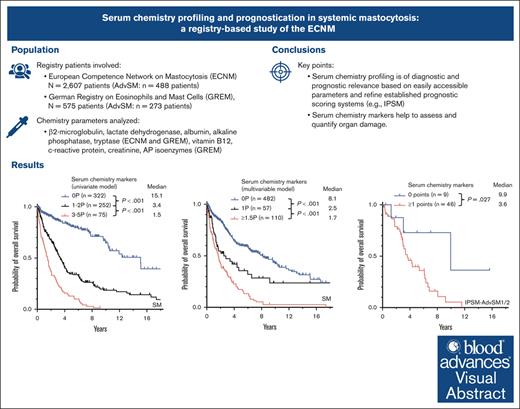
Serum chemistry profiling is of diagnostic and prognostic relevance based on easily accessible parameters.
Serum chemistry markers help to assess and quantify organ damage.
Visual Abstract
Certain laboratory abnormalities correlate with subvariants of systemic mastocytosis (SM) and are often prognostically relevant. To assess the diagnostic and prognostic value of individual serum chemistry parameters in SM, 2607 patients enrolled within the European Competence Network on Mastocytosis and 575 patients enrolled within the German Registry on Eosinophils and Mast Cells were analyzed. For screening and diagnosis of SM, tryptase was identified as the most specific serum parameter. For differentiation between indolent and advanced SM (AdvSM), the following serum parameters were most relevant: tryptase, alkaline phosphatase, β2-microglobulin, lactate dehydrogenase (LDH), albumin, vitamin B12, and C-reactive protein (P < .001). With regard to subvariants of AdvSM, an elevated LDH of ≥260 U/L was associated with multilineage expansion (leukocytosis, r = 0.37, P < .001; monocytosis, r = 0.26, P < .001) and the presence of an associated myeloid neoplasm (P < .001), whereas tryptase levels were highest in mast cell leukemia (MCL) vs non-MCL (308μg/L vs 146μg/L, P = .003). Based on multivariable analysis, the hazard-risk weighted assignment of 1 point to LDH (hazard ratio [HR], 2.1; 95% confidence interval [CI], 1.1-4.0; P = .018) and 1.5 points each to β2-microglobulin (HR, 2.7; 95% CI, 1.4-5.4; P = .004) and albumin (HR, 3.3; 95% CI, 1.7-6.5; P = .001) delineated a highly predictive 3-tier risk classification system (0 points, 8.1 years vs 1 point, 2.5 years; ≥1.5 points, 1.7 years; P < .001). Moreover, serum chemistry parameters enabled further stratification of patients classified as having an International Prognostic Scoring System for Mastocytosis–AdvSM1/2 risk score (P = .027). In conclusion, serum chemistry profiling is a crucial tool in the clinical practice supporting diagnosis and prognostication of SM and its subvariants.
Introduction
Systemic mastocytosis (SM) is characterized by expansion and accumulation of neoplastic mast cells (MCs) in various organ systems, frequently including the bone marrow (BM), skin, and the gastrointestinal tract. According to the World Health Organization (WHO) 2022 and the International Consensus Criteria (ICC) 2022, main subvariants of SM are indolent SM (ISM), smoldering SM (SSM), and advanced SM (AdvSM). BM mastocytosis (BMM) has previously been defined as a provisional subvariant of ISM and is now recognized as a separate variant of SM by the WHO. BMM is characterized by a limited degree of BM infiltration, absence of skin lesions, normal or slightly elevated serum tryptase levels, older age, male predominance, and a strong association with severe allergic reactions to hymenoptera sting. AdvSM comprises aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN)/myeloid neoplasm, and MC leukemia (MCL). In AdvSM, the majority of patients present with signs of organ damage (C-findings), particularly cytopenias and signs of liver involvement.1-4 A somatic point mutation in KIT at codon 816 (KIT D816V) is identified in >80% of all patients with SM.5-8 Although patients with indolent forms have a normal life expectancy, patients with AdvSM have a poor prognosis with shortened survival times ranging between 1 and 4 years.9-14
Serum chemistry parameters have been variably included in recently published risk-stratification scoring systems for SM. Besides serum tryptase, which is known for its outstanding value for screening and diagnosis, hypoalbuminemia and elevated alkaline phosphatase (AP) are established C-findings whereas β2-microglobulin is an unspecific but valuable prognostic marker in the Global Prognostic Score for SM (GPSM).12,15,16
Notwithstanding, thorough and systematic analyses on the diagnostic and prognostic value of those low-invasive and reproducible biomarkers are lacking. Based on data obtained from 2 registries (European Competence Network on Mastocytosis [ECNM] and the German Registry on Eosinophils and Mast Cells [GREM]), we therefore sought to establish various serum chemistry parameters for diagnosis and prognostication, the differentiation between ISM and AdvSM, and the differentiation between various subvariants of AdvSM.
Patients and methods
Patients
SM was diagnosed and classified according to the revised 2022 WHO/ICC classification. Patient characteristics are provided in supplemental Data (supplemental Table 1). The study design adhered to the tenets of the Declaration of Helsinki and was approved by the relevant institutional review boards of the participating centers. All patients gave written informed consent before registry participation.
ECNM registry
The ECNM registry was established in 2012 as a multidisciplinary, multinational cooperative initiative to analyze basic clinical, laboratory, and prognostic parameters in patients with cutaneous mastocytosis and SM.17-19 Details about the ECNM registry have been published elsewhere.19 For this analysis, we used data from a validated cohort updated in March 2019. Patients were enrolled from 26 centers in Europe (12 countries) and 1 center in the United States. We identified 2607 patients with SM with at least 1 entry regarding 1 of the following 5 serum chemistry parameters: serum tryptase, AP, β2-microglobulin, lactate dehydrogenase (LDH), and/or albumin at time of diagnosis. Three patients with MC sarcoma and 635 patients with mastocytosis in the skin were excluded. The 2607 patients were subtyped as having ISM (n = 1734, 66%), BMM (n = 327, 13%), SSM (n = 58, 2%), ASM (n = 117, 4%), SM-AHN (n = 326, 13%), and MCL (n = 45, 2%). Availability of data and disease characteristics of the overall population and a subgroup of patients with available outcome data (n = 685) are presented in supplemental Tables 1 and 2.
