Genetic deletion of Dusp1 eliminates CSF3R-induced leukemia.
Inhibition of Dusp1 induces the expression of Bim and p53 in oncogenic context, resulting in selective demise of leukemic cells.
Visual Abstract
Elevated MAPK and the JAK-STAT signaling play pivotal roles in the pathogenesis of chronic neutrophilic leukemia and atypical chronic myeloid leukemia. Although inhibitors targeting these pathways effectively suppress the diseases, they fall short in providing enduring remission, largely attributed to the cytostatic nature of these drugs. Even combinations of these drugs are ineffective in achieving sustained remission. Enhanced MAPK signaling besides promoting proliferation and survival triggers a proapoptotic response. Consequently, malignancies reliant on elevated MAPK signaling use MAPK feedback regulators to intricately modulate the signaling output, prioritizing proliferation and survival while dampening the apoptotic stimuli. Herein, we demonstrate that enhanced MAPK signaling in granulocyte colony-stimulating factor 3 receptor (CSF3R)–driven leukemia upregulates the expression of dual specificity phosphatase 1 (DUSP1) to suppress the apoptotic stimuli crucial for leukemogenesis. Consequently, genetic deletion of Dusp1 in mice conferred synthetic lethality to CSF3R-induced leukemia. Mechanistically, DUSP1 depletion in leukemic context causes activation of JNK1/2 that results in induced expression of BIM and P53 while suppressing the expression of BCL2 that selectively triggers apoptotic response in leukemic cells. Pharmacological inhibition of DUSP1 by BCI (a DUSP1 inhibitor) alone lacked antileukemic activity due to ERK1/2 rebound caused by off-target inhibition of DUSP6. Consequently, a combination of BCI with a MEK inhibitor successfully cured CSF3R-induced leukemia in a preclinical mouse model. Our findings underscore the pivotal role of DUSP1 in leukemic transformation driven by enhanced MAPK signaling and advocate for the development of a selective DUSP1 inhibitor for curative treatment outcomes.
Introduction
Enhanced MAPK (mitogen activated protein kinase) activity due to activating mutations or overexpression of pathway components is one of the hallmarks of cancer.1 Despite considerable knowledge about MAPK interactions with downstream effectors, the intricacies driving cellular transformation remain less elucidated. Depending on the cellular and genetic context, MAPK signaling can either promote cell proliferation and survival or instigate apoptotic machinery for cellular destruction.2,3 For instance, RAS (Rat Sarcoma)-MAPK signaling not only triggers proliferative pathways but also activates stress-activated MAPKs, P38, and c-Jun N-terminal kinase (JNK), implicated in apoptosis induction.4,5 However, the fate of cells, whether they undergo apoptosis or proliferate, is determined by genetic and cellular contexts. To accomplish this, most tumor cells use MAPK-negative feedback regulators to suppress the apoptotic response.6 Importantly, the magnitude, duration, and location of MAPK signaling are strictly regulated to support malignant growth.7 Both positive and negative feedback regulators are implicated in shaping the final signaling output through spatio-temporal regulation. MAPK-negative feedback loops are composed of both transcriptional (MAPK phosphatases or dual specificity phosphatases [DUSPs]) and posttranscriptional (direct phosphorylation of pathway components by ERK1/2).6 Although the mechanisms activating RAS-MAPK signaling in cancer cells are well explored, the understanding of how negative feedback regulators contribute to cellular transformation and treatment outcomes is not fully understood. Given the essential role of MAPK-negative regulators in dampening the apoptotic stimulants, we hypothesize that inhibiting these regulators could unleash robust apoptotic response that may selectively eliminate the leukemic clones.
Activating mutations in the granulocyte colony-stimulating factor 3 receptor (CSF3R) has been reported in patients with chronic neutrophilic leukemia (CNL) and acute myeloid leukemia.8 A minority of patients harbor >1 mutation (compound mutation), which causes more aggressive leukemia. Previous studies from Drucker and Tyner et al reported that CSF3R proximal mutants depend on JAK-STAT signaling, whereas the truncation mutants seemingly rely on SRC-dependent survival.8 Despite significant strides in understanding the biology of CSF3R-induced leukemogenesis, effective treatment is lacking especially for patients with high-risk CNL. Our earlier work using mouse models underscored the essential role of enhanced MAPK signaling in CSF3R-induced leukemia.9 As a result, treatment with a MEK inhibitor suppressed the leukemic progression. However, akin to JAK2 inhibitors, MEK inhibition engendered a cytostatic response. Even a combination of inhibitors targeting both JAK2 and MEK was ineffective in inducing the clonal selectivity.9 Subsequent studies, using unbiased phospho-proteomic analyses, unveiled persistent BTK signaling in CSF3R-induced leukemia.10 Nevertheless, attempts to enforce clonal selectivity through inhibition of BTK alone or in combination with JAK2 or MEK inhibitors were ineffective (unpublished).
Cancers fueled by elevated MAPK signaling induce its negative feedback regulators (MKP activity) to fine-tune the signaling output and suppress ERK-induced apoptotic activity to foster cancer progression.5,11,12 Herein, we show that enhanced MAPK activity in CSF3R-induced leukemia is associated with elevated expression of dual specificity phosphatase 1 (DUSP1). Deletion of Dusp1 is synthetic lethal to CSF3R-induced leukemia. DUSP1 depletion in the leukemic context induced the enzymatic activity of JNK1/2 that suppressed the expression of BCL2 (B cell leukemia/lymphoma 2) while inducing the expression of BIM (BCL2 like 11) and trnsformation related protein (P53). This meticulous fine-tuning of apoptotic machinery resulted in selective demise of leukemic cells. Unexpectedly, chemical inhibition of DUSP1 by BCI was ineffective in suppressing the leukemic progression. We noted that ERK1/2 rebound abrogated the treatment response to BCI due to off-target inhibition of DUSP6. As a proof of concept, ectopic overexpression of Dusp6 in leukemic cells restored the antileukemic effect of BCI. Accordingly, leukemic mice treated with a combination of BCI and trametinib, such as genetic deletion of Dusp1, selectively eradicated the CSF3R-induced leukemia. Altogether, these observations provide evidence that DUSP1 confers oncogene dependence, whereas DUSP6 functions as a tumor suppressor in CSF3R-driven leukemia. Targeting DUSP1 with a selective inhibitor could provide a curative response to CSF3R mutant–driven myeloid leukemia.
