Key Points
Younger haploidentical donor (PTCy prophylaxis) HCT was associated with improved OS compared with older MUD (conventional prophylaxis) HCT in ALL.
Younger haploidentical donors had a lower risk of chronic GVHD and NRM, but a higher relapse than older MUD.
Abstract
Haploidentical hematopoietic cell transplantation (HCT) with posttransplant cyclophosphamide (PTCy) graft-versus-host-disease (GVHD) prophylaxis yields a similar overall survival (OS) to HLA-matched unrelated donor (MUD) HCT with conventional prophylaxis. Given the prognostic implications of donor age, we investigated the impact of donor age (younger [<35 years, n = 868] vs older [≥35 years, n = 418]) and donor type (haploidentical [n = 373] vs MUD [n = 913]) on OS in adult patients with acute lymphoblastic leukemia (ALL). Older donor age was independently associated with significantly poor OS, whereas donor type was not. Next, we directly compared the outcomes of a younger haploidentical donor (n = 187) vs an older MUD (n = 232). In this cohort, more patients in the haploidentical group had B-cell immunophenotype (89% vs 77%, respectively, P < .001), poor cytogenetics (61% vs 51%, respectively, P = .44), Philadelphia chromosome–negative (53% vs 48%, respectively, P = .38), received bone marrow graft (42% vs 16%, respectively, P < .001), and reduced-intensity conditioning (45% vs 23%, respectively, P < .001). In the multivariate analysis, the older MUD group was associated with a significantly higher risk of chronic GVHD, higher nonrelapse mortality (NRM), lower relapse, and poorer OS. Despite a higher risk of relapse, younger donor haploidentical HCT with PTCy prophylaxis may be preferred over older MUD HCT with conventional prophylaxis in patients with ALL due to lower NRM and better OS. Further analysis comparing the effect of donor age in haploidentical PTCy vs MUD PTCy is warranted.
Introduction
Large-scale registry studies showed similar outcomes for haploidentical hematopoietic cell transplantations (HCTs) with posttransplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis vs HLA-matched unrelated donor (MUD) HCT with conventional GVHD prophylaxis in patients with acute myeloid leukemia1 or acute lymphoblastic leukemia (ALL).2 Although the use of PTCy has increased in patients undergoing HLA-matched donor HCT,3-7 the combination of a calcineurin inhibitor and methotrexate remains the current standard of care.5,7,8 Abundant evidence also indicates that there is a detrimental effect of increasing donor age on HCT outcomes.9-18
Therefore, we reasoned that if haploidentical (PTCy prophylaxis) and MUD (conventional prophylaxis) HCT have similar outcomes,1,2 and if a younger donor is associated with improved outcomes,9-17,19 a younger haploidentical donor (PTCy prophylaxis) may be associated with better overall survival (OS) than an older MUD (conventional prophylaxis). We tested this hypothesis using the Center for International Blood and Marrow Transplant Research (CIBMTR) data set from a previously published study2 and reported the results of our analysis in patients with ALL. We used this particular data set because it contained several ALL-specific key prognostic variables. Similar analysis in patients with acute myeloid leukemia will be reported separately.
Methods
The inclusion criteria were adult patients (age ≥18 years) with ALL in complete remission (CR) who underwent haploidentical or MUD HCT between 2013 and 2017. Donors <35 years of age were categorized as “younger,” whereas donors ≥35 years were classified as “older.” A donor age of 35 years was selected as a cutoff based on previous publications that showed a survival advantage with younger donors between the ages of 30 and 36 years than older donors.9,11,12,15-17 All haploidentical HCT were T-cell replete and included PTCy prophylaxis, whereas all MUD HCT used conventional calcineurin inhibitor–based GVHD prophylaxis. Patients who received antithymocyte globulin or alemtuzumab, matched siblings, umbilical cord blood HCT, and MUD HCT with PTCy prophylaxis were excluded. The primary outcome of interest was OS, defined as the time from HCT to death from any cause. Secondary outcomes included grade 2 to 4 acute GVHD (aGVHD);20 grade 3 to 4 aGVHD;20 chronic GVHD (cGVHD);21 relapse; nonrelapse mortality (NRM), defined as death without recurrence of underlying malignancy; and progression-free survival (PFS), defined as the time from HCT to treatment failure due to relapse or death from any cause. The MD Anderson Institutional Review Board approved this study (protocol: 2022-0684). This study was conducted following the Declaration of Helsinki.
