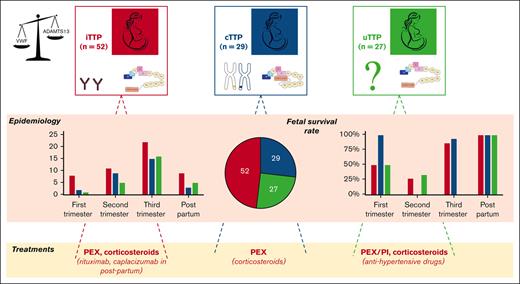
Three distinct entities of pregnancy-onset TTP are singled out: iTTP, uTTP, and cTTP, with fetal outcome closely linked to gestational age.
In the context of pregnancy, most iTTP and uTTP are shown to have an open ADAMTS13 conformation at acute phase.
Visual Abstract
Pregnancy-onset thrombotic thrombocytopenic purpura (TTP) is a rare and life-threatening disease of which diagnosis and management requires experienced multidisciplinary teams. The mechanisms responsible for a deficiency in the disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13) leading to pregnancy-onset TTP may be congenital or acquired, and studying ADAMTS13 conformation could be of interest. The differential diagnosis between TTP and other pregnancy-associated thrombotic microangiopathies (TMA) is often challenging. Our retrospective multicenter study highlights the significance and the challenges associated with pregnancy-onset TTP and childbirth in terms of diagnosis, obstetric management, and follow-up aspects. Among 1174 pregnancy-onset TMA enrolled in the French Registry for TMA from 2000 to 2020, we identified 108 pregnancy-onset TTP: 52 immune-mediated TTP (iTTP, 48.1%), 27 acquired TTP of unidentified mechanism (uTTP, 25%), and 29 congenital TTP (cTTP, 26.9%). Data show that maternal outcome is good (survival rate: 95%) and fetal outcome is linked to the gestational age at the onset of the disease (survival rate: 75.5%). Three distinct entities with different natural histories emerged: pregnancy-onset iTTP appears similar to idiopathic iTTP, with an open ADAMTS13 conformation, and is marked by a relapse risk independent of subsequent pregnancies; pregnancy-onset uTTP appears to have a different pathophysiology with an unexpected open ADAMTS13 conformation and a very low relapse risk independent of subsequent pregnancies; finally, pregnancy-onset cTTP is characterized by the necessity of pregnancy as a systematic and specific trigger and a need for prophylactic plasmatherapy for subsequent pregnancies. This trial was registered at www.clinicaltrials.gov as #NCT00426686, and at the Health Authority and the French Ministry of Health (P051064/PHRC AOM05012).
Introduction
Pregnancy and postpartum are known as high-risk periods for developing thrombotic microangiopathy (TMA) syndromes.1 In rare cases, pregnancy-related TMA correspond to thrombotic thrombocytopenic purpura (TTP) with predominant hematological and neurological involvement.2,3 Pregnancy-onset TTP accounts for ∼10% of all TTP cases with an overall incidence of ∼1 in 200 000 pregnancies.4-7 The clinical features of pregnancy-onset TTP may overlap with other more common TMA, such as preeclampsia (PE) especially with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome (specific for pregnancy), or TMA resulting from disseminated intravascular coagulation, antiphospholipid syndrome, and the hemolytic uremic syndrome (nonspecific for pregnancy). Consequently, the differential diagnosis of pregnancy-onset TTP with other pregnancy-onset TMAs remains very challenging.8-12
In physiological conditions, von Willebrand factor (VWF) levels increase from the early stages of pregnancy and can reach levels >200 IU/dL, returning to normal values 1 to 3 weeks postpartum.13 This increase in VWF during pregnancy is associated with a moderate decrease in the activity of its specific cleaving protease ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) of 20% to 40% compared with prepregnancy levels.13 Pregnancy-onset TTP is defined by a severe ADAMTS13 deficiency leading to the accumulation of the most hemostatically active ultralarge multimers of VWF that spontaneously bind to platelets to form platelet-rich microthrombi obstructing the microcirculation and resulting in thrombocytopenia and hemolytic anemia.14-16
In the vast majority of pregnancy-onset TTP cases, the severe functional deficiency in ADAMTS13 is acquired (aTTP) with a recovery of an ADAMTS13 activity detectable during remission (>20 IU/dL). aTTP is most often immune mediated (iTTP) by anti-ADAMTS13 autoantibodies.17,18 In up to 20% of cases, aTTP may not be associated with detectable anti-ADAMTS13 autoantibodies. In such cases, the term TTP of unidentified pathophysiology (uTTP) is now used.19 Another specificity of pregnancy is the relatively high frequency of the otherwise extremely rare late-onset congenital TTP (cTTP; Upshaw-Schulman syndrome), which accounts for 24% to 66% of the published cohorts of pregnancy-onset TTP.4-6,20 cTTP stems from recessively biallelic deleterious variants of the ADAMTS13 gene, cloned for the first time in 2001.21 The clinical distinction between cTTP and aTTP (either iTTP or uTTP) at the acute phase is practically impossible without rapid turnover of anti-ADAMTS13 autoantibodies or genetic analysis; in contrast, it is crucial to adapt management and subsequent follow-up.1,22
The exact risk of relapse during pregnancy in women who have recovered from iTTP or uTTP is unknown. This risk primarily relies on the level of ADAMTS13 activity at the start of pregnancy, with an increased risk if the activity remains undetectable.23 In cTTP, the risk of relapse in subsequent pregnancies is 100% in the absence of prophylactic plasma therapy.23,24
Beyond the knowledge of the VWF/ADAMTS13 system as a highly specific enzyme–substrate complex25 in which the flexibility of ADAMTS13 is the key to its proteolytic cleavage of VWF,26 ADAMTS13 conformation was recently shown to play a specific role in TTP pathophysiology. Physiologically, ADAMTS13 closed form is maintained by interactions between its CUB (complement C1r/C1s, Uegf, Bmp1) and spacer domains.27 When ADAMTS13 binds via its CUB domains to the VWF D4 domain, a change in the CUB interface occurs, which leads to the uncoupling of the CUB-spacer domains. As a consequence, ADAMTS13 adopts an open conformation.28 Additional binding of an exosite within the ADAMTS13 disintegrin domain to VWF triggers a conformational shift in the metalloprotease domain, which opens the active site and results in an active metalloprotease.29 In pathology, an open ADAMTS13 conformation was shown to be a hallmark of both acute and subclinical iTTP.30,31
In this study based on our 21 years of experience, we focused on pregnancy-onset TTP. Our aim was to understand the mechanisms leading to a severe ADAMTS13 deficiency during pregnancy and how such mechanisms influence diagnosis and therapeutic management, maternal and fetal outcomes, relapses, and subsequent pregnancies.