GREM registry
The first patients were enrolled in 2009. An updated cohort in May 2019 (n = 575; ISM, n = 224, 39%; BMM, n = 55, 10%; SSM, n = 23, 4%; ASM, n = 32, 5%; SM-AHN, n = 233, 41%; and MCL, n = 8, 1%) was used for complementary analyses on parameters that were primarily not included in the ECNM database (AP isoenzymes, C-reactive protein [CRP], and creatinine, and vitamin B12). A patient flow diagram is provided as supplemental Figure 1.
Statistical analyses
In this retrospective study, statistical analyses were performed on clinical, laboratory, and molecular parameters that were obtained at the time of diagnosis/first referral to the servicing center and throughout the disease course. Differences in the distribution of continuous variables between categories were analyzed by the Mann-Whitney U test. For categorical variables, Fisher exact test was used. Overall survival (OS) analysis was considered from the date of diagnosis to date of death for any reasons (event) or last documented visit. Deaths unrelated to disease progression were observed in <0.1% of the patients. Censoring was applied for patients who did not experience death or were lost to follow-up within the study period (type-1 right-censored data). OS probabilities were calculated by the Kaplan-Meier method and compared by the log-rank test. For multivariable analysis of serum chemistry markers, a Cox proportional hazard model was used. The proportional hazards assumption was tested by the correlation of scaled Schoenfeld residuals with time.20 Power calculation for the comparison of survival curves between 2 groups were performed according to Freedman.21 Receiver operating characteristic (ROC) analyses with calculation of the area under the ROC curve were performed to determine optimal cutoff values for the several laboratory parameters. The Youdens index was given as the summary measure of the ROC curve (sensitivity + specificity − 1). The Pearson and Spearman correlation coefficients were calculated to assess linear or monotonic relationships between 2 variables. P values were further adjusted by the Holm-Bonferroni method. In general, a test result with P < .05 has been considered as statistically significant. Statistical analyses were performed using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). For graphical output, GraphPad Prism version 9 was used (GraphPad Software Inc, San Diego, CA).
The study was approved by our local ethics committee led by Harald Klüter (2020-593N), and all patients gave written informed consent.
Results
ECNM registry–based analyses
Comparison of baseline serum parameters in ISM vs AdvSM
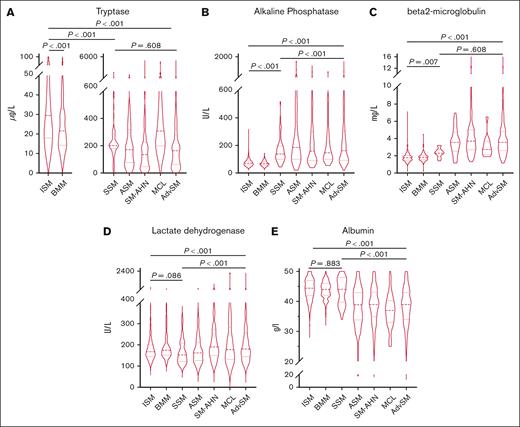
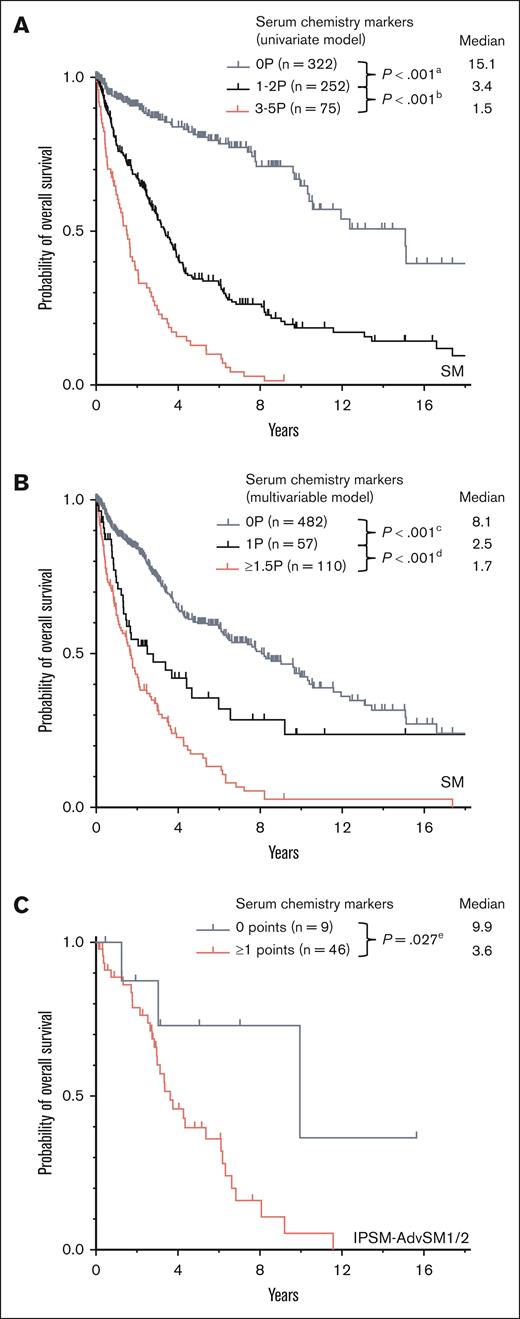
In AdvSM, significantly higher median serum levels were observed for tryptase (164 μg/Lvs 29 μg/L, P < .001, Mann-Whitney U test), AP (162 U/L vs 71 U/L, P < .001, Mann-Whitney U test), β2-microglobulin (3.6 mg/L vs 1.8 mg/L, P < .001, Mann-Whitney U test), and LDH (181 U/L vs 168 U/L, P < .001, Mann-Whitney U test), whereas significantly lower median serum levels were observed for albumin (39 g/dL vs 44 g/dL, P < .001, Mann-Whitney U test; Figure 1; supplemental Table 1). ROC analyses identified optimal cutoff values for discrimination between ISM and AdvSM for serum tryptase at 125 μg/L, for AP at 150 U/L, for β2-microglobulin at 2.5 mg/L, for LDH at 260 U/L, and for albumin at 34 g/dL. In 649 patients with diagnosis of SM and available outcome data, the log-rank test identified all respective markers as adverse prognostic variables regarding OS (Table 1). Assignment of 1 point each to 5 individual parameters allowed to establish a serum score through which patients at low, intermediate, and high risk can be defined (median OS 15.1 vs 3.4 vs 1.5 years, P < .001, log-rank test; Figure 2A). Multivariable analysis identified β2-microglobulin (hazard ratio [HR], 2.7; 95% confidence interval [CI], 1.4-5.4; P = .004), LDH (HR, 2.1; 95% CI, 1.1-4.0; P = .018), and albumin (HR, 3.3; 95% CI, 1.7-6.5; P = .001) of prognostic significance. Based on the multivariable analysis, we built an HR–weighted score and applied 1 point to patients with LDH of ≥260 U/L, and 1.5 points each to patients with β2-microglobulin of ≥2.5 mg/L and albumin of ≥34 g/dL (Figure 2B).