Materials and methods
Plasmids and inhibitors
Retroviral plasmids expressing CSF3R mutants (CSF3R-WT, CSF3RT618I, CSF3RQ741∗, CSF3RW791∗, CSF3RT618I/Q741∗, and CSF3RT618I/W791) were described earlier.9 Ruxolitinib (JAK2 inhibitor) and trametinib (MEK1/2 inhibitor) were purchased from AdooQ Biosciences. DUSP1 inhibitor BCI [(2E)-2-benzylidene-3-(cyclohexylamino)-3H-inden-1-one], was custom synthesized by Tocris (Bio-Techne Inc).
Cell lines
BaF3 cell line, a growth factor dependent murine pro–B-cell line, was transduced using retroviral vectors expressing CSF3R mutants and venus as described earlier.13,14 After 24 hours of viral transduction, venus-positive cells were sorted by fluorescence-activatedcellsorting (FACS) and grown without interleukin-3 (IL3). Retroviral production and transduction were performed as described earlier.9 BaF3 cells were grown in RPMI 1640) supplemented with 10% FBS (fetal bovine serum) and 100 μg/mL streptomycin, 100 IU/mL penicillin, and 2mM L-glutamine. HEK293T were grown in Dulbecco's Modified Eagle Medium (DMEM) containing 10% FBS and 100 IU/mL penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine. For in vitro proliferation assays, BaF3 parental cells or BaF3 cells expressing CSF3R mutants were grown in RPMI supplemented with 10% FBS, 100 μg/mL streptomycin, 100 IU/mL penicillin, 2mM L-glutamine, and recombinant murine IL-3 (10 ng/mL) from Peprotech for 1 week. All cell lines were evaluated for mycoplasma contamination.
Immunoblotting
Six million BaF3 cells expressing CSF3R variants were suspended in lysis buffer followed by sonication. Composition of lysis buffer has been described earlier.9 Lysates were separated by 10% SDS-PAGE and transferred to supported nitrocellulose membrane (Bio-rad) and probed with anti-HA tag, phospho-MEK1/2, phospho-ERK1/2, phospho-STAT3, phospho-STAT5 MEK1/2, ERK1/2, STAT5, P53, BIM, BCL2, phospho-JNK1/2, JNK1/2, phospho-P38, and P38 antibodies (Cell Signaling Technology). Anti-DUSP1 antibody was purchased from Santacruz biotechnology. All primary antibodies were used at dilutions as recommended by the manufacturer. Anti-mouse or anti-rabbit immunoglobulin G HRP conjugated secondary antibodies (GE Healthcare) were used at a 1:5000 dilution. HRP conjugated β-actin antibody was purchased from Cell Signaling Technology. Immunoblots were developed using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific) followed by scanning on ChemiDoc Touch Imaging system (Bio-Rad). All western blots were replicated twice.
Hematopoietic colony forming cell assays
Hematopoietic progenitors Kit+ cells from the bone marrow (BM) of C57Bl/6 or Dusp1-/- mice were isolated using the CD117 MicroBead Kit (Miltenyi Biotec, Inc) according to the manufacturer’s instructions. Cells were cultured in IMDM supplemented with 10% FBS and 100 μg/mL streptomycin, 100 IU/mL penicillin, and 2 mM L-glutamine with the addition of mSCF (50 ng/mL), mTPO (50 ng/mL), mFLT3-L (20 ng/mL), mIL-6 (10 ng/mL), and mIL-3 (10 ng/mL; R&D systems). After 12 hours of stimulation, cells were transduced with retroviruses expressing CSF3R mutants and venus using home-made retronectin. A total of 35 000 venus-positive cells (isolated by FACS) were plated in triplicate in MethoCult GF M3434 (STEMCELL Technologies) with ruxolitinib (1 μM), trametinib (5 nM), BCI (400 nM), ruxolitinib + BCI (1 μM + 400 nM), and trametinib + BCI (5 nM + 400 nM). Colonies were enumerated after 1 week of incubation at 37°C.
BM transduction and transplantation
BM transduction and transplantations were performed as described earlier.9 Kit+ BM cells were isolated from the wild-type (C57Bl/6) or Dusp1-/- mice and transduced with retroviruses expressing vector (pMSCV-Ires-Venus, pMIV), pMSCV-CSF3RT618I-Ires-Venus, and pMSCV-CSF3RT618I/Q741∗-Ires-Venus. Five mice for each construct received transplantation with 100 000 venus-positive cells mixed with 0.2 to 0.5 million helper BM cells into each lethally irradiated mouse through tail vein injection. Sample sizes were chosen on the basis of previous experience and published transplantation data. The experiment was repeated 3 times with similar results. After 2 weeks of transplantation, engraftments were determined by analyzing the venus-positive cells from the peripheral blood (PB) using (flow activated cell sorting) FACS. These mice were monitored for leukemia progression and survival, and leukemic burden (venus-positive cells) and white blood cell numbers were determined weekly up to 16 to 18 weeks in surviving mice. All mouse work was performed with approval of the Institutional Animal Care and Use Committee.
Flow cytometry
Twenty μL of PB were collected from the mice that received transplantation via tail bleeding. Cells were lysed using RBC lysis buffer (BD Technologies), and the total mononuclear cells were pelleted by centrifugation. The cell pellets were washed once with cold phosphate-buffered saline, followed by blocking for 10 minutes at room temperature using mouse Fc Receptor (FcR) blocking reagent (Miltenyi biotec, inc). Antibody staining and FACS analysis were performed as described earlier.9
Drug preparation and in vivo treatments
All drug stocks were made in dimethyl sulfoxide to 10 mM and stored in –20°C until use. For in vivo injection, dimethyl sulfoxide drug stocks of ruxolitinib, trametinib, and BCI were diluted in phosphate-buffered saline. After 2 weeks of transplantation, venus percentage was measured to determine the leukemic engraftment and chimerism. The mice were grouped, and drugs were orally administered or injected through intraperitoneal injection. Ruxolitinib (50 mg/kg twice a day) and trametinib (10 mg/kg once daily) were given by oral gavage. BCI (10 mg/kg twice) was administered through intraperitoneal injection as described earlier.14
Statistical analysis
Statistical analyses were performed using Prism software v9.0 (GraphPad Software). The median survival was calculated by log-rank test. For in vitro studies, statistical significance was determined by the 2-tailed unpaired t test. A P value <.05 was considered statistically significant; for all figures: NS, not significant; ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001. Unless otherwise indicated, all data represent the mean ± standard deviation from 3 technical replicates.