Statistical analysis
Baseline characteristics were summarized using descriptive statistics with median and interquartile range (IQR) for continuous variables and numbers/percentages for categorical variables. These were compared between the groups using the χ2 test for categorical variables and Mann-Whitney U test for continuous variables. The Kaplan-Meier method was used to estimate OS and PFS probabilities. The cumulative incidence estimate for NRM was calculated using relapse as a competing risk, whereas that for relapse was calculated using NRM as a competing risk. Death was considered as a competing risk for aGVHD or cGVHD. Factors considered in the univariate analysis included patient age (younger [<40 years] vs older [≥40 years]22), patient sex, sex mismatch (female donor to a male recipient vs others23), conditioning intensity (total body irradiation [TBI]–based myeloablative conditioning [MAC] vs chemotherapy-based MAC vs reduced-intensity conditioning/nonmyeloablative), graft source (bone marrow vs peripheral blood), disease status (first CR [CR1]-minimal residual disease (MRD)–negative vs CR1-MRD–positive vs CR1-MRD missing vs CR2 vs ≥CR3), cytogenetics (normal vs poor), immunophenotype (T cell vs B cell), Philadelphia chromosome status (positive vs negative), HCT-specific Comorbidity Index score (0-2 vs ≥3), Karnofsky performance score (<90 vs ≥90), recipient cytomegalovirus serostatus (positive vs negative), and interval from diagnosis to HCT (<6 months, 6-12 months, >12 months). Multivariable Cox proportional hazards (PH) regression analysis for outcomes (OS, PFS, aGVHD, cGVHD, relapse, and NRM) and the Fine-Gray model for competing risk outcomes were used. A backward stepwise model selection was used to identify all the significant variables. Because donor type (younger haploidentical vs older MUD) was the primary variable of interest, it was included in all the multivariate analyses. The factors that were significant at the 5% level were retained in the final model. Interactions between covariates and the main test variable (donor type) were tested using the Cox regression analysis. All variables were tested for affirmation of the PH assumption by assessing them graphically and by including a time interaction of the variable(s) in the model. Factors that violated the PH assumption were adjusted through stratification. If the main variable of interest (donor type) violated the PH assumption, the analyses were conducted before and after the time when the hazards crossed. All analyses were performed at a 2-sided significance level of P < .05. All statistical analyses were performed using STATA/MP 17.0 (StataCorp LLC, College Station, TX)
Results
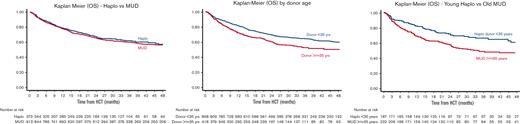
Our principal aim was to directly compare the outcomes of a younger haploidentical donor (PTCy prophylaxis) vs an older MUD (conventional prophylaxis). Before conducting the main analyses, we performed supplementary analyses to assess the relative impact of donor age (younger [<35 years, n = 868] vs older [≥35 years, n = 418]) and donor type (haploidentical [n = 373] vs MUD [n = 913]) in the entire cohort (supplemental Table 1). The Kaplan-Meier estimates of OS by donor age are shown in supplemental Figures 1 and 2 and by donor type in supplemental Figure 3. No statistical interactions were noted between donor age and type. In a multivariate analysis that included donor age and donor type along with other covariates, older donor age was found to be an independent significant predictor of poor OS (hazard ratio [HR], 1.29; 95% confidence interval [CI], 1.01-1.66; P = .043), whereas donor type was not (supplemental Table 2). Next, we restricted the study population to only those with younger donors and compared haploidentical to MUD (supplemental Table 3); and then restricted the study population to only those with older donors and compared haploidentical to MUD (supplemental Table 4). In both subgroup analyses, where the donor age groups were forced to be equalized (either young or old), multivariate analyses did not reveal any differences in OS between the haploidentical and MUD groups. These analyses substantiated the rationale for our primary analysis of interest: the direct comparison of younger haploidentical donor vs an older MUD, the results of which are shown below. This comparison was meant to inform the common clinical question faced in selecting donors for a patient in need of HCT.
Primary analysis
We included 419 patients: 232 in the older MUD cohort and 187 in the younger haploidentical cohort. The median patient age at the time of HCT was 42.7 years (IQR, 31.3-56.7) vs 41.4 years (IQR, 29.2-55), respectively, P = .29. The median donor age was 42.4 years (IQR, 38.4-46.3) vs 25.5 years (IQR, 21.3-30), respectively, P < .001. The majority were male in both groups (58% vs 56%, respectively, P = .59), but the MUD group had a slightly lower proportion of male recipients with a female donor (16%) than the haploidentical group (23%), P = .05. Most patients were in CR1 with either minimal residual disease (MRD)–negative CR1: 42% vs 36%, MRD-positive CR1: 32% vs 30%, or CR1 with missing MRD data: 3% vs 2%. A significantly higher proportion of patients in the MUD group had T-cell immunophenotypes (14% vs 3%, P < .001). Among patients with B-cell ALL, approximately half were Philadelphia chromosome–positive (52% vs 47%, respectively, P = .38). Most patients had poor-risk cytogenetics in both groups (51% vs 61%, respectively, P = .44). TBI-based MAC was used more commonly in the MUD (60% vs 40%), whereas reduced-intensity conditioning/nonmyeloablative was used more frequently in the haploidentical (45%) than in the MUD group (23%), P < .001. Peripheral blood graft was used more commonly in the MUD (84%) than in the haploidentical (58%) group, P < .001. Most patients had cytomegalovirus-seropositive status (62% vs 72%, respectively, P = .03) and Karnofsky performance score >90 (59% vs 62%, respectively, P = .49). The HCT-specific Comorbidity Index score was ≥3 in 52% (MUD) vs 42% (haploidentical), P = .05. The median year of HCT was 2015 vs 2016, respectively, and the median follow-up among survivors was 36.6 months vs 31.5 months, respectively, P = .009 (Table 1).
