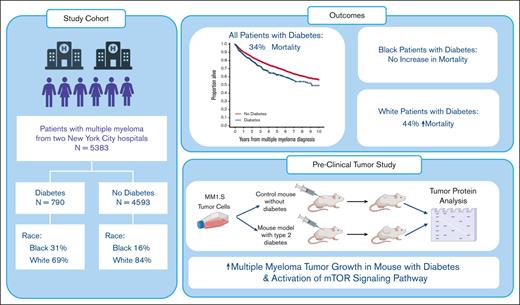
In myeloma, diabetes is more prevalent in Black (25%) compared with White patients (12%) and is associated with worse survival (P < .001).
In a type 2 diabetes mouse model, the progression of MM xenografts is faster in mice with diabetes than in mice without diabetes (P < .05).
Visual Abstract
Multiple myeloma (MM) is twice as common in Black individuals compared with in White individuals, and diabetes mellitus (DM) disproportionately affects Black patients. Although numerous studies have shown a correlation between DM and MM, this has not been studied in the context of race and in vivo mechanisms. We conducted a retrospective clinical study of 5383 patients with MM of which 15% had DM (White, 12% and Black, 25%). Multivariable Cox models showed reduced overall survival (OS) for patients with DM (hazard ratio, 1.27; 95% confidence interval, 1.11-1.47; P < .001). This appeared to be driven by a marked difference in OS between White patients with and without DM but not in Black patients. In contrast, obesity was associated with better OS in Black patients but not in White patients. To complement this analysis, we assessed MM growth in a genetically engineered immunocompromised nonobese diabetic (Rag1−/−/muscle creatinine kinase promoter expression of a human IGF1R [M] with a lysine [K] to arginine [R] point mutation) mouse model to evaluate the mechanisms linking DM and MM. MM.1S xenografts grew in more Rag1−/−/MKR mice and grew more rapidly in the Rag1−/−/MKR mice compared with in controls. Western blot analysis found that MM1.S xenografts from Rag1−/−/MKR mice had higher phosphorylated S6 ribosomal protein (Ser235/236) levels, indicating greater activation of the mammalian target of rapamycin pathway. Our study is, to our knowledge, the first to evaluate racial differences in DM prevalence and survival in MM, as well as the effect of DM on tumor growth in mouse models. Our results suggest that DM may contribute to the higher incidence of MM in Black patients; and to improve survival in MM, DM management cannot be ignored.
Introduction
The prevalence of diabetes mellitus (DM) is rising in adults in the United States, according to the Centers for Disease Control and Prevention.1,2 The prevalence differs, however, by racial/ethnic group. It affects significantly more non-Hispanic Black adults (16.4%) compared with Hispanic (14.7%), non-Hispanic White (11.9%), and non-Hispanic Asian (14.9%) adults.1 In the United Kingdom, cancer has now surpassed cardiovascular disease as the leading cause of death in individuals with DM, and this trend is anticipated to be mirrored in other countries in the near future.3
Multiple myeloma (MM) is the second most common hematologic malignancy and also disproportionately affects non-Hispanic Black adults, in whom it is the most common hematologic malignancy. In the United States, the age-adjusted incidence of MM was 7.7 per 100 000 individuals per year in 2019 based on the Surveillance, Epidemiology and End Results Program from the National Cancer Institute. This incidence was 15.5 per 100 000 Black individuals and 7 per 100 000 White individuals.4 Therefore, MM is more than twice as common in Black adults when compared with White adults. The greater risk has been attributed to several factors, including metabolic conditions.5
DM has been associated with an increased risk of MM in multiple large epidemiologic studies from the United States (odds ratio [OR] 2, 1.1-3.8),6 Israel (in men: hazard ratio [HR], 1.8; 95% confidence interval [CI], 1.52-2.14; and in women: HR, 1.58; 95% CI, 1.30-1.92),7 Canada (HR, 1.15; 95% CI, 1.09-1.20),8 and Sweden (OR, 1.30; 95% CI, 1.22-1.39),9 although this risk seems to be time dependent with the highest risk seen within the first 6 months of diagnosis of DM.9 It is also not consistently seen in all studies (OR, 1.05; 95% CI, 0.83-1.33).10 Therefore, it is possible that this risk is partially attributable to a detection bias. More consistently, DM and poor glucose tolerance have been associated with increased mortality in patients with MM in epidemiologic studies from Canada (HR, 1.38; 95% CI, 1.28-1.50)8 and the United States (HR, 3.06; 95% CI, 1.05-8.93).11 Additionally, DM has been associated with a worse overall survival (OS) in patients with MM in retrospective studies from hospitals in Taiwan (HR, 1.5; 95% CI, 1.02-2.23),12 Israel (HR, 1.38; 95% CI, 0.96-1.99),13 and the United States (steroid induced DM: HR, 1.62; 95% CI, 1.33-1.97).14 The study from Israel also showed that individuals with DM had shorter time to second-line treatment (HR, 1.31; 95% CI, 1.0-1.72).13
However, there is a paucity of data on racial differences in DM prevalence and mortality in patients with MM. Given the higher prevalence of DM in Black individuals compared with White individuals, the focus of this study was to investigate the impact of DM on OS in patients with MM in the context of race, from 2 academic institutions in the New York Metropolitan area, the Memorial Sloan Kettering Cancer Center (MSK) and the Icahn School of Medicine at Mount Sinai (ISMMS). We find that DM is more prevalent in Black patients with MM but that DM has a marked negative impact on OS in White patients. Additionally, despite epidemiologic evidence for the association between DM and MM, this has not been studied in animal models, and the mechanisms driving this association have not been elucidated. Our study evaluates the effects of DM on MM growth in a well-characterized transgenic mouse model of type 2 DM that supports DM as a potential contributing factor to the development of MM that disproportionately affects the Black population.
Methods
Retrospective clinical data analysis
We obtained the data from 2 centers, and the study was approved by the institutional review boards (IRBs) of MSK (IRB18-143) and ISMMS (IRB #11-1433). Patients not specified as White or Black race were excluded. Patients were considered Black if their race was recorded as Black or African American. In the ISMMS cohort, details on Jamaican, Ugandan, or Nigerian race was available and included under Black race. Patients with MM were identified with the International Classification of Diseases (ICD)10 code C90.00 and ICD9 code 203.0 in the institutional databases and electronic medical records (EMRs) from January 2010 until December 2020. DM (type 1 and 2) was identified based on ICD10 codes E08, E09, E10.1-E10.9, E11.1-E11.9, E13.1-E13.9, and ICD9: 250, or presence of hemoglobin A1c (HbA1c) ≥6.5% before MM diagnosis. DM was ascertained by elevated HbA1c in 26% of patients and by ICD code in 74% of patients. Following a landmark approach, patients with DM diagnosis after MM were considered unexposed for purposes of our primary survival analysis. Information on duration of DM or treatment for DM was not available. Body mass index (BMI) was calculated from height and weight recorded closest to MM diagnosis date but no later than 3 months after the MM diagnosis date. BMI was classified into: underweight (<18.5 kg/m2), normal (18.5 kg/m2 to <25 kg/m2), overweight (25 kg/m2 to <30 kg/m2), and obese (≥30 kg/m2).15 Consequently, 48 (1.3%) patients in the MSK cohort and 68 (4%) patients of ISMMS cohort with missing BMI were excluded from analysis. Demographic and clinical covariates were ascertained from retrospective chart review. OS was defined as time from diagnosis to death, or last follow-up for those who survived. EMRs were used for extracting laboratory and BMI data, and ICD 10 codes. Institutional registry and EMRs were used to ascertain OS. Autologous stem cell transplant status and International Staging System stage were only available in the database for ISMMS patients through an institutional database.
