Key Points
Despite higher risk of early CMV reactivation using PTCy, 1-year viral burden and CMV disease are comparable with MTX-based prophylaxis.
MMF is associated with higher risk of early and late CMV reactivation and higher 1-year viral burden than MTX-based prophylaxis.
Abstract
The kinetics of early and late cytomegalovirus (CMV) reactivation after hematopoietic cell transplantation using various methods of graft-versus-host-disease (GVHD) prophylaxis are poorly defined. We retrospectively compared CMV reactivation and disease among 780 seropositive patients given HLA-matched peripheral blood stem cell (PBSC) grafts and calcineurin inhibitor plus posttransplantation cyclophosphamide (PTCy; n = 44), mycophenolate mofetil (MMF; n = 414), or methotrexate (MTX; n = 322). Transplantation occurred between 2007 and 2018; CMV monitoring/management followed uniform standard practice. Hazards of CMV reactivation at various thresholds were compared. Spline curves were fit over average daily viral load and areas under the curve (AUC) within 1 year were calculated. PTCy and MMF were associated with an increased risk of early (day ≤100) CMV reactivation ≥250 IU/mL after multivariate adjustment. The viral load AUC at 1 year was highest with MMF (mean difference = 0.125 units vs MTX group) and similar between PTCy and MTX (mean difference = 0.016 units vs MTX group). CMV disease risk was similar across groups. There was no interaction between GVHD prophylaxis and CMV reactivation on chronic GVHD risk. Despite PTCy-associated increased risk of early CMV reactivation, the CMV disease risk by 1 year was low in HLA-matched PBSC transplant recipients. In contrast, MMF was associated with higher overall CMV viral burden in the 1 year posttransplant. Although different mechanisms of immunosuppressive agents may affect CMV reactivation risk, effective prevention of GVHD may reduce corticosteroid exposure and mitigate infection risk over time.
Introduction
Cytomegalovirus (CMV) reactivation at any level after allogeneic stem cell transplantation is strongly associated with inferior overall and nonrelapse mortality. The differential impact of various pharmacologic graft-versus-host disease (GVHD) prophylaxis regimens on CMV infection is poorly defined. In recent years, the use of posttransplantation cyclophosphamide (PTCy) has been shown to significantly reduce both severe acute and chronic GVHD risk after HLA-haploidentical (haplo)1 and HLA-matched related and unrelated donor stem cell transplantation.2 Single-center retrospective studies have reported a higher incidence of CMV infection after haplo transplantation using PTCy than historically experienced with HLA-matched transplantation using other methods of GVHD prophylaxis.3,4 These studies have prompted adoption of more aggressive CMV prevention strategies for haplo transplantation, such as the use of letermovir prophylaxis.5 However, it has yet to be determined whether the increased CMV reactivation risk after haplo transplantation is attributable to donor/recipient HLA-disparity or intrinsic to the effect of PTCy. A recent large registry analysis also showed an increased risk of CMV infection after PTCy compared with calcineurin inhibitor–based prophylaxis in HLA-matched sibling transplants;6 however, detailed analysis of virologic kinetics was not included. Furthermore, very little has been published on the risk of CMV reactivation and disease with mycophenolate mofetil (MMF) relative to PTCy and methotrexate (MTX).7
We sought to compare CMV viral kinetics between patients given PTCy vs other GVHD prophylaxis regimens in a uniform cohort of CMV-seropositive patients receiving HLA-matched related and unrelated donor peripheral blood stem cell (PBSC) grafts. We specifically aimed to analyze the impact of different periods of CMV risk and whether CMV affected chronic GVHD risk, especially in PTCy recipients.6
Methods
Patients
We retrospectively analyzed patients who were CMV-seropositive and were undergoing their first HLA-matched unrelated or HLA-identical sibling donor PBSC transplantation for hematologic malignancies in which GVHD prophylaxis included a calcineurin inhibitor backbone between July 2007 and September 2018 at the Fred Hutchinson Cancer Center. Letermovir prophylaxis was implemented at our center in October 2018; thus, patients receiving letermovir were excluded from this cohort. Patients receiving antithymocyte globulin, alemtuzumab, ex vivo T-cell–depleted grafts, or investigational GVHD prophylaxis were excluded to avoid potential confounders for infection. Patients receiving investigational agents as part of the conditioning regimen were also excluded. Conditioning intensity (myeloablative, reduced-intensity, and nonmyeloablative) was defined according to previously published criteria.8 The Fred Hutchinson Cancer Center Institutional Review Board approved the use of patient data in this study. The study was conducted in accordance with the Declaration of Helsinki.