Patients and methods
Patients
Since 2000, all patients with a presumptive diagnosis of TMA (microangiopathic hemolytic anemia, severe thrombocytopenia, and organ ischemia) have been prospectively enrolled in the registry of the French Reference Center for TMA qualified by the National Plan for Rare Diseases of the French Health Ministry. ADAMTS13 investigation (activity, antigen, anti-ADAMTS13 immunoglobulin G [IgG], ADAMTS13 gene sequencing) is carried out in the central French reference laboratory. This study is a retrospective observational multicenter study focusing on patients presenting with their first TTP episode (ADAMTS13 activity of <10 IU/dL, associated or not with anti-ADAMTS13 IgG or bi-allelic deleterious ADAMTS13 sequence variations) during either pregnancy, postpartum, or in the aftermath of an abortion, from 1 January 2000 to 30 September 2020. All women with a history of TTP who became pregnant afterward were intentionally excluded from the study. We also investigated the follow-up (including future pregnancies) of the patients included in the study over the same time period. Our strategy for the differential diagnosis between cTTP and aTTP (iTTP and uTTP) is depicted in supplemental Figure 1. Clinical and biological data at acute phase and during follow-up, with a specific focus on subsequent pregnancies, were gathered through hospital reports. Placental histology reports, when available, were also collected. Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. This study was approved by the ethics committee of Hospital Pitié-Salpêtrière (Paris, France), and is registered at www.clinicaltrials.gov under identifier NCT00426686 and at the Health Authority and the French Ministry of Heath under the number P051064/PHRC AOM05012.
Blood collections and laboratory assays
Venous blood was drawn at the acute phase of TTP before any treatment and during follow-up, and, for phenotypic analysis, collected to a final volume of 1:10 into a tube containing 3.8% sodium citrate tube, or, for genetic analysis, into an EDTA tube, as previously described.32 Plasma samples and DNA were available thanks to the Biobank of the French Reference Center for TMA (CRB-LRB, biobank BB-0033-00064 certified NFS 96-900, Lariboisière Hospital, Assistance Publique Hôpitaux de Paris Nord, Paris, France). All blood samples were collected prospectively as part of routine care.
ADAMTS13 assays
Reference ADAMTS13 activity was measured in the context of care using our in-house FRETS-VWF73 assay7 (Peptide Institute Inc, Osaka, Japan) adapted from Kokame et al.33 The normal range is 50 to 150 IU/dL.
Anti-ADAMTS13 IgG were detected and titered using the Technozym ADAMTS13 INH (inhibitor) enzyme-linked immunosorbent assay (ELISA) assay (Technoclone, Vienna, Austria) with a positivity threshold at 15 U/mL according to the manufacturer’s instructions.
ADAMTS13 antigen and conformation were measured using in-house ELISA assays34 based on murine anti-ADAMTS13 antibody–labeled 3H9- and 1C4-targeting epitopes located in the metalloprotease and spacer domains, respectively.30,35 The normal range of ADAMTS13 antigen is 0.930 to 1.350 μg/mL.30,36 A minimal antigen level of 0.03 μg/mL is required to determine a conformation index reflecting the increase of 1C4 binding induced by the opening of ADAMTS13 by a murine anti-CUB1 antibody–labeled 17G2.30 A conformation index of ≤0.5 corresponds to a closed ADAMTS13 whereas a conformation index of >0.5 corresponds to an open ADAMTS13.30
ADAMTS13 (NM_139025.5) genotype was determined using high throughput sequencing of the coding sequence with IDT probes (Integrated DNA Technologies Inc, Basel, Switzerland), and row data were analyzed by SOPHiA DDM (Sophia Genetics). The deleterious status of ADAMTS13 sequences variations was determined using recommendations by The American College of Medical Genetics and Genomics and the American Association of Molecular Pathology.37
Statistics
Quantitative parameters are reported as median and interquartile range (IQR) values and were compared with the nonparametric Mann-Whitney U test or Kruskal-Wallis test when appropriate. Qualitative parameters are reported as numbers and proportions, with 95% confidence intervals (95% CIs) for survival rates. Statistical significance was set at P < .05. Statistical analysis was performed using Prism version 7.00 (GraphPad Software, La Jolla, CA).
Results
Inaugural acute phase presentation and outcome
Over a period of 21 years, 1174 patients with pregnancy-onset TMA were prospectively enrolled in our registry and were investigated for ADAMTS13, which allowed the diagnosis of 108 (9%) patients with pregnancy-onset TTP (Figure 1). In the same timeframe, a total of 567 women (pregnant or not) aged 18 to 45 were diagnosed with TTP, indicating that obstetrical TTP represents 19% (108/567) of TTP in women of child-bearing age. Subsequent investigation, including anti-ADAMTS13 antibodies and ADAMTS13 sequencing, led to the identification of 52 iTTP cases (52/108, 48.1%), 27 uTTP cases (27/108, 25.0%), and 29 cTTP cases (29/108, 26.9%). Sixty-four patients had their inaugural TTP episode during their first pregnancy (64/108, 59.3%; 27 iTTP, 18 uTTP, and 19 cTTP). The remaining 44 patients previously experienced at least 1 pregnancy before TTP episode (Table 1). The main ethnic groups were Caucasian (69/108, 63.8%), Afro-Caribbean (21/108, 19.4%), and North-African (16/108, 14.8%). Most patients (72/108, 66.7%) had no other clinical context associated. Noteworthy contexts were systemic autoimmune diseases (18/108, 16.7%), infections (17/108, 15.7%), ovarian stimulation for in vitro fertilization (5 of 108, 4.6%), or abortion (2/108, 1.9%). The demographic, clinical, and biologic features of our 108 patients are presented in Table 1. The median age reported was 28 years (IQR, 8 years), with patient age ranging from 17 to 44 years, and was similar within the 3 subgroups. Median term during which TTP episode occurred was 30 weeks of gestation (WG; IQR, 10 weeks), from 5 WG to 4 weeks postpartum. Seven patients (7/108, 6.5%) had twin pregnancies. Most patients experienced their TTP episode during the last third of their pregnancy (53 patients) or during postpartum (17 patients). Thirteen patients were diagnosed with TTP during their first trimester (including 2 patients who developed TTP in the aftermath of an abortion), and the 25 remaining patients experienced TTP during the second trimester of their pregnancy, with no differences between the 3 subgroups. Maternal outcome was generally favorable, with 103 of 108 survivals (95% survival rate; 95% CI, 90-98). Overall, after excluding 2 pregnancies that ended in voluntary abortions, of 106 pregnancies, 80 live births were reported (80/106; 75.5% survival rate; 95% CI, 67-83). Main clinical features reported include fever (28/108, 25.9%), neurologic symptoms (50 of 108, 46.3%), high blood pressure (29 of 108, 26.8%), abdominal pain (42/108, 38.9%), and acute kidney injury (AKI; 44 of 108, 40.7%). Of interest, high blood pressure and AKI were statistically more frequent in the uTTP group (P < .05 for both). The median platelet count was 18 x109/L (IQR, 20 x109/L) and was significantly higher (P < .0001) in the uTTP subgroup (34 x109/L) than in the iTTP and cTTP subgroups (13 and 19 x109/L, respectively). Similarly, although median serum creatinine was 94 μmol/L (IQR, 119 μmol/L) for the whole cohort, it was statistically higher (P < .005) in the uTTP subgroup (202 μmol/L) than in the iTTP and cTTP subgroups (84 and 79 μmol/L, respectively). The median hemoglobin level was 7.7 g/dL (IQR, 2.1 g/dL) and median lactate dehydrogenase level was 1400 U/L (IQR, 1992 U/L), with no discrepancy between groups. Fetal outcome appears to be highly dependent on the pregnancy term (P < .0001): of the 11 pregnancies who experienced TTP during their first trimester, 5 fetal deaths were reported (5/11; 45.5% survival rate; 95% CI, 21.3-72), 19 fetal deaths (18 intrauterine fetal death [IUFD] and 1 medical termination of pregnancy) were reported of the 25 second-trimester pregnancies (6/25; 24% survival rate; 95% CI, 11.5-43.4), 5 fetal deaths were reported of the 53 patients who experienced TTP during their third trimester (48/53; 90.6% survival rate; 95% CI, 79.7-95.9), and no fetal death was reported of the 17 postpartum TTP cases (Table 2). Nine placental pathology reports (iTTP, n = 2; uTTP, n = 2; and cTTP, n = 5) were available and described severe placental ischemia with numerous intervillous thrombi of various ages.
Flowchart of the enrollment of patients with pregnancy-onset TTP in the cohort study. From 1 January 2000 to 30 September 2020, 1174 patients with pregnancy onset-TMA were enrolled and investigated for ADAMTS13. In total, 108 patients had a severe functional deficiency in ADAMTS13 (activity of <10 IU/dL) and were subsequently diagnosed with pregnancy-onset TTP. Of those 108 patients, 52 had iTTP (anti-ADAMTS13 IgG of >15 U/mL), 27 had uTTP (anti-ADAMTS13 IgG of <15 U/mL), and 29 had cTTP.
Flowchart of the enrollment of patients with pregnancy-onset TTP in the cohort study. From 1 January 2000 to 30 September 2020, 1174 patients with pregnancy onset-TMA were enrolled and investigated for ADAMTS13. In total, 108 patients had a severe functional deficiency in ADAMTS13 (activity of <10 IU/dL) and were subsequently diagnosed with pregnancy-onset TTP. Of those 108 patients, 52 had iTTP (anti-ADAMTS13 IgG of >15 U/mL), 27 had uTTP (anti-ADAMTS13 IgG of <15 U/mL), and 29 had cTTP.
Demographic, clinical, and biological characteristics of the first episode of pregnancy-onset TTP in 108 patients
| Demographic features . | iTTP (n = 52) . | uTTP (n = 27) . | cTTP (n = 29) . | Total (N = 108) . | P value . |
|---|---|---|---|---|---|
| Age, y | 29 (8) | 28 (11) | 27 (7) | 28 (8) | ns |
| Gestational age (WG) | 30 (11) | 34 (11) | 30 (8) | 30 (10) | ns |
| Gravidity | 2 (2) | 2 (2) | 1 (1) | 2 (2) | ns |
| Parity | 1 (2) | 1 (1) | 1 (1) | 1 (2) | ns |
| Twin pregnancies | 2/52 (3.8%) | 4/27 (14.8%) | 1/29 (3.5%) | 7/108 (6.5%) | ns |
| Maternal survival | 48/52 (92.3%) | 26/27 (96.2%) | 29/29 (100%) | 103/108 (95%) | ns |
| Fetal survival | 35/50∗ (70%) | 21/27 (77.8%) | 21/29 (72.4%) | 80/106 (75.5%) | ns |
| Clinical features | |||||
| Fever | 17/52 (32.7%) | 9/27 (33.3%) | 2/29 (6.8%) | 28/108 (25.9%) | ns |
| Neurologic symptoms | 30/52 (57.7%) | 13/27 (48.2%) | 7/29 (24%) | 50/108 (46.3%) | ns |
| High blood pressure | 7/52 (13.5%) | 14/27 (51.8%) | 8/29 (27.6%) | 29/108 (26.8%) | <.05 |
| Abdominal pain | 19/52 (36.5%) | 12/27 (44.4%) | 11/29 (37.9%) | 42/108 (38.9%) | ns |
| AKI | 17/52 (32.7%) | 18/27 (66.7%) | 9/29 (31%) | 44/108 (40.7%) | <.05 |
| Biological features | |||||
| Platelet count (×109/L) | 13 (9) | 34 (34) | 19 (12) | 18 (20) | <.0001 |
| Hemoglobin level (g/dL) | 7.2 (2.1) | 8.1 (1.8) | 7.9 (2.2) | 7.7 (2.1) | ns |
| Serum creatinine (μmol/L) | 80 (87) | 202 (300) | 79 (48) | 94 (24-500, 119) | <.005 |
| LDH (U/L) | 1588 (1460) | 1400 (2400) | 1000 (2325) | 1400 (1992) | ns |
| Demographic features . | iTTP (n = 52) . | uTTP (n = 27) . | cTTP (n = 29) . | Total (N = 108) . | P value . |
|---|---|---|---|---|---|
| Age, y | 29 (8) | 28 (11) | 27 (7) | 28 (8) | ns |
| Gestational age (WG) | 30 (11) | 34 (11) | 30 (8) | 30 (10) | ns |
| Gravidity | 2 (2) | 2 (2) | 1 (1) | 2 (2) | ns |
| Parity | 1 (2) | 1 (1) | 1 (1) | 1 (2) | ns |
| Twin pregnancies | 2/52 (3.8%) | 4/27 (14.8%) | 1/29 (3.5%) | 7/108 (6.5%) | ns |
| Maternal survival | 48/52 (92.3%) | 26/27 (96.2%) | 29/29 (100%) | 103/108 (95%) | ns |
| Fetal survival | 35/50∗ (70%) | 21/27 (77.8%) | 21/29 (72.4%) | 80/106 (75.5%) | ns |
| Clinical features | |||||
| Fever | 17/52 (32.7%) | 9/27 (33.3%) | 2/29 (6.8%) | 28/108 (25.9%) | ns |
| Neurologic symptoms | 30/52 (57.7%) | 13/27 (48.2%) | 7/29 (24%) | 50/108 (46.3%) | ns |
| High blood pressure | 7/52 (13.5%) | 14/27 (51.8%) | 8/29 (27.6%) | 29/108 (26.8%) | <.05 |
| Abdominal pain | 19/52 (36.5%) | 12/27 (44.4%) | 11/29 (37.9%) | 42/108 (38.9%) | ns |
| AKI | 17/52 (32.7%) | 18/27 (66.7%) | 9/29 (31%) | 44/108 (40.7%) | <.05 |
| Biological features | |||||
| Platelet count (×109/L) | 13 (9) | 34 (34) | 19 (12) | 18 (20) | <.0001 |
| Hemoglobin level (g/dL) | 7.2 (2.1) | 8.1 (1.8) | 7.9 (2.2) | 7.7 (2.1) | ns |
| Serum creatinine (μmol/L) | 80 (87) | 202 (300) | 79 (48) | 94 (24-500, 119) | <.005 |
| LDH (U/L) | 1588 (1460) | 1400 (2400) | 1000 (2325) | 1400 (1992) | ns |
Categorical variables are presented as n/number of patients and percentage. Continuous variables are presented as median (IQR).