Descriptive statistics were used to summarize patient characteristics by DM status, for the entire cohort and separately for the Black and White cohorts. Distributions of patient characteristics were compared between patients with and without diabetes using the χ2 test. The Kaplan-Meier method was used to estimate distributions of OS. The log-rank test was used to compare OS distributions by DM status. Multivariable Cox proportional hazards regression models were used to estimate HRs for the association between DM and OS. Center-specific models for the entire cohort were adjusted for race, gender, age, and BMI. Additionally, sensitivity analyses using inverse probability of treatment weighting (IPTW) was used as an alternative to multivariable modeling to adjust for confounders. We first estimated propensity scores (PSs) through logistic regression, with DM status as the response variable, and confounders of race, gender, age, and BMI as predictors. The average treatment effect (ATE) was estimated using the weighted HR between those with diabetes and those without diabetes, with weights equal to 1/PS for those with diabetes and 1/(1 − PS) for those without. Moreover, the ATE among the treated (ATT) was estimated with weights equal to 1 for those with diabetes and PS/(1 − PS) for those without. Finally, the ATE among the controls (ATU) was estimated with weights equal to 1 for the individuals without diabetes and PS/(1 − PS) for those with diabetes. ATE-, ATT-, and ATU-weighted HRs are presented in a supplemental Table 3 with corresponding 95% CIs derived using robust standard errors. Consistency of results across methods was evaluated. Log HRs, estimated from multivariable and IPTW Cox proportional hazard models, and their 95% CIs were pooled across centers with a random effects meta-analysis. The inverse variance method was used for pooling standard errors, and was implemented with the meta package in R. All statistical analyses were performed with SAS software (version 9.4; SAS Institute, Cary, NC) and the R package (version 3.2.1; R Foundation for Statistical Computing, Vienna, Austria). Hypothesis testing was 2-sided and conducted at the 5% level of significance.
In vivo and in vitro preclinical studies
The muscle creatinine kinase promoter expression of a human IGF1R (M) with a lysine (K) to arginine (R) point mutation mouse has been well-characterized and described in previous publications. It is a transgenic mouse that, under the muscle (M) creatinine kinase promoter, expresses the human insulin-like growth factor 1 receptor (IGF-1R) with a lysine-to-arginine mutation.16 The immunodeficient MKR mouse was generated by crossing the recombination activating gene 1 (Rag1) knockout (Rag1−/−) mouse to generate homozygous Rag1−/−/MKR mice. The metabolic phenotype of the Rag1−/−/MKR male and female mice on the Friend virus B background have been described previously.17 Briefly, the male Rag1−/−/MKR mice develop type 2 DM, with insulin resistance, hyperinsulinemia, and hyperglycemia, but are not obese and have lower leptin levels than control Rag1−/− mice. Female Rag1−/−/MKR mice develop hyperinsulinemia and insulin resistance but are not hyperglycemic.17
All animal studies were performed at the ISMMS Center for Comparative Medicine and Surgery and were in compliance with the current standards specified in the Guide of the Care and Use of Laboratory Animals, provided by the Association for Assessment and Accreditation of Laboratory Animal Care, and approved by the ISMMS Institutional Animal Care and Use Committee. Mice were housed 4 to 5 per cage and given free access to regular laboratory chow (PicoLab 5053, Brentwood, MO) and water, and kept on a 12-hour light/dark cycle.
MM1.S cells were obtained from American Type Culture Collection and authenticated via short tandem repeats. They were cultured in RPMI 1640 medium (Corning, NY) supplemented with 10% fetal bovine serum (Invitrogen, Life Technologies, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin (Mediatech, Manassas, VA). Cells were propagated at 37°C in 5% carbon dioxide. Cells were authenticated, and tested negative for mycoplasma. Three million MM1.S cells were mixed with 50% Matrigel (BD Biosciences, Franklin Lakes, NJ) and injected subcutaneously into the right flank of 8- to 12-week-old male Rag1−/−/MKR and control Rag1−/− mice. Tumor growth was measured using calipers, and the volume was calculated using the formula: volume = 4/3 × pi × (length/2) × (width/2) × (depth/2). Studies were stopped when the mice reached a humane end point.
For in vitro cell stimulation, MM1.S cells were grown as described earlier, and then serum starved overnight in RPMI 1640 with 0.1% free fatty acid free bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO). Cells were aliquoted into 1.5 mL microcentrifuge tubes, in 1 mL of RPMI 1640 with 0.1% BSA, and stimulated with 10 nM insulin in 0.1% BSA or control (0.1% BSA) at 37°C for 60 minutes. After 60 minutes, tubes were placed on ice, washed twice with ice-cold phosphate-buffered saline, and centrifuged at 450g to pellet the cells before freezing on dry ice.
Western blot analysis: tumor tissue and cells were lysed in ice-cold lysis buffer, as previously described.17 Denatured and reduced protein lysates were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis Tris-glycine gels (Invitrogen, Life Technologies) and transferred to nitrocellulose membranes. Membranes were incubated overnight with primary antibodies at 4°C, followed by incubation with secondary antibodies: goat anti-rabbit near infrared (IRDye) 800W or donkey anti-mouse IRDye 680RD (Li-Cor Biosciences, Lincoln, NE). Membranes were scanned using the Li-Cor infrared imaging system, and quantified using the Li-Cor Image Studio software.
Primary antibodies and dilutions used were as follows: anti–phosphorylated IGF-1Rβ (Tyr1150/1151)/phosphorylated insulin receptor β (pIRβ) (Tyr1135/1136) (#3024, 1:1000, Cell Signaling Technology [CST], Danvers, MA), total IGF-1R (1:1000, #3027, CST), total IRβ (1:200, C-19, Santa Cruz Biotechnology, Santa Cruz, Dallas, TX), phosphorylated Akt (pAkt) (Ser473) (1:1000, #9271, CST), total Akt (1:2000, #2920, CST), phosphorylated S6 ribosomal protein (pS6rp) (Ser235/236) (1:1000, #2211, CST), total S6 ribosomal protein (1:1000, #2317, CST), and β-actin (1:10 000, A228, Sigma-Aldrich).