CMV monitoring and preemptive therapy
Our institutional standard practice recommends that CMV DNA polymerase chain reaction (PCR) is collected weekly, beginning on day 0 until day 100 posttransplant. After day 100, weekly CMV PCR is obtained from patients treated for CMV before day 100 or who are on steroids or other immunosuppressive agents for treatment of acute or chronic GVHD. Surveillance beyond 100 days is changed to every other week if the patient is on <0.5 mg/kg per day of prednisone or prednisone equivalent and on stable or tapering doses of other immunosuppressive agents after ensuring 3 consecutive negative surveillance tests. Thereafter, surveillance is stopped entirely after 2 negative tests if tapering of immunosuppression continues. Weekly CMV surveillance testing is resumed if treatment with immunosuppression is increased or reinitiated for GVHD.
The CMV viral load threshold for preemptive therapy was ≥150 IU/mL within 100 days posttransplant and ≥500 IU/mL after day 100 posttransplant in this cohort; in those receiving ≥1 mg/kg of prednisone or its equivalent, the threshold was ≥50 IU/mL. Further details regarding guidelines for preemptive therapy have been previously published.9
Statistical methods
Fine and Gray versions of the Cox proportional hazard model were used to estimate unadjusted and adjusted hazard ratios (HRs) of CMV reactivation at >0, ≥250, or ≥1000 IU/mL and CMV disease within 100 days and 1 year posttransplant between groups. Death was treated as a competing risk in the model. Linear regression was used to evaluate associations between risk factors and normalized mean area under the curve (AUC) of log10 of CMV viral load. A spline curve fitted over the average CMV viral load in log10 scale stratified by 3 groups of GVHD prophylaxis was presented for data visualization. The probabilities of CMV reactivation and disease were estimated by cumulative incidence and compared using the Gray model, treating death as a competing risk. All statistical analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC).
AUC was calculated using the trapezoidal method, then normalized by dividing the AUC by the number of days alive up to 1 year posttransplant for patients whose last available CMV test was negative. For patients in whom the last available CMV test was positive, the denominator was the number of days from transplant to last positive CMV PCR. This allowed us to reduce the risk of underestimating the AUC in those whose last PCR was positive because of a lack of available test results and falsely assigning a low AUC to patients who died “early.” The CMV viral load was log transformed (after adding 1 to the viral load, allowing the logarithm of observations with a viral load of 0).
Results
Patient and transplant characteristics
Seven hundred eighty patients met study inclusion criteria. Demographic and transplant characteristics by type of GVHD prophylaxis (MTX, n = 322; MMF, n = 414; and PTCy, n = 44) are summarized in Table 1. No patients in the PTCy group received MMF. The conditioning intensity was predominantly myeloablative in the MTX and PTCy groups, whereas nonmyeloablative conditioning was most common in the MMF group. The median age was older in the MMF group than in the MTX and PTCy groups because of preferential use of MMF following lower intensity conditioning. Median donor age was also higher in the MMF group, likely because of sibling donor age approximating patient age. Donor CMV seropositivity rates were similar across the 3 groups. Acute myeloid leukemia and myelodysplastic syndrome/myeloproliferative neoplasm were the most common indications for transplantation in the MTX and MMF groups; acute myeloid leukemia and acute lymphoblastic leukemia were the most common indications in the PTCy group. The PTCy group had a higher proportion of unrelated donors.
