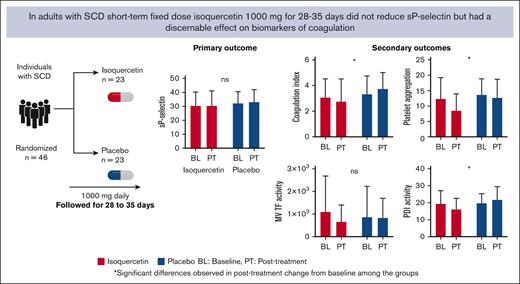
Short-term fixed-dose isoquercetin did not lower plasma soluble P-selectin in adults with steady-state SCD.
Isoquercetin treatment attenuated blood coagulation, platelet aggregation, and inducible tissue factor gene expression in adults with SCD.
Visual Abstract
Data from a small trial in patients with cancer suggest that isoquercetin (IQ) treatment lowered thrombosis biomarkers and prevented clinical thrombosis, but, to our knowledge, no studies of IQ have been conducted to target thromboinflammation in adults with sickle cell disease (SCD). We conducted a randomized, double-blind, placebo-controlled trial in adults with steady-state SCD (hemoglobin SS [HbSS], HbSβ0thal, HbSβ+thal, or HbSC). The primary outcome was the change in plasma soluble P-selectin (sP-selectin) after treatment compared with baseline, analyzed in the intention-to-treat population. Between November 2019 and July 2022, 46 patients (aged 40 ± 11 years, 56% female, 75% under hydroxyurea treatment) were randomized to receive IQ (n = 23) or placebo (n = 23). IQ was well tolerated and all the adverse events (AEs; n = 21) or serious AEs (n = 14) recorded were not attributable to the study drug. The mean posttreatment change for sP-selectin showed no significant difference between the treatment groups (IQ, 0.10 ± 6.53 vs placebo, 0.74 ± 4.54; P = .64). In patients treated with IQ, whole-blood coagulation (P = .03) and collagen-induced platelet aggregation (P = .03) were significantly reduced from the baseline. Inducible mononuclear cell tissue factor gene expression and plasma protein disulfide isomerase reductase activity were also significantly inhibited (P = .003 and P = .02, respectively). Short-term fixed-dose IQ in patients with SCD was safe with no off-target bleeding and was associated with changes from the baseline in the appropriate direction for several biomarkers of thromboinflammation. The trial was registered at www.clinicaltrials.gov as #NCT04514510.
Introduction
Sickle cell disease (SCD) is an inherited hemoglobin (Hb) disorder wherein a single-nucleotide change in the β-globin gene leads to production of variant HbS (βGlu6Val), which, under hypoxic conditions, polymerizes causing “sickling” of red blood cells.1,2 Disease manifestations characteristically include episodic disease flares, termed acute vaso-occlusive crises (VOCs), that accumulate over time and lead to vascular complications, end-organ damage, and a reduction in adult life expectancy. Although the backbone of treatment for SCD is hydroxyurea, 3 new drugs (crizanlizumab, L-glutamine, and voxelotor) were recently approved by the US Food and Drug Administration. Yet, most of these disease-modifying therapies fail to substantially reduce VOC frequency and are not widely available to the global sickle cell community, leaving patients with this disease vulnerable to devastating complications.
HbS polymerization is seen as the primary driver of SCD pathophysiology, but a cascade of interrelated events including hemolysis; activation and adhesion of neutrophils, monocytes, platelets, and endothelial cells; sterile inflammation; and abnormal coagulation are increasingly recognized as important contributors to clinical disease.3 An inherent hypercoagulable state heightens the risk for arterial and venous thrombosis in patients with SCD, both outcomes that are associated with higher mortality.4-6 Unfortunately, even patients treated with hydroxyurea experience recurrent venous thromboembolism (VTE) necessitating lifelong anticoagulation, which increases the risk of life-threatening bleeding.7 Thus, treatments targeting thromboinflammatory pathophysiology in SCD with agents that lack bleeding side effects and are widely implementable are a priority to investigate.
Accumulated evidence points to tissue factor (TF)-initiated thromboinflammation, in both patients with SCD8,9 and animal models of SCD, which favors systemic thrombin generation,10 stasis,11 and end-organ damage.12 Patients with SCD display “blood-borne” TF on the surface of monocytes13 and vascular endothelial cells,8 and on microvesicles (MVs) derived from these cells,14 which increases further during VOC. TF-initiated coagulation is also posttranslationally regulated by a vascular thiol-isomerase, protein disulfide isomerase (PDI).15 In animal models, release of endothelial- and platelet-derived PDI into the vasculature after vessel injury, as occurs during thromboinflammation, facilitates TF-initiated thrombosis.16 Our in vitro studies demonstrate robust inhibition of monocyte and endothelial cell-surface TF expression and cell-surface PDI reductase activity by a flavonoid, quercetin. Because isoquercetin (IQ), the oral bioavailable glucoside form of quercetin, improved thrombosis biomarkers in patient with cancer without inducing bleeding17 we tested its safety and efficacy in modulating thromboinflammatory pathophysiology in SCD.
Methods
Study design
This was an investigator-initiated single-center, randomized, double-blind, placebo-controlled phase 2 study conducted between 19 November 2019 and 7 July 2022. The trial consisted of a 4-week screening phase and a 4-week (28-35 days) blinded treatment phase, followed by a 4-week follow-up phase for assessing safety and adverse events (AEs). The study protocol was approved by the National Institutes of Health (NIH) Institutional Review Board and a US Food and Drug Administration investigational new drug (IND no. 150896) application and was registered at www.clinicaltrials.gov as #NCT04514510.
Study participants
Participants were aged ≥18 years with SCD defined by Hb electrophoresis (HbSS, HbSß0thal, HbSβ+thal, or HbSC) who were in their steady state defined as having no significant complications (VOC, or acute condition requiring hospitalization) or blood transfusion occurring within 2 months of the baseline visit. Patients receiving hydroxyurea were required to be on a stable dose for ≥12 weeks before the baseline visit. Participants with a history of a recent VOC or blood transfusion (<2 months), a VTE event (<3 months), or actively receiving crizanlizumab therapy were excluded.
Randomization and blinding
Participants meeting eligibility criteria were randomly assigned in a 1:1 ratio to receive 28 to 35 doses of either 1000 mg IQ or identically matching placebo. The randomization allocation was prepared by a statistician who was not part of the study team and was shared directly with the NIH pharmacy team who assumed responsibility for dispensing study drug per assigned allocation. Participants and the study team remained blinded throughout study conduct and during analysis of results. Unblinding of treatment allocations occurred after the data were curated by the study team.