AKI, acute kidney injury; LDH, lactate dehydrogenase; ns, nonsignificant.
Two pregnancies were ended (abortion). P from Mann-Whitney U test or Kruskal-Wallis test for count data.
Survival rate as a function of gestational age at the inaugural acute phase of TTP in 108 patients
| . | iTTP (n = 50)∗ . | uTTP (n = 27) . | cTTP (n = 29) . | Total (N = 106) . |
|---|---|---|---|---|
| First trimester | 4/8 (50.0%) | 1/1 (100.0%) | 1/2 (50.0%) | 6/11 (54.5%) |
| Second trimester | 3/11 (27.2%) | 0/5 (0.0%) | 3/9 (33.3%) | 6/25 (24.0%) |
| Third trimester | 19/22 (86.4%) | 15/16 (93.8%) | 14/15 (93.3%) | 48/53 (90.6%) |
| Postpartum | 9/9 (100.0%) | 5/5 (100.0%) | 3/3 (100.0%) | 17/17 (100.0%) |
| . | iTTP (n = 50)∗ . | uTTP (n = 27) . | cTTP (n = 29) . | Total (N = 106) . |
|---|---|---|---|---|
| First trimester | 4/8 (50.0%) | 1/1 (100.0%) | 1/2 (50.0%) | 6/11 (54.5%) |
| Second trimester | 3/11 (27.2%) | 0/5 (0.0%) | 3/9 (33.3%) | 6/25 (24.0%) |
| Third trimester | 19/22 (86.4%) | 15/16 (93.8%) | 14/15 (93.3%) | 48/53 (90.6%) |
| Postpartum | 9/9 (100.0%) | 5/5 (100.0%) | 3/3 (100.0%) | 17/17 (100.0%) |
The results are presented as number of live births per number of pregnancies (survival rate). Trimesters and postpartum refer to pregnancies complicated by a TTP episode: first trimester, before 15 WG; second trimester, between 16-28 WG; and third trimester, after 28 WG but before birth.
There was no significant association between the subtype of TTP and the survival rate. The second trimester is significantly associated with lower survival rate (P < .0001).
Two pregnancies were ended (abortion) and are therefore not reported in this table.
Genotypic analysis of patients with cTTP
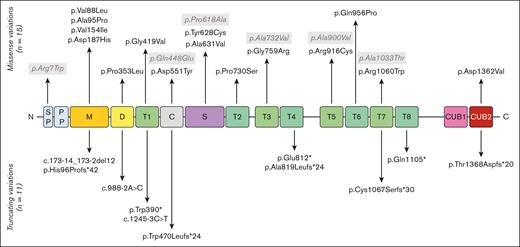
Most of the patients with cTTP in this study were previously reported by our group.5,7,38 A total of 26 deleterious sequence variations, 15 missense, and 11 truncating variants (5 frameshift, 3 nonsense, and 3 splicing variants) are reported (supplemental Table 1). The missense variant p.Arg1060Trp (R1060W) is overwhelmingly frequent, being found in 21 patients (21/29, 72.4%), 6 of which were homozygous, which represents 27 alleles of 58 (46.7%). Overall, 19 patients (65.5%) were compound heterozygous, including 1 with 3 variations. Three patients only had 1 deleterious variant, and 1 patient was hemizygous for the ADAMTS13 gene, with no variation on her remaining allele but with 4 single-nucleotide polymorphisms. Five single-nucleotide polymorphisms in the ADAMTS13 gene were found much more frequently in our cohort: p.Arg7Trp, p.Gln448Glu, p.Pro618Ala, p.Ala732Val, and p.Ala900Val than in the general population (gnomAD project). An illustration of the ADAMTS13 sequence variations found in our cohort of patients with cTTP is presented in Figure 2. The variants found span the entire gene with no obvious hot spot except the predominant R1060W deleterious variant in the TSP1-7 domain.
ADAMTS13 sequence variations reported in the 29 cases of pregnancy-onset cTTP. ADAMTS13 consists of several domains: a signal peptide (SP), a propeptide (PP), a metalloprotease domain (M), a disintegrin-like domain (D), a first thrombospondin type-1 repeat (T1), a cysteine-rich domain (C), a spacer domain (S), 7 additional thrombospondin type-1 repeats (T2-T8), and 2 CUB domains. Deleterious sequence variations of the ADAMTS13 gene appear in black whereas single-nucleotide polymorphism (SNPs) appear in gray.
ADAMTS13 sequence variations reported in the 29 cases of pregnancy-onset cTTP. ADAMTS13 consists of several domains: a signal peptide (SP), a propeptide (PP), a metalloprotease domain (M), a disintegrin-like domain (D), a first thrombospondin type-1 repeat (T1), a cysteine-rich domain (C), a spacer domain (S), 7 additional thrombospondin type-1 repeats (T2-T8), and 2 CUB domains. Deleterious sequence variations of the ADAMTS13 gene appear in black whereas single-nucleotide polymorphism (SNPs) appear in gray.
