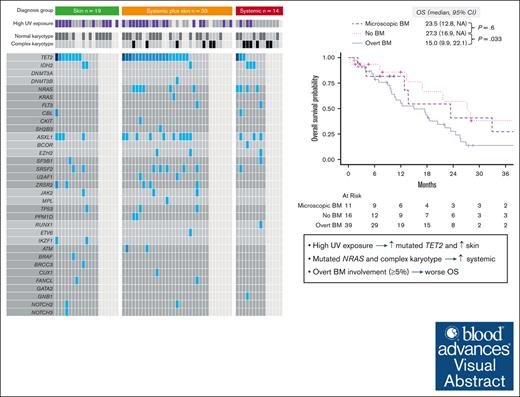
High UV exposure is associated with mutated TET2 and skin involvement in BPDCN, whereas mutated NRAS is associated with systemic involvement.
Overt BM involvement (≥5% BPDCN cells in the BM) at BPDCN diagnosis is independently associated with worse OS.
Visual Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) can involve skin, bone marrow (BM), central nervous system (CNS), and non-CNS extramedullary sites. Preclinical models demonstrated clonal advantage of TET2-mutated plasmacytoid dendritic cells exposed to UV radiation. However, whether sun exposure, disease characteristics, and patient survival are clinically related is unclear. We classified organ involvement in 66 patients at diagnosis as skin only (n = 19), systemic plus skin (n = 33), or systemic only (n = 14). BM involvement was absent, microscopic (<5%), or overt (≥5%). UV exposure was based on clinical and demographic data. Patients with skin only BPDCN were more frequently aged ≥75 years (47% vs 19%; P = .032) and had lower rates of complex karyotype (0 vs 32%, P = .022) and mutated NRAS (0 vs 29%, P = .044). Conversely, those without skin involvement had lower UV exposure (23% vs 59%, P = .03) and fewer TET2 mutations (33% vs 72%, P = .051). The median overall survival (OS) was 23.5, 20.4, and 17.5 months for skin only, systemic plus skin, and systemic only, respectively. Patients with no BM involvement had better OS vs overt involvement (median OS, 27.3 vs 15.0 months; P = .033) and comparable with microscopic involvement (27.3 vs 23.5 months; P = .6). Overt BM involvement remained significant for OS when adjusted for baseline characteristics and treatment received. In summary, BPDCN clinical characteristics are associated with disease genetics and survival, which together may impact prognosis and indicate informative disease subtypes for future research.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, aggressive hematologic neoplasm that arises from malignant plasmacytoid dendritic cells (pDCs) or their precursors.1,2 Most patients (80%-90%) present with characteristic, deep purple skin tumors or plaques. Remarkably, despite being a hematologic cancer, half of patients with BPDCN have disease restricted to the skin at diagnosis, without detectable disease in the marrow, blood, or lymph nodes.3 Recent findings show that migration of pDC progenitors to and from the skin, where they may be exposed to UV light, contributes to the development of BPDCN.4 Whether UV exposure influences likelihood of a cutaneous presentation of BPDCN, is associated with specific disease genetics, or influences prognosis is unknown.
In addition to skin, BPDCN can involve the bone marrow (BM), central nervous system (CNS), and non-CNS extramedullary disease (EMD) sites.5-7 Conflicting data exist as to whether the pattern of anatomic involvement affects prognosis. Specifically, the role of low burden, nonovert BM involvement (>0% but <5%, known as “microscopic” or “minimal” disease) remains unclear, because trials have only regarded ≥5% as evidence of BPDCN. Furthermore, BPDCN genetics have not been fully incorporated into prognostic models that include sites of disease.8-10
To address these questions, we conducted a retrospective cohort study to characterize the associations between genetics, clinicopathological characteristics, and UV exposure in BPDCN and to determine the impact of these disease features on clinical outcomes.