Intervention
IQ dose and exposure duration were determined based on prior clinical experience from a phase 2 clinical trial of IQ in cancer-induced hypercoagulability.17 The duration of study drug exposure was reduced to 4 weeks to minimize confounding by the occurrence of frequent VOCs. Participants in the intervention group took oral IQ (Quercis Pharma AG, Zug, Switzerland) 1000 mg administered orally, once daily for at least 4 weeks, ranging from 28 days to a maximum of 35 days. IQ was supplied as capsules of 250 mg active dosage strength IQ blended with 62 mg of vitamin C/ascorbic acid and 5 mg of vitamin B3/nicotinic acid. Participants in the control group took identically formulated placebo containing 62 mg of vitamin C/ascorbic acid and 5 mg of vitamin B3/nicotinic acid.
Sample collection
Blood samples for the primary and secondary end points were obtained at baseline and after treatment. Platelet-free plasma (PFP) was prepared from citrated anticoagulated blood by double centrifugation at 2500g for 15 minutes within 30 minutes of sample collection and stored at −80°C until batch analysis. Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-anticoagulated blood by gradient centrifugation (Histopaque-1077, Sigma-Aldrich) and stored at −80°C until analysis.
Primary outcome
The primary end point of the trial was the change in plasma soluble P-selectin (sP-selectin) from baseline after 4 weeks of treatment in the IQ group compared with placebo, assessed by enzyme-linked immunosorbent assay (ELISA; Human P-Selectin/CD62P Quantikine ELISA Kit, R&D Systems, no. SPSE00). To adhere to the intention-to-treat principle, for every patient that was randomized, we attempted to obtain 28- to 35-day end point measurements despite occurrence of expected and/or unexpected events due to the underlying disease.
Secondary outcomes
Safety
Safety assessments performed during screening (visit number 1), baseline (visit number 2), after treatment (visit number 3), and at the end of study (visit number 4) included SCD-focused medical history, concomitant medication use, side effects, physical examination, vital signs, AEs and serious AEs (SAEs), and clinical laboratory tests (comprehensive metabolic panel, complete and differential blood count, and urinalysis). The medical records of SAEs were obtained and reviewed to determine study relatedness. The principal investigator and study team reviewed the safety parameters and determined whether the intervention required modification as defined in the study protocol.
Biomarkers of thromboinflammatory pathophysiology
Whole-blood coagulability
Thromboelastography (TEG) was performed in citrate-anticoagulated whole blood within 30 minutes of phlebotomy using the TEG 5000 Analyzer (Haemonetics UK Ltd, Coventry, United Kingdom). The parameters of interest included reaction time, clot kinetics, rate of clot formation (α-angle), maximal amplitude, and coagulation index (CI); CI of more than +3.0 indicates hypercoagulability, and CI of less than −3.0 indicates hypocoagulability.18
Platelet aggregation
Platelet aggregometry was performed using whole-blood optical lumiaggregometry (Model 700 Whole Blood/Optical Lumi-aggregometer, CHRONO-LOG). Maximal platelet aggregation using electrical impedance in whole-blood samples was determined after stimulation by various platelet agonists under conditions of continuous stirring (1200 rpm).19
Plasma TF-positive (TF+) MVs
TF+ MVs in PFP were detected and enumerated by flow cytometry using a high-resolution flow cytometer (CytoFLEX, Beckman Coulter Inc, San Jose, CA) equipped with a 405-nm laser (violet), using published methods with minor modifications.14 MVs isolated from plasma were visualized by scanning electron microscopy to confirm their vesicular structure.
MV–associated TF procoagulant activity
MVs isolated from PFP (20 000g for 60 minutes) were used to determine MV–associated TF procoagulant activity using a more sensitive fluorogenic substrate (Pefafluor Fxa), as described previously.20
Thrombin and fibrin generation
D-dimer levels were measured using a latex- immunoturbidimetric assay (STA-Liatest D-Di/Diagnostica Stago). Thrombin-antithrombin complexes, were measured by ELISA following the manufacturer’s instructions (Human thrombin-antithrombin complex ELISA kit, no. ab108907, Abcam,).
Plasma PDI antigen and activity
Mononuclear cell TF messenger RNA (mRNA) expression
Lipopolysaccharide (LPS)–induced TF gene expression was assessed using stored PBMCs obtained at baseline and after treatment only in participants from the IQ group (n = 22). Briefly, PBMCs were suspended in RPMI 1640 with 10% fetal bovine serum at a concentration of 3 × 106 cells per mL and stimulated with 100 ng LPS (Escherichia coli O26:B6, Invitrogen) for 3 hours. Subsequently, the total cell-derived RNA was extracted using Trizol (no. 15596-026, Invitrogen, Life Technologies) and subjected to quantitative reverse transcription polymerase chain reaction (iTaq Universal SYBR Green One-Step Kit, no. 1725150; Bio-Rad, Hercules, CA) according to the kit instructions. The TF gene mRNA level was normalized to glyceraldehyde-3-phosphate dehydrogenase (primer sequences available in supplemental Table 2) and TF gene expression was compared with unstimulated mononuclear cell TF gene expression, and presented as the fold change using the 2−ΔΔCT method.23
Adherence and plasma quercetin measurement
Patient adherence was enhanced using an electronic pill dispenser and was objectively determined by pill counts performed by the research team. Using random nonfasting blood samples obtained after treatment, plasma quercetin levels were determined by liquid chromatography–tandem mass spectrometry, as described previously.24
Statistical analysis
We hypothesized a 25% reduction in sP-selectin (ie, a 7.25-ng/mL decline from an average value of 29 ng/mL) as the treatment effect for IQ to achieve a clinically meaningful reduction in thrombosis risk using basal plasma sP-selectin levels in banked samples from patients with steady-state SCD (n = 29) recruited under NIH protocol (17-H-0056). Under these conditions, a total of 40 participants (20 per group) were required to obtain the power of 90% using an analysis of covariance (ANCOVA) model. This number was increased to 46 to account for potential diluting effects of possible treatment noncompliance and/or study dropouts, and to provide adequate power to test our hypothesis in the subgroup of patients, per protocol, who avoided acute crises that could have distorted their plasma sP-selectin and other measurements. The statistical analysis was performed on an intention-to-treat principle. The primary end point was the change in plasma sP-selectin when comparing the baseline with the posttreatment level after 28 days among patients in the IQ group vs those in the placebo group. We used an ANCOVA model with follow-up sP-selectin measurements as the dependent variable, with baseline measurements and treatment assignment as the covariates. To address missing data from the participant/s lost to follow-up, a multiple imputation procedure was developed without knowledge of the treatment assignment and performed for the primary end point. A per-protocol analysis was also conducted for the primary end point after the exclusion of participant/s failing to remain in steady state during the intervention period (experienced VOC, infection, and/or received red blood cell transfusion) or those not receiving the intervention. Significance was evaluated using a 2-sided test with an α level of .05. Differences in posttreatment measures of secondary end points were assessed with ANCOVA, and, for non-Gaussian–distributed data, either with the Wilcoxon rank-sum test or the Spearman test. In vitro study end point differences were analyzed using analysis of variance, t test, and paired t tests.
