a best overall response of CR (n = 4, HCT; n = 6, SOT) or PR (n = 3, HCT; n = 4, SOT; Table 2). The estimated 1-year and 2-year OS rates were both 70.0% (95% CI, 46.5-84.7) in all patients, both 61.5% (95% CI, 30.8-81.8) in the HCT cohort, and both 81.5% (95% CI, 43.5-95.1) in the SOT cohort (Figure 2, Table 3). No deaths were reported between 1 and 2 years. The median (range) follow-up time for OS was 8.2 months (95% CI, 1.0-26.2) in all patients, 2.8 months (95% CI, 1.0-25.3) in the HCT cohort, and 22.5 months (95% CI, 2.6-26.2) in the SOT cohort. Of 26 patients, 7 (26.9%) patients died before 9 months (n = 5, HCT; n = 2, SOT), and 19 of 26 (73.1%) were censored (n = 9, HCT; n = 10, SOT), including 7 patients censored before 12 months (n = 5, HCT; n = 2, SOT). Two patients were lost to follow-up; 6 discontinued for other reasons.
ORRs
| . | HCT (n = 14) . | SOT (n = 12) . | Total (N = 26) . |
|---|---|---|---|
| Best overall response, n (%) | |||
| CR | 4 (28.6) | 6 (50.0) | 10 (38.5) |
| PR | 3 (21.4) | 4 (33.3) | 7 (26.9) |
| SD | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| PD | 4 (28.6) | 1 (8.3) | 5 (19.2) |
| NE | 1 (7.1)∗ | 0 | 1 (3.8)∗ |
| Responders, n (%) | 7 (50.0) | 10 (83.3) | 17 (65.4) |
| 95% CI | 23.0-77.0 | 51.6-97.9 | 44.3-82.8 |
| . | HCT (n = 14) . | SOT (n = 12) . | Total (N = 26) . |
|---|---|---|---|
| Best overall response, n (%) | |||
| CR | 4 (28.6) | 6 (50.0) | 10 (38.5) |
| PR | 3 (21.4) | 4 (33.3) | 7 (26.9) |
| SD | 2 (14.3) | 1 (8.3) | 3 (11.5) |
| PD | 4 (28.6) | 1 (8.3) | 5 (19.2) |
| NE | 1 (7.1)∗ | 0 | 1 (3.8)∗ |
| Responders, n (%) | 7 (50.0) | 10 (83.3) | 17 (65.4) |
| 95% CI | 23.0-77.0 | 51.6-97.9 | 44.3-82.8 |
A patient is considered a responder if the best overall response is either CR or PR.
NE, not evaluable; PD, progressive disease.
No disease assessment obtained.
Overall survival by transplant type and responder status. Kaplan-Meier plot of OS in (A) all patients with PTLD in the HCT and SOT cohorts and (B) responders and nonresponders in combined cohorts.
Overall survival by transplant type and responder status. Kaplan-Meier plot of OS in (A) all patients with PTLD in the HCT and SOT cohorts and (B) responders and nonresponders in combined cohorts.
OS rates
| . | HCT (n = 14) . | SOT (n = 12) . | Total (N = 26) . |
|---|---|---|---|
| Status, n (%) | |||
| Death | 5 (35.7) | 2 (16.7) | 7 (26.9)∗ |
| Censored | 9 (64.3) | 10 (83.3) | 19 (73.1) |
| OS estimate (KM) (mo) | |||
| Median (95% CI) | NE (1.5-NE) | NE (8.2-NE) | NE (8.2-NE) |
| OS rate (KM) (95% CI), % | |||
| 6 mos | 61.5 (30.8-81.8) | 91.7 (53.9-98.8) | 75.8 (53.8-88.3) |
| 12 mos | 61.5 (30.8-81.8) | 81.5 (43.5-95.1) | 70.0 (46.5-84.7) |
| 24 mos | 61.5 (30.8-81.8) | 81.5 (43.5-95.1) | 70.0 (46.5-84.7) |
| Follow-up time (mo) | |||
| Median (range) | 2.8 (1.0-25.3)† | 22.5 (2.6-26.2) | 8.2 (1.0-26.2) |
| . | HCT (n = 14) . | SOT (n = 12) . | Total (N = 26) . |
|---|---|---|---|
| Status, n (%) | |||
| Death | 5 (35.7) | 2 (16.7) | 7 (26.9)∗ |
| Censored | 9 (64.3) | 10 (83.3) | 19 (73.1) |
| OS estimate (KM) (mo) | |||
| Median (95% CI) | NE (1.5-NE) | NE (8.2-NE) | NE (8.2-NE) |
| OS rate (KM) (95% CI), % | |||
| 6 mos | 61.5 (30.8-81.8) | 91.7 (53.9-98.8) | 75.8 (53.8-88.3) |
| 12 mos | 61.5 (30.8-81.8) | 81.5 (43.5-95.1) | 70.0 (46.5-84.7) |
| 24 mos | 61.5 (30.8-81.8) | 81.5 (43.5-95.1) | 70.0 (46.5-84.7) |
| Follow-up time (mo) | |||
| Median (range) | 2.8 (1.0-25.3)† | 22.5 (2.6-26.2) | 8.2 (1.0-26.2) |
KM, Kaplan-Meier; NE, not evaluable.