Methods
Patients
We retrospectively identified consecutive patients with biopsy-proven BPDCN seen at the Dana-Farber Cancer Institute between 2006 and 2022. Patient, disease, and treatment characteristics were extracted from electronic medical records. UV exposure was dichotomized as high vs low through a comprehensive analysis of each patient’s medical, occupational, and social histories by an independent investigator (C.J.F.), who was blinded to study outcomes. A patient was assigned to the high UV exposure category if they satisfied at least 1 of the following criteria: documented presence of severely sun-damaged skin by a dermatologist, history of blistering sunburns, documented employment or recreational activities necessitating a majority of time outdoors, or history of residing in geographical regions with substantially heightened sun exposure for >10 cumulative years. Next-generation sequencing was performed on blood or BM at diagnosis.11 BM involvement at diagnosis was classified as overt (≥5% BPDCN cells on aspirate smear, flow cytometry, or core biopsy), microscopic (>0% but <5% identifiable clonal BPDCN cells), or absent (no evidence of any BPDCN cells). Based on organ involvement at diagnosis, we classified 3 organ involvement groups: “systemic without skin” (absence of skin involvement, with either overt BM and/or EMD involvement), “skin only” (skin involvement, with no EMD and absent or microscopic BM involvement), or “skin and systemic” (skin involvement, with either overt BM involvement and/or EMD involvement; supplemental Figure 1). Due to paucity of data on CNS involvement at diagnosis, particularly from earlier years before CNS evaluation was standard in BPDCN, anatomic groups were classified irrespective of CNS involvement.
Outcomes
Overall survival (OS) was calculated from day 1 of treatment until death or last follow-up. Responses were documented as the best response achieved per line of therapy and classified as complete remission (CR; <5% disease cells in the BM and no EMD, CNS, or skin involvement documented), partial response (<5% disease cells in the BM and no EMD and CNS, improvement in skin lesion), or progressive disease (overt BM disease or EMD or CNS disease, progression or new skin lesions).
Statistics
Categorical variables are presented as numbers and percentages, and comparisons were performed by Fisher exact test. Continuous variables are presented as median and range or interquartile range (IQR), and comparisons were performed by Wilcoxon rank-sum or Kruskal-Wallis tests. All survival data and duration of response (DOR) were calculated with the Kaplan-Meier method and reported as medians plus 95% confidence interval (CI). Survival comparisons were made by the log-rank test. Cumulative incidence of relapse (CIR) was performed with death as competing risk, and comparisons were conducted by the Gray test. CIR and DOR were only calculated for patients who achieved CR after the first line of therapy. Univariable and multivariable Cox regression models were fit for association with OS, with allogeneic hematopoietic stem cell transplantation (allo-SCT) as a time-varying covariate. Mutated genes were univariable model candidates if prevalent in ≥10% of patients. All analyses were conducted using R, version 4.3.
This study was conducted with the approval of the institutional review board at the Dana-Farber Cancer Institute.
Results
Patients
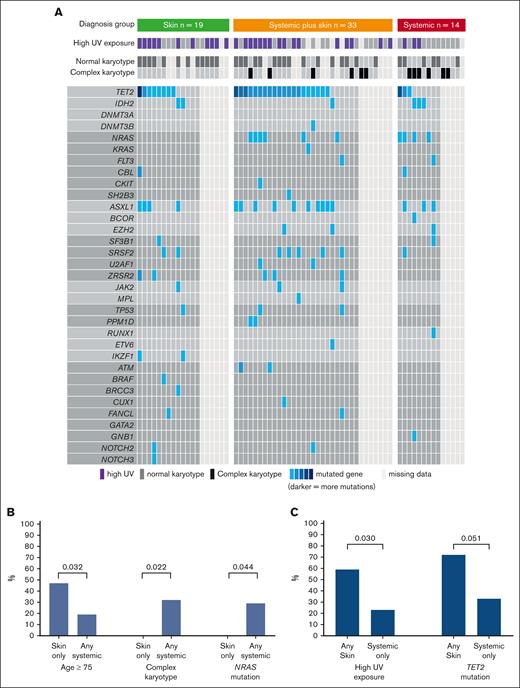
Overall, we included 66 patients (median age, 68 years; IQR, 61-76), who were predominantly male (n = 57 [86%]; Table 1). Disease distribution at diagnosis involved skin (n = 52 [79%]), BM (n = 50 [76%]; 39 [59%] with overt involvement and 11 [17%] with microscopic involvement), and EMD (n = 30 [45%]). Based on the aforementioned criteria, patients were classified into 3 diagnostic organ groups: skin only (n = 19 [29%]), skin plus systemic (n = 33 [50%]), and systemic only (n = 14 [21%]; Figure 1). Ten patients (15%) had prior myeloid disease, most commonly myelodysplastic syndrome, with no statistically significant difference in prior myelodysplastic syndrome between organ groups (skin only, n = 5 [26%]; skin plus systemic, n = 2 [6%]; systemic only, n = 3 [21%]; P = .1). Eleven patients (17%) had prior nonmelanoma skin cancer, and the same number (n = 11 [17%]) had another solid tumor. UV exposure history was assessable for 59 of 66 patients (89%), of whom 30 (51%) were classified as high UV exposure. Among patients with known karyotype, normal and complex karyotypes at diagnosis were seen in 34 (68%) and 12 of 50 (24%), respectively. TET2 was the most commonly mutated gene found in the BM/blood at diagnosis (31/48 [65%] patients with molecular data available; Figure 2A). The median time interval from first symptom to diagnosis was 61 days (IQR, 35-134) and from diagnosis to day 1 of treatment was 30 days (IQR, 20-46). The first therapeutic regimen was tagraxofusp (n = 30 [45%]), AML–based (n = 12 [18%]), or ALL–based (n = 12 [18%]) chemotherapy regimens or other (n = 12 [18%]). Overall, patients received a median of 2 lines of therapy (range, 0-6).