Results
Induced expression of Dusp1 in CSF3R-induced leukemia
Earlier, we have shown that the expression of CSF3R wild-type or truncation mutants (Q741∗ or W791∗) were unable to induce leukemia despite persistent granulocytic hyperplasia.9 A comparative whole-genome expression profiling of leukemic and nonleukemic CSF3R mutants revealed induced expression of 23 MAPK pathway genes in leukemic cells.9 Consequently, MAPK-negative regulators are induced to prevent the apoptotic stimulation associated with elevated MAPK activity. Although we noted differential expression of Dusp family members in cells expressing CSF3R mutants compared with vector control, expression of Dusp1 is specifically induced only in cells expressing CSF3R mutants (Figure 1A). Quantitative expression analysis by quantitative polymerase chain reaction of Lineage-negative, KIT+, and SCA1+ cells revealed significant induction of Dusp1 in cells expressing CSF3R variants compared with CSF3R-WT and vector control (Figure 1B). Notably, cells expressing leukemic CSF3R variants displayed higher Dusp1 expression (Figure 1B). Next, to determine the role of Dusp1 in leukemic transformation by CSF3R mutants, BaF3 cells expressing control Sc-shRNA (scrambled- short hairpin RNA) and Dusp1-shRNA14 were transduced with retroviruses expressing CSF3R mutants, followed by an analysis of growth kinetics and downstream MAPK signaling. The expression of CSF3R mutants renders BaF3 cells with growth factor independence, although with differing transformation potential, as follows: compound mutations > proximal mutation > truncation mutation > wild-type.9 As reported earlier,14 expression of Dusp1-shRNA resulted in ∼60% to 70% knockdown of DUSP1 protein (Figure 1C). Depletion of Dusp1 did not show any effect on survival and proliferation of parental BaF3 or BaF3 cells expressing CSF3R mutants when grown with IL-3 (Figure 1D; supplemental Figure 1A). However, in the absence of growth factor, IL-3, Dusp1 depletion significantly reduced the survival and proliferation of CSF3R mutants (Figure 1E; supplemental Figure 1B). Biochemical analysis of MAPK signaling by western blotting revealed that DUSP1 depletion resulted in enhanced JNK1/2 activation and suppression of pERK1/2 compared with controls (cells expressing vector and nonleukemic CSF3R truncation mutants, CSF3RQ741∗ and CSF3RW791∗). In contrast, induction of phospho-P38 levels were noted indiscriminately in all CSF3R mutants, leukemic and nonleukemic (Figure 1C). Altogether, these results suggest that DUSP1 confers dependence to CSF3R-driven leukemia by modulating the MAPK-JNK1/2 signaling output.
Induced expression of Dusp1 in CSF3R mutant expressing cells. (A) Heat map showing deregulated expression of MAPK pathway genes in BM-derived Kit+ cells expressing different CSF3R mutants. Total RNA from the venus-positive Kit cells after 24 hours of transduction were subjected to RNA-seq analysis.9 Total RNA from the vector (pMSCV-Ires-venus) transduced Kit+ cells was used to filter out differentially expressing cells. Differences in the levels of expression were measured using t test between the samples from the leukemic variants (CSF3RT618I and CSF3RT618I/Q741∗) and nonleukemic truncation mutants (CSF3RQ741∗ and CSF3RW791∗). (B) A bar graph showing the relative expression of Dusp1 in Lineage-negative, KIT+, and SCA1+ cells expressing CSF3R mutants. (C) Immunoblots from the total protein extracts of control (sc-shRNA) and Dusp1-depleted BaF3 cells expressing CSF3R mutants grown without IL-3 showing reduced p-ERK1/2 and induced activation of p-JNK1/2 upon Dusp1 knockdown in cell expressing leukemic CSF3R mutants (CSF3RT618I, CSF3RT618I/Q741∗, and CSF3RT618I/W791). In contrast, the nonleukemic CSF3R truncation mutations exhibit modest elevation in p-ERK1/2 without any alteration in p-JNK1/2 levels. Expression levels were quantified and normalized to the control condition (pMIV-Vector expressing Sc-ShRNA normalized to to β-actin). The resulting normalized values are presented below each blot for reference. BaF3 cells expressing Dusp1-shRNA resulted in ∼6% to 70% knockdown at protein level and control sc-shRNA has been described earlier.14 (D) A cell proliferation growth curve of Dusp1-depleted BaF3 cells expressing CSF3R mutants showing normal growth when grown with IL-3. (E) Growth curve showing significantly reduced proliferation upon Dusp1 depletion in the absence of IL3. This assay revealed that the transformation potential of CSF3R is compromised upon Dusp1 depletion. Presented data are from 2 independent experiments, shown as means ± standard deviation (SD). ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001. RNA-seq, RNA sequencing.