None were treatment-related per investigator assessment.
Of 14 HCT recipients, 9 had OS follow-up of <4.5 months because of either death (n = 5) or study discontinuation (n = 4). Of the remaining 5 patients, 3 survived up to the 2-year study completion and 2 were censored between 8 and 13 months, with 1 exiting the study 5 months after treatment discontinuation because of start of subsequent therapy and 1 achieving maximal response. Maximum follow-up for the HCT cohort was 25.3 months, enabling the computation of OS rate estimates up to 24 months, including 95% CIs.
Of 17 responders, 12 responded after 1 cycle of tabelecleucel treatment, 4 after 2 cycles, and 1 after 6 cycles of tabelecleucel treatment, with a median of 1 cycle (range, 1-6 cycles). Median time to response in all responders was 1.0 month (range, 0.6-7.1 months). Sixteen responders achieved response either without any restriction switch or before the first restriction switch; the patient who responded after 6 cycles of treatment achieved response after 2 restriction switches. Among responders, median OS was not reached, only 1 of 17 (5.9%) patient died, and the estimated 1-year and 2-year OS rates were both 94.1% (95% CI, 65.0-99.1; Figure 2B). Among nonresponders, median OS was 2.4 months (range, 1.2-8.2 months), 6 of 9 (66.7%) patients died, and 1-year and 2-year OS rates were both 0% (Figure 2B).
ORRs were similar among subgroups within HCT and SOT cohorts (by age, sex, race, and ethnicity; supplemental Figure 1). Additionally, overall efficacy was generally consistent across risk stratification (ORR 60.0% in high-risk patients), whereas ORR was higher in pediatric patients (83.3%) than in the general study population.
Safety
All patients experienced at least 1 TEAE (Table 4). The most common TEAEs (≥20% of all patients) included diarrhea and pyrexia (34.6% each); aspartate aminotransferase increased, alanine aminotransferase increased, cough, hyponatremia, and fatigue (30.8% each); white blood cell count decrease (26.9%); and pneumonia and disease progression (23.1% each). Grade ≥3 TEAEs were reported in 73.1% of patients (Table 4). Treatment-related TEAEs were reported for 34.6% of patients (n = 9), with 15.4% (n = 4) at grade ≥3 (Table 4); abdominal pain (including abdominal pain, abdominal discomfort, and abdominal pain lower) was the only treatment-related event reported in >1 patient (n = 4, 15.4%; Table 5). A total of 65.4% of patients (n = 17) experienced treatment-emergent serious AEs (TESAEs). Grade ≥3 treatment-related TESAEs were reported in 11.5% of patients (n = 3; Table 4) and included abdominal pain, colitis, acute GVHD of the gastrointestinal tract, acute GVHD of the liver, and pneumonitis (3.8% for each). Of 7 patients who died during the study, 5 (19.2%) had fatal TESAEs (n = 3, disease progression; n = 1, cardiac arrest; and n = 1, multiple organ dysfunction syndrome) and 2 (3.8%) had deaths that occurred outside of the AE collection period. All 7 fatalities were considered unrelated to treatment by investigators (Table 4), and 4 deaths overall were caused by disease progression.