ADAMTS13 conformation at acute phase of TTP
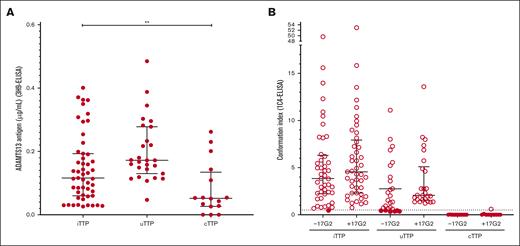
ADAMTS13 antigen was decreased in all 108 patients with acute TTP (0.116 μg/mL [IQR, 0.133 μg/mL] in iTTP; 0.172 μg/mL [IQR, 0.148] in uTTP; and 0.052 μg/mL [IQR, 0.108 μg/mL] in cTTP38 groups). Interestingly, ADAMTS13 antigen was significantly lower in the iTTP group than in the uTTP group (P < .05; Figure 3A).
In acute pregnancy-onset TTP, ADAMTS13 may adopt several profiles of conformation. (A) ADAMTS13 antigen levels (3H9-ELISA) in iTTP (n = 52), uTTP (n = 27), and cTTP (n = 29). ADAMTS13 antigen was decreased (lower than the normal range) in all 108 patients. Median ADAMTS13 antigen level was 0.116 μg/mL (IQR, 0.133 μg/mL) in the iTTP group, 0.172 μg/mL (IQR, 0.148 μg/mL) in the uTTP group, and 0.052 μg/mL (IQR, 0.108 μg/mL) in the cTTP group as previously reported by Joly et al.38 Only plasma samples containing ADAMTS13 antigen of ≥0.03 μg/mL could be tested for ADAMTS13 conformation. (B) ADAMTS13 conformation (1C4-ELISA) in iTTP (n = 46), uTTP (n = 26), and cTTP (n = 11). The activating murine monoclonal antibody 17G2 was used as a positive control in all samples tested. ADAMTS13 conformation was found open in all but 1 patient with iTTP tested (45 of 46, 97.8%) and open in the majority of patients with uTTP tested (20 of 26, 76.9%). As previously reported by Joly et al, ADAMTS13 conformation was closed in all but 1 patient with cTTP.
In acute pregnancy-onset TTP, ADAMTS13 may adopt several profiles of conformation. (A) ADAMTS13 antigen levels (3H9-ELISA) in iTTP (n = 52), uTTP (n = 27), and cTTP (n = 29). ADAMTS13 antigen was decreased (lower than the normal range) in all 108 patients. Median ADAMTS13 antigen level was 0.116 μg/mL (IQR, 0.133 μg/mL) in the iTTP group, 0.172 μg/mL (IQR, 0.148 μg/mL) in the uTTP group, and 0.052 μg/mL (IQR, 0.108 μg/mL) in the cTTP group as previously reported by Joly et al.38 Only plasma samples containing ADAMTS13 antigen of ≥0.03 μg/mL could be tested for ADAMTS13 conformation. (B) ADAMTS13 conformation (1C4-ELISA) in iTTP (n = 46), uTTP (n = 26), and cTTP (n = 11). The activating murine monoclonal antibody 17G2 was used as a positive control in all samples tested. ADAMTS13 conformation was found open in all but 1 patient with iTTP tested (45 of 46, 97.8%) and open in the majority of patients with uTTP tested (20 of 26, 76.9%). As previously reported by Joly et al, ADAMTS13 conformation was closed in all but 1 patient with cTTP.
Because a minimal ADAMTS13 antigen level of 0.03 μg/mL is necessary to determine ADAMTS13 conformation, 7 patients were excluded from ADAMTS13 analysis. ADAMTS13 conformation with and without preincubation with the opening 17G2 antibody in 101 patients are presented in Figure 3B. ADAMTS13 conformation was open in virtually all patients with iTTP (45/46, 97.8%); only 1 patient with iTTP exhibited a borderline conformation index at 0.46. Usually, ADAMTS13 conformation was closed in nonpregnancy-onset uTTP.19 Surprisingly, ADAMTS13 conformation was also open in most patients with pregnancy-onset uTTP (20/26, 76.9%). ADAMTS13 conformation in adult-onset cTTP was previously reported by our group to be folded in all tested patients.38
Management
Therapeutic management of our patients is presented in Table 3.
Therapeutic management of the first episode of pregnancy-onset TTP in 108 patients
| . | iTTP (n = 52) . | uTTP (n = 27) . | cTTP (n = 29) . | Total (N = 108) . |
|---|---|---|---|---|
| PEX | 50/52 (96.2%) | 19/27 (70.3%) | 16/29 (55.2%) | 85/ 108 (78.7%) |
| FFP infusion (no PEX) | 1/52 (1.9%) | 0/27 (0%) | 11/29 (37.9%) | 12/108 (11.1%) |
| Corticosteroids | 42/52 (80.8%) | 19/27 (70.3%) | 15/29 (51.7%) | 76/108 (70.4%) |
| Rituximab | 29/52 (55.8%) | 1/27 (3.7%) | 3/29 (10.3%) | 33/108 (30.6%) |
| Caplacizumab∗ | 4/52 (7.7%) | 0/27 (0%) | 0/29 (0%) | 4/108 (3.7%) |
| Platelet concentrates | 9/52 (17.3%) | 3/27 (11.1%) | 3/29 (10.3%) | 15/108 (13.9%) |
| Antihypertensive drugs | 1/52 (1.9%) | 5/27 (18.5%) | 6/29 (20.6%) | 12/108 (11.1%) |
| Splenectomy | 1/52 (1.9%) | 0/27 (0%) | 0/29 (0%) | 1/108 (0.9%) |
| Cyclophosphamide | 1/52 (1.9%) | 0/27 (0%) | 0/29 (0%) | 1/108 (0.9%) |
| IV immunoglobulin | 3/52 (5.8%) | 0/27 (0%) | 0/29 (0%) | 3/108 (2.8%) |
| Heparin | 3/52 (5.8%) | 1/27 (3.7%) | 0/29 (0%) | 4/108 (3.7%) |
| Aspirin | 6/52 (11.5%) | 1/27 (3.7%) | 0/29 (0%) | 7/108 (6.5%) |
| . | iTTP (n = 52) . | uTTP (n = 27) . | cTTP (n = 29) . | Total (N = 108) . |
|---|---|---|---|---|
| PEX | 50/52 (96.2%) | 19/27 (70.3%) | 16/29 (55.2%) | 85/ 108 (78.7%) |
| FFP infusion (no PEX) | 1/52 (1.9%) | 0/27 (0%) | 11/29 (37.9%) | 12/108 (11.1%) |
| Corticosteroids | 42/52 (80.8%) | 19/27 (70.3%) | 15/29 (51.7%) | 76/108 (70.4%) |
| Rituximab | 29/52 (55.8%) | 1/27 (3.7%) | 3/29 (10.3%) | 33/108 (30.6%) |
| Caplacizumab∗ | 4/52 (7.7%) | 0/27 (0%) | 0/29 (0%) | 4/108 (3.7%) |
| Platelet concentrates | 9/52 (17.3%) | 3/27 (11.1%) | 3/29 (10.3%) | 15/108 (13.9%) |
| Antihypertensive drugs | 1/52 (1.9%) | 5/27 (18.5%) | 6/29 (20.6%) | 12/108 (11.1%) |
| Splenectomy | 1/52 (1.9%) | 0/27 (0%) | 0/29 (0%) | 1/108 (0.9%) |
| Cyclophosphamide | 1/52 (1.9%) | 0/27 (0%) | 0/29 (0%) | 1/108 (0.9%) |
| IV immunoglobulin | 3/52 (5.8%) | 0/27 (0%) | 0/29 (0%) | 3/108 (2.8%) |
| Heparin | 3/52 (5.8%) | 1/27 (3.7%) | 0/29 (0%) | 4/108 (3.7%) |
| Aspirin | 6/52 (11.5%) | 1/27 (3.7%) | 0/29 (0%) | 7/108 (6.5%) |
The results are presented as number of patients who received the therapeutic options per number of patients (percentage).