Induced expression of Dusp1 in CSF3R mutant expressing cells. (A) Heat map showing deregulated expression of MAPK pathway genes in BM-derived Kit+ cells expressing different CSF3R mutants. Total RNA from the venus-positive Kit cells after 24 hours of transduction were subjected to RNA-seq analysis.9 Total RNA from the vector (pMSCV-Ires-venus) transduced Kit+ cells was used to filter out differentially expressing cells. Differences in the levels of expression were measured using t test between the samples from the leukemic variants (CSF3RT618I and CSF3RT618I/Q741∗) and nonleukemic truncation mutants (CSF3RQ741∗ and CSF3RW791∗). (B) A bar graph showing the relative expression of Dusp1 in Lineage-negative, KIT+, and SCA1+ cells expressing CSF3R mutants. (C) Immunoblots from the total protein extracts of control (sc-shRNA) and Dusp1-depleted BaF3 cells expressing CSF3R mutants grown without IL-3 showing reduced p-ERK1/2 and induced activation of p-JNK1/2 upon Dusp1 knockdown in cell expressing leukemic CSF3R mutants (CSF3RT618I, CSF3RT618I/Q741∗, and CSF3RT618I/W791). In contrast, the nonleukemic CSF3R truncation mutations exhibit modest elevation in p-ERK1/2 without any alteration in p-JNK1/2 levels. Expression levels were quantified and normalized to the control condition (pMIV-Vector expressing Sc-ShRNA normalized to to β-actin). The resulting normalized values are presented below each blot for reference. BaF3 cells expressing Dusp1-shRNA resulted in ∼6% to 70% knockdown at protein level and control sc-shRNA has been described earlier.14 (D) A cell proliferation growth curve of Dusp1-depleted BaF3 cells expressing CSF3R mutants showing normal growth when grown with IL-3. (E) Growth curve showing significantly reduced proliferation upon Dusp1 depletion in the absence of IL3. This assay revealed that the transformation potential of CSF3R is compromised upon Dusp1 depletion. Presented data are from 2 independent experiments, shown as means ± standard deviation (SD). ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001. RNA-seq, RNA sequencing.
Genetic deletion of Dusp1 is synthetic lethal to CSF3R-induced leukemia
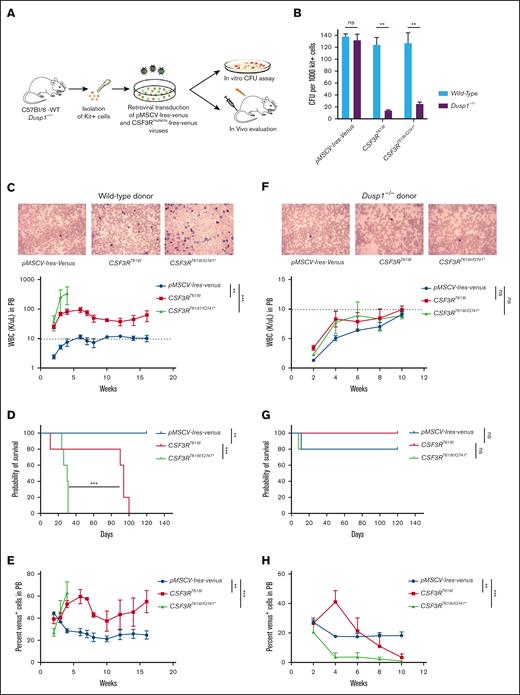
Although BaF3 cellular transformation and shRNA knockdown studies are useful surrogate model for functional studies, it does not fully recapitulate in vivo disease development. To determine the role Dusp1 in CSF3R-induced leukemogenesis, BM-derived Kit+ cells from C57Bl/6 WT and Dusp1-/- mice were transduced with CSF3R-Ires-venus retroviruses for in vitro CFU assays and in vivo leukemogenesis (Figure 2A). Genetic deletion of Dusp1 significantly reduced the CSF3R-induced CFUs (∼ 90%) compared with that of vector control cells expressing MSCV-Ires-venus (Figure 2B). As described earlier, CSF3R proximal (CSF3RT618I) and compound mutants (CSF3RT618I/Q741∗) were used for in vivo leukemogenesis assay. Mice received transplantation with 80 000 venus-positive Dusp1-deficient and -proficient Kit+ cells expressing leukemic CSF3R mutants, CSF3RT618I and CSF3RT618I/Q741∗, or MSCV-Ires-venus vector control. Mice that received transplantation with wild-type Kit+ cells expressing leukemic CSF3R mutants developed fatal leukemia with a disease latency of 2 to 3 weeks for CSF3RT618I/Q741∗ and 13 to 15 weeks for the proximal-mutant CSF3RT618I (Figure 2C-E). Notably, mice recipients of Dusp1 deficient Kit+ cells did not develop leukemia and exhibited disease-free survival (Figure 2F-H). Strikingly, Dusp1-deficient Kit+ cells expressing CSF3R mutants were gradually removed from the mice that received transplantation, whereas control cells (expressing MSCV-Ires-venus vector) were maintained, suggesting that Dusp1 deletion is synthetic lethal to CSF3R mutants (Figure 2E,H). Next, we performed secondary transplantation to confirm that the leukemic cells are completely eradicated from the BM, and mice were cured of the disease. Mice recipients of primary wild-type BM cells expressing CSF3R variants developed robust leukemia with a shorter disease latency (2-6 weeks) than those of primary transplantation. In contrast, mice that received transplantation with Dusp1-deficient primary BM cells exhibited leukemia-free survival without any trace of leukemic cells determined by venus-positive cells (supplemental Figure 2A-H). Altogether, these data provide evidence that Dusp1 deletion confers synthetic lethality to CSF3R-induced leukemia.