Safety profile
| . | HCT (n = 14) . | SOT (n = 12) . | All (N = 26) . |
|---|---|---|---|
| All TEAEs | 14 (100) | 12 (100) | 26 (100) |
| Grade ≥3 TEAEs | 12 (85.7) | 7 (58.3) | 19 (73.1) |
| TEAEs leading to study discontinuation | 4 (28.6) | 4 (33.3) | 8 (30.8) |
| All TR-TEAEs | 4 (28.6) | 5 (41.7) | 9 (34.6) |
| Grade ≥3 TR-TEAEs | 2 (14.3) | 2 (16.7) | 4 (15.4) |
| TR-TEAEs leading to study discontinuation | 0 | 1 (8.3) | 1 (3.8) |
| All TESAEs | 9 (64.3) | 8 (66.7) | 17 (65.4) |
| Grade ≥3 TESAEs | 9 (64.3) | 7 (58.3) | 16 (61.5) |
| Fatal TESAEs | 4 (28.6) | 1 (8.3) | 5 (19.2)∗ |
| All TR-TESAEs | 1 (7.1) | 2 (16.7) | 3 (11.5) |
| Grade ≥3 TR-TESAEs | 1 (7.1) | 2 (16.7) | 3 (11.5) |
| Fatal TR-TESAEs | 0 | 0 | 0 |
| . | HCT (n = 14) . | SOT (n = 12) . | All (N = 26) . |
|---|---|---|---|
| All TEAEs | 14 (100) | 12 (100) | 26 (100) |
| Grade ≥3 TEAEs | 12 (85.7) | 7 (58.3) | 19 (73.1) |
| TEAEs leading to study discontinuation | 4 (28.6) | 4 (33.3) | 8 (30.8) |
| All TR-TEAEs | 4 (28.6) | 5 (41.7) | 9 (34.6) |
| Grade ≥3 TR-TEAEs | 2 (14.3) | 2 (16.7) | 4 (15.4) |
| TR-TEAEs leading to study discontinuation | 0 | 1 (8.3) | 1 (3.8) |
| All TESAEs | 9 (64.3) | 8 (66.7) | 17 (65.4) |
| Grade ≥3 TESAEs | 9 (64.3) | 7 (58.3) | 16 (61.5) |
| Fatal TESAEs | 4 (28.6) | 1 (8.3) | 5 (19.2)∗ |
| All TR-TESAEs | 1 (7.1) | 2 (16.7) | 3 (11.5) |
| Grade ≥3 TR-TESAEs | 1 (7.1) | 2 (16.7) | 3 (11.5) |
| Fatal TR-TESAEs | 0 | 0 | 0 |
Data are given as number (%).
TR-TEAE, treatment-related TEAE; TR-TESAE, treatment-related TESAE.
Three of 5 deaths were due to disease progression (1 in a pediatric patient); 1 was due to cardiac arrest, and 1 was due to multiple organ dysfunction syndrome. Deaths due to other causes (eg, other than fatal TESAEs) occurred in 2 additional patients (1 with diffuse alveolar hemorrhage and hypoxic respiratory failure, and 1 with disease progression).
Treatment-related TEAEs reported
| . | HCT (n = 14) . | SOT (n = 12) . | All (N = 26) . |
|---|---|---|---|
| Abdominal pain∗ | 0 (0) | 4 (33.3) | 4 (15.4) |
| Abdominal distension | 0 (0) | 1 (8.3) | 1 (3.8) |
| Anemia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Colitis | 0 (0) | 1 (8.3) | 1 (3.8) |
| Dizziness | 1 (7.1) | 0 (0) | 1 (3.8) |
| Fatigue | 0 (0) | 1 (8.3) | 1 (3.8) |
| Febrile neutropenia | 0 (0) | 1 (8.3) | 1 (3.8) |
| General physical health deterioration | 1 (7.1) | 0 (0) | 1 (3.8) |
| GVHD in gastrointestinal tract | 1 (7.1) | 0 (0) | 1 (3.8) |
| GVHD in liver | 1 (7.1) | 0 (0) | 1 (3.8) |
| Hypocalcemia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Hyponatremia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Pneumonitis | 0 (0) | 1 (8.3) | 1 (3.8) |
| Pyrexia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Rash maculo-papular | 1 (7.1) | 0 (0) | 1 (3.8) |
| Tumor pain | 0 (0) | 1 (8.3) | 1 (3.8) |
| White blood cell count increased | 0 (0) | 1 (8.3) | 1 (3.8) |
| . | HCT (n = 14) . | SOT (n = 12) . | All (N = 26) . |
|---|---|---|---|
| Abdominal pain∗ | 0 (0) | 4 (33.3) | 4 (15.4) |
| Abdominal distension | 0 (0) | 1 (8.3) | 1 (3.8) |
| Anemia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Colitis | 0 (0) | 1 (8.3) | 1 (3.8) |
| Dizziness | 1 (7.1) | 0 (0) | 1 (3.8) |
| Fatigue | 0 (0) | 1 (8.3) | 1 (3.8) |
| Febrile neutropenia | 0 (0) | 1 (8.3) | 1 (3.8) |
| General physical health deterioration | 1 (7.1) | 0 (0) | 1 (3.8) |
| GVHD in gastrointestinal tract | 1 (7.1) | 0 (0) | 1 (3.8) |
| GVHD in liver | 1 (7.1) | 0 (0) | 1 (3.8) |
| Hypocalcemia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Hyponatremia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Pneumonitis | 0 (0) | 1 (8.3) | 1 (3.8) |
| Pyrexia | 0 (0) | 1 (8.3) | 1 (3.8) |
| Rash maculo-papular | 1 (7.1) | 0 (0) | 1 (3.8) |
| Tumor pain | 0 (0) | 1 (8.3) | 1 (3.8) |
| White blood cell count increased | 0 (0) | 1 (8.3) | 1 (3.8) |
Data are given as number (%).