Patient demographics
| Variables . | Categories . | MTX (N = 322) . | MMF (N = 414) . | PTCy (N = 44) . | Total (N = 780) . |
|---|---|---|---|---|---|
| Recipient age, y | Median (range) | 51.4 (3.3-69.4) | 61.5 (18.2-80.9) | 45.6 (3.0-70.0) | 56.1 (3.0-80.9) |
| Donor age, y | Median (range) | 35.9 (15.8-77.4) | 41.1 (18.1-76.6) | 29.3 (16.4-63.3) | 38.1 (15.8-77.4) |
| Gender, n (%) | Female | 140 (43) | 185 (45) | 16 (36) | 341 (44) |
| Male | 182 (57) | 229 (55) | 28 (64) | 439 (56) | |
| Disease, n (%) | ALL | 43 (13) | 22 (5) | 12 (27) | 77 (10) |
| AML | 125 (39) | 135 (33) | 20 (45) | 280 (36) | |
| CLL/PLL | 4 (1) | 41 (10) | 0 (0) | 45 (6) | |
| MDS/MPN | 130 (40) | 97 (23) | 9 (20) | 236 (30) | |
| MM/PCL | 6 (2) | 53 (13) | 1 (2) | 60 (8) | |
| NHL/HL | 14 (4) | 66 (16) | 2 (5) | 82 (11) | |
| Conditioning intensity, n (%) | Myeloablative | 303 (94) | 62 (15) | 36 (82) | 401 (51) |
| Nonmyeloablative | 0 (0) | 321 (78) | 4 (9) | 325 (42) | |
| RIC | 19 (6) | 31 (7) | 4 (9) | 54 (7) | |
| Donor CMV serostatus, n (%) | + | 148 (46) | 202 (49) | 22 (50) | 372 (48) |
| − | 174 (54) | 212 (51) | 22 (50) | 408 (52) | |
| Donor relationship, n (%) | Unrelated | 189 (59) | 236 (57) | 35 (80) | 460 (59) |
| Sibling | 133 (41) | 178 (43) | 9 (20) | 320 (41) | |
| Sirolimus, n (%) | No | 322 (100) | 351 (85) | 36 (82) | 709 (91) |
| Yes | 0 (0) | 63 (15) | 8 (18) | 71 (9) | |
| GVHD, n (%) | Acute grade 2-4 | 233 (72) | 254 (61) | 30 (68) | 517 (66) |
| Acute grade 3-4 | 36 (11) | 41 (10) | 1 (2) | 78 (10) | |
| Chronic∗ | 150 (47) | 221 (53) | 14 (32) | 385 (49) |
| Variables . | Categories . | MTX (N = 322) . | MMF (N = 414) . | PTCy (N = 44) . | Total (N = 780) . |
|---|---|---|---|---|---|
| Recipient age, y | Median (range) | 51.4 (3.3-69.4) | 61.5 (18.2-80.9) | 45.6 (3.0-70.0) | 56.1 (3.0-80.9) |
| Donor age, y | Median (range) | 35.9 (15.8-77.4) | 41.1 (18.1-76.6) | 29.3 (16.4-63.3) | 38.1 (15.8-77.4) |
| Gender, n (%) | Female | 140 (43) | 185 (45) | 16 (36) | 341 (44) |
| Male | 182 (57) | 229 (55) | 28 (64) | 439 (56) | |
| Disease, n (%) | ALL | 43 (13) | 22 (5) | 12 (27) | 77 (10) |
| AML | 125 (39) | 135 (33) | 20 (45) | 280 (36) | |
| CLL/PLL | 4 (1) | 41 (10) | 0 (0) | 45 (6) | |
| MDS/MPN | 130 (40) | 97 (23) | 9 (20) | 236 (30) | |
| MM/PCL | 6 (2) | 53 (13) | 1 (2) | 60 (8) | |
| NHL/HL | 14 (4) | 66 (16) | 2 (5) | 82 (11) | |
| Conditioning intensity, n (%) | Myeloablative | 303 (94) | 62 (15) | 36 (82) | 401 (51) |
| Nonmyeloablative | 0 (0) | 321 (78) | 4 (9) | 325 (42) | |
| RIC | 19 (6) | 31 (7) | 4 (9) | 54 (7) | |
| Donor CMV serostatus, n (%) | + | 148 (46) | 202 (49) | 22 (50) | 372 (48) |
| − | 174 (54) | 212 (51) | 22 (50) | 408 (52) | |
| Donor relationship, n (%) | Unrelated | 189 (59) | 236 (57) | 35 (80) | 460 (59) |
| Sibling | 133 (41) | 178 (43) | 9 (20) | 320 (41) | |
| Sirolimus, n (%) | No | 322 (100) | 351 (85) | 36 (82) | 709 (91) |
| Yes | 0 (0) | 63 (15) | 8 (18) | 71 (9) | |
| GVHD, n (%) | Acute grade 2-4 | 233 (72) | 254 (61) | 30 (68) | 517 (66) |
| Acute grade 3-4 | 36 (11) | 41 (10) | 1 (2) | 78 (10) | |
| Chronic∗ | 150 (47) | 221 (53) | 14 (32) | 385 (49) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma; PCL, plasma cell leukemia; PLL, prolymphocytic leukemia; RIC, reduced-intensity conditioning.