Results
Of 168 eligible individuals with SCD who were approached for study participation, 52 did not meet the eligibility criteria, and 70 were not enrolled for other reasons (supplemental Figure 1). This resulted in 46 participants randomly allocated to receive either 1000 mg of IQ (n = 23) or placebo (n = 23) daily for a minimum of 28 days to a maximum of 35 days (supplemental Figure 1). Attrition because of screen failure in the IQ group (n = 1) and loss to follow-up in the placebo group (n = 1) resulted in 22 participants per study group providing posttreatment measurements. At baseline, clinical and laboratory parameters and thrombosis biomarkers were relatively well balanced between the study groups (Table 1). The mean age of the study participants was 40 ± 11 years, and 56% were female. Most participants had HbSS genotype and received disease-modifying therapy with hydroxyurea (75%; average dose = 18 ± 8 mg/kg). A subgroup of patients reported prior history of thrombosis (venous thrombosis, n = 13; arterial thrombosis, n = 6) and while on study received treatment with either systemic anticoagulants (n = 4) or aspirin (n = 6). During the intervention period, 22% of the participants experienced acute VOCs (5 in each study group) that occasionally required blood transfusions (n = 2).
Demographics and clinical characteristics of enrolled patients at baseline
| Parameters . | IQ (n = 23) . | Placebo (n = 23) . |
|---|---|---|
| n (%) . | n (%) . | |
| Age (y) | 43 ± 12 | 38 ± 12 |
| Body mass index (kg/m2)∗ | 26.1 ± 4.9 | 24.4 ± 3.2 |
| Sex† | ||
| Female | 12 (52) | 14 (61) |
| Male | 11 (48) | 9 (39) |
| Genotype† | ||
| SS | 18 (78) | 22 (96) |
| Others | 5 (23) | 1 (4) |
| Race† | ||
| Black or African American | 21 (91) | 21 (95.5) |
| Multiple races | 2 (9) | 1 (4.5) |
| Ethnicity† | ||
| Non-Latino or Hispanic | 22 (96) | 19 (86.5) |
| Latino or Hispanic | 1 (4) | 2 (9) |
| Unknown | 0 | 1 (4.5) |
| History of VTE and VOC† | ||
| VOCs (per year for the past 3 y) | 3 (14) | 3 (14) |
| VTE | 5 (26) | 8 (36) |
| Therapy | ||
| Hydroxyurea | 18 (78) | 18 (82) |
| DOACs | 2 (9) | 2 (9) |
| Aspirin | 3 (13) | 3 (13.6) |
| Hematological parameters∗ | ||
| Hb (g/dL) | 8.9 ± 1.4 | 8.8 ± 1.6 |
| HbS (%) | 72.6 ± 13.8 | 77.9 ± 9.0 |
| HbF (%) | 11.1 ± 11.3 | 15.3 ± 9.9 |
| Reticulocytes (103/μL) | 217.8 ± 108.8 | 174 ± 124 |
| Neutrophils (103/μL) | 4.2 ± 2.0 | 4.0 ± 2.0 |
| Monocytes (103/μL) | 0.6 ± 0.7 | 0.6 ± 0.3 |
| Platelets (103/μL) | 217.8 ± 111.2 | 354.1 ± 120 |
| Biochemical parameters∗ | ||
| hsCRP (mg/L) | 7.5 ± 5.8 | 6.0 ± 6.0 |
| LDH (U/L) | 392 ± 172 | 404 ± 135 |
| Bilirubin total (mg/dL) | 2.6 ± 2.0 | 2.5 ± 1.6 |
| Biomarkers of thrombotic risk∗ | ||
| sP-selectin (ng/mL) | 30 ± 9.9 | 32 ± 8.3 |
| D-dimer (μg/mL)‡ | 1.5 ± 0.8 | 1.9 ± 2.3 |
| TAT (ng/mL)‡ | 2.7 ± 1.4 | 2.5 ± 0.9 |
| Parameters . | IQ (n = 23) . | Placebo (n = 23) . |
|---|---|---|
| n (%) . | n (%) . | |
| Age (y) | 43 ± 12 | 38 ± 12 |
| Body mass index (kg/m2)∗ | 26.1 ± 4.9 | 24.4 ± 3.2 |
| Sex† | ||
| Female | 12 (52) | 14 (61) |
| Male | 11 (48) | 9 (39) |
| Genotype† | ||
| SS | 18 (78) | 22 (96) |
| Others | 5 (23) | 1 (4) |
| Race† | ||
| Black or African American | 21 (91) | 21 (95.5) |
| Multiple races | 2 (9) | 1 (4.5) |
| Ethnicity† | ||
| Non-Latino or Hispanic | 22 (96) | 19 (86.5) |
| Latino or Hispanic | 1 (4) | 2 (9) |
| Unknown | 0 | 1 (4.5) |
| History of VTE and VOC† | ||
| VOCs (per year for the past 3 y) | 3 (14) | 3 (14) |
| VTE | 5 (26) | 8 (36) |
| Therapy | ||
| Hydroxyurea | 18 (78) | 18 (82) |
| DOACs | 2 (9) | 2 (9) |
| Aspirin | 3 (13) | 3 (13.6) |
| Hematological parameters∗ | ||
| Hb (g/dL) | 8.9 ± 1.4 | 8.8 ± 1.6 |
| HbS (%) | 72.6 ± 13.8 | 77.9 ± 9.0 |
| HbF (%) | 11.1 ± 11.3 | 15.3 ± 9.9 |
| Reticulocytes (103/μL) | 217.8 ± 108.8 | 174 ± 124 |
| Neutrophils (103/μL) | 4.2 ± 2.0 | 4.0 ± 2.0 |
| Monocytes (103/μL) | 0.6 ± 0.7 | 0.6 ± 0.3 |
| Platelets (103/μL) | 217.8 ± 111.2 | 354.1 ± 120 |
| Biochemical parameters∗ | ||
| hsCRP (mg/L) | 7.5 ± 5.8 | 6.0 ± 6.0 |
| LDH (U/L) | 392 ± 172 | 404 ± 135 |
| Bilirubin total (mg/dL) | 2.6 ± 2.0 | 2.5 ± 1.6 |
| Biomarkers of thrombotic risk∗ | ||
| sP-selectin (ng/mL) | 30 ± 9.9 | 32 ± 8.3 |
| D-dimer (μg/mL)‡ | 1.5 ± 0.8 | 1.9 ± 2.3 |
| TAT (ng/mL)‡ | 2.7 ± 1.4 | 2.5 ± 0.9 |
LDH, lactate dehydrogenase; DOACs, direct oral anticoagulants; hsCRP, high-sensitivity C-reactive protein; TAT, thrombin-antithrombin complex.
Mean ± SD.
Number (percentage).
TAT and D-dimer values were obtained in n = 22 in each group.