FFP, fresh frozen plasma; PEX, plasma exchange.
Caplacizumab was used postpartum.
The majority of patients with iTTP and uTTP (70/79, 88.6%) received curative plasma therapy, either as plasma exchange (PEX) or as fresh frozen plasma infusions. One patient with iTTP died before plasma therapy could be initiated, and 8 patients with uTTP did not received plasma therapy in the context of hypertension or associated HELLP syndrome, which had no impact on maternal survival but resulted in 2 fetal losses. Corticosteroids were also widely used (61/79, 77.2%). Rituximab was chosen as an immunosuppressive therapy for 30 patients (30/79, 38.0%), mostly in the iTTP group (29/52, 55.8%), the remaining patient being 1 with uTTP (1/27, 3.7%). Out of 29 patients with iTTP who received rituximab, the treatment was implemented after either birth or fetal extraction in 22 patients and during pregnancy in 7 patients. In the latter 7 patients, 5 pregnancies ended in fetal loss (1 abortion, 1 termination of pregnancy, and 3 IUFDs) and 2 pregnancies (rituximab at 13 and 18 WG) ended well for the mother and the newborn, with no specific side effects reported for both newborns. The only patient with uTTP received rituximab after fetal extraction.
Four patients with iTTP in our cohort (4/52, 7.7%), who were among the most recently recruited, received caplacizumab, after delivery (Table 3), with no reported side effects. Other therapies used in association with plasma therapy and corticosteroids were platelet concentrates (15/108, 13.9%) used to assist a cesarean delivery (11/15, 73.3%) but in some cases administered inappropriately (4/15, 26.7%), antihypertensive drugs (12 of 108, 11.1%), bolus of cyclophosphamide (1/108, 0.9%), and splenectomy (1/108, 0.9%) as salvage therapies23 (Table 3).
According to international guidelines, a prophylactic plasma therapy was initiated and increased from 10 to 20 mL/kg every 14 to 7 days as soon as they become pregnant in all patients with cTTP except for 2, to maintain a normal platelet count without hemolysis; 1 of whom had PEX throughout her pregnancy. No patient with cTTP experienced relapse. Three patients with cTTP received steroids before the diagnosis of cTTP could be made, and 2 patients with cTTP received rituximab after fetal extraction, whereas 1 patient with cTTP received rituximab at 22 WG, with a good maternal and fetal outcome.
Follow-up
The median length of follow-up for iTTP and uTTP was 2 years (IQR, 7 years; Figure 4). Only 1 patient with uTTP developed anti-ADAMTS13 IgG during follow-up. A TTP relapse, outside of pregnancy, was reported in 11 of 48 surviving patients with iTTP (22.9%), and in the only 1 patient (of 26 [3.8%] surviving patients with uTTP) who developed anti-ADAMTS13 IgG during follow-up. A total of 24 subsequent pregnancies were recorded in 14 women (12 iTTP; 2 uTTP).
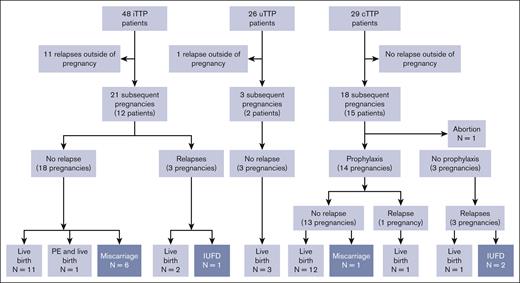
Flowchart of the subgroup of patients with subsequent relapses and pregnancies as a function of pregnancy-onset TTP. A total of 12 relapses outside of pregnancies and 42 subsequent pregnancies were reported. Eleven relapses outside of pregnancies were observed in the 48 surviving patients with iTTP, 1 in the surviving 26 patients with uTTP, and none in the 29 surviving patients with cTTP. The 21 subsequent pregnancies reported in the iTTP group can be broken down into a no TTP–relapse group (18 pregnancies: 11 live births, 6 miscarriages, and 1 live birth complicated by PE) and a TTP-relapse group (3 pregnancies: 2 live births and 1 IUFD). All 3 of the subsequent pregnancies reported in the uTTP group ended in live births without relapses. Finally, 18 pregnancies were monitored in the cTTP group. One ended in an abortion and 14 were led with prophylactic plasmatherapy preventing a relapse in 13 pregnancies (12 live births and 1 miscarriage). Despite prophylactic plasmatherapy, 1 TTP relapse was reported, ending with a live birth. The final 3 were carried out without prophylactic plasmatherapy and were all marked by a TTP relapse, with 2 IUFD and 1 live birth.
Flowchart of the subgroup of patients with subsequent relapses and pregnancies as a function of pregnancy-onset TTP. A total of 12 relapses outside of pregnancies and 42 subsequent pregnancies were reported. Eleven relapses outside of pregnancies were observed in the 48 surviving patients with iTTP, 1 in the surviving 26 patients with uTTP, and none in the 29 surviving patients with cTTP. The 21 subsequent pregnancies reported in the iTTP group can be broken down into a no TTP–relapse group (18 pregnancies: 11 live births, 6 miscarriages, and 1 live birth complicated by PE) and a TTP-relapse group (3 pregnancies: 2 live births and 1 IUFD). All 3 of the subsequent pregnancies reported in the uTTP group ended in live births without relapses. Finally, 18 pregnancies were monitored in the cTTP group. One ended in an abortion and 14 were led with prophylactic plasmatherapy preventing a relapse in 13 pregnancies (12 live births and 1 miscarriage). Despite prophylactic plasmatherapy, 1 TTP relapse was reported, ending with a live birth. The final 3 were carried out without prophylactic plasmatherapy and were all marked by a TTP relapse, with 2 IUFD and 1 live birth.