Includes abdominal pain, abdominal discomfort, and abdominal pain lower.
Because tabelecleucel consists of partially HLA-matched, human-derived products using healthy donor peripheral blood mononuclear cells as starting material, several AEs of special interest, including GVHD, TID, IRR, and CRS, were monitored. No events of TID, IRR, or CRS were reported in this study. Tumor flare reaction (TFR), which is the only identified risk for tabelecleucel, was not reported in these patients. Furthermore, no HCT graft loss or SOT rejection events were reported. Four events of acute GVHD were reported in 3 HCT recipients. Two events of liver GVHD (grade 4) and gastrointestinal GVHD (grade 4) occurred in 1 patient with a history of GVHD and were reported as possibly related to tabelecleucel. This patient died ∼3 weeks later from EBV+ PTLD progression while the GVHD events were resolving. In another patient with a history of GVHD, 1 event of gut GVHD (grade 2) started before tabelecleucel administration and was not considered treatment-related. This GVHD event resolved; however, the patient also died ∼1 month later from complications of PTLD. The third patient, with no history of GVHD, developed a grade 3 maculopapular rash in the context of sun and chlorine exposure that was considered as possible GVHD and was reported as possibly related to tabelecleucel. Skin biopsy was inconclusive, and the event recovered within a few days with topical steroids. GVHD events led to study drug interruption in 2 of 3 patients. In pediatric patients (n = 6), no grade ≥3 treatment-related TEAEs, no treatment-related TESAEs, and no AEs of special interest were reported. A fatal TEAE was reported in 1 (16.7%) pediatric patient (disease progression); it was not deemed treatment-related.
Discussion
This report describes results in the subset of 26 patients treated for R/R EBV+ PTLD after HCT or SOT as part of a multicenter, open-label, single-arm expanded access protocol of tabelecleucel. The majority of patients had high- or intermediate-risk clinical features by PTLD prognostic index. Consistent with current literature,16,33,50,51 the most commonly reported EBV+ PTLD histology in this study was diffuse large B-cell lymphoma, a monomorphic classification that has been associated with more aggressive disease.4,52 Additionally, median time from transplant to diagnosis of EBV+ PTLD across both HCT and SOT cohorts was consistent with the known epidemiology and natural history of the disease. Thus, this study enrolled patients representative of a typical EBV+ PTLD population and course of disease.