Patient characteristics
| . | N (%) (N = 66) . |
|---|---|
| Sex (male) | 57 (86) |
| Age, median, IQR, y | 68.3 (61.2, 75.5) |
| Age groups | |
| <60 y | 15 (23) |
| 60 y to <75 y | 33 (50) |
| ≥75 y | 18 (27) |
| Prior neoplasms | |
| Myeloid disease | 10 (15) |
| Non-melanoma skin cancer | 11 (17) |
| Other solid cancer | 11 (17) |
| ECOG∗ | |
| 0 | 43 (66) |
| 1 | 21 (32) |
| 2 | 1 (2) |
| UV exposure† | |
| High | 30 (51) |
| Low | 29 (49) |
| Cytogenetics‡ | |
| Complex karyotype | 12 (24) |
| Normal karyotype | 34 (68) |
| Other | 4 (8) |
| First treatment | |
| Tagraxofusp | 30 (45) |
| AML-like intensive chemotherapy regimen | 12 (18) |
| ALL-like intensive chemotherapy regimen | 12 (18) |
| Others | 12 (19) |
| Selected time intervals | |
| Diagnosis to first treatment (d, median, IQR) | 29.5 (20.0, 46.0) |
| First symptom to first treatment (d, median, IQR) | 108 (64.2, 161.0) |
| . | N (%) (N = 66) . |
|---|---|
| Sex (male) | 57 (86) |
| Age, median, IQR, y | 68.3 (61.2, 75.5) |
| Age groups | |
| <60 y | 15 (23) |
| 60 y to <75 y | 33 (50) |
| ≥75 y | 18 (27) |
| Prior neoplasms | |
| Myeloid disease | 10 (15) |
| Non-melanoma skin cancer | 11 (17) |
| Other solid cancer | 11 (17) |
| ECOG∗ | |
| 0 | 43 (66) |
| 1 | 21 (32) |
| 2 | 1 (2) |
| UV exposure† | |
| High | 30 (51) |
| Low | 29 (49) |
| Cytogenetics‡ | |
| Complex karyotype | 12 (24) |
| Normal karyotype | 34 (68) |
| Other | 4 (8) |
| First treatment | |
| Tagraxofusp | 30 (45) |
| AML-like intensive chemotherapy regimen | 12 (18) |
| ALL-like intensive chemotherapy regimen | 12 (18) |
| Others | 12 (19) |
| Selected time intervals | |
| Diagnosis to first treatment (d, median, IQR) | 29.5 (20.0, 46.0) |
| First symptom to first treatment (d, median, IQR) | 108 (64.2, 161.0) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ECOG, Eastern Cooperative Oncology Group.
ECOG is missing for 1 patient.
UV exposure data is missing for 7 patients.
Cytogenetic data is missing for 16 patients.
Patients’ organ involvement and group allocation. ∗Defined as ≥5% in BM. In addition, 11 patients had measurable microscopic disease (<5%) in BM: 7 in the skin only group; 2 in the systemic plus skin group; 2 in the systemic only group.
Patients’ organ involvement and group allocation. ∗Defined as ≥5% in BM. In addition, 11 patients had measurable microscopic disease (<5%) in BM: 7 in the skin only group; 2 in the systemic plus skin group; 2 in the systemic only group.
UV exposure, cytogenetics, and molecular abnormalities by diagnostic organ groups. (A) Molecular and cytogenetic samples taken from BM or blood at diagnosis. (B) Comparison between skin only group vs any systemic involvement (defined as systemic only or systemic plus skin). (C) Comparison between systemic-only group vs any skin involvement (defined as skin only group or systemic plus skin).
UV exposure, cytogenetics, and molecular abnormalities by diagnostic organ groups. (A) Molecular and cytogenetic samples taken from BM or blood at diagnosis. (B) Comparison between skin only group vs any systemic involvement (defined as systemic only or systemic plus skin). (C) Comparison between systemic-only group vs any skin involvement (defined as skin only group or systemic plus skin).