ECNM. Violin plots on the distribution of various serum chemistry markers throughout the systemic mastocytosis subtypes. The P values refer to the Mann-Whitney U test. AdvSM, advanced systemic mastocytosis; ASM, aggressive systemic mastocytosis; BMM, bone marrow mastocytosis; ISM, indolent systemic mastocytosis; MCL, mast cell leukemia; SM-AHN, systemic mastocytosis with an associated hematologic neoplasm; SSM, smoldering systemic mastocytosis.
ECNM. Violin plots on the distribution of various serum chemistry markers throughout the systemic mastocytosis subtypes. The P values refer to the Mann-Whitney U test. AdvSM, advanced systemic mastocytosis; ASM, aggressive systemic mastocytosis; BMM, bone marrow mastocytosis; ISM, indolent systemic mastocytosis; MCL, mast cell leukemia; SM-AHN, systemic mastocytosis with an associated hematologic neoplasm; SSM, smoldering systemic mastocytosis.
Univariate and multivariable analysis regarding OS of 685 patients with SM enrolled within the ECNM
Characteristics . | Univariate . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Tryptase (n = 625) ≥/<125μg/L | 3.4 (2.6-4.4) | <.001 | 1.8 (0.9-3.6) | .078 |
| AP (n = 565) ≥/<150U/L | 3.5 (2.7-4.4) | <.001 | 1.4 (0.7-3.0) | .334 |
| β2-microglobulin (n = 158) ≥/<2.5mg/L | 4.8 (2.9-7.9) | <.001 | 2.7 (1.4-5.4) | .004 |
| LDH (n = 533) ≥/<260 U/L | 2.0 (1.5-2.7) | <.001 | 2.1 (1.1-4.0) | .018 |
| Albumin (n = 508) </≥34g/dL | 3.7 (2.7-4.9) | <.001 | 3.3 (1.7-6.5) | .001 |
Characteristics . | Univariate . | Multivariable . | ||
|---|---|---|---|---|
| HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Tryptase (n = 625) ≥/<125μg/L | 3.4 (2.6-4.4) | <.001 | 1.8 (0.9-3.6) | .078 |
| AP (n = 565) ≥/<150U/L | 3.5 (2.7-4.4) | <.001 | 1.4 (0.7-3.0) | .334 |
| β2-microglobulin (n = 158) ≥/<2.5mg/L | 4.8 (2.9-7.9) | <.001 | 2.7 (1.4-5.4) | .004 |
| LDH (n = 533) ≥/<260 U/L | 2.0 (1.5-2.7) | <.001 | 2.1 (1.1-4.0) | .018 |
| Albumin (n = 508) </≥34g/dL | 3.7 (2.7-4.9) | <.001 | 3.3 (1.7-6.5) | .001 |
Analysis included variables with receiver operating characteristic-assessed cutoffs.
AP, alkaline phosphatase; CI, confidence interval; HR, hazard ratio; LDH, lactate dehydrogenase
ECNM. (A) Kaplan-Meier estimates of overall survival (OS) in patients with systemic mastocytosis (SM) with 0 points, 1 to 2 points, vs 3 to 5 points by assignment of 1 point each to serum tryptase ≥125 μg/L, alkaline phosphatase (AP) ≥150 U/L, lactate dehydrogenase (LDH) ≥260 U/L, albumin ≤34 mg/dL, and β2-microglobulin ≥2.5 mg/L; apower =1.000, bpower = 1.000. (B) Kaplan-Meier estimates of OS in patients with SM based on the multivariable model with 0 points, 1.5 points, vs ≥1.5 points by assignment of 1 point to LDH ≥260 U/L, and 1.5 points each to albumin ≤34 mg/dL and β2-microglobulin ≥2.5 mg/L; cpower = 1.000, dpower = 0.998. (C) Kaplan-Meier estimates of OS in patients weighted as International Prognostic Scoring System for Mastocytosis-advanced SM1/2 with 0 points vs ≥1 points. epower = 0.441. The P values refer to the log-rank test.
ECNM. (A) Kaplan-Meier estimates of overall survival (OS) in patients with systemic mastocytosis (SM) with 0 points, 1 to 2 points, vs 3 to 5 points by assignment of 1 point each to serum tryptase ≥125 μg/L, alkaline phosphatase (AP) ≥150 U/L, lactate dehydrogenase (LDH) ≥260 U/L, albumin ≤34 mg/dL, and β2-microglobulin ≥2.5 mg/L; apower =1.000, bpower = 1.000. (B) Kaplan-Meier estimates of OS in patients with SM based on the multivariable model with 0 points, 1.5 points, vs ≥1.5 points by assignment of 1 point to LDH ≥260 U/L, and 1.5 points each to albumin ≤34 mg/dL and β2-microglobulin ≥2.5 mg/L; cpower = 1.000, dpower = 0.998. (C) Kaplan-Meier estimates of OS in patients weighted as International Prognostic Scoring System for Mastocytosis-advanced SM1/2 with 0 points vs ≥1 points. epower = 0.441. The P values refer to the log-rank test.