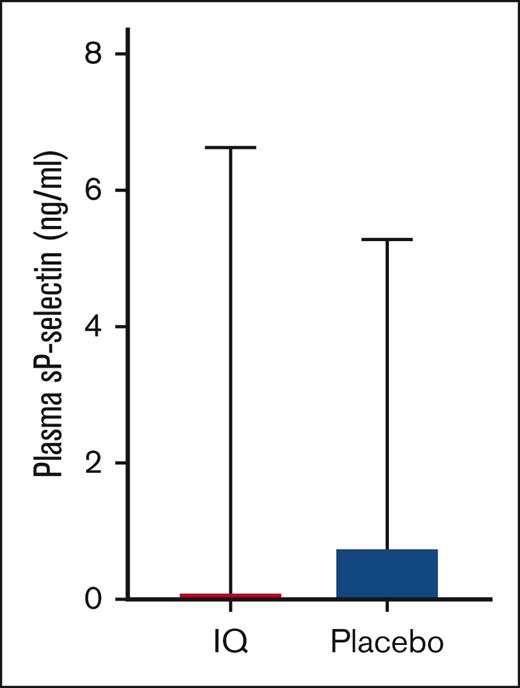
Patients with steady state SCD in both groups had comparable sP-selectin levels (ng/mL, IQ: 30 ± 9.9 vs placebo: 32 ± 8.3; P = .56) that were significantly elevated when compared with ethnic-matched healthy controls (supplemental Figure 2). After 28 to 35 days of treatment, plasma sP-selectin levels remained elevated (IQ: 30 ± 10.3 ng/mL vs placebo: 33 ± 9.2; P = .42), and the primary analysis revealed no differences in mean change from baseline after treatment (mean change from baseline ± standard deviation [SD]: IQ: 0.10 ± 6.53 ng/mL vs placebo: 0.74 ± 4.54; P = .64; Figure 1). Per protocol, analysis conducted after excluding patients that experienced VOC (n = 10; 5 in each study group) or failed to receive the intervention (n = 2) also did not reveal differences (P = .61). Although the sample size was small, a sensitivity analysis conducted for the presence of a treatment interaction with hydroxyurea or prior history of VTE revealed no evidence of interactions (P = .48 and P = .90 respectively).
Effect of IQ on plasma sP-selectin. Mean change from baseline in plasma sP-selectin level in participants from the IQ group and placebo group reveals no significant differences (IQ, n = 23; placebo, n = 23; ANCOVA, P = .64).
Effect of IQ on plasma sP-selectin. Mean change from baseline in plasma sP-selectin level in participants from the IQ group and placebo group reveals no significant differences (IQ, n = 23; placebo, n = 23; ANCOVA, P = .64).
A total of 21 AEs were reported in 15 patients, 10 in 8 patients treated with IQ, and 11 in 7 patients that received placebo (Table 2). The majority of AEs in the IQ group were moderate, except 1 that was severe. Two severe grade AEs were reported in the placebo group. None of the AEs in either the IQ or the placebo group were attributable to study drug. Fourteen SAEs were reported in 10 patients, including 8 in 6 patients from the IQ group, and 6 in 4 patients in the placebo group. All SAEs except 1 were because of VOC and were attributable to the underlying SCD and not to study drug exposure. One incident of retinal detachment occurring in the IQ group was deemed secondary to high myopia. Comparison of the baseline and posttreatment clinical and laboratory parameters (Table 3) did not reveal any organ toxicity. Importantly, there were no off-target bleeding side effects detected. Overall, short-term fixed-dose IQ was safe and well tolerated.
AEs and SAEs observed in enrolled study patients
| . | SAEs (total 14 SAEs in 10 patients) . | |
|---|---|---|
| IQ (n = 23) . | Placebo (n = 23) . | |
| (8 SAEs in 6 patients) . | (6 SAEs in 4 patients) . | |
| SCA with VOC | 5 | 4∗ (1 had 2 VOCs) |
| COVID-19 | 1∗ (also had SCA with VOC) | 0 |
| Lung infection | 0 | 1∗ (also had SCA with VOC) |
| Priapism | 1∗ (also had SCA with VOC) | 0 |
| Retinal detachment | 1 | 0 |
| AEs (total 21 AEs in 15 patients) | ||
| 10 AEs in 8 patients | 11 AEs in 7 patients | |
| SCA with VOC | 2 | 2 |
| Chronic sickle cell pain | 1 | 0 |
| General disorder: pain (not otherwise specified) | 0 | 1 |
| Infections and infestations: Other specify: COVID-19 | 1 | 1 |
| CPK increased | 1 | 1∗ (also had COVID-19) |
| Gallbladder pain | 1 | 0 |
| Rash acneiform | 1 | 0 |
| Reproductive system and breast disorders: Penile pain | 1 | 0 |
| Abdominal pain | 1∗ (also had SCA with VOC) | 0 |
| Colitis | 1∗ (also had gallbladder pain) | 0 |
| Bloating | 0 | 1 |
| Headache | 0 | 1∗ (also had bloating) |
| Blurred vision | 0 | 1 |
| Retinopathy | 0 | 1∗ (also had blurred vision and dysmenorrhea) |
| Dysmenorrhea | 0 | 1∗ (also had retinopathy and blurred vision) |
| Diarrhea | 0 | 1 |
| . | SAEs (total 14 SAEs in 10 patients) . | |
|---|---|---|
| IQ (n = 23) . | Placebo (n = 23) . | |
| (8 SAEs in 6 patients) . | (6 SAEs in 4 patients) . | |
| SCA with VOC | 5 | 4∗ (1 had 2 VOCs) |
| COVID-19 | 1∗ (also had SCA with VOC) | 0 |
| Lung infection | 0 | 1∗ (also had SCA with VOC) |
| Priapism | 1∗ (also had SCA with VOC) | 0 |
| Retinal detachment | 1 | 0 |
| AEs (total 21 AEs in 15 patients) | ||
| 10 AEs in 8 patients | 11 AEs in 7 patients | |
| SCA with VOC | 2 | 2 |
| Chronic sickle cell pain | 1 | 0 |
| General disorder: pain (not otherwise specified) | 0 | 1 |
| Infections and infestations: Other specify: COVID-19 | 1 | 1 |
| CPK increased | 1 | 1∗ (also had COVID-19) |
| Gallbladder pain | 1 | 0 |
| Rash acneiform | 1 | 0 |
| Reproductive system and breast disorders: Penile pain | 1 | 0 |
| Abdominal pain | 1∗ (also had SCA with VOC) | 0 |
| Colitis | 1∗ (also had gallbladder pain) | 0 |
| Bloating | 0 | 1 |
| Headache | 0 | 1∗ (also had bloating) |
| Blurred vision | 0 | 1 |
| Retinopathy | 0 | 1∗ (also had blurred vision and dysmenorrhea) |
| Dysmenorrhea | 0 | 1∗ (also had retinopathy and blurred vision) |
| Diarrhea | 0 | 1 |
CPK, creatinine phosphokinase.