Results
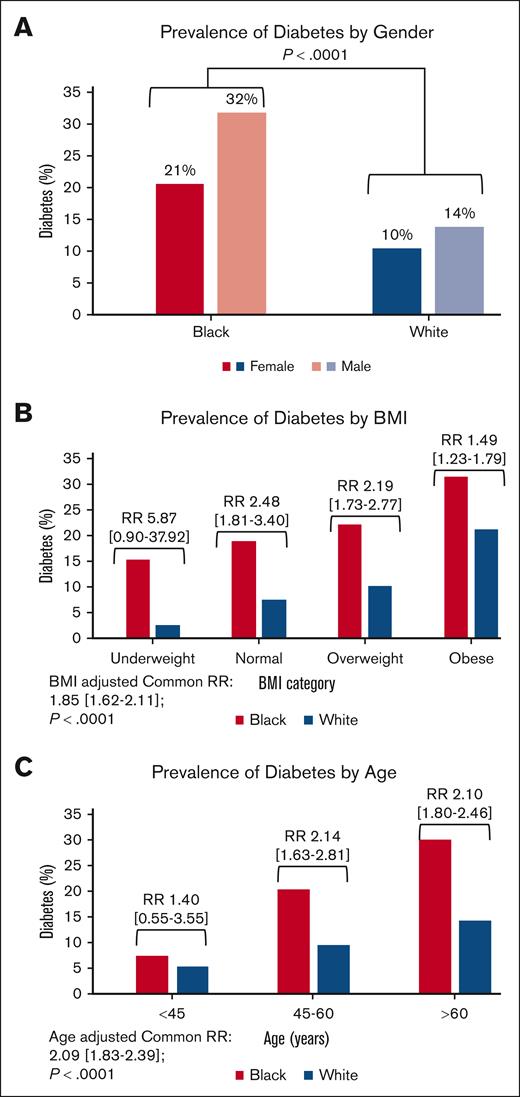
Descriptive characteristics of the entire cohort are provided in Table 1, and by institution in supplemental Table 1. The total cohort included 5383 patients, of which 790 (15%) had DM (MSK, 16% and ISMMS, 11%). Only 0.4% of DM cases were type 1 DM in the MSK cohort and these data are not available for the ISMMS cohort. The cohort was predominantly White (81%), male (56%), aged >60 years (65%), and with an elevated BMI (68%). The Black patients were younger than White patients, with 43% of the Black population and 33% of the White population aged ≤60 years. Despite being younger, Black patients had twice the rate of DM (25%) compared with the White patients (12%), with the highest prevalence in Black males (32%; Figure 1A). The prevalence of DM increased in both Black and White patients with higher BMI categories and advancing age (Figure 1B-C). However, notably, the prevalence of DM was almost as high in Black patients with normal weight (19%), as in White patients with obesity (21%), and almost a third of Black patients (32%) with obesity had DM (Figure 1B). Additionally, DM affected 20% of Black patients aged between 45 and 60 years, far exceeding the prevalence of DM in White patients aged >60 years (14%; Figure 1C). These results show that DM is much more prevalent in Black patients with MM compared with White patients, and disproportionately affects Black patients with MM who are younger and who have a normal weight.
Patient characteristics by race and diabetes status at MM diagnosis
| Entire cohort, N (%) . | Total 5383 (100%) . | Nondiabetic 4593 (85.3%) . | Diabetic 790 (14.7%) . | P value∗ . |
|---|---|---|---|---|
| Race, n (%) | <.0001 | |||
| Black | 1001 (18.6%) | 754 (16.4%) | 247 (31.3%) | |
| White | 4382 (81.4%) | 3839 (83.6%) | 543 (68.7%) | |
| Gender, n (%) | .0486 | |||
| Female | 2381 (44.2%) | 2057 (44.8%) | 324 (41.0%) | |
| Male | 3002 (55.8%) | 2536 (55.2%) | 466 (59.0%) | |
| Age (y), n (%) | <.0001 | |||
| <45 | 326 (6.1%) | 307 (6.7%) | 19 (2.4%) | |
| 45-60 | 1558 (28.9%) | 1373 (29.9%) | 185 (23.4%) | |
| >60 | 3499 (65.0%) | 2913 (63.4%) | 586 (74.2%) | |
| BMI, n (%) | <.0001 | |||
| Underweight | 89 (1.7%) | 85 (1.9%) | 4 (0.5%) | |
| Normal | 1612 (29.9%) | 1460 (31.8%) | 152 (19.2%) | |
| Overweight | 2079 (38.6%) | 1823 (39.7%) | 256 (32.4%) | |
| Obese | 1603 (29.8%) | 1225 (26.7%) | 378 (47.8%) |
| Entire cohort, N (%) . | Total 5383 (100%) . | Nondiabetic 4593 (85.3%) . | Diabetic 790 (14.7%) . | P value∗ . |
|---|---|---|---|---|
| Race, n (%) | <.0001 | |||
| Black | 1001 (18.6%) | 754 (16.4%) | 247 (31.3%) | |
| White | 4382 (81.4%) | 3839 (83.6%) | 543 (68.7%) | |
| Gender, n (%) | .0486 | |||
| Female | 2381 (44.2%) | 2057 (44.8%) | 324 (41.0%) | |
| Male | 3002 (55.8%) | 2536 (55.2%) | 466 (59.0%) | |
| Age (y), n (%) | <.0001 | |||
| <45 | 326 (6.1%) | 307 (6.7%) | 19 (2.4%) | |
| 45-60 | 1558 (28.9%) | 1373 (29.9%) | 185 (23.4%) | |
| >60 | 3499 (65.0%) | 2913 (63.4%) | 586 (74.2%) | |
| BMI, n (%) | <.0001 | |||
| Underweight | 89 (1.7%) | 85 (1.9%) | 4 (0.5%) | |
| Normal | 1612 (29.9%) | 1460 (31.8%) | 152 (19.2%) | |
| Overweight | 2079 (38.6%) | 1823 (39.7%) | 256 (32.4%) | |
| Obese | 1603 (29.8%) | 1225 (26.7%) | 378 (47.8%) |
| Black cohort, n (%) . | Total 1001 (100%) . | Nondiabetic 754 (75.3%) . | Diabetic 247 (24.7%) . | P value . |
|---|---|---|---|---|
| Gender, n (%) | .7162 | |||
| Female | 549 (54.8%) | 416 (55.2%) | 133 (53.8%) | |
| Male | 452 (45.2%) | 338 (44.8%) | 114 (46.2%) | |
| Age (y), n (%) | <.0001 | |||
| <45 | 81 (8.1%) | 75 (9.9%) | 6 (2.4%) | |
| 45-60 | 351 (35.1%) | 280 (37.1%) | 71 (28.7%) | |
| >60 | 569 (56.8%) | 399 (52.9%) | 170 (68.8%) | |
| BMI, n (%) | .0014 | |||
| Underweight | 13 (1.3%) | 11 (1.5%) | 2 (0.8%) | |
| Normal | 253 (25.3%) | 205 (27.2%) | 48 (19.4%) | |
| Overweight | 374 (37.4%) | 291 (38.6%) | 83 (33.6%) | |
| Obese | 361 (36.1%) | 247 (32.8%) | 114 (46.2%) |
| Black cohort, n (%) . | Total 1001 (100%) . | Nondiabetic 754 (75.3%) . | Diabetic 247 (24.7%) . | P value . |
|---|---|---|---|---|
| Gender, n (%) | .7162 | |||
| Female | 549 (54.8%) | 416 (55.2%) | 133 (53.8%) | |