Baseline characteristics
| . | MUD (donor ≥35 y) . | Haploidentical (donor <35 y) . | P . |
|---|---|---|---|
| n = 232 . | n = 187 . | ||
| Patient age, median (IQR), y | 42.7 (31.3-56.7) | 41.4 (29.2-55) | .29 |
| Donor age, median (IQR), y | 42.4 (38.4-46.3) | 25.5 (21.3-30) | <.001 |
| Patient age, n (%), y | .69 | ||
| <40 | 106 (46) | 89 (48) | |
| ≥40 | 126 (54) | 98 (52) | |
| Sex, n (%) | .59 | ||
| Male | 135 (58) | 104 (56) | |
| Female | 97 (42) | 83 (44) | |
| Sex mismatch, n (%) | .05 | ||
| Female-to-male | 36 (16) | 43 (23) | |
| Others | 195 (84) | 144 (77) | |
| Race/ethnicity, n (%) | <.001 | ||
| White, non-Hispanic | 20 (9) | 54 (29) | |
| White, Hispanic | 159 (69) | 64 (34) | |
| Black | 13 (6) | 31 (17) | |
| Asian | 15 (6) | 14 (7) | |
| Other/not specified | 25 (11) | 24 (13) | |
| Remission status, n (%) | .31 | ||
| CR1-MRD–negative | 98 (42) | 68 (36) | |
| CR1-MRD–positive | 75 (32) | 57 (30) | |
| CR1-MRD missing | 6 (3) | 3 (2) | |
| CR2 | 47 (20) | 50 (27) | |
| ≥CR3 | 6 (3) | 9 (5) | |
| Immunophenotype, n (%) | <.001 | ||
| B cell | 178 (77) | 166 (89) | |
| T cell | 33 (14) | 5 (3) | |
| Unspecified | 21 (9) | 16 (9) | |
| Philadelphia chromosome–positive/BCR-ABL1 status∗, n (%) | .38 | ||
| No | 86 (48) | 88 (53) | |
| Yes | 92 (52) | 78 (47) | |
| Cytogenetics, n (%) | .44 | ||
| Normal | 49 (21) | 39 (21) | |
| Poor | 119 (51) | 115 (61) | |
| Missing | 64 (28) | 33 (18) | |
| Conditioning, n (%) | <.001 | ||
| MAC-TBI | 140 (60) | 74 (40) | |
| MAC chemotherapy | 38 (16) | 29 (16) | |
| Reduced-intensity conditioning/nonmyeloablative | 54 (23) | 84 (45) | |
| Graft, n (%) | <.001 | ||
| Bone marrow | 37 (16) | 79 (42) | |
| Peripheral blood | 195 (84) | 108 (58) | |
| Cytomegalovirus donor/recipient, n (%) | .02 | ||
| Positive/positive | 78 (34) | 88 (47) | |
| Positive/negative | 34 (15) | 14 (7) | |
| Negative/positive | 64 (28) | 46 (25) | |
| Negative/negative | 54 (23) | 39 (21) | |
| Karnofsky performance scale, n (%) | .49 | ||
| <90 | 93 (41) | 68 (36) | |
| ≥90 | 138 (59) | 116 (62) | |
| HCT-specific Comorbidity Index, n (%) | .05 | ||
| 0-2 | 112 (48) | 108 (58) | |
| ≥3 | 120 (52) | 79 (42) | |
| GVHD prophylaxis, n (%) | <.001 | ||
| PTCy + CNI ± mycophenolate mofetil | 0 | 187 (100) | |
| CNI + methotrexate ± others | 26 (11) | 0 | |
| CNI + mycophenolate mofetil ± others | 170 (73) | 0 | |
| CNI ± others | 36 (16) | 0 | |
| Time from diagnosis to HCT, n (%) | .02 | ||
| 0-5 mo | 98 (42) | 68 (36) | |
| 6-11 mo | 86 (37) | 58 (31) | |
| ≥12 mo | 48 (21) | 51 (33) | |
| Y of transplant, median (IQR) | 2015 (2014-2016) | 2016 (2015-2017) | <.001 |
| Follow-up among survivors, median (IQR), mo | 36.3 (24.2-49.4) | 31.5 (23.6-41.8) | .009 |
| . | MUD (donor ≥35 y) . | Haploidentical (donor <35 y) . | P . |
|---|---|---|---|
| n = 232 . | n = 187 . | ||
| Patient age, median (IQR), y | 42.7 (31.3-56.7) | 41.4 (29.2-55) | .29 |
| Donor age, median (IQR), y | 42.4 (38.4-46.3) | 25.5 (21.3-30) | <.001 |