Deletion of Dusp1 is synthetically lethal to CSF3R-induced leukemia. (A) Experimental design for evaluating the role of Dusp1 in CNL/aCML. (B) Percent CFUs from the C57Bl/6-WT and Dusp1-/- Kit+ cells expressing leukemic CSF3RT618I (proximal) and CSF3RT618I/Q741∗(compound mutation). The data shown are the mean colony number from 2 independent experiments ± SD. (C-E) Shown are the leukemia development in mice that received transplantation with wild-type BM-derived Kit+ cells expressing CSF3RT618I and CSF3RT618I/Q741∗. (C) PB smear (top panel) and white blood cell (WBC) levels determined biweekly (bottom panel). (D) Survival curve of leukemic mice that received transplantation with CSF3RT618I and CSF3RT618I/Q741∗ expressing Kit+ cells. (E) Shown is the venus-positive cells as a surrogate leukemic burden from the PB. Dotted lines represent normal WBC levels. Representative data are from the 2 independent transplant experiments. (F-H) Mice that received transplantation with Dusp1-deficient BM-derived Kit+ cells expressing CSF3RT618I and CSF3RT618I/Q741∗ are gradually eradicated from the BM. (F) PB smear (top) and WBC levels determined biweekly (bottom) do not show any elevation of WBC levels. (G) Survival curve showing prolong survival of mice that received transplantation with Dusp1-deficient Kit+ cells expressing CSF3RT618I and CSF3RT618I/Q741∗. (H) Kit cells expressing CSF3RT618I and CSF3RT618I/Q741∗ are progressively removed from the PB while maintaining the vector-expressing cells. Representative data are from 2 independent experiments (5 mice per group), shown as the means ± SD. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Deletion of Dusp1 is synthetically lethal to CSF3R-induced leukemia. (A) Experimental design for evaluating the role of Dusp1 in CNL/aCML. (B) Percent CFUs from the C57Bl/6-WT and Dusp1-/- Kit+ cells expressing leukemic CSF3RT618I (proximal) and CSF3RT618I/Q741∗(compound mutation). The data shown are the mean colony number from 2 independent experiments ± SD. (C-E) Shown are the leukemia development in mice that received transplantation with wild-type BM-derived Kit+ cells expressing CSF3RT618I and CSF3RT618I/Q741∗. (C) PB smear (top panel) and white blood cell (WBC) levels determined biweekly (bottom panel). (D) Survival curve of leukemic mice that received transplantation with CSF3RT618I and CSF3RT618I/Q741∗ expressing Kit+ cells. (E) Shown is the venus-positive cells as a surrogate leukemic burden from the PB. Dotted lines represent normal WBC levels. Representative data are from the 2 independent transplant experiments. (F-H) Mice that received transplantation with Dusp1-deficient BM-derived Kit+ cells expressing CSF3RT618I and CSF3RT618I/Q741∗ are gradually eradicated from the BM. (F) PB smear (top) and WBC levels determined biweekly (bottom) do not show any elevation of WBC levels. (G) Survival curve showing prolong survival of mice that received transplantation with Dusp1-deficient Kit+ cells expressing CSF3RT618I and CSF3RT618I/Q741∗. (H) Kit cells expressing CSF3RT618I and CSF3RT618I/Q741∗ are progressively removed from the PB while maintaining the vector-expressing cells. Representative data are from 2 independent experiments (5 mice per group), shown as the means ± SD. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Off-target inhibition of DUSP6 abrogates the antileukemic response to DUSP1 inhibition by BCI
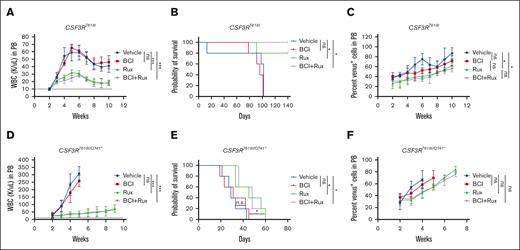
To determine the potential of DUSP1 targeting, a small molecule DUSP1 inhibitor (BCI) was evaluated in vitro and in vivo assays either as a single agent or in combination with JAK2 inhibitor (ruxolitinib). Primary BM Kit+ cells expressing CSF3R variants treated with BCI and ruxolitinib alone indiscriminately suppressed the CFU formation in normal and leukemic cells (supplemental Figure 3). However, a combination of BCI and ruxolitinib showed significant suppression of CSF3R-induced CFUs compared with vector control (supplemental Figure 3). Next, we examined the in vivo efficacy of BCI alone and with ruxolitinib using the retroviral transduction and transplantation model described above. Drug treatments were started after 2 weeks of transplantation. As reported earlier, ruxolitinib treatment suppressed the white blood cell levels but lacked clonal selectivity.9 All mice that received transplantation showed progressive leukemia and eventually succumbed to the disease in both the models of CSF3R-induced leukemia, CSF3RT618I (Figure 3 A-C) and CSF3RT618I/Q741∗ (Figure 3 D-F). Mice treated with BCI alone or in combination with ruxolitinib did not show any improvement in leukemic progression or reduction in leukemic burden compared with the ruxolitinib treatment group (Figure 3A-F). Altogether, these data suggest that treatment with BCI alone or in combination with ruxolitinib is ineffective in vivo.
Chemical inhibition of DUSP1 by BCI is ineffective. (A-C) Mice that received transplantation with wild-type Kit+ cells expressing CSF3RT618I. Graphs showing the total WBC levels (A), survival (B), and percent venus-positive cells as a surrogate leukemic burden (C). (D-F) Leukemic progression in mice that received transplantation with CSF3RT618I/Q741∗ expressing Kit+ cells. Graphs showing the total WBC levels (D), survival (E), and percent venus-positive cells as a surrogate leukemic burden (F). Dotted lines represent normal WBC levels. Treatment with BCI alone or with ruxolitinib is ineffective in suppressing the disease in both models of CSF3R induced leukemia. Representative data are from 2 independent experiments (3 mice per group), shown as the means ± SD. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
Chemical inhibition of DUSP1 by BCI is ineffective. (A-C) Mice that received transplantation with wild-type Kit+ cells expressing CSF3RT618I. Graphs showing the total WBC levels (A), survival (B), and percent venus-positive cells as a surrogate leukemic burden (C). (D-F) Leukemic progression in mice that received transplantation with CSF3RT618I/Q741∗ expressing Kit+ cells. Graphs showing the total WBC levels (D), survival (E), and percent venus-positive cells as a surrogate leukemic burden (F). Dotted lines represent normal WBC levels. Treatment with BCI alone or with ruxolitinib is ineffective in suppressing the disease in both models of CSF3R induced leukemia. Representative data are from 2 independent experiments (3 mice per group), shown as the means ± SD. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