| Male | 452 (45.2%) | 338 (44.8%) | 114 (46.2%) | |
| Age (y), n (%) | <.0001 | |||
| <45 | 81 (8.1%) | 75 (9.9%) | 6 (2.4%) | |
| 45-60 | 351 (35.1%) | 280 (37.1%) | 71 (28.7%) | |
| >60 | 569 (56.8%) | 399 (52.9%) | 170 (68.8%) | |
| BMI, n (%) | .0014 | |||
| Underweight | 13 (1.3%) | 11 (1.5%) | 2 (0.8%) | |
| Normal | 253 (25.3%) | 205 (27.2%) | 48 (19.4%) | |
| Overweight | 374 (37.4%) | 291 (38.6%) | 83 (33.6%) | |
| Obese | 361 (36.1%) | 247 (32.8%) | 114 (46.2%) |
| White cohort, n (%) . | Total 4382 (100%) . | Nondiabetic 3839 (87.6%) . | Diabetic 543 (12.4%) . | P value . |
|---|---|---|---|---|
| Gender, n (%) | .0008 | |||
| Female | 1832 (41.8%) | 1641 (42.7%) | 191 (35.2%) | |
| Male | 2550 (58.2%) | 2198 (57.3%) | 352 (64.8%) | |
| Age (y), n (%) | <.0001 | |||
| <45 | 245 (5.6%) | 232 (6.0%) | 13 (2.4%) | |
| 45-60 | 1207 (27.5%) | 1093 (28.5%) | 114 (21.0%) | |
| >60 | 2930 (66.9%) | 2514 (65.5%) | 416 (76.6%) | |
| BMI, n (%) | <.0001 | |||
| Underweight | 76 (1.7%) | 74 (1.9%) | 2 (0.4%) | |
| Normal | 1359 (31.0%) | 1255 (32.7%) | 104 (19.2%) | |
| Overweight | 1705 (38.9%) | 1532 (39.9%) | 173 (31.9%) | |
| Obese | 1242 (28.3%) | 978 (25.5%) | 264 (48.6%) |
| White cohort, n (%) . | Total 4382 (100%) . | Nondiabetic 3839 (87.6%) . | Diabetic 543 (12.4%) . | P value . |
|---|---|---|---|---|
| Gender, n (%) | .0008 | |||
| Female | 1832 (41.8%) | 1641 (42.7%) | 191 (35.2%) | |
| Male | 2550 (58.2%) | 2198 (57.3%) | 352 (64.8%) | |
| Age (y), n (%) | <.0001 | |||
| <45 | 245 (5.6%) | 232 (6.0%) | 13 (2.4%) | |
| 45-60 | 1207 (27.5%) | 1093 (28.5%) | 114 (21.0%) | |
| >60 | 2930 (66.9%) | 2514 (65.5%) | 416 (76.6%) | |
| BMI, n (%) | <.0001 | |||
| Underweight | 76 (1.7%) | 74 (1.9%) | 2 (0.4%) | |
| Normal | 1359 (31.0%) | 1255 (32.7%) | 104 (19.2%) | |
| Overweight | 1705 (38.9%) | 1532 (39.9%) | 173 (31.9%) | |
| Obese | 1242 (28.3%) | 978 (25.5%) | 264 (48.6%) |
χ2P value testing association between patient characteristic and diabetes status within race.
Bar graphs showing diabetes prevalence by race in various subgroups. (A) Gender, (B) BMI, and (C) age. RR is relative risk and 95% CIs. P value for all associations (DM, BMI, and age) by race is <.0001, as indicated.
Bar graphs showing diabetes prevalence by race in various subgroups. (A) Gender, (B) BMI, and (C) age. RR is relative risk and 95% CIs. P value for all associations (DM, BMI, and age) by race is <.0001, as indicated.
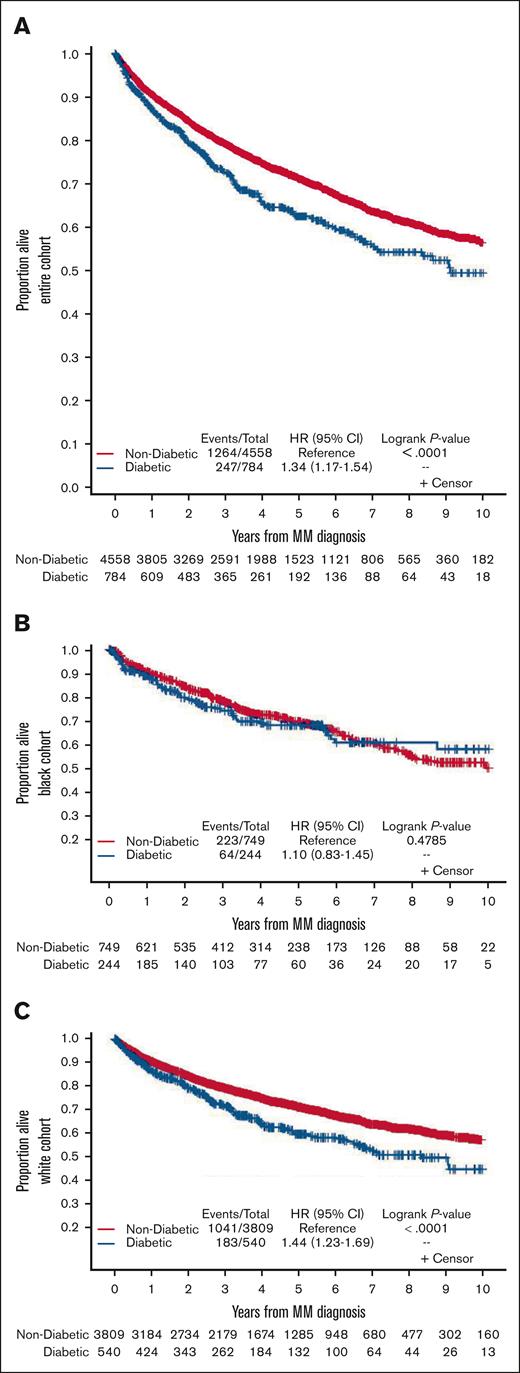
The median follow-up time for the population was 4.62 years (range, 0.003-11.99 years). On univariate analysis, pooled Kaplan-Meier curves show that in the entire cohort, patients with DM had a worse OS compared with those without DM. There were 247 deaths in 784 patients with DM, and there were 1264 deaths in 4558 patients without DM (HR, 1.34; 95% CI, 1.17-1.54; P < .0001; Figure 2A). Similar results were seen in White patients, with 183 deaths in 540 patients with DM, and 1041 deaths in 3809 patients without DM (HR, 1.44; 95% CI, 1.23-1.69; P < .0001; Figure 2C) but not in Black patients, with 64 deaths in 244 patients with DM, and 223 deaths in 749 patients without DM (HR, 1.10; 95% CI, 0.83-1.45; P = .48; Figure 2B). Multivariable Cox regression analyses adjusting for race, gender, age (categorized), and BMI revealed findings similar to those from univariate analyses. There was a significantly reduced OS for patients with DM on pooled analysis in the entire cohort (HR, 1.27; 95% CI, 1.11-1.47; P < .001) and in White patients (HR, 1.35; 95% CI, 1.15-1.59; P < .001), but not in Black patients (HR, 1.08; 95% CI, 0.81-1.44; P = .584; Table 2). Overall, White patients may have slightly improved survival compared with Black patients on pooled multivariable analysis irrespective of DM status, although this did not achieve statistical significance (HR, 0.88; 95% CI, 0.77-1.01; P = .059).
Kaplan-Meier curves show OS by diabetes status in newly diagnosed MM. (A) Pooled entire cohort, (B) pooled Black cohort, and (C) pooled White cohort.
Kaplan-Meier curves show OS by diabetes status in newly diagnosed MM. (A) Pooled entire cohort, (B) pooled Black cohort, and (C) pooled White cohort.