| Patient age, n (%), y | .69 | ||
| <40 | 106 (46) | 89 (48) | |
| ≥40 | 126 (54) | 98 (52) | |
| Sex, n (%) | .59 | ||
| Male | 135 (58) | 104 (56) | |
| Female | 97 (42) | 83 (44) | |
| Sex mismatch, n (%) | .05 | ||
| Female-to-male | 36 (16) | 43 (23) | |
| Others | 195 (84) | 144 (77) | |
| Race/ethnicity, n (%) | <.001 | ||
| White, non-Hispanic | 20 (9) | 54 (29) | |
| White, Hispanic | 159 (69) | 64 (34) | |
| Black | 13 (6) | 31 (17) | |
| Asian | 15 (6) | 14 (7) | |
| Other/not specified | 25 (11) | 24 (13) | |
| Remission status, n (%) | .31 | ||
| CR1-MRD–negative | 98 (42) | 68 (36) | |
| CR1-MRD–positive | 75 (32) | 57 (30) | |
| CR1-MRD missing | 6 (3) | 3 (2) | |
| CR2 | 47 (20) | 50 (27) | |
| ≥CR3 | 6 (3) | 9 (5) | |
| Immunophenotype, n (%) | <.001 | ||
| B cell | 178 (77) | 166 (89) | |
| T cell | 33 (14) | 5 (3) | |
| Unspecified | 21 (9) | 16 (9) | |
| Philadelphia chromosome–positive/BCR-ABL1 status∗, n (%) | .38 | ||
| No | 86 (48) | 88 (53) | |
| Yes | 92 (52) | 78 (47) | |
| Cytogenetics, n (%) | .44 | ||
| Normal | 49 (21) | 39 (21) | |
| Poor | 119 (51) | 115 (61) | |
| Missing | 64 (28) | 33 (18) | |
| Conditioning, n (%) | <.001 | ||
| MAC-TBI | 140 (60) | 74 (40) | |
| MAC chemotherapy | 38 (16) | 29 (16) | |
| Reduced-intensity conditioning/nonmyeloablative | 54 (23) | 84 (45) | |
| Graft, n (%) | <.001 | ||
| Bone marrow | 37 (16) | 79 (42) | |
| Peripheral blood | 195 (84) | 108 (58) | |
| Cytomegalovirus donor/recipient, n (%) | .02 | ||
| Positive/positive | 78 (34) | 88 (47) | |
| Positive/negative | 34 (15) | 14 (7) | |
| Negative/positive | 64 (28) | 46 (25) | |
| Negative/negative | 54 (23) | 39 (21) | |
| Karnofsky performance scale, n (%) | .49 | ||
| <90 | 93 (41) | 68 (36) | |
| ≥90 | 138 (59) | 116 (62) | |
| HCT-specific Comorbidity Index, n (%) | .05 | ||
| 0-2 | 112 (48) | 108 (58) | |
| ≥3 | 120 (52) | 79 (42) | |
| GVHD prophylaxis, n (%) | <.001 | ||
| PTCy + CNI ± mycophenolate mofetil | 0 | 187 (100) | |
| CNI + methotrexate ± others | 26 (11) | 0 | |
| CNI + mycophenolate mofetil ± others | 170 (73) | 0 | |
| CNI ± others | 36 (16) | 0 | |
| Time from diagnosis to HCT, n (%) | .02 | ||
| 0-5 mo | 98 (42) | 68 (36) | |
| 6-11 mo | 86 (37) | 58 (31) | |
| ≥12 mo | 48 (21) | 51 (33) | |
| Y of transplant, median (IQR) | 2015 (2014-2016) | 2016 (2015-2017) | <.001 |
| Follow-up among survivors, median (IQR), mo | 36.3 (24.2-49.4) | 31.5 (23.6-41.8) | .009 |
CNI, calcineurin inhibitor.
In patients with B-cell ALL.
GVHD
The cumulative incidence of grade 2 to 4 aGVHD on day 100 was 21.5 (95% CI, 15.0-28.7) in the older MUD vs 16.3 (95% CI, 10.9-22.7) in the younger haploidentical group. The cumulative incidence of grade 3 to 4 aGVHD at day 100 was 8.4% (95% CI, 4.9-13.1) vs 4.2 (95% CI, 1.8-8.0), respectively. The cumulative incidence of cGVHD at 2 years was 54.4% (95% CI, 47.5-60.8) vs 31.5% (95% CI, 24.8-38.4), respectively (Table 2). The univariate analyses are shown in supplemental Table 5.