Multiple events in a single patient.
Summary of results obtained in the phase 2 IQ clinical trial
| Laboratory biomarkers . | IQ (n = 22) . | Placebo (n = 22) . | ||
|---|---|---|---|---|
| Baseline . | After treatment . | Baseline . | After treatment . | |
| Plasma sP-selectin levels (ng/mL)∗ | 30.3 ± 9.9 | 30.4 ± 10.6 | 32.1 ± 8.4 | 32.8 ± 9.2 |
| TEG∗ | ||||
| Clot formation time (min) | 4.6 ± 1.4 | 4.8 ± 1.3 | 4.3 ± 1.3 | 4.0 ± 1.0 |
| Reaction time (min) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.7 | 1.0 ± 0.2 |
| α-angle (degree) | 73.4 ± 3.4 | 72.5 ± 4.0 | 73.1 ± 5.5 | 75.2 ± 3.3 |
| Maximal amplitude (mm) | 71.3 ± 5.1 | 70.4 ± 6.7 | 71.1 ± 5.8 | 72.1 ± 5.6 |
| CI (AU)† | 3.0 ± 1.5 | 2.7 ± 1.7 | 3.3 ± 1.5 | 3.7 ± 1.3 |
| Whole-blood platelet aggregation (impedance, ohms)∗ | ||||
| Thrombin (0.1 U) | 31 ± 14.5 | 31.7 ± 13.3 | 35.8 ± 10.3 | 33.6 ± 12.9 |
| ADP (10 μM) | 13.1 ± 6.4 | 12.9 ± 6.2 | 17.7 ± 7.4 | 14.1 ± 7.2 |
| Arachidonic acid (5 mM) | 14.1 ± 7.7 | 12.7 ± 8.1 | 12.2 ± 6.9 | 12.9 ± 7.1 |
| Collagen (1 μg/mL)† | 12.4 ± 6.9 | 8.5 ± 5.6 | 13.6 ± 5.2 | 12.6 ± 6.2 |
| Collagen (5 μg/mL) | 14.2 ± 6.1 | 14.8 ± 4.9 | 18 ± 6.2 | 17.2 ± 5.9 |
| TF‡ | ||||
| TF+ MVs (number per mL) | 1551 (404, 6379) | 1723 (516, 7181) | 2468 (603, 6827) | 1515 (440, 3830) |
| TF+ MVs PCA (fmol) | 257 (148, 927) | 362 (166, 834) | 414 (122, 859) | 498 (307, 923) |
| PBMC TF mRNA expression (fold change)§,† | 1.41 (0.46, 6.11) | 0.43 (0.29, 2.19) | NA | NA |
| PDI‡ | ||||
| Plasma PDI reductase activity (pmol/min per μL)† | 16 (14, 27) | 16 (13, 21) | 19 (16, 23) | 20 (17, 25) |
| Plasma PDI antigen (ng/mL) | 2.14 (1.61, 2.96) | 2.18 (1.77, 3.31) | 2.08 (1.88, 2.31) | 2.23 (1.75, 2.72) |
| Other markers of coagulation∗ | ||||
| D-dimer (μg/mL) | 1.5 ± 0.8 | 1.7 ± 1.1 | 1.6 ± 1.1 | 1.9 ± 1.8 |
| Thrombin–antithrombin complexes (ng/mL) | 2.7 ± 1.4 | 2.7 ± 1.7 | 2.5 ± 0.9 | 2.4 ± 1.1 |
| Plasma quercetin levels (ng/mL)∗ | NA | 253 ± 330 | NA | 15 ± 17 |
| Laboratory biomarkers . | IQ (n = 22) . | Placebo (n = 22) . | ||
|---|---|---|---|---|
| Baseline . | After treatment . | Baseline . | After treatment . | |
| Plasma sP-selectin levels (ng/mL)∗ | 30.3 ± 9.9 | 30.4 ± 10.6 | 32.1 ± 8.4 | 32.8 ± 9.2 |
| TEG∗ | ||||
| Clot formation time (min) | 4.6 ± 1.4 | 4.8 ± 1.3 | 4.3 ± 1.3 | 4.0 ± 1.0 |
| Reaction time (min) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.7 | 1.0 ± 0.2 |
| α-angle (degree) | 73.4 ± 3.4 | 72.5 ± 4.0 | 73.1 ± 5.5 | 75.2 ± 3.3 |
| Maximal amplitude (mm) | 71.3 ± 5.1 | 70.4 ± 6.7 | 71.1 ± 5.8 | 72.1 ± 5.6 |
| CI (AU)† | 3.0 ± 1.5 | 2.7 ± 1.7 | 3.3 ± 1.5 | 3.7 ± 1.3 |
| Whole-blood platelet aggregation (impedance, ohms)∗ | ||||
| Thrombin (0.1 U) | 31 ± 14.5 | 31.7 ± 13.3 | 35.8 ± 10.3 | 33.6 ± 12.9 |
| ADP (10 μM) | 13.1 ± 6.4 | 12.9 ± 6.2 | 17.7 ± 7.4 | 14.1 ± 7.2 |
| Arachidonic acid (5 mM) | 14.1 ± 7.7 | 12.7 ± 8.1 | 12.2 ± 6.9 | 12.9 ± 7.1 |
| Collagen (1 μg/mL)† | 12.4 ± 6.9 | 8.5 ± 5.6 | 13.6 ± 5.2 | 12.6 ± 6.2 |
| Collagen (5 μg/mL) | 14.2 ± 6.1 | 14.8 ± 4.9 | 18 ± 6.2 | 17.2 ± 5.9 |
| TF‡ | ||||
| TF+ MVs (number per mL) | 1551 (404, 6379) | 1723 (516, 7181) | 2468 (603, 6827) | 1515 (440, 3830) |
| TF+ MVs PCA (fmol) | 257 (148, 927) | 362 (166, 834) | 414 (122, 859) | 498 (307, 923) |
| PBMC TF mRNA expression (fold change)§,† | 1.41 (0.46, 6.11) | 0.43 (0.29, 2.19) | NA | NA |
| PDI‡ | ||||
| Plasma PDI reductase activity (pmol/min per μL)† | 16 (14, 27) | 16 (13, 21) | 19 (16, 23) | 20 (17, 25) |
| Plasma PDI antigen (ng/mL) | 2.14 (1.61, 2.96) | 2.18 (1.77, 3.31) | 2.08 (1.88, 2.31) | 2.23 (1.75, 2.72) |
| Other markers of coagulation∗ | ||||
| D-dimer (μg/mL) | 1.5 ± 0.8 | 1.7 ± 1.1 | 1.6 ± 1.1 | 1.9 ± 1.8 |
| Thrombin–antithrombin complexes (ng/mL) | 2.7 ± 1.4 | 2.7 ± 1.7 | 2.5 ± 0.9 | 2.4 ± 1.1 |
| Plasma quercetin levels (ng/mL)∗ | NA | 253 ± 330 | NA | 15 ± 17 |
Differences in posttreatment measures were assessed with ANCOVA and, for non-Gaussian–distributed data, either with the Wilcoxon rank-sum test or the Spearman test. Posttreatment value comparisons were intentionally not performed based on our a priori statistical analytical plan.