Multivariable Cox regression HRs for all-cause mortality
| . | Pooled . | MSK . | ISMMS . | ||||
|---|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Entire cohort | |||||||
| Diabetes | |||||||
| Nondiabetic | 1299/4593 | Reference | Reference | Reference | |||
| Diabetic | 253/790 | 1.27 (1.11-1.47) | <.001 | 1.26 (1.06-1.48) | .008 | 1.31 (1.00-1.71) | .048 |
| Race | |||||||
| Black | 295/1001 | Reference | Reference | Reference | |||
| White | 1257/4382 | 0.88 (0.77-1.01) | .059 | 0.92 (0.77-1.09) | .331 | 0.83 (0.68-1.02) | .077 |
| Gender | |||||||
| Female | 630/2381 | Reference | Reference | Reference | |||
| Male | 922/3002 | 1.23 (1.10. 1.39) | <.001 | 1.29 (1.14-1.47) | <.001 | 1.14 (0.95-1.36) | .163 |
| Age (y) | |||||||
| <45 | 55/326 | Reference | Reference | Reference | |||
| 45-60 | 362/1558 | 1.52 (1.13-2.04) | .006 | 1.59 (1.13-2.25) | <.001 | 1.33 (0.75-2.35) | .007 |
| >60 | 1135/3499 | 2.41 (1.82-3.20) | <.001 | 2.51 (1.81-3.50) | .009 | 2.15 (1.23-3.74) | .330 |
| BMI | |||||||
| Normal | 499/1612 | Reference | Reference | Reference | |||
| Overweight | 588/2079 | 0.83 (0.70-0.99) | .035 | 0.77 (0.66-0.90) | .001 | 0.92 (0.75-1.13) | .415 |
| Obese | 442/1603 | 0.82 (0.71-0.93) | .003 | 0.81 (0.69-0.96) | .013 | 0.83 (0.66-1.05) | .116 |
| Underweight | 23/89 | 1.08 (0.69-1.69) | .725 | 1.09 (0.63-1.89) | .756 | 1.07 (0.50-2.28) | .871 |
| Black cohort | |||||||
| Diabetes | |||||||
| Nondiabetic | 67/247 | Reference | Reference | Reference | |||
| Diabetic | 228/754 | 1.08 (0.81-1.44) | .584 | 1.01 (0.70-1.46) | .942 | 1.21 (0.76-1.91) | .428 |
| Gender | |||||||
| Female | 147/549 | Reference | Reference | Reference | |||
| Male | 148/452 | 1.30 (1.03-1.65) | .028 | 1.19 (0.86-1.64) | .286 | 1.44 (1.02-2.03) | .040 |
| Age (y) | |||||||
| <45 | 16/81 | Reference | Reference | Reference | |||
| 45-60 | 182/569 | 1.59 (0.92-2.73) | .095 | 1.59 (0.84-3.01) | .030 | 1.58 (0.56-4.42) | .126 |
| >60 | 97/351 | 2.03 (1.20-3.42) | .008 | 1.97 (1.07-3.62) | .154 | 2.20 (0.80-6.05) | .387 |
| BMI | |||||||
| Normal | 96/253 | Reference | Reference | Reference | |||
| Overweight | 104/374 | 0.68 (0.52-0.90) | .007 | 0.63 (0.43-0.92) | .016 | 0.75 (0.50-1.14) | .181 |
| Obese | 89/361 | 0.62 (0.46-0.83) | .002 | 0.57 (0.38-0.86) | .007 | 0.68 (0.43-1.05) | .083 |
| Underweight | 6/13 | 1.88 (0.81-4.33) | .139 | 1.82 (0.65-5.11) | .255 | 1.99 (0.48-8.27) | .347 |
| White cohort | |||||||
| Diabetes | |||||||
| Nondiabetic | 186/543 | Reference | Reference | Reference | |||
| Diabetic | 1071/3839 | 1.35 (1.15-1.59) | <.001 | 1.33 (1.11-1.61) | .003 | 1.41 (1.02-1.96) | .040 |
| Gender | |||||||
| Female | 483/1832 | Reference | Reference | Reference | |||
| Male | 774/2550 | 1.16 (0.93-1.46) | .194 | 1.29 (1.12-1.49) | <.001 | 1.02 (0.82-1.26) | .878 |
| Age (y) | |||||||
| <45 | 39/245 | Reference | Reference | Reference | |||
| 45-60 | 953/2930 | 1.51 (1.06-2.14) | .023 | 1.63 (1.08-2.46) | <.001 | 1.21 (0.61-2.41) | .032 |
| >60 | 265/1207 | 2.51 (1.79-3.53) | <.001 | 2.69 (1.81-4.00) | .022 | 2.07 (1.07-4.03) | .592 |
| BMI | |||||||
| Normal | 403/1359 | Reference | Reference | Reference | |||
| Overweight | 484/1705 | 0.87 (0.70-1.10) | .248 | 0.79 (0.67-0.94) | .006 | 1.00 (0.78-1.27) | .970 |
| Obese | 353/1242 | 0.88 (0.76-1.02) | .095 | 0.86 (0.72-1.03) | .095 | 0.93 (0.71-1.21) | .575 |
| Underweight | 17/76 | 0.93 (0.55-1.57) | .794 | 0.95 (0.50-1.82) | .880 | 0.90 (0.37-2.21) | .816 |
| . | Pooled . | MSK . | ISMMS . | ||||
|---|---|---|---|---|---|---|---|
| Events/patients . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | HR (95% CI) . | P value . | |
| Entire cohort | |||||||
| Diabetes | |||||||
| Nondiabetic | 1299/4593 | Reference | Reference | Reference | |||
| Diabetic | 253/790 | 1.27 (1.11-1.47) | <.001 | 1.26 (1.06-1.48) | .008 | 1.31 (1.00-1.71) | .048 |
| Race | |||||||
| Black | 295/1001 | Reference | Reference | Reference | |||
| White | 1257/4382 | 0.88 (0.77-1.01) | .059 | 0.92 (0.77-1.09) | .331 | 0.83 (0.68-1.02) | .077 |
| Gender | |||||||
| Female | 630/2381 | Reference | Reference | Reference | |||
| Male | 922/3002 | 1.23 (1.10. 1.39) | <.001 | 1.29 (1.14-1.47) | <.001 | 1.14 (0.95-1.36) | .163 |
| Age (y) | |||||||
| <45 | 55/326 | Reference | Reference | Reference | |||
| 45-60 | 362/1558 | 1.52 (1.13-2.04) | .006 | 1.59 (1.13-2.25) | <.001 | 1.33 (0.75-2.35) | .007 |
| >60 | 1135/3499 | 2.41 (1.82-3.20) | <.001 | 2.51 (1.81-3.50) | .009 | 2.15 (1.23-3.74) | .330 |
| BMI | |||||||
| Normal | 499/1612 | Reference | Reference | Reference | |||
| Overweight | 588/2079 | 0.83 (0.70-0.99) | .035 | 0.77 (0.66-0.90) | .001 | 0.92 (0.75-1.13) | .415 |
| Obese | 442/1603 | 0.82 (0.71-0.93) | .003 | 0.81 (0.69-0.96) | .013 | 0.83 (0.66-1.05) | .116 |
| Underweight | 23/89 | 1.08 (0.69-1.69) | .725 | 1.09 (0.63-1.89) | .756 | 1.07 (0.50-2.28) | .871 |
| Black cohort | |||||||
| Diabetes | |||||||
| Nondiabetic | 67/247 | Reference | Reference | Reference | |||
| Diabetic | 228/754 | 1.08 (0.81-1.44) | .584 | 1.01 (0.70-1.46) | .942 | 1.21 (0.76-1.91) | .428 |
| Gender | |||||||
| Female | 147/549 | Reference | Reference | Reference | |||
| Male | 148/452 | 1.30 (1.03-1.65) | .028 | 1.19 (0.86-1.64) | .286 | 1.44 (1.02-2.03) | .040 |
| Age (y) | |||||||
| <45 | 16/81 | Reference | Reference | Reference | |||