Clinicopathological associations with organ involvement group at diagnosis
Patients in the skin only group (n = 19) vs those with any systemic involvement (n = 47; composed of systemic only and systemic plus skin groups) were more frequently aged ≥75 years (9/19 [47%] vs 9/47 [19%]; P = .032), had lower rates of complex karyotype (0 vs 12 [32%]; P = .022), and had lower rates of activated signaling mutations (NRAS, KRAS, FLT3, CBL, CKIT, and SH2B3; 1 [8%] vs 14 [40%]; P = .04), mainly driven by lower NRAS (0 in skin only vs 10 [29%] with any systemic; P = .044; Figure 2B). In contrast, those with any skin involvement (n = 52; composed of skin only and systemic plus skin groups) had higher rates of UV exposure (27 [59%] vs 3 [23%]; P = .03) and TET2 mutations (28 [72%] vs 3 [33%]; P = .051) than those with no skin involvement (Figure 2C).
To determine whether patients with overt BM (n = 39; ≥5%) differ from those with microscopic BM involvement (n = 11; >0 but <5%), we compared those patients’ baseline characteristics (supplemental Table 1). Patients with overt BM disease had shorter period between diagnosis and treatment (median, 25 days; IQR, 15-37; vs median, 45 days; IQR, 34-69; P = .019), higher rates of complex karyotype (40% vs 0%; P = .036), and lower rates of normal karyotype (50% vs 100%; P = .007) than patients with microscopic BM involvement.
Frequent involvement of the CNS in patients with BPDCN was increasingly recognized in recent years; thus, systematic evaluation of the CNS at diagnosis was not performed in all patients throughout the entire study period. However, among the 16 patients who were diagnosed in 2021 and later, 15 had CNS evaluation at diagnosis; 3 of the 15 (20%) were positive. Among the 58 patients who had at least 1 CNS evaluation by lumbar puncture at any time, 16 (28%) had evidence of BPDCN cells in the cerebrospinal fluid. The rates of CNS disease at any time point were higher among those with systemic only disease at presentation (7/13 [54%]) than those who had any skin involvement (either skin only, 3/17 [18%]; or systemic plus skin, 6/28 [21%]; P = .031).
Response, relapse, and allo-SCT
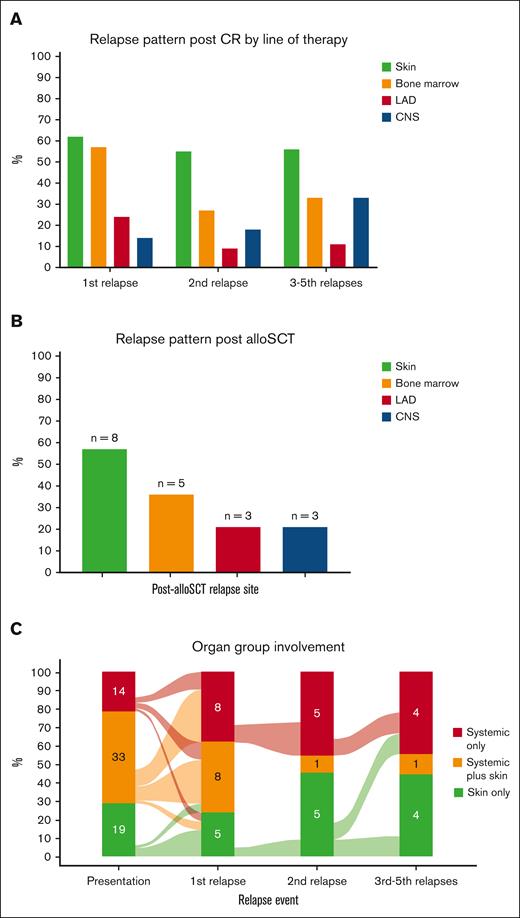
Complete and partial remissions after first treatment were achieved in 35 (57%) and 13 (21%) patients, respectively. After a median of 6.1 months (IQR, 2.4-8.1) from the first CR achievement, 22 of 35 (63%) who had achieved CR relapsed (Table 2), with skin as the most common relapse site (n = 13 of 21 evaluated patients; 62%), followed by overt BM (n = 12 [57%]), EMD (n = 5 [24%]), and CNS (n = 3 [14%]; Figure 3A). Although CRs were also achieved after subsequent lines of therapy (CR achievement after second line, 18/35; after third line, 7/20; after fourth line, 2/12; after fifth line, 2/4; after sixth line, 2/3; Table 2), those responses were not durable and most patients relapsed, with skin consistently being the most common organ involved in relapse (Figure 3A).