Comparison of baseline serum parameters in patients with ISM, BMM, and SSM
Median serum tryptase and AP levels were significantly higher in SSM (202 μg/L and 138 U/L, respectively) and ISM (32 μg/L and 72 U/L, respectively) vs BMM (21μg/L and 67U/L, respectively; P < .001 for all comparisons, Mann-Whitney U test). SSM was further associated with higher β2-microglobulin levels compared with BMM (2.3 mg/L vs 1.8 mg/L, P = .010, Mann-Whitney U test). No differences were seen regarding the median albumin level (44 g/dL in all subgroups). Surprisingly, BMM (175 U/L) showed higher LDH levels than SSM (153 U/L, P = .002) and ISM (167 U/L, P = .001, Mann-Whitney U test). Patients with serum tryptase levels of ≥125 μg/L were associated with an adverse median OS (15.1 vs 9.7 years, P = .045, log-rank test; supplemental Table 1).
Comparison of baseline serum parameters within subvariants of AdvSM
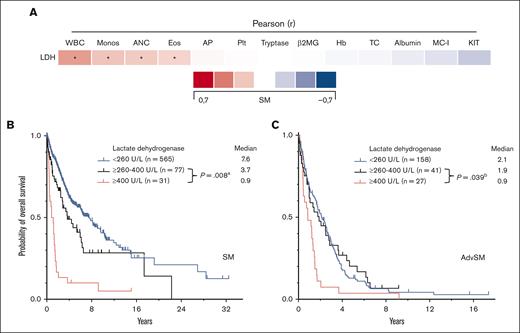
Serum tryptase levels were significantly higher in MCL (308 μg/L) than ASM (171μg/L, P = .006, Mann-Whitney U test) and SM-AHN (135 μg/L, P = .002, Mann-Whitney U test). An elevated LDH level of ≥260 U/L was associated with the presence of an AHN (77 of 236 [33%] vs 199 of 1715 [12%]; P < .001, Mann-Whitney U test) and with a more aggressive clinical course (Table 2; Figure 3B). The median LDH levels were significantly higher in SM-AHN than in ASM (191 vs 162 U/L, P < .001, Mann-Whitney U test) (Figure 1D). Pearson correlation of LDH levels with AHN-related parameters revealed modest correlations with the absolute numbers of leukocytes (r=0.37, P < .001; Figure 3A). Within AdvSM, patients with LDH levels of ≥260 U/L (n = 68), the threshold of ≥400 U/L or <400 U/L significantly affected the OS in this cohort (0.9 vs 1.9 years, P = .039, log-rank test; Figure 3C).
Patients with systemic mastocytosis (ECNM) stratified according to lactate dehydrogenase levels
| . | LDH ≥260 U/L . | LDH <260 U/L . | P value . |
|---|---|---|---|
| Number of patients at diagnosis, n (%) | 236 (12) | 1715 (88) | |
| Age, years; median (range) | 55 (18-90) | 50 (5-87) | <.001 |
| Male, n (%) | 149 (63) | 789 (46) | <.001 |
| C-findings | |||
| Hemoglobin, g/dL; median (range) | 13 (4-18) | 14 (4-18) | <.001 |
| <10 g/dL, n (%) | 44 (19) | 107 (6) | <.001 |
| Platelets, ×109/L; median (range) | 227 (0-958) | 251 (5-893) | NS |
| <100 ×109/L, n (%) | 51 (22) | 141 (8) | <.001 |
| ANC, ×109/L; median (range) | 4 (0-9) | 4 (0-76) | <.001 |
| <1 ×109/L, n (%) | 7 (3) | 20 (1) | .025 |
| Alkaline phosphatase, U/L; median (range) | 84 (34-1696) | 77 (20-1407) | .001 |
| >150 U/L, n (%) | 58 (27) | 206 (13) | <.001 |
| Albumin level, g/L; median (range) | 41 (16-57) | 44 (20-57) | <.001 |
| <34 g/L, n (%) | 26 (14) | 58 (4) | <.001 |
| Ascites, n (%) | 23 (10) | 97 (6) | .020 |
| Portal hypertension, n (%) | 7 (3) | 42 (3) | NS |
| Weight loss (>10% over last 6 months), n (%) | 44 (19) | 172 (10) | <.001 |
| Osteolytic lesions, n (%) | 7 (3) | 47 (3) | NS |
| B-findings | |||
| Dysmyelopoiesis, n (%) | 64 (28) | 206 (13) | <.001 |
| BM MC infiltration, %; median (range) | 15 (1-90) | 10 (0-100) | NS |
| Serum tryptase level, μg/L; median (range) | 43 (1-4530) | 36 (2-4980) | NS |
| >125 μg/L, n (%) | 59 (27) | 330 (20) | .020 |
| Splenomegaly, n (%) | 65 (28) | 291 (17) | <.001 |
| Hepatomegaly, n (%) | 58 (234) | 249 (1662) | <.001 |
| Lymphadenopathy, n (%) | 32 (210) | 148 (10) | .009 |
| Other relevant findings | |||
| Leukocytes, ×109/L; median (range) | 7.7 (0.6-129.3) | 6.7 (1.0-97.3) | <.001 |
| >16 ×109/L, n (%) | 47 (20) | 55 (3) | <.001 |
| Monocytes, ×109/L; median (range) | 0.5 (0.0-18.7) | 0.4 (0.0-8.2) | <.001 |
| >0.8 ×109/L | 44 (58) | 16 (12) | <.001 |
| Eosinophils, ×109/L; median (range) | 0.2 (0.0-35.0) | 0.1 (0.0-18.5) | .006 |
| >1.5 ×109/L | 17 (23) | 6 (4) | <.001 |
| KIT D816V positive, n (%) | 163 (82) | 1308 (88) | .029 |
| BM MC burden in smears, %; median (range) | 5 (0-88) | 2 (0-100) | .041 |
| Outcome | |||