| 45-60 | 182/569 | 1.59 (0.92-2.73) | .095 | 1.59 (0.84-3.01) | .030 | 1.58 (0.56-4.42) | .126 |
| >60 | 97/351 | 2.03 (1.20-3.42) | .008 | 1.97 (1.07-3.62) | .154 | 2.20 (0.80-6.05) | .387 |
| BMI | |||||||
| Normal | 96/253 | Reference | Reference | Reference | |||
| Overweight | 104/374 | 0.68 (0.52-0.90) | .007 | 0.63 (0.43-0.92) | .016 | 0.75 (0.50-1.14) | .181 |
| Obese | 89/361 | 0.62 (0.46-0.83) | .002 | 0.57 (0.38-0.86) | .007 | 0.68 (0.43-1.05) | .083 |
| Underweight | 6/13 | 1.88 (0.81-4.33) | .139 | 1.82 (0.65-5.11) | .255 | 1.99 (0.48-8.27) | .347 |
| White cohort | |||||||
| Diabetes | |||||||
| Nondiabetic | 186/543 | Reference | Reference | Reference | |||
| Diabetic | 1071/3839 | 1.35 (1.15-1.59) | <.001 | 1.33 (1.11-1.61) | .003 | 1.41 (1.02-1.96) | .040 |
| Gender | |||||||
| Female | 483/1832 | Reference | Reference | Reference | |||
| Male | 774/2550 | 1.16 (0.93-1.46) | .194 | 1.29 (1.12-1.49) | <.001 | 1.02 (0.82-1.26) | .878 |
| Age (y) | |||||||
| <45 | 39/245 | Reference | Reference | Reference | |||
| 45-60 | 953/2930 | 1.51 (1.06-2.14) | .023 | 1.63 (1.08-2.46) | <.001 | 1.21 (0.61-2.41) | .032 |
| >60 | 265/1207 | 2.51 (1.79-3.53) | <.001 | 2.69 (1.81-4.00) | .022 | 2.07 (1.07-4.03) | .592 |
| BMI | |||||||
| Normal | 403/1359 | Reference | Reference | Reference | |||
| Overweight | 484/1705 | 0.87 (0.70-1.10) | .248 | 0.79 (0.67-0.94) | .006 | 1.00 (0.78-1.27) | .970 |
| Obese | 353/1242 | 0.88 (0.76-1.02) | .095 | 0.86 (0.72-1.03) | .095 | 0.93 (0.71-1.21) | .575 |
| Underweight | 17/76 | 0.93 (0.55-1.57) | .794 | 0.95 (0.50-1.82) | .880 | 0.90 (0.37-2.21) | .816 |
Bold values indicate P < 0.05.
Elevated BMI was associated with improved OS in the pooled multivariable Cox regression models compared with normal weight (obesity HR, 0.82; 95% CI, 0.71-0.93; P = .003; and overweight HR, 0.83; 95% CI, 0.70-0.99; P = .035). Patients who are underweight had similar OS to patients with normal weights patients (HR, 1.08; 95% CI, 0.69-1.69; P = .725). When analyzed by race, elevated BMI was protective in Black patients (obesity HR, 0.62; 95% CI, 0.46-0.83; P = .002; and overweight 0.68; 95% CI, 0.52-0.90, P = .007) but not in White patients (obesity HR, 0.88; 95% CI, 0.76-1.02; P = .095; and overweight HR, 0.87; 95% CI, 0.70-1.10; P = .248; Table 2). Other predictors of decreased OS were age >60 years, which was associated with worse OS in the entire cohort, and in the Black and White patients separately. Male gender was associated with decreased OS in the Black population only. These results show that apart from age, other factors including DM, BMI, and gender have differing associations with OS in the Black and White populations.
Additional adjustment for transplant status and International Staging System stage did not substantively change the magnitude of the HRs in the entire cohort (HR, 1.27; 95% CI, 0.97-1.66; P = .088) and in the White cohort (HR, 1.36; 95% CI, 0.98-1.90; P = .067), which still showed worse OS in those with DM compared with those without DM (supplemental Table 2). Results of IPTW sensitivity analyses were consistent with those from multivariable Cox regression models (supplemental Table 3).
To evaluate the mechanisms linking DM and MM progression, we examined the growth of MM1.S xenografts in diabetic Rag1−/−/MKR and control Rag1−/− mice. Tumors grew in more of the Rag1−/−/MKR mice compared with in control mice (50% [5 of 10] vs 83% [10 of 12]) and grew more rapidly in the Rag1−/−/MKR mice compared with in controls (Figure 3A). Plasma insulin concentrations were measured in the Rag1−/− and Rag1−/−/MKR mice at the end of the study (Figure 3B). Western blot analysis of the tumor xenograft protein found that the MM1.S xenografts expressed IRβ at similar levels between Rag1−/− control and Rag1−/−/MKR mice (Figure 3C-D). Tumors from Rag1−/−/MKR mice had greater phosphorylation of S6 ribosomal protein (Ser235/236) compared with controls (Figure 3C,E), indicating activation of the mammalian target of rapamycin (mTOR) pathway in the tumors from the diabetic mice. To determine whether this pathway was activated by insulin in the MM1.S cells, we performed in vitro cell stimulation, and found that insulin stimulation led to activation of the IR/IGF-1R, Akt, mTOR signaling pathway, as manifested by higher levels of phosphorylated (p)IRβ (Y1150/1151), pIGF-1Rβ (Y1135/1136), pAKT(S473) and pS6RP(S235/236) (Figure 3F-I).
Progression of myeloma xenografts is faster in a mouse model of type 2 diabetes than without diabetes. (A) Representative MM1.S tumor xenograft growth trajectories from Rag1−/−(Rag wild-type [WT]), and Rag1−/−/MKR (Rag MKR) male mice; n = 3 to 5 per group. (B) Plasma insulin concentrations in Rag1−/− and Rag1−/−/MKR male mice; n = 3 to 5 per group. (C) Representative western blot analysis of MM1.S tumor xenograft protein lysates from Rag1−/− and Rag1−/−/MKR mice, as indicated. (D-E) Quantification of total insulin receptor expression corrected for β actin, and S6rp phosphorylation, relative to total S6rp protein levels. Results are expressed as relative difference to that of Rag1−/− mice. (F) Representative western blot analysis of MM1.S tumor cell protein lysates from with and without insulin stimulation, as indicated. (G-I) Quantification of pIR/IGF-1R, pAkt, and pS6rp relative to total protein levels; n = 3 per group. ∗P < .05 between groups; ∗∗∗P < .001, as indicated.