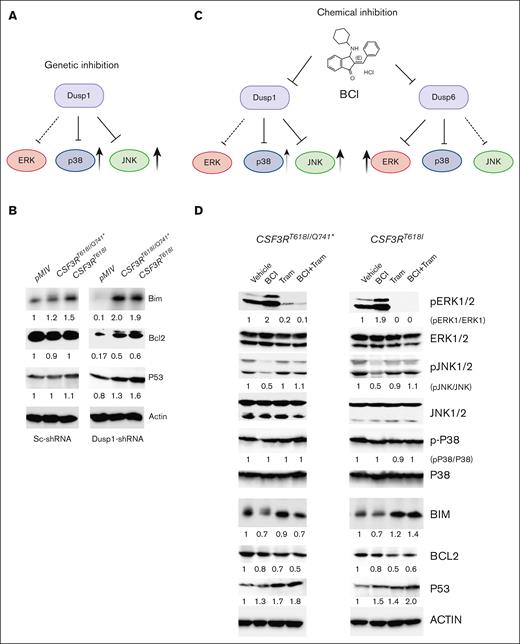
Next, we sought to understand how Dusp1 deletion confers lethality to CSF3R mutants and why its chemical inhibition is ineffective. Because BCI inhibits both DUSP1 and DUSP6, we reasoned that inhibition of DUSP6 likely abolished DUSP1 dependence. We reasoned that a comprehensive analysis of DUSP1/DUSP6 substrates and their downstream targets implicated in mediating apoptosis (BIM, BCL2, and P53) will illuminate the underlying mechanisms driving synthetic lethality and why BCI treatment is ineffective (Figure 4A,C). Genetic knockdown of Dusp1 revealed elevated p-JNK levels with a modest increase in p-P38 levels, whereas pERK1/2 levels were suppressed in cells expressing leukemic CSF3R variants compared with controls, pMIV-Sc-ShRNA and pMIV-Dusp1-ShRNA (Figure 1C). Consequently, proapoptotic protein BIM and P53 levels were significantly increased with a notable reduction in the levels of antiapoptotic protein, BCL2, compared with Sc-shRNA control cells (Figure 4B). Because BCI also inhibits DUSP6, we reasoned that concomitant inhibition of DUSP6 would activate its preferred substrate ERK1/2 (Figure 4C). Elevated ERK1/2 activity beside promoting proliferation and survival modulates the turnover of BIM and BCL2.15-17 ERK1/2-mediated phosphorylation of BIM is targeted for proteasome-dependent degradation, whereas the phosphorylation of BCL2 prevents its degradation.18,19 As envisioned, BCI treatment resulted in increased phosphorylation of pERK1/2 with a marked reduction in p-JNK1/2, whereas p-P38 levels were unchanged (Figure 4C-D). In contrast to the genetic inhibition of DUSP1, BCI treatment reduced the BIM levels, suggesting that its turnover is directly controlled by ERK1/2. Similar to the genetic inhibition of DUSP1, BCI treatment induced the expression of P53 while decreasing the BCL2 levels. Interestingly, cells treated with BCI + trametinib exhibited greater P53 expression and suppression of BCL2 possibly due to the restoration of JNK1/2 activity. Nonetheless, a negative cross talk between ERK and JNK has been reported, in which sustained ERK activity suppressed JNK activation.20 Next, leukemic cells were transduced with retroviruses expressing Dusp6 to determine whether activation of ERK1/2 by BCI is due to inhibition of DUSP6 or some other MAPK phosphatases. Ectopic expression of Dusp6 in primary BM cells significantly suppressed the CSF3R-induced CFUs compared with control (supplemental Figure 4 A). Interestingly, BCI treatment of Dusp6-overexpressing leukemic cells fully suppressed the CSF3R-dependent CFUs (supplemental Figure 4A). This suggests that the inhibition of pERK1/2 may restore BCI sensitivity and result in selective eradication of leukemic cells, as noted with Dusp1 deletion. Altogether these data provide evidence that off-target inhibition of DUSP6 by BCI resulted in higher ERK1/2 expression, which abrogated its antileukemic response. These results suggest that inhibition of ERK1/2 may provide an effective response to BCI treatment.
Activation of p-ERK1/2 due to off-target inhibition of DUSP6 by BCI abrogated its antileukemic response. (A) A model depicting JNK1/2 and P38 activation in leukemic cells upon Dusp1 knockdown. (B) Consequently, the expression of proapoptotic proteins BIM and P53 are induced, whereas the expression of antiapoptotic protein BCL2 was significantly reduced. Expression levels were quantified and normalized to the control condition (pMIV-Vector expressing Sc-ShRNA normalized to β-actin). The resulting normalized values are presented below each blot for reference. (C) A model depicting chemical inhibition of DUSP1 and DUSP6 by BCI-activated p-ERK1/2 that suppressed JNK1/2-mediated apoptotic response. (D) Immunoblots showing the activation of p-ERK1/2 upon BCI treatment resulting to inhibition of JNK1/2 and reduced expression of BIM, whereas the levels of P53 and BCL2 were unaffected. Treatment with trametinib alone restored JNK1/2 activation and the level of BIM with modest reduction on BCL2 levels. Interestingly, cells treated with BCI + Tram exhibit induced expression of P53 with reduced BCL2 levels. Representative blots are from 2 independent experiments. Expression levels were assessed and normalized to control condition (vehicle treatment normalized to β-actin). The resulting normalized values are indicated below each blot. Tram, trametinib.
Activation of p-ERK1/2 due to off-target inhibition of DUSP6 by BCI abrogated its antileukemic response. (A) A model depicting JNK1/2 and P38 activation in leukemic cells upon Dusp1 knockdown. (B) Consequently, the expression of proapoptotic proteins BIM and P53 are induced, whereas the expression of antiapoptotic protein BCL2 was significantly reduced. Expression levels were quantified and normalized to the control condition (pMIV-Vector expressing Sc-ShRNA normalized to β-actin). The resulting normalized values are presented below each blot for reference. (C) A model depicting chemical inhibition of DUSP1 and DUSP6 by BCI-activated p-ERK1/2 that suppressed JNK1/2-mediated apoptotic response. (D) Immunoblots showing the activation of p-ERK1/2 upon BCI treatment resulting to inhibition of JNK1/2 and reduced expression of BIM, whereas the levels of P53 and BCL2 were unaffected. Treatment with trametinib alone restored JNK1/2 activation and the level of BIM with modest reduction on BCL2 levels. Interestingly, cells treated with BCI + Tram exhibit induced expression of P53 with reduced BCL2 levels. Representative blots are from 2 independent experiments. Expression levels were assessed and normalized to control condition (vehicle treatment normalized to β-actin). The resulting normalized values are indicated below each blot. Tram, trametinib.