| Follow-up, years; median (range) | 1.34 (0.0-22.3) | 2.1 (0.0-28.6) | NS |
| Death, n (%) | 65 (30) | 177 (14) | <.001 |
| . | LDH ≥260 U/L . | LDH <260 U/L . | P value . |
|---|---|---|---|
| Number of patients at diagnosis, n (%) | 236 (12) | 1715 (88) | |
| Age, years; median (range) | 55 (18-90) | 50 (5-87) | <.001 |
| Male, n (%) | 149 (63) | 789 (46) | <.001 |
| C-findings | |||
| Hemoglobin, g/dL; median (range) | 13 (4-18) | 14 (4-18) | <.001 |
| <10 g/dL, n (%) | 44 (19) | 107 (6) | <.001 |
| Platelets, ×109/L; median (range) | 227 (0-958) | 251 (5-893) | NS |
| <100 ×109/L, n (%) | 51 (22) | 141 (8) | <.001 |
| ANC, ×109/L; median (range) | 4 (0-9) | 4 (0-76) | <.001 |
| <1 ×109/L, n (%) | 7 (3) | 20 (1) | .025 |
| Alkaline phosphatase, U/L; median (range) | 84 (34-1696) | 77 (20-1407) | .001 |
| >150 U/L, n (%) | 58 (27) | 206 (13) | <.001 |
| Albumin level, g/L; median (range) | 41 (16-57) | 44 (20-57) | <.001 |
| <34 g/L, n (%) | 26 (14) | 58 (4) | <.001 |
| Ascites, n (%) | 23 (10) | 97 (6) | .020 |
| Portal hypertension, n (%) | 7 (3) | 42 (3) | NS |
| Weight loss (>10% over last 6 months), n (%) | 44 (19) | 172 (10) | <.001 |
| Osteolytic lesions, n (%) | 7 (3) | 47 (3) | NS |
| B-findings | |||
| Dysmyelopoiesis, n (%) | 64 (28) | 206 (13) | <.001 |
| BM MC infiltration, %; median (range) | 15 (1-90) | 10 (0-100) | NS |
| Serum tryptase level, μg/L; median (range) | 43 (1-4530) | 36 (2-4980) | NS |
| >125 μg/L, n (%) | 59 (27) | 330 (20) | .020 |
| Splenomegaly, n (%) | 65 (28) | 291 (17) | <.001 |
| Hepatomegaly, n (%) | 58 (234) | 249 (1662) | <.001 |
| Lymphadenopathy, n (%) | 32 (210) | 148 (10) | .009 |
| Other relevant findings | |||
| Leukocytes, ×109/L; median (range) | 7.7 (0.6-129.3) | 6.7 (1.0-97.3) | <.001 |
| >16 ×109/L, n (%) | 47 (20) | 55 (3) | <.001 |
| Monocytes, ×109/L; median (range) | 0.5 (0.0-18.7) | 0.4 (0.0-8.2) | <.001 |
| >0.8 ×109/L | 44 (58) | 16 (12) | <.001 |
| Eosinophils, ×109/L; median (range) | 0.2 (0.0-35.0) | 0.1 (0.0-18.5) | .006 |
| >1.5 ×109/L | 17 (23) | 6 (4) | <.001 |
| KIT D816V positive, n (%) | 163 (82) | 1308 (88) | .029 |
| BM MC burden in smears, %; median (range) | 5 (0-88) | 2 (0-100) | .041 |
| Outcome | |||
| Follow-up, years; median (range) | 1.34 (0.0-22.3) | 2.1 (0.0-28.6) | NS |
| Death, n (%) | 65 (30) | 177 (14) | <.001 |
A total of 1951 patients with systemic mastocytosis from the ECNM database were stratified according to lactate dehydrogenase levels of ≥260 U/L or <260 U/L.
ANC, absolute neutrophil count; BM, bone marrow; MC, mast cell; NS, not significant.
ECNM. (A) Pearson correlation of the lactate dehydrogenase (LDH) levels with other disease parameters. ∗Significant P values adjusted by Holm-Bonferroni method. (B) Kaplan-Meier estimates of overall survival (OS) in patients with systemic mastocytosis (SM) stratified according to LDH levels (260-400 U/L vs ≥400 U/L); apower = 0.995. (C) Kaplan-Meier estimates of OS in patients with AdvSM according to LDH levels (260-400 U/L vs ≥400 U/L); bpower = 0.720. The P values refer to the log-rank test. ANC, absolute neutrophil count; β2MG, β2-microglobulin; Eos, eosinophil count; Hb, hemoglobin; KIT, KIT D816V allele burden; MC-I, mast cell infiltration; Monos, monocyte count; Plt, platelets; WBC, white blood cells.
ECNM. (A) Pearson correlation of the lactate dehydrogenase (LDH) levels with other disease parameters. ∗Significant P values adjusted by Holm-Bonferroni method. (B) Kaplan-Meier estimates of overall survival (OS) in patients with systemic mastocytosis (SM) stratified according to LDH levels (260-400 U/L vs ≥400 U/L); apower = 0.995. (C) Kaplan-Meier estimates of OS in patients with AdvSM according to LDH levels (260-400 U/L vs ≥400 U/L); bpower = 0.720. The P values refer to the log-rank test. ANC, absolute neutrophil count; β2MG, β2-microglobulin; Eos, eosinophil count; Hb, hemoglobin; KIT, KIT D816V allele burden; MC-I, mast cell infiltration; Monos, monocyte count; Plt, platelets; WBC, white blood cells.