National Institutes of Health consensus–defined chronic GVHD10 requiring systemic immunosuppressive treatment or late acute GVHD treated with systemic immunosuppression by 1 year posttransplant.
CMV testing
Most patients in each group had ≥1 CMV PCR test each week posttransplant through day 100 (supplemental Figure 1). Around day 100, most patients were discharged from our transplant center to their local physicians. Thus, after day 100, CMV PCR data are available only for patients who continued to receive care at our institution. Despite this limitation, the proportion of patients with ≥1 CMV PCR each week posttransplant from days 100 to 365 was similar across the 3 groups, alleviating concerns of differential sampling across the groups (supplemental Figure 1).
Early CMV reactivation after transplant
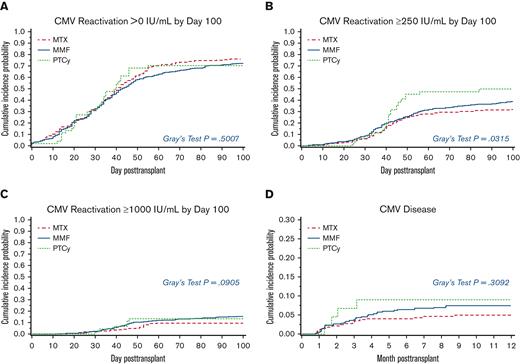
We first looked at the impact of GVHD prophylaxis, donor type, donor CMV serostatus, and conditioning regimen intensity on time to first CMV reactivation >0, ≥250, and ≥1000 IU/mL before day 100 posttransplant by univariate analysis (supplemental Table 1). No factors were associated with significantly increased risk of CMV reactivation >0 IU/mL. At higher thresholds of reactivation, PTCy and unrelated donor (for ≥250 IU/mL) and MMF, unrelated donor, and myeloablative conditioning (for ≥1000 IU/mL) were significantly associated with risk of CMV reactivation (supplemental Table 1). The cumulative incidence of viral load ≥250 IU/mL at day 100 in the PTCy group was higher than the MTX group; PTCy: 50% (95% confidence interval [CI], 35-65), MTX: 32% (95% CI, 27-37), and MMF: 39% (95% CI, 34-43; P = .032) (Figure 1).
Cumulative incidence of CMV reactivation by day 100 posttransplant and CMV disease by 1 year. The probabilities of CMV reactivation and disease were compared using the Gray model, treating death as a competing risk. (A) CMV reactivation >0 IU/mL; P = .5007 (B) CMV reactivation ≥250 IU/mL; P = .0315 (C) CMV reactivation ≥1000 IU/mL; P = .0905 (D) CMV disease; P = .3092.
Cumulative incidence of CMV reactivation by day 100 posttransplant and CMV disease by 1 year. The probabilities of CMV reactivation and disease were compared using the Gray model, treating death as a competing risk. (A) CMV reactivation >0 IU/mL; P = .5007 (B) CMV reactivation ≥250 IU/mL; P = .0315 (C) CMV reactivation ≥1000 IU/mL; P = .0905 (D) CMV disease; P = .3092.
By multivariate analysis, PTCy was associated with significantly higher risk of CMV reactivation to ≥250 IU/mL after adjusting for recipient and donor age at transplant, conditioning regimen, donor CMV serostatus, and donor relationship (PTCy vs MTX: HR, 1.64; 95% CI, 1.03-2.61; P = .039) (Table 2). The HR for MMF vs MTX after the same adjustment was 1.50 (95% CI, 0.97-2.32; P = .067). The risk of CMV reactivation at ≥250 IU/mL with an unrelated donor was 44% higher than that with a sibling donor in the multivariate model. There were also numerical increases in the risk of CMV reactivation at the higher threshold of ≥1000 IU/mL associated with the use of PTCy and MMF and with transplantation from an unrelated donor (Table 2). Overall, these results suggest a higher risk of early CMV reactivation for PTCy than for MTX, even after adjustment for certain donor and transplant variables.