CRs and post-CR relapse after each treatment
| . | N, % . | |||||
|---|---|---|---|---|---|---|
| Line of therapy | First line | Second line | Third line | Fourth line | Fifth line | Sixth line |
| Treated | 66 | 38∗ | 21† | 14‡ | 4 | 3 |
| Achieved complete response | 35 (53%) | 18 (51%) | 7 (35%) | 2 (17%) | 2 (50%) | 2 (67%) |
| Relapsed after complete response | 22/35 (63%) | 11/18 (61%) | 6/7 (86%) | 2/2 (100%) | 1/2 (50%) | 0/2 (0%) |
| Time from previous line (IQR), mo | NR | 3.4 (2.7-7.6) | 3.1 (1.9,6.1) | 3.9 (2.3-6.6) | 1.4 (0.5-5.9) | 2.7 (2.1-3.9) |
| . | N, % . | |||||
|---|---|---|---|---|---|---|
| Line of therapy | First line | Second line | Third line | Fourth line | Fifth line | Sixth line |
| Treated | 66 | 38∗ | 21† | 14‡ | 4 | 3 |
| Achieved complete response | 35 (53%) | 18 (51%) | 7 (35%) | 2 (17%) | 2 (50%) | 2 (67%) |
| Relapsed after complete response | 22/35 (63%) | 11/18 (61%) | 6/7 (86%) | 2/2 (100%) | 1/2 (50%) | 0/2 (0%) |
| Time from previous line (IQR), mo | NR | 3.4 (2.7-7.6) | 3.1 (1.9,6.1) | 3.9 (2.3-6.6) | 1.4 (0.5-5.9) | 2.7 (2.1-3.9) |
NR, not relevant.
Three patients missing evaluation post second line.
One patient missing evaluation post third line.
Two patients missing evaluation post fourth line.
Organ involvement at relapse. (A) Involvement by lines of therapy. (B) Involvement after allo-SCT. (C) Involvement classified by diagnostic organ groups. CR, complete response; LAD, lymphadenopathy.
Organ involvement at relapse. (A) Involvement by lines of therapy. (B) Involvement after allo-SCT. (C) Involvement classified by diagnostic organ groups. CR, complete response; LAD, lymphadenopathy.
To evaluate whether the pattern of relapse differs after allo-SCT, we evaluated all patients who underwent transplantation. Overall, 35 patients (53%) received allo-SCT, most commonly in CR1 (28/35 [80%]), with reduced intensity conditioning in 54% and a matched unrelated donor in 46% (supplemental Table 2). After a median of 5.9 months (IQR, 2.8-17.7) following allo-SCT, relapse occurred in 14 of 35 patients (40%) who received transplantation. Similar to the aforementioned relapse pattern, skin was also the most common relapse site after allo-SCT (n = 8 [57%]), followed by overt BM (n = 5 [36%]), EMD (n = 3 [21%]), and CNS (n = 3 [21%]; Figure 3B).
When evaluated by organ involvement groups, both first treatment CR rates (skin only 50%; systemic only 57%; systemic plus skin 61%; P = .7) and relapse rates (skin only 50%; systemic only 75%; systemic plus skin 63%; P = .6) were comparable. The CR rates with tagraxofusp vs other treatments were numerically lower in those with skin disease (30% vs 83%; P = .12) and in those with systemic disease (41% vs 71%; P = .063), but these results did not reach statistical significance. When we evaluated the pattern of post-CR relapse by organ groups, the diagnostic group tended to predict organ group at relapse: those who were classified as skin only at diagnosis tended to relapse as skin only (5/8 [63%]); those classified as systemic only at diagnosis relapsed as systemic only (4/9 [44%]) or systemic plus skin (3/9 [33%]); and those with systemic plus skin had a more heterogenous pattern of relapse (Figure 3C).