Further risk stratification in patients with IPSM-AdvSM1/2
Risk stratification was performed by using the previously published International Prognostic Scoring System for Mastocytosis (IPSM),11 a multiparametric (age of ≥60 year/<60 years, serum tryptase, ≥125 ng/mL/<125 ng/mL, leukocytes ≥16/nL/<16/nL, hemoglobin of ≥11g/dL/<11g/dL, and platelets ≥100/nL/<100/nL), with +1 point for each of these parameters, and −1 point for skin involvement resulting in a 3-tired risk model (consisting of a total score between −1 and 5 points) scoring system. In 55 patients with AdvSM with an IPSM risk score of IPSM-AdvSM1/2 (ie, −1 to 1 IPSM score points), assignment of 1 point to each of 5 serum parameters exceeding the described cutoff values (serum tryptase, AP, β2-microglobulin, LDH, and albumin; Table 1) allowed a further risk stratification with a median OS of 9.9 vs 3.6 years, respectively (P = .027, log-rank test), for patients with low (0 points, n = 9) vs high-risk (2-4 points, n = 46) disease (Figure 2C).
Complementary GREM-based analyses
AP isoenzymes
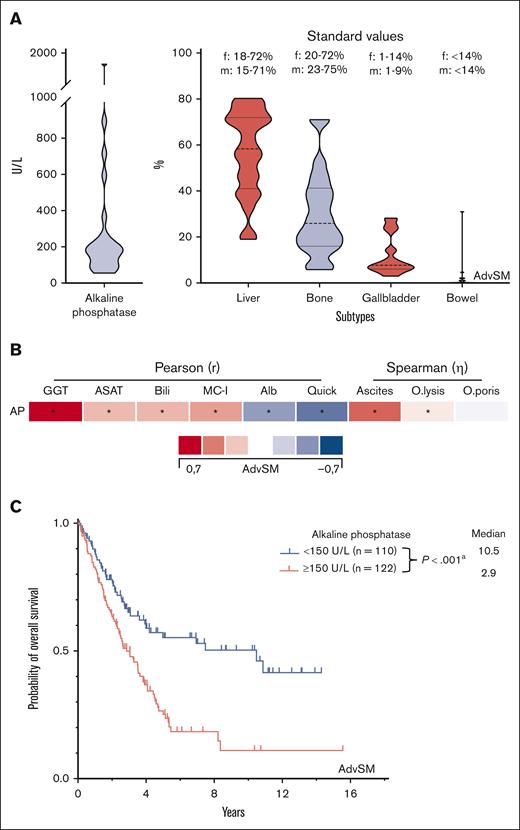
AP isoenzymes of the liver, gallbladder, bowel, and BM were measured in 24 patients with AdvSM. Compared with standard values, increased AP isoenzymes were predominantly derived from the liver and gallbladder (Figure 4A). The most robust, yet still moderate, correlations were seen with other liver parameters (γ-glutamyl transferase, r = 0.67, P < .001; albumin, r = −0.37, P < .001; international normalized ratio, r = −0.49, P < .001 all Pearson correlation) and the (relatively) poor correlation with bone and BM parameters (BM MC infiltration, r = 0.35, P < .001, Pearson correlation; osteoporosis, η = 0.09, P < .001; osteolysis, η = 0.16, P < .001, both Spearman correlation) further substantiated the strong association between elevated AP and liver involvement/damage (Figure 4B). AP levels of ≥150 U/L (optimal cutoff assessed per ROC analysis) significantly affected OS (10.5 vs 2.9 years, P < .001, log-rank test; Figure 4C).
GREM. (A) Violin plots on the distribution of the alkaline phosphatase (AP) subtypes in 24 patients with advanced systemic mastocytosis (AdvSM). (B) Pearson and Spearman correlations of the AP levels with other (liver and bone marrow) specific parameters. ∗Significant P values adjusted by Holm-Bonferroni method. (C) Kaplan-Meier estimates of overall survival in patients with AdvSM stratified according to AP levels ≥150 U/L/<150 U/L. apower = 0.974. Alb, albumin; ASAT, aspartate aminotransferase; Bili, bilirubin; MC-I, BM mast cell infiltration; O.lysis, osteolysis; O.poris, osteoporosis.
GREM. (A) Violin plots on the distribution of the alkaline phosphatase (AP) subtypes in 24 patients with advanced systemic mastocytosis (AdvSM). (B) Pearson and Spearman correlations of the AP levels with other (liver and bone marrow) specific parameters. ∗Significant P values adjusted by Holm-Bonferroni method. (C) Kaplan-Meier estimates of overall survival in patients with AdvSM stratified according to AP levels ≥150 U/L/<150 U/L. apower = 0.974. Alb, albumin; ASAT, aspartate aminotransferase; Bili, bilirubin; MC-I, BM mast cell infiltration; O.lysis, osteolysis; O.poris, osteoporosis.
Vitamin B12
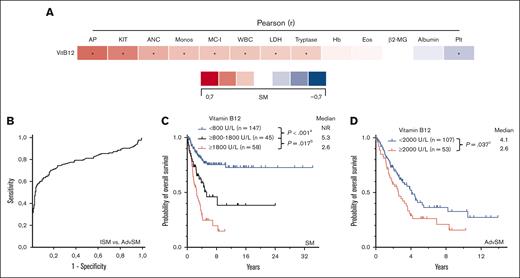
Median vitamin B12 levels (n = 334) were significantly higher in AdvSM than ISM (1275 U/L vs 401 U/L, P < .001, Mann-Whitney U test) or SSM (353 U/L, P < .001, Mann-Whitney U test; Figure 5B). Elevated vitamin B12 levels correlated with markers of advanced disease (eg, high KIT D816V allele burden, r = 0.45, P < .001; elevated AP, r = 0.51, P < .001, both Pearson correlation; Figure 5A). The area under the curve for predicting AdvSM was 0.795 (95% CI, 0.744-0.847; P < .001, ROC analysis) and the optimal cutoff value was 400 U/L with a sensitivity of 82% (Youden index, 0.307; Figure 5B). In AdvSM, vitamin B12 levels of ≥2000U/L conferred into worse outcome (median OS, 4.1 vs 2.6 years, P = .037, log-rank test; Figure 5D).