In these patients for whom there are no other PTLD-specific approved therapies, ORR was 65.4% for the entire cohort, 50.0% for the HCT cohort, and 83.3% for the SOT cohort. The ORR observed in the HCT cohort is encouraging relative to the historically dismal prognosis of HCT recipients with PTLD that fails to respond to rituximab. Although sample sizes were small, ORRs were similar among age, sex, race, and ethnicity subgroups in both cohorts. Although SOT ORR was higher than HCT ORR with rationale for this difference likely being multifactorial, overall ORR is consistent with larger overall tabelecleucel clinical experience including previous phase 2 studies (supplemental Figure 1).37 Similarly, estimated long-term OS among patients was consistent with previously reported data across tabelecleucel clinical studies,37 with both 1-year and 2-year OS rates of 61.5% for the HCT cohort and both 81.5% for the SOT cohort (no deaths were reported between 1 and 2 years). This study also supports previous findings demonstrating that patients responding to tabelecleucel had longer survival than nonresponders. These results suggest tabelecleucel imparts substantial clinical benefit after failure of standard-of-care therapies.35,36
The results presented here confirm findings from previous studies with adoptively transferred third-party EBV-CTLs42-45 and prior experience in the single-center setting demonstrating response to tabelecleucel and promising 2-year survival in HCT and SOT recipients with R/R EBV+ PTLD.37
Also consistent with the single-center analysis,37 tabelecleucel was well tolerated in this multicenter study of immunocompromised patients with EBV+ PTLD after HCT or SOT. There was no graft loss, transplanted-organ rejection, or fatal TESAEs assessed as related to tabelecleucel, and there were no reports of CRS or TFR. Four acute GVHD events occurred in 3 (11.5%) HCT recipients; 3 events were deemed possibly related to tabelecleucel, but these cases had confounding factors. These results are in line with prior experience showing that tabelecleucel has been safe and well tolerated in >180 patients with R/R EBV+ PTLD, with TFR as the only identified risk (data on file, Atara Biotherapeutics).
Given these results and the favorable toxicity profile, tabelecleucel could help address a key unmet need in patients with R/R EBV+ PTLD, including special patient populations included in this study. For example, tabelecleucel demonstrates efficacy in pediatric patients aged <16 years (responses in 5 of 6 patients; ORR, 83.3%), with an overall safety profile that was comparable with that of adults. Another special population with high unmet need are patients with central nervous system (CNS) EBV+ PTLD, who typically have extremely poor outcomes with therapies because of limited drug penetration across the blood–brain barrier.41 However, in this study, both patients with R/R CNS EBV+ PTLD had disease that responded to tabelecleucel (1 CR, 1 PR), perhaps indicating an ability of EBV-specific T cells to cross the blood–brain barrier.42 These results support findings from previous studies in patients with CNS involvement whose disease responded to EBV-CTL therapy.42,43,53 Finally, patients considered high risk per the PTLD–adapted prognostic index may benefit from treatment with tabelecleucel; the ORR was 60.0%, even in the 5 high-risk patients included here.
Patients with EBV+ PTLD whose disease does not experience a CR to initial therapy with rituximab with/without chemotherapy have poor long-term outcomes,26,29,32,54 because of, in part, the toxicity,11,14,47 GVHD,14,26 and graft rejection11,12,26 typically associated with alternative therapies. In contrast, results from the multicenter expanded access protocol presented here, supported by those from the prior single-center experience, demonstrate clinically meaningful outcomes across a broad population treated with tabelecleucel. Additionally, this study supports the use of adoptive immunotherapy with off-the-shelf allogeneic EBV-CTLs such as tabelecleucel to achieve disease-specific responses without the potentially serious toxicities of CRS and neurologic complications that have been described with chimeric antigen receptor (CAR) T-cell therapies.46,55,56
In addition to efficacy and safety, the tabelecleucel manufacturing process and storage for off-the-shelf use overcomes much of the logistical complexity, clinical delays, and other concerns associated with autologous cell therapies that are currently US Food and Drug Administration approved for oncologic indications.55,57 In addition to these logistical challenges, for many of these patients, current or prior immunosuppression has significant impact on the feasibility of manufacturing autologous CARs at all. In contrast, the inventory of allogeneic tabelecleucel is ready in advance of patient need, with well-characterized HLA restriction facilitating the goal of rapid delivery to patients within days, compared with the weeks-long period for apheresis/acquisition of starting material and new manufacturing of donor-directed or autologous CTLs, autologous CARs, tumor-infiltrating lymphocytes, or other adoptive cell therapies.26,47,56-58 Allogeneic T cells collected from 1 donor and stored in third-party banks can also be used to create many doses and therefore treat a large volume of patients.57-59 Lastly, third-party EBV-CTLs are easily administered in an infusion clinic setting on an outpatient basis because of minimal toxicities and without the need for any lymphodepleting chemotherapy.43
Given the poor survival associated with this ultrarare and aggressive disease, further clinical investigation and confirmation in larger trials is warranted. Results from the first phase 3, multicenter, open-label, global registrational trial of tabelecleucel in EBV+ PTLD after HCT and SOT (ALLELE; NCT03394365; the only currently recruiting phase 3 EBV-CTL trial for PTLD) have confirmed outcomes from this study.60 Additionally, tabelecleucel is being investigated in other EBV+ diseases within NCT04554914; study cohorts include EBV+ primary immunodeficiency-LPD, EBV+ acquired immunodeficiency-LPD, EBV+ sarcoma (including leiomyosarcoma), EBV+ PTLD with CNS involvement, and EBV+ PTLD in which standard first-line therapy is inappropriate. Limitations of this current study include small sample size owing, in part, to the rarity of PTLD, as well as variable duration of follow-up because of the expanded access nature of the study. Additionally, the Kaplan-Meier estimated OS function may be limited after 24 months because of small sample size and censoring caused by early study discontinuation.