Unadjusted cumulative incidences/estimates
| . | Older MUD (donor ≥35 y) . | Younger haploidentical (donor <35 y) . | ||
|---|---|---|---|---|
| Estimate (%) . | 95% CI . | Estimate (%) . | 95% CI . | |
| aGVHD grade 2-4, d 100 | 21.5 | 15.0-28.7 | 16.3 | 10.9-22.7 |
| aGVHD grade 3-4, d 100 | 8.4 | 4.9-13.1 | 4.2 | 1.8-8.0 |
| cGVHD | ||||
| 1 y | 48.6 | 41.8-55.1 | 26.4 | 20.3-32.9 |
| 2 y | 54.4 | 47.5-60.8 | 31.5 | 24.8-38.4 |
| 3 y | 55.1 | 48.1-61.5 | 33.9 | 26.7-41.2 |
| 4 y | 56.0 | 49.0-62.5 | 33.9 | 26.7-41.2 |
| NRM | ||||
| 1 y | 22.5 | 17.3-28.1 | 15.1 | 14.0-16.2 |
| 2 y | 28.1 | 22.4-34.2 | 19.0 | 17.8-20.2 |
| 3 y | 33.5 | 27.1-40.0 | 21.2 | 19.9-22.6 |
| 4 y | 35.2 | 28.5-42.0 | 22.4 | 21.0-23.9 |
| Relapse | ||||
| 1 y | 18.1 | 13.4-23.3 | 23.0 | 17.1-29.4 |
| 2 y | 21.8 | 16.7-27.4 | 32.7 | 25.7-39.8 |
| 3 y | 23.2 | 17.8-29.1 | 39.4 | 31.4-47.2 |
| 4 y | 24.1 | 18.5-30.2 | 41.7 | 32.8-50.3 |
| PFS | ||||
| 1 y | 59.4 | 52.8-65.5 | 67.5 | 60.2-73.8 |
| 2 y | 49.5 | 42.8-55.9 | 54.3 | 46.5-61.4 |
| 3 y | 42.8 | 35.9-49.6 | 45.9 | 37.7-53.8 |
| 4 y | 40.2 | 33.2-47.2 | 41.1 | 31.4-50.5 |
| OS | ||||
| 1 y | 71.5 | 65.2-76.8 | 82.2 | 75.9-87.0 |
| 2 y | 58.7 | 51.9-64.8 | 71.8 | 64.5-77.9 |
| 3 y | 50.2 | 43.1-56.8 | 66.3 | 58.2-73.2 |
| 4 y | 47.1 | 39.9-54.0 | 60.5 | 50.6-69.1 |
| . | Older MUD (donor ≥35 y) . | Younger haploidentical (donor <35 y) . | ||
|---|---|---|---|---|
| Estimate (%) . | 95% CI . | Estimate (%) . | 95% CI . | |
| aGVHD grade 2-4, d 100 | 21.5 | 15.0-28.7 | 16.3 | 10.9-22.7 |
| aGVHD grade 3-4, d 100 | 8.4 | 4.9-13.1 | 4.2 | 1.8-8.0 |
| cGVHD | ||||
| 1 y | 48.6 | 41.8-55.1 | 26.4 | 20.3-32.9 |
| 2 y | 54.4 | 47.5-60.8 | 31.5 | 24.8-38.4 |
| 3 y | 55.1 | 48.1-61.5 | 33.9 | 26.7-41.2 |
| 4 y | 56.0 | 49.0-62.5 | 33.9 | 26.7-41.2 |
| NRM | ||||
| 1 y | 22.5 | 17.3-28.1 | 15.1 | 14.0-16.2 |
| 2 y | 28.1 | 22.4-34.2 | 19.0 | 17.8-20.2 |
| 3 y | 33.5 | 27.1-40.0 | 21.2 | 19.9-22.6 |
| 4 y | 35.2 | 28.5-42.0 | 22.4 | 21.0-23.9 |
| Relapse | ||||
| 1 y | 18.1 | 13.4-23.3 | 23.0 | 17.1-29.4 |
| 2 y | 21.8 | 16.7-27.4 | 32.7 | 25.7-39.8 |
| 3 y | 23.2 | 17.8-29.1 | 39.4 | 31.4-47.2 |
| 4 y | 24.1 | 18.5-30.2 | 41.7 | 32.8-50.3 |
| PFS | ||||
| 1 y | 59.4 | 52.8-65.5 | 67.5 | 60.2-73.8 |
| 2 y | 49.5 | 42.8-55.9 | 54.3 | 46.5-61.4 |
| 3 y | 42.8 | 35.9-49.6 | 45.9 | 37.7-53.8 |
| 4 y | 40.2 | 33.2-47.2 | 41.1 | 31.4-50.5 |
| OS | ||||
| 1 y | 71.5 | 65.2-76.8 | 82.2 | 75.9-87.0 |
| 2 y | 58.7 | 51.9-64.8 | 71.8 | 64.5-77.9 |
| 3 y | 50.2 | 43.1-56.8 | 66.3 | 58.2-73.2 |
| 4 y | 47.1 | 39.9-54.0 | 60.5 | 50.6-69.1 |
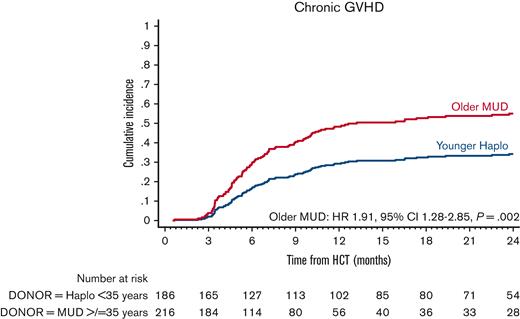
In multivariate analysis, the risk of grade 2 to 4 aGVHD (HR, 1.83; 95% CI, 0.90-3.71; P = .10) and grade 3 to 4 aGVHD (HR, 2.89; 95% CI, 0.86-9.77; P = .09) in older MUD was not statistically different from that in the younger haploidentical group. However, the risk of cGVHD was significantly higher in the older MUD (HR, 1.91; 95% CI, 1.28-2.85; P = .002) (Figure 1; Table 3). Patients aged ≥40 years were at a higher risk of cGVHD (HR, 1.56; 95% CI, 1.01-2.41; P = .04) than younger patients.
Adjusted cGVHD after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis. Haplo, haploidentical.
Adjusted cGVHD after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis. Haplo, haploidentical.