GREM. (A) Pearson correlation of the vitamin B12 levels with other disease specific parameters. ∗Significant P values adjusted by Holm-Bonferroni method. (B) Receiver operating characteristic-curve for optimal cutoff assessment regarding diagnosis of indolent systemic mastocytosis (ISM) vs advanced SM (AdvSM). (C) Kaplan-Meier estimates of overall survival (OS) in patients with SM according to vitamin B12 levels (<800 U/L vs ≥800-1800 U/L vs ≥1800 U/L); apower = 0.948, bpower = 0.627. (D) Kaplan-Meier estimates of OS in patients with AdvSM (≥2000 U/L vs <2000 U/L); cpower = 0.586. The P values refer to the log-rank test. ANC, absolute neutrophil count; β2MG, β2-microglobulin; Eos, eosinophil count; Hb, hemoglobin; KIT, KIT D816V allele burden; MC-I, mast cell infiltration; Monos, monocyte count; Plt, platelets; WBC, white blood cells.
GREM. (A) Pearson correlation of the vitamin B12 levels with other disease specific parameters. ∗Significant P values adjusted by Holm-Bonferroni method. (B) Receiver operating characteristic-curve for optimal cutoff assessment regarding diagnosis of indolent systemic mastocytosis (ISM) vs advanced SM (AdvSM). (C) Kaplan-Meier estimates of overall survival (OS) in patients with SM according to vitamin B12 levels (<800 U/L vs ≥800-1800 U/L vs ≥1800 U/L); apower = 0.948, bpower = 0.627. (D) Kaplan-Meier estimates of OS in patients with AdvSM (≥2000 U/L vs <2000 U/L); cpower = 0.586. The P values refer to the log-rank test. ANC, absolute neutrophil count; β2MG, β2-microglobulin; Eos, eosinophil count; Hb, hemoglobin; KIT, KIT D816V allele burden; MC-I, mast cell infiltration; Monos, monocyte count; Plt, platelets; WBC, white blood cells.
CRP
The median CRP levels (n = 405) were markedly increased in patients with AdvSM compared with either ISM (10.0 mg/dL vs 2.9 mg/dL, P < .001, Mann-Whitney U test) or SSM (3.7 mg/dL, P < .001, Mann-Whitney U test). Within AdvSM subvariants, the highest levels were found in SM-AHN (11 mg/dL) vs ASM (3.5 mg/dL, P = .057) or MCL (7.6 mg/dL, P = .008, Mann-Whitney U test). Concomitant infections or autoimmune diseases as confounding conditions could be excluded in 390 of 405 (96%) patients.
Creatinine-adjusted β2-microglobulin
Elevated β2-microglobulin levels were strongly associated with poor OS. Median OS was similarly affected in patients with AdvSM with β2-microglobulin levels of ≥2.5 mg/L independently whether or not the levels were adjusted for sex and age-matched serum creatinine values of >ULN (not adjusted: median OS was 4.1 years vs not reached, P < .001; median OS was 3.9 years vs not reached, P = .010, log-rank test).
Discussion
Diagnosis and prognostication of ISM and AdvSM warrants application of comprehensive clinical (eg, organomegaly and weight loss), morphological (eg, BM MC infiltration and blood counts), serological (eg, tryptase and AP), and molecular (eg, KIT D816V and additional somatic mutations) studies.5,6,13,15,22-28 Because of the frequent absence of skin involvement and of typical MC mediator–related symptoms in AdvSM, many patients are misdiagnosed or the diagnosis may be significantly delayed,15,24 unless disease-specific features and markers, such as BM MC infiltration, serum tryptase, or KIT D816V are considered and comprehensively studied. Although often used as a serological marker for screening of SM, an elevated serum tryptase may also be detected in patients with kidney failure, hereditary α-tryptasemia, obesity, and other myeloid neoplasms.16,29-33 While there is a certain correlation between serum tryptase levels and the subtype of SM, the differentiation between ISM, SSM, or AdvSM is not straightforward. Therefore, additional serologic parameters have been analyzed in the context of SM. One example is AP, which is typically elevated in patients with AdvSM with liver involvement. However, only a few comprehensive studies have systematically analyzed multiple serum chemistry parameters in patients with SM. Based on 2 robust patient registries, the ECNM registry and the GREM registry, we here show that a thorough and careful investigation and interpretation of certain serum chemistry parameters substantially supports the clinician in establishing the correct diagnosis, subclassification, and prognostication of patients with SM.
Although serum tryptase levels were highest in MCL, a level below 125 μg/L in indolent phase disease was clearly associated with a more favorable outcome in patients with ISM, BMM, and SSM thus verifying the established cutoff level for differentiation between ISM and BMM.34 In addition to serum tryptase, most valuable serum parameters include an elevated AP and hypoalbuminemia, which are widely included as C-findings in diagnostic criteria, recently established prognostic scoring systems, and response criteria.10,11,35 Based on AP isoform analyses, increased levels should be interpreted as liver involvement/damage by MC infiltration rather than as derived from bone involvement, for example, indicating osteosclerosis or osteolyzes. An elevated LDH correlated moderately with leukocytosis, monocytosis, and eosinophilia, indicating presence of an AHN.36 The careful cross-assessment of serum tryptase representing the MC component, LDH (plus monocytes/eosinophils) representing the AHN component, and the KIT D816V variant allele frequency potentially indicative for both components may therefore allow a rapid and thorough interpretation regarding the contribution of SM or AHN to relevant organ damage such as cytopenias and clinical parameters of liver dysfunction. It offers a first estimation of a sensible use of KIT-targeted treatment.4,13
Elevated vitamin B12 is a known, unspecific finding in various (myeloid) neoplasms.37-39 ROC analyses revealed that a cutoff level of ≥400 U/L (in the absence of supplementation) is indicative of AdvSM, and that a level of ≥2000 U/L correlated with adverse prognosis allowing its complimentary use for assessment of response and progression. Significant weight loss may result from malabsorption and malnutrition but also from chronic inflammation, and it was regularly accompanied by hypoalbuminemia and elevated CRP, with median CRP values being higher in AdvSM than in ISM (GREM cohort). Of interest, disease-associated symptoms such as night sweats or weight loss as well as the aforementioned serum chemistry parameters, responded significantly on targeted treatment with midostaurin or avapritinib in most patients.40-43
Several prognostic scoring systems have recently been established for patients with SM and its subtypes. Whereas some scores are based on clinical parameters with inclusion of serum chemistry parameters, for example, tryptase (IPSM), AP (GPSM), and β2-microglobulin (GPSM), and the WHO classification, others are based on additional molecular abnormalities. By now, no serum chemistry–specific score has been developed. In this study, assignment of 1 point each to 5 individual serum parameters based on ROC analyses defined cutoff values for tryptase, AP, β2-microglobulin, LDH, and albumin allowed to create a serum score with differentiation between patients with low-, intermediate-, and high-risk SM (median OS, 15.1 vs 3.4 vs 1.5 years, respectively, P < .001). Further adjunction of serum markers in patients with IPSM AdvSM1/2-weighted disease identified patients at higher risk in these otherwise favorable AdvSM subgroups. However, given the limited power of the log-rank test, a possible overestimation of the observed effect size could not be excluded with complete certainty, necessitating verification in a larger, higher-powered analysis. Unfortunately, information on β2-microglobulin was missing in 78% of patients from the ECNM cohort. We therefore strongly recommend the routine assessment of β2-microglubulin in the diagnostic workup of SM.