Survival
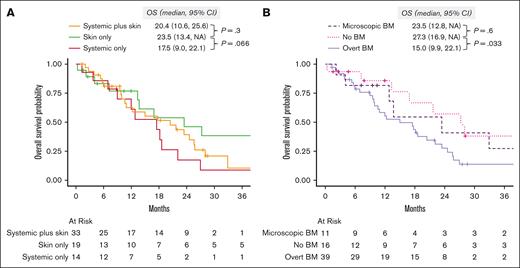
After a median follow-up of 41.6 months (95% CI, 28-81), the median OS was 18.2 months (95% CI, 13-24). When evaluated by organ involvement group, the median OS was 23.5 months (95% CI, 13.4 to not available [NA]) in the skin only group, 20.4 months (95% CI, 10.6-25.6) in the skin plus systemic group (P = .3, compared with the skin only group), and 17.5 months (95% CI, 9.0-22.1) in the systemic only group (P = .066, vs skin only group; Figure 4A). When we evaluated survival by the type of BM involvement (none vs microscopic vs overt), those with no BM involvement (median OS, 27.3 months; 95% CI, 16.9 to NA) had better OS than those with overt BM involvement (median OS, 15.0 months; 95% CI, 9.9-22.1; P = .033), and comparable with those with microscopic BM involvement (median OS, 23.5 months; 95% CI, 12.8 to NA; P = .6; Figure 4B).
Overall survival. (A) Stratified by diagnostic organ group. (B) Stratified by BM involvement: none vs microscopic (defined as >0 and < 5% of BPDCN cells) vs overt (defined as ≥5% BPDCN cells in the BM). NA, not available.
Overall survival. (A) Stratified by diagnostic organ group. (B) Stratified by BM involvement: none vs microscopic (defined as >0 and < 5% of BPDCN cells) vs overt (defined as ≥5% BPDCN cells in the BM). NA, not available.
In a multivariable analysis, age <60 years (vs 60-75 years) was associated with improved OS (hazard ratio [HR], 0.35; 95% CI, 0.13-0.94; P = .038), whereas BM involvement ≥5% (but not >0 to <5% involvement) was independently associated with worse OS (HR, 2.65; 95% CI, 1.1-6.36; P = .029; Table 3). When we conducted univariable and multivariable analyses in patients who were transplant eligible by age (≤75 years, n = 48), we found that allo-SCT as a time-varying covariate was associated with better OS (HR, 0.4; 95% CI, 0.17-0.94; P = .036). In contrast, similar to the entire cohort, overt BM involvement at diagnosis was associated with worse survival even in those receiving allo-SCT (HR, 3.34; 95% CI, 0.97-11.5; P = .055; supplemental Table 3).
Univariate and multivariate Cox regression OS analysis
| Covariate . | Univariable analysis, HR (95% CI) . | P value . | Multivariable analysis, HR (95% CI) . | P value . |
|---|---|---|---|---|
| Age (relative to 60 y to <75 y) | ||||
| <60 y | 0.30 (0.12-0.75) | .0097 | 0.35 (0.13-0.94) | .038 |
| ≥75 y | 1.87 (0.94-3.68) | .072 | 1.74 (0.78-3.89) | .17 |
| Sex (relative to female) | 1.83 (0.65-5.13) | .25 | ||
| Prior neoplasm | ||||
| Prior myeloid neoplasm | 1.25 (0.56-2.83) | .59 | ||
| Prior solid cancer | 0.63 (0.25-1.60) | .33 | ||
| ECOG (1 or 2 relative to 0)∗ | 1.83 (0.97-3.44) | .061 | ||
| Cytogenetics† | ||||
| Complex karyotype | 1.16 (0.52-2.57) | .72 | ||
| Normal karyotype | 0.59 (0.29-1.20) | .15 | ||
| Organ involvement at diagnosis | ||||
| Skin at diagnosis | 0.58 (0.29-1.13) | .11 | ||
| BM at diagnosis | ||||
| Microscopic (<5% involvement) | 1.36 (0.46-4.06) | .58 | 1.09 (0.35-3.42) | .89 |
| Overt (≥5% involvement) | 2.40 (1.04-5.54) | .039 | 2.65 (1.10-6.36) | .029 |
| EMD at diagnosis | 1.37 (0.75-2.51) | .31 | ||
| Molecular‡ | ||||
| ASXL1 | 1.46 (0.71-3.02) | .30 | ||
| IDH2 | 0.95 (0.33-2.76) | .93 | ||
| NRAS | 1.26 (0.53-2.96) | .60 | ||
| SRSF2 | 1.71 (0.64-4.57) | .29 | ||
| TET2 | 1.95 (0.89-4.31) | .097 | ||
| ZRSR2 | 1.78 (0.67-4.76) | .25 | ||
| First treatment (SL401 relative to no SL401) | 0.92 (0.50-1.68) | .78 | ||
| Allo-SCT as time-varying covariate | 0.34 (0.17-0.68) | .0024 | 0.55 (0.24-1.30) | .17 |
| Covariate . | Univariable analysis, HR (95% CI) . | P value . | Multivariable analysis, HR (95% CI) . | P value . |
|---|---|---|---|---|
| Age (relative to 60 y to <75 y) | ||||
| <60 y | 0.30 (0.12-0.75) | .0097 | 0.35 (0.13-0.94) | .038 |
| ≥75 y | 1.87 (0.94-3.68) | .072 | 1.74 (0.78-3.89) | .17 |
| Sex (relative to female) | 1.83 (0.65-5.13) | .25 | ||
| Prior neoplasm | ||||
| Prior myeloid neoplasm | 1.25 (0.56-2.83) | .59 | ||
| Prior solid cancer | 0.63 (0.25-1.60) | .33 | ||
| ECOG (1 or 2 relative to 0)∗ | 1.83 (0.97-3.44) | .061 | ||
| Cytogenetics† | ||||