Based on the demonstrated safety and efficacy reported in this multicenter expanded access protocol, tabelecleucel is a novel, first-in-class, potentially practice-changing treatment for patients with treatment-refractory EBV+ PTLD after HCT or SOT. Tabelecleucel is rapidly available and has a favorable safety profile, allowing prompt outpatient administration, making it an appealing treatment option, especially in this posttransplant population with high unmet need. These clinically meaningful outcomes support tabelecleucel as a potentially transformative treatment advance for R/R EBV+ PTLD.
Acknowledgments
The authors gratefully acknowledge the patients who participated in these studies, their families, and the participating institutions. Medical writing assistance was provided by Tricia Gallagher AMICULUM Ltd, funded by Atara Biotherapeutics.
This study was funded by Atara Biotherapeutics.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for B-Cell Lymphomas version 1.2024 National Comprehensive Cancer Network, Inc, 2024.38 All rights reserved; accessed 21 March 2024. The most recent and complete version of the guideline is available online at: NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use, or application; and disclaims any responsibility for their application or use in any way.
Authorship
Contribution: All authors contributed to writing, reviewing, and approving the manuscript.
Conflict-of-interest disclosure: S.N. receives consultancy fees from GlaxoSmithKline, Iovance, and Kite/Gilead. J.S.W. receives consultancy fees from Mallinkrodt and Orchard Therapeutics, and is employed by VOR Biopharma. R.R. serves in a consultant or advisory role with Atara Biotherapeutics, Gilead Sciences, Takeda, Instil Bio, Regeneron, TScan, Synthekine, Orca, MidaTech, Capstan, and Jasper; serves in an expert witness role with Bayer; and receives research funding from Atara Biotherapeutics, Sanofi, Immatics, Takeda, Gilead Sciences, CareDx, TScan, Synthekine, Bristol Myers Squibb, Johnson & Johnson, and Precision Biosciences. D.E.T. reports receiving employment income and stock options from Loxo Oncology. K.M.M. receives support for the conduct of sponsored clinical trials from Atara Biotherapeutics. K.V.B. receives research funding from Miltenyi, Precision Biosciences, ORCA Biotherapeutics, Calibr, and Bristol Myers Squibb; is a shareholder and advisory board member for Hemogenyx; receives honorarium from Pfizer; and receives consultancy fees from Glycostem, Autolus, ADC Therapeutics, Gamida Biosciences, Intellia, and CTI. S.D.N. receives research funding from Genentech/Roche, Millenium/Takeda, Rafael, Debiopharm, and Pharmacyclics. S.P. is a coinventor of intellectual property licensed to Atara Biotherapeutics with all rights to this intellectual property assigned to Memorial Sloan Kettering Cancer Center (MSK) and has no personal financial interests in Atara Biotherapeutics, and received research funding in support of sponsored clinical trials from Atara Biotherapeutics, Jasper Therapeutics, and AlloVir through MSK. MSK has financial interests in Atara Biotherapeutics and intellectual property interests relevant to the work that is related to this study. A.M., J.W., L.G., and R.D. are employees and shareholders of Atara Biotherapeutics. W.Z., Y.S., and F.G. were employees of Atara Biotherapeutics at the time of the studies; the studies were funded by Atara Biotherapeutics starting in 2015. The remaining authors declare no competing financial interests.
Correspondence: Susan Prockop, Dana-Farber Cancer Institute/Boston Children's Hospital Cancer and Blood Disorders Center, Department of Pediatrics, Harvard Medical School, 450 Brookline Ave, SW331D, Boston, MA 02115; email: susan.prockop@childrens.harvard.edu.
References
Author notes
Individual participant data will not be shared.
The full-text version of this article contains a data supplement.