The 12 patients with iTTP total 21 subsequent pregnancies:
Out of 21 pregnancies, 18 pregnancies did not relapse, and 11 of 18 who were monitored regularly on their ADAMTS13 activity, which remained in normal range, ended with a live birth. Of particular interest, 3 additional patients (3 of 18 pregnancies) received immunosuppressive therapy: 1 received rituximab as a preemptive therapy after a biological relapse that occurred 5 months before her pregnancy; 1 received azathioprine, hydroxychloroquine, and corticosteroid throughout her pregnancy because of a preexisting lupus; and 1 received corticosteroids in postpartum also because of a preexisting lupus. Two patients (2 of 18 pregnancies) received aspirin and low-molecular weight heparin during their pregnancy. Six pregnancies (6 of 18; 2 patients, 1 of which also had lupus) ended in miscarriage; in 4 cases, ADAMTS13 activity was normal. One pregnancy (1 of 18) ended in PE, and a live birth but no TTP relapse (ADAMTS13 activity decreased at 16 IU/dL).
The final 3 of 21 pregnancies were marked by a TTP relapse, ending with live birth (n = 2) and IUFD (n = 1). In all 3 cases, ADAMTS13 activity measured at the beginning of the pregnancy was very low (≤20 IU/dL).
The 2 patients with uTTP total 3 subsequent pregnancies, during which ADAMTS13 activity remained normal and no relapse occurred.
In the cTTP group, median length of follow-up was 6.5 years (IQR, 6.5 years). No TTP relapse outside of pregnancy were reported for the 29 patients with cTTP in our cohort. Eighteen pregnancies were reported in 15 patients. One pregnancy ended in abortion, 3 pregnancies were led without prophylaxis and subsequently were complicated in each case by a TTP relapse, ending in 2 IUFD and 1 live birth. The remaining 14 other pregnancies were all carried out with prophylactic plasma therapy: 1 pregnancy resulted in IUFD but no relapse; 12 pregnancies ended in a live birth with no relapse; and 1 pregnancy was marked by a TTP relapse with a live birth.
Discussion
This study reports, to our knowledge, the world-wide largest cohort of patients with pregnancy-onset TTP enrolled on a national scale and exhaustively characterized at the first episode and during follow-up.
The 19% frequency of pregnancy-onset TTP within the overall group of women of child-bearing age with TTP highlights the importance of pregnancy as a major TTP trigger.39 The high frequency of cTTP in this pregnancy-onset TTP cohort (27%) is also consistent with previous studies.5,6,40 Although the majority (65%) of the patients in our cohort experienced their TTP episode in the last third of their pregnancy (third trimester or postpartum), a sizable portion (35%) of patients were diagnosed during the first or second trimester. This suggests that, although rare, TTP should always be considered when dealing with pregnancy-onset TMA regardless of the pregnancy term. Maternal outcome proved good and indicates that the past 2 decades have seen substantial improvement in the diagnosis and management of this overall very rare disease.41,42 Another confirmation of this study is the close relationship between fetal outcome and pregnancy term. Although it appears rational that fetal outcome is excellent in third trimester or postpartum pregnancy-onset TTP, the discrepancy between what we observe in first trimester and second trimester onset TTP is more surprising. A possible explanation, supported by placental pathology reports gathered in this study or in others,6,9 is the severe placental ischemia with numerous intervillous thrombi caused by TTP. This is also consistent with the unexpected number of patients with elevated sFlt-1:PlGF ratio in pregnancy-onset TTP previously published by our team.43
The different aspects of this study (genotypic analysis, ADAMTS13 conformation, clinical history, management, and follow-up) allow us to distinguish 3 different subtypes of pregnancy-onset TTP with different mechanisms and evolution.
The first and most common subtype of pregnancy-onset TTP remains iTTP (48.1% of our cohort) sharing similarities with idiopathic iTTP cohorts in terms of clinical presentation (pregnancy aside) and autoimmunity against ADAMTS13,7 risk of relapse,22,44 functional deficiency in ADAMTS13, and its open conformation blocked by anti-ADAMTS13 autoantibodies.30,31 The implications in term of management of iTTP are multiple and quite well illustrated by our study. PEX was necessary for almost all patients with pregnancy-onset iTTP. Immunosuppressive therapies were also very often necessary to control the disease. Rituximab use during pregnancy raises concern, and whereas we report a total of 3 patients who received rituximab with pregnancies ending in live births with no side effects, this very small number of patients should lead to caution. Regarding caplacizumab, there are obviously very few cases as of 202345-47 and it should be used only in more severe cases.48 The follow-up of these patients with iTTP shows a risk of relapse of ∼23% that can occur independently of a subsequent pregnancy and, as such, these patients need lifetime monitoring. ADAMTS13 activity measurement during the preconception phase has prognostic value, particularly for patients who begin their pregnancy with normal activity, because they typically maintain it throughout the entire pregnancy. As previously reported,3,41 we believe that beginning a pregnancy with low or undetectable ADAMTS13 activity is associated with a high risk of relapse, whereas normal (or normalized by preemptive rituximab) ADAMTS13 activity strongly decreases the likelihood of a TTP relapse.