Given the unavailability of molecular analyses for nonhematologists and the multidisciplinary management of patients with SM by dermatologists, gastroenterologists, and immunologists/endocrinologists, it is of substantial relevance to identify easily available “red flags” pointing out on a more thorough diagnostic workup, for example, BM biopsy and molecular analyses, and the potential need for more aggressive treatment.
We conclude that beside its value for diagnosis according to WHO/ICC (albumin, tryptase, and AP) classifications and various risk scoring systems (β2-microglobulin and AP), routine serum chemistry should also include vitamin B12, LDH, and CRP, individually contributing to improved diagnosis, subclassification, and prognostication. Those easily accessible parameters combine advantages regarding availability, reliability, and observer-objectivity, and may therefore serve as feasible tools in the clinical practice of medical doctors from all specialties involved in the management of patients with SM.
Acknowledgments
J.L., A.R., and J.S. are supported by the Wilhelm Sander-Stiftung (grant no. 2023.120.1). P.V. was supported by the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung), grant numbers: F4704-B20 and P32470-B. E.H. was supported by the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung): P32470-B. M.N. and A.G. were supported by the Medical University of Gdańsk, grant ST 02-0141/07/231. K.H. was supported by the Swiss National Science Foundation, grant number: 310030_207705. M.L. was supported by the Medical University of Gdańsk, grant 02-10022/0000701. V.S. is a senior clinical researcher of the Research Foundation Flanders/Fonds Wetenschappelijk Onderzoek (1804518N). L.M. was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (Investigator grant no. 20125; AIRC 5×1000 project no. 21267); and Cancer Research UK, Fundacion Cientifica de la Asociacion Espanola Contra el Cancer, and AIRC under the International Accelerator Award Program (project no. C355/A26819 and no. 22796).
Authorship
Contribution: J.L., W.R.S., P.V., A.R., and J.S. conceptualized and designed the study; all authors provided study materials, and collected and assembled data; J.L., A.R., and J.S. analyzed and interpreted data; and all authors wrote the manuscript and approved the final version of the manuscript and are accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no conflict of interest within this study and declare the following conflict of interests outside of this study. D.C. received consulting fees from Blueprint Medicines, Novartis, Pfizer and BeiGene, honoraria from Blueprint Medicines, Novartis and AstraZeneca and travel support from Amgen and Janssen. H.N.G.O.E. serves on the advisory board of, and received honoraria from, Blueprint Medicines. R.Z. serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, and Cogent Biosciences. P.B. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis. K.S. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis; and is a member of the study steering committee for the CGT9486 Study of Cogent Biosciences. C.E. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Gilead. K.B. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis. V.S. serves on the advisory board of Blueprint Medicines and Novartis. K.H. received research funding from ThermoFisher; and serves on the advisory board of, and received honoraria from ALK-Abello, Allergopharma, Blueprint, Deciphera, Leo Pharma, Menarini, Novartis, Pfizer, Sanofi, Takeda, and ThermoFisher. M.T. serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, and Cogent. J.G. received research grants (funds for administration of clinical trials) from Novartis, Blueprint Medicines, and Cogent Biosciences; serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, Deciphera, and Cogent Biosciences; and received reimbursement of travel expenses from Novartis and Blueprint Medicines. M.A. received research grants from Blueprint Medicines; and serves on the advisory board of, and received honoraria from, AB Science, Blueprint Medicines, Novartis, and ThermoFisher. H.C.K.-N. served as a nonpaid independent monitoring committee member of the avapritinib study. J.P. serves on the advisory board of, and received honoraria from, Blueprint Medicines, Novartis, and Deciphera; is a member of the study steering committee for the HARBOR Study of BLU-263 for indolent systemic mastocytosis. W.R.S. serves on the advisory board of, and received honoraria from, AbbVie, Novartis, Bristol Myers Squibb/Celgene, Pfizer, Teva, and Stemline. P.V. serves on the advisory board of, and received honoraria from, Novartis, Blueprint, Bristol Myers Squibb/Celgene, Pfizer, Incyte, AOP Orphan, Cogent, and Stemline. A.R. is a member of the study steering committee for the global trial of midostaurin in AdvSM (Novartis); is a member of the response adjudication committee for studies of avapritinib in AdvSM (Blueprint Medicines), and the study steering committee for the phase 2 trial of ripretinib in AdvSM (Deciphera Pharmaceuticals); has received funding for the conduct of these trials; and has received honoraria and reimbursement of travel expenses from Novartis, Blueprint Medicines, and Deciphera Pharmaceuticals. J.S. serves on the advisory board of, and received honoraria from, Blueprint Medicines and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Juliana Schwaab, Department of Hematology and Oncology, University Hospital Mannheim, Heidelberg University, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Germany; email: juliana.schwaab@medma.uni-heidelberg.de.
References
Author notes
The data sets used and/or analyzed during this study are available on request from the corresponding author, Juliana Schwaab (juliana.schwaab@medma.uni-heidelberg.de).
The full-text version of this article contains a data supplement.