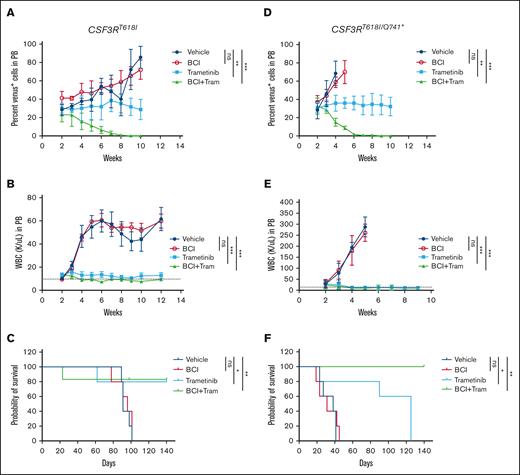
A combination of BCI and trametinib cured the mice of leukemia
To test whether inhibition of ERK1/2 would restore the antileukemic efficacy of BCI, we performed in vitro CFU assays with trametinib alone and in combination with BCI. As reported earlier, trametinib alone suppressed the CSF3R mutant–induced CFUs. However, it also equally suppressed the control cells, expressing vector pMSCV-Ires-venus (supplemental Figure 4B). Strikingly, the combination of BCI and trametinib fully suppressed CSF3R-induced CFUs without any noted toxicity for control cells compared with cells treated with trametinib alone (supplemental Figure 4B). Next, we examined the in vivo efficacy of BCI with trametinib using retroviral transduction and transplantation model. After 2 weeks of transplantation, leukemic engraftments and percent chimerism were determined by analyzing the venus-positive cells from the PB using FACS. Mice were randomized; 5 mice per group were treated with vehicle, trametinib alone, and BCI + trametinib for 8 weeks. As reported earlier, treatment with trametinib alone suppressed the leukemic burden and prolonged the survival of mice but lacked clonal selectivity. Although trametinib treatment prevented disease-related death in mice that received transplantation with CSF3RT618I, its efficacy was significantly reduced against the compound mutant (CSF3RT618I/Q741∗) because almost all treated mice succumbed to the disease. Strikingly, treatment with BCI + trametinib for 6 weeks resulted in the complete suppression of disease progression and rescued ∼90% of mice from leukemia induced by both CSF3R-proximal and -compound mutant (Figure 5A-F). We could not detect minimal residual disease determined by examining the venus-expressing cells from the PB (Figure 5A,D).
BCI in combination with trametinib eradicated the leukemic clones and cured the leukemic mice. (A-C) Mice that received transplantation with wild-type Kit+ cells expressing CSF3RT618I treated with BCI and trametinib alone or in combination. Graphs showing the percent venus-positive cells as a surrogate leukemic burden (A), total WBC levels (B), and survival (C). (D-F) Mice that received transplantation with compound mutation treated with BCI and trametinib alone or in combination. Graphs showing the percent venus-positive cells as a surrogate leukemic burden (D), total WBC level (E), and survival (F). Treatment with trametinib suppresses leukemia but lacked clonal selectivity resulting to cytostatic response. Treatment with BCI lacked both antileukemic response and clonal selectivity. However, a combination of trametinib and BCI not only suppressed the WBC levels but also effectively eradicated the leukemic clones, resulting in cure in both models of CSF3R-induced leukemia. Representative data are from 2 independent experiments (3 mice per group), shown as the means ± SD. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
BCI in combination with trametinib eradicated the leukemic clones and cured the leukemic mice. (A-C) Mice that received transplantation with wild-type Kit+ cells expressing CSF3RT618I treated with BCI and trametinib alone or in combination. Graphs showing the percent venus-positive cells as a surrogate leukemic burden (A), total WBC levels (B), and survival (C). (D-F) Mice that received transplantation with compound mutation treated with BCI and trametinib alone or in combination. Graphs showing the percent venus-positive cells as a surrogate leukemic burden (D), total WBC level (E), and survival (F). Treatment with trametinib suppresses leukemia but lacked clonal selectivity resulting to cytostatic response. Treatment with BCI lacked both antileukemic response and clonal selectivity. However, a combination of trametinib and BCI not only suppressed the WBC levels but also effectively eradicated the leukemic clones, resulting in cure in both models of CSF3R-induced leukemia. Representative data are from 2 independent experiments (3 mice per group), shown as the means ± SD. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001.
To confirm that the treated mice were cured, secondary BM transplantations were performed using whole BM cells from the vehicle and drug-treated mice (supplemental Figure 5). Mice recipients of vehicle, BCI, and trametinib-treated primary BM cells exhibited aggressive leukemic development with shorter disease latency (6 weeks for CSF3RT618I and 3 weeks for CSF3RT618I/Q741∗) than mice that received primary transplantation (15 weeks for CSF3RT618I and 4-5 weeks for CSF3RT618I/Q741∗), supplemental Figure 5). As expected, mice recipients of BCI + trametinib–treated primary BM cells did not show any sign of leukemia and minimal residual disease cells determined by enumerating the venus-positive cells by FACS (supplemental Figure 5). Altogether, these data validate DUSP1dependence in CSF3R-induced leukemia and support developing a DUSP1-selective inhibitor for effective treatment outcomes.
Discussion
Both CNL and atypical chronic myeloid leukemia (aCML) pose significant clinical challenge due to poor prognosis.21 A vast majority of patients with CNL harbor mutations in CSF3R, activating both JAK-STAT and MAPK signaling pathways.8,9,21,22 Conversely, mutations activating the RAS-MAPK pathway are seemingly more common in aCML.23,24 The efficacy of JAK2 inhibitor treatment were observed primarily with the CSF3R-proximal mutant (CSF3RT618I), whereas the CSF3R compound mutation (CSF3RT618I/Q741∗), associated with more aggressive leukemia, proved refractory to ruxolitinib treatment.9,22,25
Recent genomic and proteomic studies have revealed that, regardless of the genetic mutations they harbor, both CNL and aCML are fueled by enhanced MAPK signaling.9,10,12,26 Consequently, the efficacy of trametinib was noted in patients with leukemia and preclinical mouse models of CNL and aCML.9,27 As noted in the preclinical model, treatment with MAPK or JAK-STAT inhibitors suppresses leukemic progression but failed to induce a durable response.9 Both trametinib and ruxolitinib exert cytostatic response and are rarely selective to leukemic clones. As a result, treatment outcomes to cytostatic drugs are short-lived, and loss of therapeutic response and emergence of resistance is inevitable. Therefore, treatment strategies targeting leukemic clones are needed for durable and curative response. Herein, we show that DUSP1 confers oncogene dependence in CSF3R-induced leukemia. Both genetic and pharmacological inhibition of DUSP1 selectively eradicated the leukemic cells and cured the mice of leukemia. Given that DUSP1 is not required for normal development, therapeutic targeting of DUSP1 in CNL/aCML would impart a curative response.