ADP, adenosine 5′-diphosphate; AU, arbitrary units; NA, not applicable.
Mean ± SD.
Statistically significant differences observed among the IQ and placebo groups.
Median (interquartile range [IQR]).
n = 20.
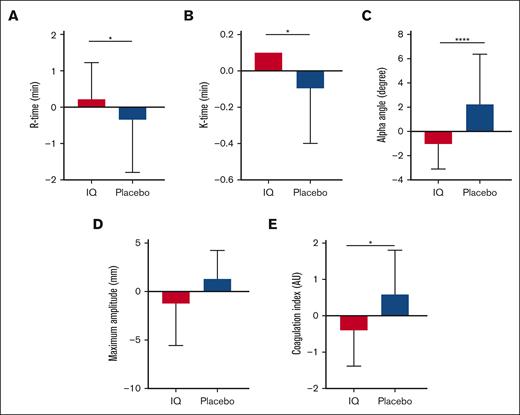
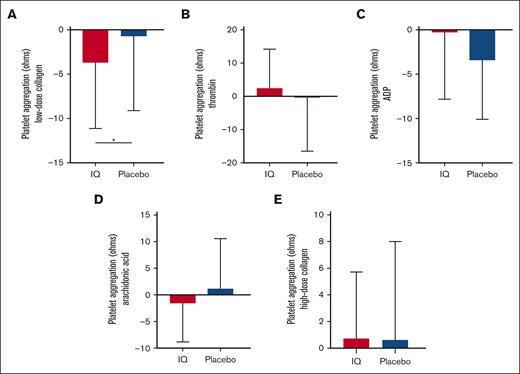
Consistent with prior reports,18 TEG-determined whole-blood coagulation in patients from both study groups was significantly elevated compared with ethnic-matched healthy controls (CI, IQ: 3.0 ± 1.5 and placebo: 3.3 ± 1.5 vs healthy ethnic-matched controls: 2.5 ± 0.8; P = .02). Compared with that of the placebo group, almost all TEG parameters demonstrated a significant change in the appropriate direction from the baseline after IQ treatment (mean change in CI from baseline ± SD: IQ: −0.29 ± 1.30, placebo: 0.43 ± 1.35; P = .03; Figure 2; Table 3). IQ treatment also significantly reduced platelet aggregation responses after activation with low-dose collagen (mean change from baseline in impedance ± SD: IQ: −3.71 ± 7.30 ohms, placebo: −0.71 ± 8.39 ohms; P = .03) although platelet aggregation induced by more potent platelet agonists was unaffected (Figure 3; Table 3). The effects of IQ treatment on whole-blood coagulation and collagen-induced platelet aggregation persisted after exclusion of participants receiving either anticoagulants (TEG; IQ, n = 2; placebo, n = 2) or aspirin (platelet aggregation; IQ, n = 3; placebo, n = 3). Plasma D-dimer levels reflective of the sickle hypercoagulable state were elevated at baseline above the normal range (Table 1) but IQ treatment did not affect either D-dimers (mean change from baseline ± SD: IQ: 0.15 ± 0.92 μg/L; placebo: −0.10 ± 2.1 μg/L; P = .81) or thrombin–antithrombin complexes (mean change from baseline ± SD: IQ: 0.09 ± 1.6 ng/mL; placebo: −0.05 ± 1.3 ng/mL; P = .91; Table 3).
Effects of IQ on whole-blood coagulation. Mean change from baseline in (A) clot initiation time (reaction-time or R-time) and (B) clot formation time (K time) showing significant prolongation from the baseline value after IQ treatment (ANCOVA, ∗P = .04 and ∗P = .02, respectively). (C) Fibrin crosslinking determined by the α-angle acuteness signified by the maximal amplitude showing significant reduction from the baseline value after IQ treatment (ANCOVA, ∗∗∗∗P = .0001). However, (D) clot strength (MA) was not affected by IQ treatment (P = .09). (E) Mean change from the baseline in whole-blood CI assessed by TEG showed a significant reduction in the IQ group compared with in the placebo group (ANCOVA, ∗P = .03; IQ, n = 22; placebo, n = 22). AU, arbitrary units; MA, maximum amplitude.
Effects of IQ on whole-blood coagulation. Mean change from baseline in (A) clot initiation time (reaction-time or R-time) and (B) clot formation time (K time) showing significant prolongation from the baseline value after IQ treatment (ANCOVA, ∗P = .04 and ∗P = .02, respectively). (C) Fibrin crosslinking determined by the α-angle acuteness signified by the maximal amplitude showing significant reduction from the baseline value after IQ treatment (ANCOVA, ∗∗∗∗P = .0001). However, (D) clot strength (MA) was not affected by IQ treatment (P = .09). (E) Mean change from the baseline in whole-blood CI assessed by TEG showed a significant reduction in the IQ group compared with in the placebo group (ANCOVA, ∗P = .03; IQ, n = 22; placebo, n = 22). AU, arbitrary units; MA, maximum amplitude.
Effects of IQ on platelet aggregation. Mean agonist-induced platelet aggregation shown as change from the baseline in impedance whole-blood aggregometry from patients from the IQ group (n = 22) and the placebo group (n = 22). IQ treatment only shows a significant effect on platelet aggregation after exposure to (A) low doses of collagen (ANCOVA, ∗P = .02; IQ, n = 21; placebo, n = 21); however, after standard platelet aggregation, agonists such as (B) thrombin, (C) adenosine 5′-diphosphate (ADP), (D) arachidonic acid, and (E) high dose of collagen (ANCOVA, P = .93, P = .72, P = .63, and P = .66; respectively) did not show any effect.
Effects of IQ on platelet aggregation. Mean agonist-induced platelet aggregation shown as change from the baseline in impedance whole-blood aggregometry from patients from the IQ group (n = 22) and the placebo group (n = 22). IQ treatment only shows a significant effect on platelet aggregation after exposure to (A) low doses of collagen (ANCOVA, ∗P = .02; IQ, n = 21; placebo, n = 21); however, after standard platelet aggregation, agonists such as (B) thrombin, (C) adenosine 5′-diphosphate (ADP), (D) arachidonic acid, and (E) high dose of collagen (ANCOVA, P = .93, P = .72, P = .63, and P = .66; respectively) did not show any effect.