Progression of myeloma xenografts is faster in a mouse model of type 2 diabetes than without diabetes. (A) Representative MM1.S tumor xenograft growth trajectories from Rag1−/−(Rag wild-type [WT]), and Rag1−/−/MKR (Rag MKR) male mice; n = 3 to 5 per group. (B) Plasma insulin concentrations in Rag1−/− and Rag1−/−/MKR male mice; n = 3 to 5 per group. (C) Representative western blot analysis of MM1.S tumor xenograft protein lysates from Rag1−/− and Rag1−/−/MKR mice, as indicated. (D-E) Quantification of total insulin receptor expression corrected for β actin, and S6rp phosphorylation, relative to total S6rp protein levels. Results are expressed as relative difference to that of Rag1−/− mice. (F) Representative western blot analysis of MM1.S tumor cell protein lysates from with and without insulin stimulation, as indicated. (G-I) Quantification of pIR/IGF-1R, pAkt, and pS6rp relative to total protein levels; n = 3 per group. ∗P < .05 between groups; ∗∗∗P < .001, as indicated.
Discussion
The underlying basis for increased incidence of MM in Black patients is not known. Genome-wide association studies account for ∼15% of the heritable risk. Unique loci in Black individuals have not been identified,18 suggesting factors beyond genetics including DM, and obesity may be contributing, given that they affect the Black population to a greater degree than the White population. In our study, we saw twice the prevalence of DM in the Black population compared with the White population with MM. The higher prevalence of DM in Black patients, our preclinical data from mouse models, and the known increased risk for MM in patients with DM suggest that DM may be a risk factor contributing to the increased development of MM in Black individuals compared with in White individuals.
Patients with DM had a worse OS in our study, which is consistent with previously published studies and confirms these findings.12-14 The racial differences in OS in patients with and without DM was an unexpected finding, with White patients with DM having had a worse OS compared with those without DM, but this was not seen in Black patients. The prevalence of DM usually increases with advancing age, which we observed in our Black and White cohorts; however, the prevalence of DM was 50% higher in Black patients who were younger (aged 45-60 years) than in White patients who were older (aged >60 years). Although age of >60 years was an independent risk factor for mortality in both groups, it is possible that we found no association between DM and OS in the Black population with DM because they were a younger population than the White population with DM, and therefore potentially had better tolerance to MM treatments and associated potential complications than the older White population with DM.
In contrast to other studies,19 our study did not show a worse OS in Black patients compared with White patients with MM. This is consistent with studies in which outcomes are similar or even better when access to care is the same for Black patients with MM.20-27 In the 2019 Surveillance Epidemiology, and End Results program data set, the 5-year relative survival of patients with MM was not different between Black (57.8%) and White (57.9%) patients.4
Obesity is a risk factor for several other conditions, including type 2 DM. MM is 1 of the 13 cancers associated with excess adiposity by the International Agency for Research on Cancer.28,29 Results from the CoMMpass cohort study show that a BMI of ≥35 kg/m2 was associated with trend toward worse progression-free survival and OS whereas a normal BMI between 18.5 and 24.9 had similar progression-free survival or OS as patients with a BMI of 25 to 34.9.30
However, 1 prior study of 2968 patients with MM in the Veterans Health Administration system showed that patients with an elevated BMI had lower mortality compared with patients with a normal BMI. They also showed that weight loss of ≥10% of baseline in the year before diagnosis was associated with increased mortality and made the association between increased BMI and survival nonsignificant.31 In our study we see an improved OS in the multivariable model for patients with an elevated BMI (overweight and obese), this improvement was seen in Black patients but not White patients. The inverse associations of overweight/obesity with OS in patients with MM seen in this analysis may reflect weight loss associated with more advanced disease at diagnosis (because BMI was ascertained at diagnosis). This suggests that obesity and DM play different roles in MM progression, and/or treatment responses. The “obesity paradox” has previously been described in other cancers.32 In our study, we did not have weight trajectories before the initial visit for MM treatment. It is possible that Black individuals with lower body mass indices had lost weight in the period before presentation and were therefore more cachectic than those with higher body mass indices. BMI as a measure of obesity has limitations across racial/ethnic groups. It does not take into account body composition, thus it is possible that higher BMI in the younger Black population was associated with higher lean mass rather than adipose tissue mass, which may contribute to better tolerance to treatment, or treatment response in certain patients with MM. It is also possible that obesity is associated with less aggressive MM as has been previously reported in solid cancers and may lead to racial differences in the impact of elevated BMI on survival.33
To our knowledge, this is the first study to show the mechanistic association between type 2 DM and MM progression in an in vivo model. The mouse model is nonobese, and therefore separates the metabolic effects of DM from obesity. The mTOR signaling pathway is an important mediator of IR signaling, and also a regulator of glucose homeostasis in cancer cells.34 A previous preclinical study showed that inhibiting PI3K–AKT–mTOR signaling in MM-associated mesenchymal stem cells impedes the proliferation of MM cells.35 Further studies to separate the effects of hyperinsulinemia from hyperglycemia in the activation of this pathway in different models of MM will be critical to optimize treatment strategies in individuals with DM and MM.
Our study has several potential limitations including its retrospective nature, potential bias in self-reported racial identification, and referral bias of populations seen at 2 large academic centers. Additionally, there is a potential for underdiagnosing DM in our study because the retrospective electronic review relied on ICD codes and HbA1c testing within the study period. Another variable we did not study was the impact of DM care on the outcome, which may be pursued in future studies. Strengths of the study lie in the multi-institutional data, with a large sample size, and similar treatment infrastructure and patterns between institutions. Moreover, to our knowledge, this study is the first to evaluate the effect of DM on MM tumor growth and survival in an established transgenic mouse model to validate and provide a mechanistic basis to confirming the association between DM and MM seen in our and prior studies.
Because patients with MM live longer than ever before given a rapidly changing treatment landscape because of the approval of novel therapies,36 inadequate DM management can lead to delays in diagnostic tests and the initiation of treatments, higher risks of complications from treatments, and deaths from cancer and noncancer causes. Our data suggest that to further improve OS in our patients with MM, modifiable risk factors such as DM can no longer be ignored as we improve the chemotherapeutic management of this common hematologic neoplasm. Pharmacological and nonpharmacological measures such as dietary intervention need to be investigated in future studies to improve outcomes in MM.37-45
Acknowledgments
The authors thank Frank Lewis and Marina Kerpelev, data engineers in the Digital Informatics and Technology Solutions (DigITs) at Memorial Sloan Kettering Cancer Center who identified and retrieved the data from the institutional database for this study.
This study is funded, in part, through the National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center support grants, Memorial Sloan Kettering grant P30 CA008748, and Tisch Cancer Institute grant P30 CA196521. U.A.S. received research support from the NCI Memorial Sloan Kettering Cancer Center (MSK) Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746, the American Society of Hematology Scholar Award, the Parker Institute for Cancer Immunotherapy at MSK Career Development Award, and the International Myeloma Society Career Development Award. She also received research funding from the Paula and Rodger Riney Foundation, HealthTree Foundation, the Allen Foundation Inc, the Willow Foundation, the David Drelich, Irrevocable Trust Transdisciplinary Research in Energetics and Cancer Research Training Workshop R25CA203650 (principal investigator: Melinda Irwin), and the American Society of Hematology Clinical Research Training Institute. E.J.G. reports research support and salary support from NIH/NCI R37CA266853. S.P. reports grant support from NIH/NCI R01CA252222, NIH/NCI R01CA244899, and NIH/NCI UH2 CA271390.
Authorship
Contribution: E.J.G., U.A.S., Y.H., and S.P. designed the study and initiated this work; E.M. and A.D. provided biostatistical support and analyzed the data; E.J.G. performed the in vivo mouse experiments; U.A.S., E.J.G., E.M., A.D., and S.P. wrote the manuscript; and all authors made substantial contributions to acquisition of data, critically revised the manuscript, and gave final approval of the manuscript to be submitted.