Multivariate analysis
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| aGVHD grade 2-4, d 100 | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.83 | 0.90-3.71 | .10 |
| Race/ethnicity | |||
| White, non-Hispanic | Reference | ||
| White, Hispanic | 0.48 | 0.19-1.23 | .13 |
| Black | 1.88 | 0.70-5.07 | .21 |
| Asian | 1.91 | 0.71-5.14 | .20 |
| aGVHD grade 3-4, d 100 | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 2.89 | 0.86-9.77 | .09 |
| Remission status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 1.49 | 0.46-4.78 | .51 |
| CR2 | NE | NE | NE |
| ≥CR3 | 7.71 | 1.24-47.78 | .03 |
| Race/ethnicity | |||
| White, non-Hispanic | Reference | ||
| White, Hispanic | 0.19 | 0.06-0.56 | .003 |
| Black | 0.36 | 0.03-4.05 | .41 |
| Asian | 0.46 | 0.07-3.02 | .42 |
| cGVHD, 2 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.91 | 1.28-2.85 | .002 |
| Patient age, y | |||
| <40 | Reference | ||
| ≥40 | 1.56 | 1.01-2.41 | .04 |
| NRM, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 2.75 | 1.51-4.99 | .001 |
| Relapse, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 0.50 | 0.31-0.82 | .006 |
| Disease status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 2.03 | 1.14-3.64 | .02 |
| CR2 | 2.73 | 1.38-5.38 | .004 |
| ≥CR3 | 3.87 | 1.40-10.68 | .009 |
| PFS, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.09 | 0.77-1.54 | .63 |
| Patient age, y | |||
| <40 | Reference | ||
| ≥40 | 1.48 | 1.02-2.17 | .04 |
| Disease status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 1.33 | 0.88-2.00 | .18 |
| CR2 | 2.349641 | 1.43-3.87 | .001 |
| ≥CR3 | 4.608024 | 2.04-10.43 | <.001 |
| OS, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.77 | 1.16-2.71 | .008 |
| Patient age, y | |||
| <40 | Reference | ||
| ≥40 | 1.65 | 1.04-2.61 | .03 |
| Disease status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 0.79 | 0.48-1.29 | .34 |
| CR2 | 1.91 | 1.11-3.31 | .02 |
| ≥CR3 | 3.55 | 1.37-9.20 | .009 |
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| aGVHD grade 2-4, d 100 | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.83 | 0.90-3.71 | .10 |
| Race/ethnicity | |||
| White, non-Hispanic | Reference | ||
| White, Hispanic | 0.48 | 0.19-1.23 | .13 |
| Black | 1.88 | 0.70-5.07 | .21 |
| Asian | 1.91 | 0.71-5.14 | .20 |
| aGVHD grade 3-4, d 100 | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 2.89 | 0.86-9.77 | .09 |
| Remission status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 1.49 | 0.46-4.78 | .51 |
| CR2 | NE | NE | NE |
| ≥CR3 | 7.71 | 1.24-47.78 | .03 |
| Race/ethnicity | |||
| White, non-Hispanic | Reference | ||
| White, Hispanic | 0.19 | 0.06-0.56 | .003 |
| Black | 0.36 | 0.03-4.05 | .41 |
| Asian | 0.46 | 0.07-3.02 | .42 |
| cGVHD, 2 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.91 | 1.28-2.85 | .002 |
| Patient age, y | |||
| <40 | Reference | ||
| ≥40 | 1.56 | 1.01-2.41 | .04 |
| NRM, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 2.75 | 1.51-4.99 | .001 |
| Relapse, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 0.50 | 0.31-0.82 | .006 |
| Disease status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 2.03 | 1.14-3.64 | .02 |
| CR2 | 2.73 | 1.38-5.38 | .004 |
| ≥CR3 | 3.87 | 1.40-10.68 | .009 |
| PFS, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.09 | 0.77-1.54 | .63 |
| Patient age, y | |||
| <40 | Reference | ||
| ≥40 | 1.48 | 1.02-2.17 | .04 |
| Disease status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 1.33 | 0.88-2.00 | .18 |
| CR2 | 2.349641 | 1.43-3.87 | .001 |
| ≥CR3 | 4.608024 | 2.04-10.43 | <.001 |
| OS, 4 y | |||
| Donor | |||
| Haploidentical <35 y | Reference | ||
| MUD ≥35 y | 1.77 | 1.16-2.71 | .008 |
| Patient age, y | |||
| <40 | Reference | ||
| ≥40 | 1.65 | 1.04-2.61 | .03 |
| Disease status | |||
| CR1-MRD–negative | Reference | ||
| CR1-MRD–positive | 0.79 | 0.48-1.29 | .34 |
| CR2 | 1.91 | 1.11-3.31 | .02 |
| ≥CR3 | 3.55 | 1.37-9.20 | .009 |
NE, nonevaluable.
NRM
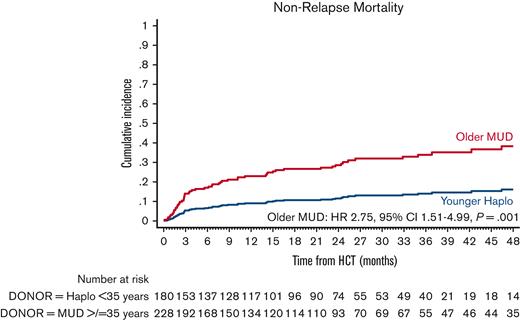
The cumulative incidence of NRM at 4 years was 35.2% (95% CI, 28.5-42.0) in the older MUD vs 22.4% (95% CI, 21.0-23.9) in the younger haploidentical group. The univariate analyses are shown in supplemental Table 6. In the multivariate analysis, older MUD was associated with a significantly higher risk of NRM (HR, 2.75; 95% CI, 1.51-4.99; P = .001) than the younger haploidentical group (Figure 2; Table 3). No other factor predicted the risk of NRM.