The second well-defined subtype is late-onset cTTP (26.9% of our cohort), which is almost exclusively triggered by pregnancy. Clinical presentation is very similar to what we observed in iTTP, and unfortunately no clinical or biological marker appears useful to discriminate these 2 forms of the disease at the acute phase. Although not all ADAMTS13 variants reported in adult-onset cTTP have been characterized, the most frequent variant R1060W (72.4% of our cohort) was reported to lead to a severe intracellular retention of an otherwise functional protein.49 In other terms, it is possible that adult-onset cTTP are linked to essentially quantitative ADAMTS13 defects that require the prolonged increase of VWF inherent to pregnancy to lead to TTP. Management data show that, to attain remission, the need in plasma is lower than in patients with iTTP. Moreover, and similarly to other studies focusing on late-onset cTTP, no TTP episode outside of pregnancy was reported in our cohort, supporting the major role played by high VWF levels during pregnancy to trigger a TTP episode in this context. Overall, our strategy, also in practice in other countries,3,24 of systematically administrating weekly or biweekly prophylactic plasma infusion initiated as soon as the pregnancy is diagnosed and close monitoring of pregnancies in those with cTTP by multidisciplinary teams appears successful. There is a perspective of alleviated treatment and high effectiveness thanks to recombinant ADAMTS13.50
The third and last subtype of pregnancy-onset TTP is uTTP (25.0% of our cohort). Clinical features vary compared with the more common iTTP, notably a higher blood pressure, higher proportion of AKI, and higher platelet count. The clinical differential diagnosis with PE is even more challenging, and the risk of underdiagnosing TTP based on PE biomarkers should be noted, because analysis of ADAMTS13 activity is usually not always available rapidly. In that regard, an elevated sFlt-1/PlGF ratio should not definitely exclude a TTP diagnosis in an obstetrical context.43 The very high proportion of patients with uTTP in whom an open ADAMTS13 conformation was found (77.8%) is quite surprising because uTTP outside of pregnancy has been associated with closed conformation of ADAMTS13,19 hence, this result raises questions. A hypothesis to consider here is that prolonged high VWF levels during pregnancy may induce an opening of ADAMTS13 in obstetrical uTTP by another mechanism than that involving anti-ADAMTS13 antibodies. In other words, open ADAMTS13 in uTTP may be linked to the prolongation of a physiological mechanism rather than to a pathologic opening of ADAMTS13 by specific autoantibodies. Another hypothesis would be the involvement of hormonal changes during pregnancy. In terms of mechanisms supporting ADAMTS13 severe deficiency in uTTP, degradation of ADAMTS13 may lead to the exposure of the cryptic binding site for 1C4 antibody in the spacer domain,26 and also explain an open ADAMTS13 conformation in most of our obstetrical uTTP. Of note, an “open,” degraded ADAMTS13 because of degradation is different than the open ADAMTS13 (which is not degraded) observed in iTTP and due to binding of autoantibodies. The ELISA technique used has limited sensitivity to detect only free IgG in vitro and not IgG trapped in immune complexes, which are quickly cleared from circulation. iTTP misdiagnosed as uTTP because of no detectable free anti-ADAMTS13 autoantibodies in vitro also remains a possibility in this subgroup of pregnancy-onset TTP, and 1 of the patients with uTTP was reclassified as having iTTP during follow-up. The management of these patients with uTTP did not rely as much on plasmatherapy or on immunosuppressive therapy as that for patient with iTTP. The follow-up also shows a very different pattern than for patients with iTTP; only 1 of 26 surviving patients with aTTP relapsed outside of pregnancy, and the 3 subsequent pregnancies reported were not marked by any decrease of ADAMTS13 activity. We believe this incredibly low relapse rate supports our hypothesis of a mechanical (quantitative defect and/or degradation of ADAMTS13) disrupting the VWF/ADAMTS13 balance rather than an immune mechanism.
Pregnancy-onset TTP remains a complex diagnosis and a challenging condition. Management of these difficult situations should be done by a multidisciplinary team involving experienced obstetricians, hematologists, and intensive care–unit specialists. Future pregnancy plans for patients with a history of TTP should be developed in collaboration with the patients themselves, and based on ADAMTS13 activity at the time of planning. Our study reports, to our knowledge, the largest cohort of patients with pregnancy onset-TTP and spans 21 years, giving a clear picture of that particular entity of TTP. Three distinct subtypes are singled, differing in key aspects such as the mechanism leading to ineffective ADAMTS13 and eventually TTP; clinical presentation; management; and, most importantly, follow-up. Beyond iTTP and cTTP, of which issues of differential diagnosis are better handled, our work highlights obstetrical uTTP as a complex entity with several unanswered questions. These data may help to better manage pregnancy-onset TTP and to offer personalized counseling adapted to each patient for subsequent pregnancies. Significant advancements in the management of pregnancy-onset TTP are promising, with 2 potential treatments, caplacizumab and recombinant ADAMTS13,50 for improving outcomes and reducing morbidity in pregnancy.
Acknowledgments
The authors thank all the physicians of the French Reference Center for Thrombotic Microangiopathies, listed in the supplemental Appendix. The authors thank Sandrine Benghezal, Sophie Capdenat, Sylvaine Savigny, Adeline Delton, Hélène Deniau, Chloé Doinel, Maria Mahieu, Sylvie Lavarde, and Raïda Bouzid for expert assistance.
This work was partly funded by a grant from the Délégation Régionale à la Recherche Clinique, Assistance Publique-Hôpitaux de Paris (PHRC AOM05012) and have been registered at www.ClinicalTrials.gov as # NCT00426686 (http://clinicaltrials.gov/show/NCT00426686, study ID number: P051064, Health Authority: France, Ministry of Health); and by a grant from the French healthcare network for rare immuno-hematological diseases (Maladies Rares Immuno-Hématologiques, MaRIH) in 2020.
Authorship
Contribution: N.B. did the cross-sectional analysis of the French Registry for Thrombotic Microangiopathies with a severe ADAMTS13 deficiency, did the phenotypic analysis, interpreted the results, and wrote the manuscript; B.S.J., A.V., and P.C. designed the study, interpreted the results, and wrote and critically reviewed the report; B.S.J. and A.V. supervised the phenotypic analysis and the database analysis; P.C., V.T., F.P., Y.D., and P.P. enrolled most patients, collected clinical and laboratory information, and critically reviewed the report; P.B. performed genetic analysis and critically reviewed the manuscript; K.V. provided monoclonal antibodies, interpreted data, and reviewed the manuscript for scientific content; and the final version of the manuscript was read and approved by all authors.
Conflict-of-interest disclosure: N.B. received speaker fees from Sanofi. V.T. is a member of the advisory board, and received speaker fees from Roche diagnosis and grants for a clinical trial (Precog Trial). P.C. is a member of advisory boards and received speaker fees from Sanofi, Alexion, Octapharma, and Takeda. Y.D. is a member of advisory boards of, and received speaker fees from, Sanofi, Takeda, and Alexion. A.V. is a member of the French advisory boards for Sanofi and Takeda and received speaker fees from Sanofi, Octapharma, LFB-Biomédicaments, and Takeda. B.S.J. received speaker fees from Sanofi, Takeda, and LFB-Biomédicaments.
A complete list of the members of the French Reference Center for Thrombotic Microangiopathies appears in the supplemental Appendix.
Correspondence: Bérangère S. Joly, Service d’Hématologie biologique, Hôpital Lariboisière, 2, Rue Ambroise Paré, 75010 Paris, France; email: berangere.joly@aphp.fr.
References
Author notes
Original anonymized data and response to specific questions are available upon reasonable request from authors Paul Coppo (paul.coppo@aphp.fr) and Bérangère S. Joly (berangere.joly@aphp.fr).
The full-text version of this article contains a data supplement.