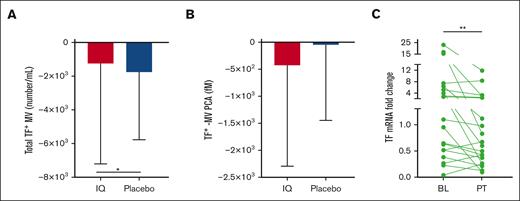
Because agonist-induced TF expression in patient-derived monocytes and cultured endothelial cells (supplemental Figure 3) was significantly inhibited by quercetin treatment in vitro, we expected a reduction in the number of plasma TF+ MVs in patients treated with IQ. Surprisingly, TF+ MVs were reduced in both groups, but the placebo- rather than the IQ-treated group demonstrated a significantly greater reduction in TF+ MVs (mean change from baseline ± SD: IQ: −1.2 × 103/mL ± 5.9 × 103/mL; placebo: −1.7 × 103/mL ± 4.0 × 10 3/mL; P = .02; Figure 4A; Table 3). However, in line with our hypothesis, TF+ MVs isolated from the plasma of patients treated with IQ accelerated coagulation in vitro less rapidly than in patients in the placebo group, although this difference was not significant (mean change from baseline ± SD: IQ: −427 ± 1868 fmol; placebo: −43 ± 1404 fmol; P = .51; Figure 4B; Table 3). To address these discrepant findings, we evaluated inducible monocyte TF mRNA expression in paired samples of PBMCs obtained from the IQ group at baseline and post-IQ treatment. Reassuringly, IQ treatment significantly attenuated TF mRNA expression ex vivo in LPS stimulated PBMCs (TF mRNA fold change mean ± SD, baseline: 4.67 ± 6.55 vs post-IQ treatment: 1.91 ± 3.03; P = .007; Figure 4C).
Effects of IQ on TF antigen, activity, and gene expression. (A) Mean change from the baseline in the number of TF+ MVs showing reduction in both IQ and placebo groups but a significantly greater reduction in the placebo group (∗P = .02). (B) Mean change from the baseline in TF+ MV procoagulant activity (PCA), which is decreased in both groups but shows no significant difference (P = .51). (C) LPS-induced TF mRNA expression in PBMCs isolated from patients with SCD in the IQ group at baseline (BL) and after treatment (PT) showed a significant reduction in inducible TF mRNA after IQ treatment (∗∗P = .003; n = 20).
Effects of IQ on TF antigen, activity, and gene expression. (A) Mean change from the baseline in the number of TF+ MVs showing reduction in both IQ and placebo groups but a significantly greater reduction in the placebo group (∗P = .02). (B) Mean change from the baseline in TF+ MV procoagulant activity (PCA), which is decreased in both groups but shows no significant difference (P = .51). (C) LPS-induced TF mRNA expression in PBMCs isolated from patients with SCD in the IQ group at baseline (BL) and after treatment (PT) showed a significant reduction in inducible TF mRNA after IQ treatment (∗∗P = .003; n = 20).
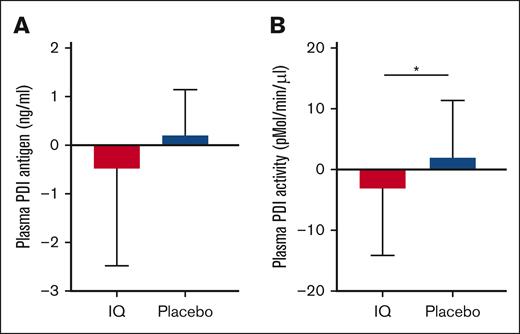
In patients with SCD, both plasma PDI antigen (ng/mL, SCD: 3.0 ± 4.5 vs ethnic matched controls: 1.2 ± 2.2; P < .0001) and PDI reductase activity (SCD: 19.4 ± 6.7 pmol/min per μL vs ethnic matched controls: 14.9 ± 1.1 pmol/min per μL; P = .04) were significantly elevated compared with ethnic-matched controls (supplemental Figure 4). Although there was no discernable effect on plasma PDI antigen (Figure 5A), IQ treatment was associated with a significantly lowered plasma PDI reductase activity compared with that of the placebo group (mean change from baseline ± SD: IQ: −3.1 ± 10.9 pmol/min per μL; placebo: 1.9 ± 9.3 pmol/min per μL; P = .02; Figure 5B).
Effect of IQ on plasma PDI antigen and plasma PDI activity. (A) Mean change from baseline in plasma PDI antigen was not different between the patients treated with IQ and those treated with placebo (ANCOVA, P = .52; IQ, n = 22; placebo, n = 22). (B) PDI reductase activity (mean change from the baseline value) is significantly decreased in the patients treated with IQ compared with those treated with placebo (ANCOVA, ∗P = .02; IQ, n = 22; placebo, n = 22).
Effect of IQ on plasma PDI antigen and plasma PDI activity. (A) Mean change from baseline in plasma PDI antigen was not different between the patients treated with IQ and those treated with placebo (ANCOVA, P = .52; IQ, n = 22; placebo, n = 22). (B) PDI reductase activity (mean change from the baseline value) is significantly decreased in the patients treated with IQ compared with those treated with placebo (ANCOVA, ∗P = .02; IQ, n = 22; placebo, n = 22).
Study drug adherence was above average and patients in both study groups exhibited similar levels of adherence (IQ = 96% vs placebo = 97%; P = .24). Consistent with these adherence data, posttreatment steady-state plasma quercetin measurements were significantly higher in the IQ-treated group than in the placebo-treated group (mean ± SD: IQ: 253 ± 330 ng/mL vs placebo: 15 ± 17; P < .0001; supplemental Figure 5).
Discussion
In this phase 2 randomized double-blind placebo-controlled trial conducted for patients with steady state SCD, we show that short-term fixed-dosage IQ is safe, well tolerated, and attenuates several biomarkers reflective of sickle thromboinflammatory pathology. Specifically, to our knowledge, this is the first report to demonstrate that IQ treatment in patients with SCD (1) does not substantially reduce basally elevated plasma sP-selectin, (2) reduces whole-blood coagulation and platelet aggregation in response to submaximal stimulation with collagen, (3) inhibits plasma PDI reductase activity, and (4) reduces LPS-induced TF mRNA expression in PBMCs.
Our safety results are in line with other phase 1 clinical trials that report no drug-linked severe adverse effects using IQ at the daily dosage of 1 g per day, validating its safety in patients with SCD.17,25 Moreover, the lack of increased frequency of VOCs in the treatment group suggests that high-dose IQ is both safe and tolerable in this patient population. Firmly establishing the safety of high-dose flavonoids in patients with SCD, paving the way for escalating-dose trials to test whether IQ can definitively attenuate thromboinflammatory pathophysiology in SCD given that a prior study has tested doses up to 5 g per day.26
Unlike the study in patients with active cancer,17 short-term fixed-dosage IQ treatment in patients with steady-state SCD neither met its primary end point of reducing basal plasma sP-selectin levels nor affected plasma D-dimers. Several reasons may explain these findings, in addition to differences in the disease population and duration of IQ exposure (28 vs 52 days). First, the anticipated effect size of treatment on sP-selectin may have been overestimated; thus, the small sample size did not provide adequate statistical power to detect a smaller, more modest change in sP-selectin from the baseline. Second, the study cohort had noticeably lower sP-selectin levels than previously reported values,27 reflecting inherent biological differences in the patient population studied. Similarly, D-dimers were lower in this steady-state cohort, suggesting that optimally dosed hydroxyurea treatment possibly attenuated sickle hypercoagulability, hindering the detection of hypothesized differences between the treatment groups.