| Complex karyotype | 1.16 (0.52-2.57) | .72 | ||
| Normal karyotype | 0.59 (0.29-1.20) | .15 | ||
| Organ involvement at diagnosis | ||||
| Skin at diagnosis | 0.58 (0.29-1.13) | .11 | ||
| BM at diagnosis | ||||
| Microscopic (<5% involvement) | 1.36 (0.46-4.06) | .58 | 1.09 (0.35-3.42) | .89 |
| Overt (≥5% involvement) | 2.40 (1.04-5.54) | .039 | 2.65 (1.10-6.36) | .029 |
| EMD at diagnosis | 1.37 (0.75-2.51) | .31 | ||
| Molecular‡ | ||||
| ASXL1 | 1.46 (0.71-3.02) | .30 | ||
| IDH2 | 0.95 (0.33-2.76) | .93 | ||
| NRAS | 1.26 (0.53-2.96) | .60 | ||
| SRSF2 | 1.71 (0.64-4.57) | .29 | ||
| TET2 | 1.95 (0.89-4.31) | .097 | ||
| ZRSR2 | 1.78 (0.67-4.76) | .25 | ||
| First treatment (SL401 relative to no SL401) | 0.92 (0.50-1.68) | .78 | ||
| Allo-SCT as time-varying covariate | 0.34 (0.17-0.68) | .0024 | 0.55 (0.24-1.30) | .17 |
ECOG, Eastern Cooperative Oncology Group.
One patient was omitted from the univariable regression analysis for ECOG due to missing data.
Sixteen patients were omitted from the univariable regression analysis for complex karyotype and normal karyotype due to missing data.
Eighteen patients were omitted from the univariable regression analysis for molecular variables due to missing data.
To evaluate for an interaction between organ groups at diagnosis, treatment modality (tagraxofusp vs others), and outcome, we conducted treatment analyses separately in the skin only group (n = 19) and in those with any systemic involvement (n = 47). There were no statistically significant differences in OS, DOR, or CIR rates between tagraxofusp vs other treatments in either the skin only group or in those with any systemic involvement (supplemental Table 4).
Discussion
BPDCN is a rare, aggressive malignant neoplasm that arises from pDCs and characteristically involves the skin but also frequently involves BM, EMD sites, and CNS.12 Recent evidence suggests a role for UV exposure in BPDCN pathogenesis.4 However, the correlation between clinicopathological characteristics, UV exposure, organ involvement at presentation, and their effect on outcomes is unclear.
Here, we demonstrate, to our knowledge, for the first time an association between skin involvement at diagnosis, high UV exposure, and TET2 mutations. Our findings are consistent with the recently demonstrated link between UV exposure and selection for TET2 mutated pDCs in BPDCN development, as well as previous findings on the association of TET2 with BPDCN.13-16 Furthermore, the high prevalence of skin involvement among patients with TET2-mutated BPDCN exposed to UV denotes that UV exposure is not only associated with the development of BPDCN but may also affect its presentation. We also showed that patients with skin only involvement at diagnosis were older. Because mutated TET2 is considered a common age-dependent clonal hematopoiesis mutation with increasing frequency at older age11 and older patients have UV exposure accumulation throughout their life,17,18 our findings are consistent with UV exposure and mutated TET2 as a combined aging-associated mechanism of BPDCN oncogenesis in older patients, particularly in those presenting with skin only disease. Because skin involvement is also seen in other myeloid neoplasms,19 future research might evaluate the role of UV radiation in these cases well. On the contrary, systemic involvement was associated with NRAS mutations and higher rates of complex karyotype. It is not clear whether systemic patients have distinct disease pathogenesis compared with those with skin disease or rather whether the acquisition of certain mutations such as in RAS or chromosomal abnormalities direct the difference in organ involvement.20 Furthermore, BPDCN-associated somatic mutations, including in TET2 and RNA splicing factors, have been implicated in impaired tumor cell cytokine production and dendritic activation phenotypes compared with normal pDCs.21,22 How these various BPDCN genotype-phenotype associations at the cellular level may be related to the features of organ involvement and clinical outcomes revealed in this study are important areas for future research.