Adjusted NRM after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Adjusted NRM after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Relapse
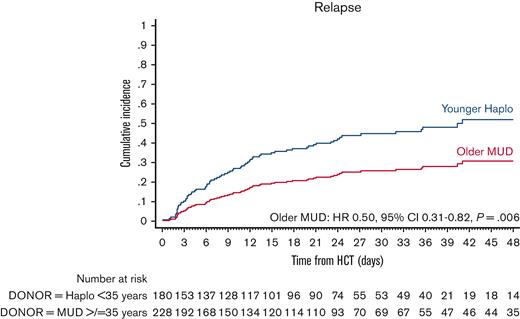
The cumulative incidence of relapse at 4 years was 24.1% (95% CI, 18.5-30.2) in the older MUD and 41.7% (95% CI, 32.8-50.3) in the younger haploidentical group (Table 2). The univariate analyses are shown in supplemental Table 6. In multivariate analysis, younger MUD had a significantly lower risk of relapse (HR, 0.50; 95% CI, 0.31-0.82; P = .006) (Figure 3; Table 3). The only other predictor of relapse was the disease status. Patients in the MRD-negative CR1 had the lowest risk of relapse compared with the other groups.
Adjusted relapse after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Adjusted relapse after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
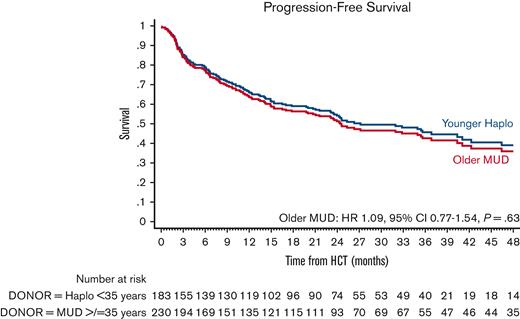
PFS
The estimated 4-year PFS was 40.2% (95% CI, 33.2-47.2) in the older MUD vs 41.1% (95% CI, 31.4-50.5) in the younger haploidentical group. The univariate analyses are shown in supplemental Table 7. In multivariate analysis, the older MUD group had similar PFS (HR, 1.09; 95% CI, 0.77-1.54; P = .63) as the younger haploidentical group (Figure 4; Table 3). Factors associated with poor PFS were patients ≥40 years old (HR, 1.48; 95% CI, 1.02-2.17; P = .04) and disease status. Patients in CR2 (HR, 2.35; 95% CI, 1.43-3.8; P = .001) and ≥CR3 (HR, 4.61; 95% CI, 2.04-10.43; P < .001) had significantly poorer PFS than those with MRD-negative CR1.
Adjusted PFS after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Adjusted PFS after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
OS
The estimate of 4-year OS was 47.1% (95% CI, 39.9-54.0) in the older MUD vs 60.5% (95% CI, 50.6-69.1) in the younger haploidentical group. The univariate analyses are shown in supplemental Table 7. In multivariate analysis, the older MUD group had a significantly inferior OS (HR, 1.77; 95% CI, 1.16-2.71; P = .008) than the younger haploidentical group (Figure 5; Table 3). Other predictors of OS were patient age ≥40 years (HR, 1.65; 95% CI, 1.04-2.61; P = .03) and disease status. Patients in CR2 (HR, 1.91; 95% CI, 1.11-3.31; P = .02) and ≥CR3 (HR, 3.55; 95% CI, 1.37-9.20; P = .009) had a significantly poorer OS than those in CR1.
Adjusted OS after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Adjusted OS after older MUD (red) vs younger haploidentical (blue) HCT. The adjusted factors are the variables listed in the multivariable regression analysis.
Discussion
In this study, we showed that HCT using a younger haploidentical donor (age <35 years) with PTCy prophylaxis was associated with significantly superior OS compared with older MUD (≥35 years) with conventional GVHD prophylaxis in adult patients with ALL in CR. Although the risk of relapse was reduced by half in the older MUD group, it was associated with an approximately twofold increased risk of cGVHD and a 2.75-fold higher risk of NRM than that in the younger haploidentical group, which resulted in a 77% higher risk of all-cause mortality in the older MUD group.
One may contest that these differences are solely related to GVHD prophylaxis rather than the effect of donor age. This can be counterargued for several reasons: (1) donor age (<35 years vs ≥35 years), but not donor type (haploidentical PTCy vs MUD conventional) was found to be an independent significant predictor of OS in multivariate analysis, and (2) we did not find differences in OS between the young haploidentical donor (PTCy) vs young MUD (conventional), and no differences were noted in OS between the older haploidentical donor (PTCy) vs and older MUD (conventional). The previous CIBMTR study showed similar OS between the MUD (conventional) and haploidentical (PTCy) HCT groups when the groups were not categorized by donor age.2 When the donor age was introduced to the equation in our study, an older MUD (conventional) was found to be associated with poorer OS than the younger haploidentical (PTCy), underscoring the significance of donor age. Despite indirect evidence, it is impossible to separate the effects of donor age from GVHD prophylaxis from our analysis. This is an important topic that should be studied further, and our study provides a rationale for such an analysis.