The sickle hypercoagulable state is accompanied by increased incident and recurrent venous thrombosis requiring treatment with anticoagulation.28 However, systemic anticoagulant use is associated with a 21% increased incidence of clinically relevant major bleeding in patients with SCD.7 By lacking off-target bleeding side effects, IQ offers some safety advantages for managing hypercoagulability in patients with SCD. Importantly, most TEG parameters previously shown to reflect the sickle hypercoagulable state18 were significantly affected by IQ treatment, although it should be recognized that TEG has not been shown to predict thrombotic risk. Similarly, IQ treatment significantly reduced platelet aggregation responses to stimulation with low-dose collagen, consistent with its known antiplatelet effect.18 Because patients with SCD in their steady state exhibit higher basal platelet activation than ethnic-matched healthy controls,29 the significance of these findings could be probed for clinical relevance in future studies of IQ treatment. Taken together, our data provide valuable safety and efficacy signals that this relatively inexpensive medication holds for managing the thromboinflammatory complications of SCD in conjunction with standard care.
Heme-induced monocyte TF expression in vitro30 mediated possibly via damage-associated molecular pattern responses, links hemolysis-induced monocyte TF expression in vivo during VOC.14,31 Patients with SCD also display monocyte activation32 and TF expression8,13 that increase further during VOC.31 Quercetin robustly inhibited heme- and LPS-induced TF expression in monocytes from patients with SCD in vitro (supplemental Figure 3A), yet short-term fixed-dose IQ treatment did not significantly affect blood-borne TF antigen assessed by the number of circulating plasma TF+ MVs, possibly reflecting the inherent variability observed with this biomarker.33 In contrast, TF procoagulant activity, a more reliable indicator of TF's functional ability to initiate coagulation in vivo, showed a nonsignificant reduction in the treatment group compared to the placebo group. Furthermore, inducible TF gene expression in peripheral blood monocytes, an important source in the blood of patients with SCD, was significantly inhibited by short-term fixed-dose IQ treatment. Taken together, these data suggest that higher doses of IQ treatment for a more prolonged duration (>28 days) could achieve clinically relevant suppression of blood-borne TF induced by heme and mitigate sickle thromboinflammatory pathophysiology.
We report plasma PDI levels with detectable plasma PDI reductase activity in patients with SCD that were significantly higher than ethnic-matched healthy controls (supplemental Figure 4). Plasma PDI is possibly elevated in SCD because of intravascular secretion of intracellular PDI from activated platelets34 and endothelial cells.35 Alternately, endothelial cell/platelet injury or red blood cell hemolysis during VOC could lead to intracellular PDI leaking into the plasma. Although the exact explanation for the source of elevated PDI in sickle plasma is presently unclear, it is of considerable interest given the role that cell-surface PDI reductase plays in sickle erythrocyte dehydration,36 platelet activation, and neutrophil recruitment in animal models of SCD.37 Akin to its effect in patients with cancer, IQ decreased plasma PDI reductase activity in patients with SCD, albeit more modestly17 because of methodological differences between the 2 studies. By targeting PDI reductase activity, which allosterically activates TF, IQ treatment can affect TF-initiated coagulation in SCD at 2 different levels: (1) at the translational level, as discussed earlier, by inhibiting inducible TF expression on the surface of monocytes; and (2) at the posttranslational level, by inhibiting PDI reductase activity.38
With respect to drug dosing, the quercetin concentrations observed in this study were comparable but noticeably lower than postsupplementation plasma quercetin concentrations observed in healthy participants (253 ± 330 ng/mL vs 427.1 ± 89.2 ng/mL) who consumed similar quantities of IQ for 28 days.39 This was probably because of a higher clearance rate in patients with SCD because of glomerular hyperfiltration but could also reflect lower dietary flavonoid intake.
This study has several strengths. First, it used a randomized, double-blind, placebo-controlled design to reduce potential biases. Second, it included a representative population of adults with steady-state SCD with hallmarks of severe disease (VOC frequency of 3 per year, and a history or prior arterial and/or venous thrombosis) receiving optimally dosed hydroxyurea treatment. Third, it assessed a relatively inexpensive oral treatment that broadly targets thromboinflammatory pathophysiology, and assessed a range of thromboinflammation biomarkers relevant to sickle cell pathobiology. However, the limitations are worth considering. Using fixed instead of escalating-dose IQ possibly hampered detection of the hypothesized differences in plasma sP-selectin. The limitations notwithstanding, this study provides an important signal of safety and efficacy upon which future clinical trials can be designed.
In conclusion, these data confirm the utility of a relatively inexpensive and safe oral flavonoid with demonstrable efficacy in reducing several thromboinflammatory biomarkers in SCD. Taken together, these findings suggest that trials of higher dose IQ for more extended treatment durations are required for patients with SCD.
Acknowledgments
The authors thank all the study participants and their families.
This study was sponsored by the intramural research program of the National Heart, Lung, and Blood Institute (ZIA #HL006256 & #HL006241). The sponsor had no role in the overall conduct of the study as well as the collection, analyses, and interpretation of the data. The content of this publication does not necessarily reflect the views or polices of the Department of Health and Human Services; nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Authorship
Contribution: M.A.L.-I. and B.P.G. managed conduct of samples and performance of laboratory assays, and contributed to data analysis and drafting of the manuscript; A.S.S. developed the protocol and, with the help of study statistician N.J., analyzed results, generated a clinical study report, and prepared the manuscript; A.S.S. was the principal investigator and provided medical oversight of the trial; A.D.-F., A.C., R.V., and D.-Y.L. conducted laboratory assays and contributed to data analysis; B.M., J.B., M.H., D.L., and R.P.-C. participated in protocol review, patient recruitment, patient management, and collection of clinical outcomes; and all authors reviewed the manuscript with opportunity to provide input.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Arun S. Shet, Sickle Cell Branch, National Heart, Lung, and Blood Institute, 10 Center Dr, Building 10, Room 6S241 MSC 1589, Bethesda, MD 20892-1589; email: arun.shet@nih.gov.
References
Author notes
∗M.A.L.-I. and B.P.G. contributed equally to this study.
Individual participant data will not be shared. Original experimental and clinical trial data can be obtained upon reasonable request from the corresponding author, Arun S. Shet (arun.shet@nih.gov).
The full-text version of this article contains a data supplement.