Conflict-of-interest disclosure: U.A.S. reports grants from MSK Paul Calabresi Career Development Award for Clinical Oncology K12CA184746, Paula and Rodger Riney Foundation, Parker Institute for Cancer Immunotherapy at MSK, HealthTree Foundation, International Myeloma Society, American Society of Hematology Scholar Award, and Allen Foundation Inc, as well as nonfinancial support from American Society of Hematology Clinical Research Training Institute and Transdisciplinary Research in Energetics and Cancer Research Training Workshop R25CA203650 (principal investigator: Melinda Irwin); other research support from Celgene/Bristol Myers Squibb (BMS) and Janssen to their institution; and personal fees from Association of Community Cancer Centers, MashUp MD, Janssen Biotech, Sanofi, BMS, MJH Life Sciences, Intellisphere, Phillips Gilmore Oncology Communications, RedMedEd, and i3Health. E.J.G. reports consulting for Novartis, Flare Therapeutics, and Seagen. S.P. reports research support from Amgen Inc, Celgene/BMS Corporation, Multiple Myeloma Research Foundation, GRAIL, and Caribou, and serves on the advisory board for GRAIL and Genentech. S.J. is a consultant for Janssen, BMS, Legend Biotech, Regeneron, Caribou, Sanofi, Takeda, and Karyopharm; is DMC chairman for Genmab and Sanofi; and has membership on an entity’s board of directors or advisory committees for International Myeloma Society, American Society of Hematology, and Society of Hematologic Oncology. C.R.T. reports research funding from Janssen and Takeda; personal fees from MJH Life Sciences; and has participated in advisory boards for Janssen and Sanofi, outside of the submitted work. M.H. reports research funding from Amgen, Daiichi Sankyo, and GlaxoSmithKline, and has received honoraria for consultancy/participated in advisory boards for Curio Science LLC, Intellisphere LLC, BMS, and GlaxoSmithKline. H.H. reports grants from Celgene, Takeda, and Janssen, outside the submitted work. S.M. reports consulting fees from Evicore, Optum, BioAscend, Janssen Oncology, and Legend Biotech. S.M. receives research funding from the National Cancer Institute, Janssen Oncology, BMS, Allogene Therapeutics, Fate Therapeutics, and Takeda Oncology, to the institution for research outside the submitted work, and has received honoraria from OncLive, Physician Education Resource, MJH Life Sciences, and Plexus Communications, outside the submitted work. K.M. reports grant support from American Society of Hematology, Multiple Myeloma Research Foundation, and International Myeloma Society. A.L. reports grants from Novartis and BMS; personal fees from Trillium Therapeutics; grants, personal fees, and nonfinancial support from Pfizer; grants and personal fees from Janssen, outside the submitted work; holds a patent US20150037346A1 with royalties paid; and serves on the data safety monitoring board for ArcellX. S.Z.U. received research funding from Amgen, Array Biopharma, BMS, Celgene, GlaxoSmithKline, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, and Takeda; is a consultant to AbbVie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GlaxoSmithKline, Janssen, Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, and TeneoBio; and is also a speaker with Amgen, BMS, Janssen, and Sanofi. N.K. reports research funding through Amgen, Janssen, Epizyme, AbbVie; consults for Clinical Care Options, OncLive, and Intellisphere Remedy Health; and participated in advisory board for Janssen. A.C. reports research support from Amgen, Array Biopharma, Celgene, GlaxoSmithKline, Janssen, Millennium/Takeda, Novartis Pharmaceuticals, Oncoceutics, Pharmacyclics, and Seattle Genetics; reports consultancy fees from Amgen, BMS, Celgene, Millennium/Takeda, Janssen, and Karyopharm; and declares membership on the scientific advisory board for Amgen, Celgene, Millennium/Takeda, Janssen, Karyopharm, Sanofi, and Seattle Genetics. H.J.C. is employed by the Multiple Myeloma Research Foundation and has research support from BMS, Takeda, and Genentech, outside the submitted work. J.R. is a consultant/adviser for Janssen, BMS, Pfizer, Karyopharm, Sanofi, Takeda, and AbbVie, and is a member of the speaker’s bureau for Janssen, BMS, Sanofi, and Adaptive Biotechnologies. A.R. is a consultant for Sanofi, BMS, Janssen, and Adaptive. C.R. is a consultant for Janssen, BMS, Takeda, Sanofi, and Artiva. The remaining authors declare no competing financial interests.
Correspondence: Urvi A. Shah, Myeloma Service, Division of Hematologic Oncology, Department of Medicine Memorial, Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; email: shahu@mskcc.org; Samir Parekh, Department of Medicine, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, Box 1079, New York, NY 10029; email: samir.parekh@mssm.edu; and Emily J. Gallagher, Division of Endocrinology, Diabetes and Bone Disease, Icahn School of Medicine at Mount Sinai, 1 Gustave L. Levy Pl, Box 1055, New York, NY 10029; email: emily.gallagher@mssm.edu.
References
Author notes
∗S.P. and E.J.G. contributed equally to this study.
For original aggregate data, please contact the corresponding authors, Urvi A. Shah (shahu@mskcc.org), Samir Parekh (samir.parekh@mssm.edu), and Emily J. Gallagher (emily.gallagher@mssm.edu). Individual participant data will not be shared.
The full-text version of this article contains a data supplement.

![Progression of myeloma xenografts is faster in a mouse model of type 2 diabetes than without diabetes. (A) Representative MM1.S tumor xenograft growth trajectories from Rag1−/−(Rag wild-type [WT]), and Rag1−/−/MKR (Rag MKR) male mice; n = 3 to 5 per group. (B) Plasma insulin concentrations in Rag1−/− and Rag1−/−/MKR male mice; n = 3 to 5 per group. (C) Representative western blot analysis of MM1.S tumor xenograft protein lysates from Rag1−/− and Rag1−/−/MKR mice, as indicated. (D-E) Quantification of total insulin receptor expression corrected for β actin, and S6rp phosphorylation, relative to total S6rp protein levels. Results are expressed as relative difference to that of Rag1−/− mice. (F) Representative western blot analysis of MM1.S tumor cell protein lysates from with and without insulin stimulation, as indicated. (G-I) Quantification of pIR/IGF-1R, pAkt, and pS6rp relative to total protein levels; n = 3 per group. ∗P < .05 between groups; ∗∗∗P < .001, as indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/1/10.1182_bloodadvances.2023010815/1/m_blooda_adv-2023-010815-gr3.jpeg?Expires=1769879620&Signature=uTmgvXNYuaQCAYCajNAwCc7ZUZqPL1R0B8zbTBRV3L44XdUxfkQOMSwteoXa~yl9wJm8efPiWLBJxrgeKXKcvrwiKVHGnTwcSaGVAGl147IWlQA2lLp0REIdoYEHC-JCGlsShjM4XT0rALR8hEKIfLVngUNiKP2Sm~tw-M-Kw5F4hbdcwLCdoUzFXhvPMINKmkf1byKyncHPdY4fm1KPrmxRYB11ggG8bPLLzSTfX32KqILbmGFCYCFWCbRpU2T5kbiRUzb3uQh3KHobNQlZ-XEok1g67PjANDK~FjuKUxmmUMOD5A1HIgAiklaER4e~hKmv0e6DU9iHztXNTul25g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)