Next, it can be debated that the use of PTCy is increasing in HLA-matched donor settings. We assert that although the safety and efficacy of PTCy are being defined in prospective clinical trials,5-7 the use of PTCy vs conventional prophylaxis with MAC in the matched donor setting remains a matter of debate. Our study included 2 HCT approaches that are currently considered standard, irrespective of conditioning intensity: haploidentical with PTCy prophylaxis and MUD with conventional prophylaxis. Hence, our results can be generalized to real-world settings. Moreover, recent data on haploidentical HCT using PTCy also suggest that donor age impacts post-HCT outcomes,18 indicating that these effects may be more broadly generalizable. Whether the use of PTCy prophylaxis with MUD HCT would negate the unfavorable effects of an older MUD remains to be determined and should be assessed in future studies. Our study has another practical application that could have affected a sizable number of patients. Although the median recruitment age for the National Marrow Donor Program donors has decreased from 35 to 28 years in the past decade,19 about half of the MUDs are still older than 30 to 35 years19,24 and ∼20% to 25% are older than 40 years,19 whereas a child (more likely to be of younger age) is the most common haploidentical donor.10
In addition to the limitations mentioned above, our study lacks data on HLA-DPB1 matching in the MUD, individual HLA allele mismatches in the haploidentical group,10 and data about donor parity and natural killer cell killer immunoglobulin-like receptors. Furthermore, our data set did not have information on some prognostic factors, such as the total white blood cell count at diagnosis, information on molecular phenotype, and causes of death. We also acknowledge the variability in the groups in terms of conditioning intensity, graft type, and immunophenotype, all of which were adjusted for in the multivariate analyses within the statistical limitations. Lastly, information on some variables that may explain differences in the outcomes between the groups, such as chimerism, cytokine release syndrome,25,26 and graft cellular composition,27 which may explain higher NRM and worse survival for patients receiving grafts from older donors or higher relapse with haploidentical donors, was not available in this registry data set. On the other hand, our data set contained several key ALL-specific prognostic characteristics, such as information on MRD status, chemotherapy- vs TBI-based MAC, immunophenotype, cytogenetics, and the presence of the Philadelphia chromosome, among other predictive factors that are often overlooked in studies that combine different types of diseases. Moreover, our study benefits from a relatively large sample size restricted to adult patients with ALL in CR who did not receive ex vivo or in vivo T-cell depletion.
The adverse outcomes associated with older donors have been shown in several studies.9-19 Our study validates the message conveyed by others that donor age is the single most important factor that determines outcomes,11,19 but builds on that by showing that donor age matters regardless of donor type, and that donor age supersedes donor type in the selection hierarchy. Our study is unique and adds to the literature by including patients exclusively with ALL, a population that has not been studied, and by investigating the effects of donor age using 2 contrasting approaches: HLA-matched vs HLA-mismatched (haploidentical) donors, which have not been previously explored. The biological rationale for why HCTs from older donor are prone to more complications than those from a younger donor is unclear but can be hypothesized to be related to several factors. First, older donors are more likely to have age-related clonal hematopoiesis, which is associated with dysregulation of cytokine signaling leading to an inflammatory cytokine milieu in HCT recipients, and an increased risk of cGVHD.28 Also, certain changes occur in the DNA methylation patterns throughout the lifespan which lead to an alteration of immune function;29 donor age is one of the most important determinants of this epigenetic aging in hematopoietic cells after HCT.30,31 Increased epigenetic age has been correlated with all-cause mortality in the general population29,32 and with a higher risk of cGVHD, deaths from infection,30,33 and inferior survival in HCT recipients.33 Furthermore, there is a significant inverse relationship between epigenetic age and the abundance of naive CD4+ T cells,29 which is largely driven by age-related thymic involution.
Conclusion
Among adult patients with ALL in CR, our data suggest that despite a higher risk of relapse, a younger haploidentical donor (age <35 years) with PTCy prophylaxis may be preferred over an older MUD (≥35 years) with conventional GVHD prophylaxis in patients for whom a younger MUD is not available. This recommendation is based on a significantly lower risk of cGVHD, lower NRM, and improved OS with a younger haploidentical donor. Whether the use of PTCy prophylaxis in MUD HCT alters these conclusions is uncertain and warrants further investigation.
Acknowledgment
The authors thank the Center for International Blood and Marrow Transplant Research staff for helping with the data set and for their feedback.
Authorship
Contribution: R.S.M. conceptualized the study design, performed the statistical analysis, interpreted the data, and wrote the manuscript; D.M. helped with statistical analysis and interpretation of data; A.A., C.G.K., R.E.C., K.R., E.J.S., K.P., S.M.G., D.W., and P.K. reviewed and interpreted the data, reviewed the manuscript, and provided critical feedback; R.S.M. and D.M. had full access to the raw data; and all authors approved the manuscript. The corresponding author had the final responsibility to submit for publication.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rohtesh S. Mehta, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: rmehta@fredhutch.org.
References
Author notes
Data are available on request from the corresponding author, Rohtesh S. Mehta (rmehta@fredhutch.org).
The full-text version of this article contains a data supplement.