Deregulated ERK activity is commonly observed in many malignancies. Its oncogenic potential is governed by modulating the apoptotic threshold, mainly by stabilizing and/or activating the antiapoptotic proteins, such as BCL-2 family members, and repressing the proapoptotic proteins, such as (BCL2 associated agonist of cell death (BAD) and BIM.3,28,29 Paradoxically, sustained ERK activity also induces robust apoptotic response that will be counterselective for cellular transformation.2,30-32 Therefore, most tumors exploit the MAPK-negative regulators (eg, PP2A and DUSP family members12) to fine-tune the signaling output to suppress ERK-induced apoptotic signaling while selectively promoting survival and proliferation.6 We observed elevated but variable expression of DUSP family members (DUSP1, 2, 4, 5, 8, 9, 10,13,14, and 22) in CSF3R-expressing primary cells. Among these only DUSP1 was consistently induced in leukemic cells. Expression of other DUSP members varied with different CSF3R mutants and half of them were noted to be induced in CSF3R wild-type cells, suggesting that they may be least likely to be engaged in leukemogenesis. Genetic deletion of Dusp1 selectively eradicated leukemic cells, further lending support to the notion that other Dusp family members are dispensable for CSF3R-induced leukemogenesis. Biochemical analysis revealed that DUSP1 selectively dampens the JNK1/2-driven apoptotic response to support leukemogenesis. Perhaps more interestingly, DUSP1-regulated inhibition of apoptotic response appears to vary depending on the specific oncogenic drivers. For instance, in the context of oncogenes; BCR-ABL and JAK2V617F-driven myeloproliferative neoplasms, suppression of apoptosis is mediated through the blockade of P38 activity,14,33 rather than JNK as noted in the context of CSF3R mutants. These observations suggest an oncogene-driven assembly of signaling complexes, leading to altered substrate selectivity in the apoptotic pathway.14,33 These findings imply an oncogene-driven orchestration of signaling complexes unique to each oncogene, resulting in altered substrate selectivity within the apoptotic pathway. Future studies will elucidate the underlying mechanisms to determine whether the altered substrate selectivity is due to spatial regulation of DUSP1, possibly modulated by scaffolding proteins, or it is caused by distinct signaling complexes uniquely orchestrated by each oncogene.
Chemical inhibition of DUSP1 by BCI failed to recapitulate genetic targeting of DUSP1. Because BCI inhibits both DUSP1 and DUSP6,14 concomitant inhibition of DUSP6 resulted in loss of inhibitory control on ERK1/2. Consequently, activated ERK suppressed the JNK activity, which we see when DUSP6 is inhibited by BCI. ERK-mediated JNK inactivation has been reported in several cancer models, although the underlying mechanism is unclear.34 Nonetheless, activation of other DUSP family members and Akt have been implicated.35 Ectopic expression of DUSP6 in CSF3R mutant cells suppressed the pERK1/2 levels and restored BCI sensitivity; thus, providing a direct evidence that the inefficacy of BCI was due to off-target inhibition of DUSP6 mediated by activated ERK1/2. Furthermore, leukemic mice treated with a combination of BCI and trametinib suppressed the pERK1/2 and restored the JNK1/2 modulated apoptotic response and selective eradication of leukemic clones, which provides additional evidence that the inefficacy of BCI treatment alone was due to ERK1/2 activation because of off-target inhibition of DUSP6. Mechanistically, BCI treatment depleted the level of BIM likely due to ERK1/2-mediated phosphorylation-induced degradation.17,36-38 In support, we show that the inhibition of pERK1/2 by trametinib stabilized the level of BIM. In contrast, JNK phosphorylated BIM follows a different fate in which it has been shown to activate BAK and BAX or neutralize BCL2, resulting in a robust apoptotic response.39,40 Perhaps more importantly, stabilization and transcriptional activation of P53 by activated JNK seemingly provides additional support to apoptotic response. For instance, phosphorylated P53 at Thr81 by JNK promotes its dimerization with P73, which induces the expression of several proapoptotic target genes, such as Puma and Bax.41 Together, our study demonstrated that the dynamic balance between ERK and JNK activation determines whether the cell survives or undergoes apoptosis. MAPK-negative regulator, DUSP1, regulates this balance to support the leukemogenesis induced by CSF3R mutants.
In conclusion, we show that DUSP1 confers oncogene dependence in CSF3R-induced leukemia. Deletion of Dusp1 selectively induces JNK1/2 activity that promotes apoptosis by stabilizing and inducing the expression of P53 and BIM while downregulating the antiapoptotic protein BCL2. In contrast, ERK1/2-negative regulator, DUSP6, functions as a tumor suppressor in CSF3R-induced leukemia that seemingly suppresses the elevated pERK1/2. Altogether, these data provide evidence that the inhibitors selectively targeting DUSP1 would exert a durable or curative response in CNL/aCML. Finally, our studies expose a new Achilles heel to malignancies fueled by enhanced MAPK signaling and support selective targeting of MAPK-negative regulators for effective treatment outcomes.
Acknowledgments
This study was supported by grants to M. Azam from the National Cancer Institute at NIH (RO1CA211594) and (RO1CA250516). M. Azam is a recipient of the Bridge award from the American Society of Hematology.
Authorship
Contribution: M.K., M. Azhar, and Z.K. performed the experiments and analyzed the data; M.K. and M. Azam designed all experiments; and M.K. and M. Azam wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohammad Azam, Cincinnati Childrens Hospital Medical Center, Experimental Hematology and Cancer Pathology, 3333 Burnett Ave, 45229 Cincinnati, OH; email: mohammad.azam@cchmc.org.
References
Author notes
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE89500).
For materials and other resources, please contact the corresponding author, Mohammad Azam (mohammad.azam@cchmc.org).
The full-text version of this article contains a data supplement.