We found that overt BM involvement (≥5% BPDCN cells in the BM at diagnosis) but not microscopic involvement (defined as presence of BPDCN cells <5% in the BM) is independently associated with worse OS when adjusted in a multivariable model. In addition, when classified by diagnostic organ groups, there was worse survival among those in the systemic only group vs skin only group. The effect of organ involvement on prognosis was evaluated in several previous studies with conflicting results.3,8,23 The difference may relate, in part, to modest cohort size or variable definitions of organ involvement or other clinicopathologic characteristics between studies. In our study, the prognostic value of BM involvement was retained even after adjustment for several other clinicopathologic characteristics, such as demographics, cytogenetics and tumor DNA sequencing, initial treatment, and allo-SCT. The effect of overt marrow involvement should be integrated into future risk models and clinical trials evaluating treatment for BPDCN.
Similar to previous studies,24,25 we demonstrated that skin is the most common site involved and is found in ∼80% of patients at diagnosis. Here, we also showed that skin is the most common relapse site, after first treatment, after subsequent lines of therapy, and after transplant. We also demonstrated that the diagnostic organ involvement patterns influence subsequent relapses sites. Despite this, given that some patients switched to a different organ involvement group at the time of relapse, these data emphasize the importance of assessing all possible disease compartments in all patients regardless of their prior organ involvement, including frequent meticulous skin evaluations. Further consideration regarding the best approaches to achieve and verify complete clearance of the skin are warranted, because beyond standard clinical examination and pathology, there are not yet established standards for determining integumentary measureable residual disease (MRD) with certainty.
Our study has several limitations. First, its retrospective nature and heterogeneity of patients spanning almost 2 decades may contribute to bias, which we partially addressed using a regression analysis that included treatment modalities. In addition, although we know today that CNS involvement is far more common than once thought,7 we had incomplete data on CNS evaluation at the time of diagnosis and whether the patients received prophylactic intrathecal treatment. However, we integrated all available data and found an association between CNS involvement at any time and systemic only disease at diagnosis. Finally, samples for next-generation sequencing were derived only from BM and/or blood; a different molecular pattern might be revealed by including genetic evaluation of other involved tissues, particularly the skin. However, because blood or BM remain the most common and feasible sites for next-generation sequencing as part of routine clinical care in patients with leukemia including BPDCN, our findings are more likely to be used in the “real world” clinical setting.
In summary, in a cohort of 66 patients with BPDCN, we found genotype-phenotype associations with disease presentation, and a correlation between organ involvement at diagnosis and subsequent relapses. We also demonstrated that overt BM involvement is independently associated with worse OS, even after adjustment for multiple clinicopathologic and molecular characteristics. Future studies should integrate organ involvement into prognostic models for BPDCN and explore potential mechanisms that delineate distinct phenotypic presentations of the disease.
Acknowledgments
This work was supported by the DFCI Hematologic Malignancies Data Repository team. Financial support was provided by the Leukemia & Lymphoma Society.
Authorship
Contribution: S.S. and A.A.L. designed the research and wrote the initial draft of the manuscript; S.S. and C.J.F. performed data extraction; S.S., J.K., and D.S.N. analyzed the data; J.K., C.J.F., M.R.L., D.S.N., N.R.L., and A.A.L. reviewed the manuscript and contributed to its final version; and all authors reviewed the final version of the manuscript and agreed on submission.
Conflict-of-interest disclosure: M.R.L. receives research funding from AbbVie, Jazz Pharmaceuticals, and Novartis. N.R.L. is a consultant and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics. A.A.L. is a Scholar of the Leukemia & Lymphoma Society; has received research funding from AbbVie and Stemline Therapeutics; received consulting fees from Cimeio Therapeutics, IDRx, Jnana Therapeutics, ProteinQure, and Qiagen; and has equity as an adviser for Medzown. The remaining authors declare no competing financial interests.
Correspondence: Andrew A. Lane, Division of Hematologic Neoplasia, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 413, Boston, MA 02215; email: andrew_lane@dfci.harvard.edu.
References
Author notes
Data that support the findings of this study are available on request from the corresponding author, Andrew A. Lane (andrew_lane@dfci.harvard.edu). The data are not publicly available due to privacy or ethical restrictions.
The full-text version of this article contains a data supplement.