Multivariate Cox regression analysis of risk factors for time to reactivation at more than or equal to 250 and 1000 IU/mL before day 100 posttransplant
| ≥250 IU/mL . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | HR (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 1.50 (0.97-2.32) | .067 | |
| PTCy | 1.64 (1.03-2.61) | .039 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 1.44 (0.99-2.08) | .054 | |
| Donor CMV serostatus | − | 1 | |
| + | 0.94 (0.74-1.21) | .643 | |
| Conditioning regimen | Nonmyeloablative/RIC | 1 | |
| Myeloablative | 1.23 (0.80-1.89) | .349 | |
| Recipient age at transplant | As continuous | 1.00 (0.99-1.01) | .576 |
| Donor age at transplant | As continuous | 0.99 (0.98-1.00) | .063 |
| ≥250 IU/mL . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | HR (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 1.50 (0.97-2.32) | .067 | |
| PTCy | 1.64 (1.03-2.61) | .039 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 1.44 (0.99-2.08) | .054 | |
| Donor CMV serostatus | − | 1 | |
| + | 0.94 (0.74-1.21) | .643 | |
| Conditioning regimen | Nonmyeloablative/RIC | 1 | |
| Myeloablative | 1.23 (0.80-1.89) | .349 | |
| Recipient age at transplant | As continuous | 1.00 (0.99-1.01) | .576 |
| Donor age at transplant | As continuous | 0.99 (0.98-1.00) | .063 |
| ≥1000 IU/mL . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | HR (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 1.36 (0.68-2.72) | .391 | |
| PTCy | 1.22 (0.49-3.03) | .663 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 1.61 (0.84-3.09) | .15 | |
| Donor CMV serostatus | − | 1 | |
| + | 0.83 (0.55-1.26) | .379 | |
| Conditioning regimen | Nonmyeloablative/RIC | 1 | |
| Myeloablative | 0.76 (0.39-1.48) | .423 | |
| Recipient age at transplant | As continuous | 1.00 (0.98-1.02) | .974 |
| Donor age at transplant | As continuous | 0.99 (0.97-1.01) | .392 |
| ≥1000 IU/mL . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | HR (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 1.36 (0.68-2.72) | .391 | |
| PTCy | 1.22 (0.49-3.03) | .663 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 1.61 (0.84-3.09) | .15 | |
| Donor CMV serostatus | − | 1 | |
| + | 0.83 (0.55-1.26) | .379 | |
| Conditioning regimen | Nonmyeloablative/RIC | 1 | |
| Myeloablative | 0.76 (0.39-1.48) | .423 | |
| Recipient age at transplant | As continuous | 1.00 (0.98-1.02) | .974 |
| Donor age at transplant | As continuous | 0.99 (0.97-1.01) | .392 |
Late CMV reactivation (from day 100 to 1 year after transplant)
To estimate the risk of late CMV reactivation posttransplant based on the GVHD prophylaxis regimen, we conducted a landmark analysis of time to reactivation from day 100 to 1 year posttransplant among patients surviving at day 100 (supplemental Table 2). This analysis was limited by the number of available test results after day 100 (discussed above in CMV testing). Within this limitation, the HRs for reactivation at every level were numerically lower in the PTCy group than in the MTX group. Notably, the number of events in the PTCy group was very low (supplemental Table 2). In contrast, the HRs for reactivation between day 100 and 1 year for MMF vs MTX were consistently above 1 at each level (supplemental Table 2). The impact of other factors (donor CMV serostatus, conditioning intensity, and donor relationship) on CMV reactivation are summarized in supplemental Table 2. The results qualitatively agree with those for reactivation up to day 100.
CMV viral kinetics
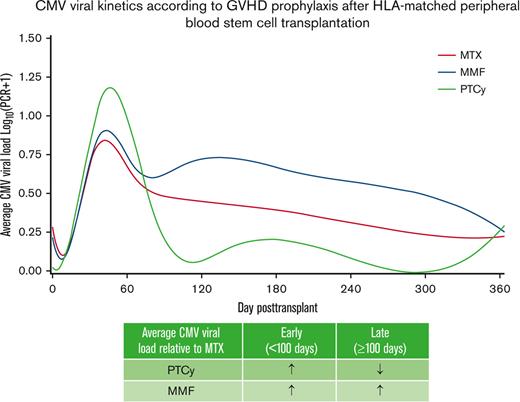
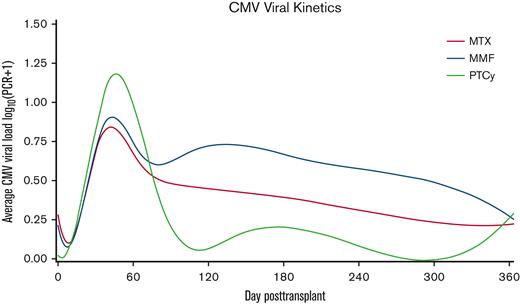
To visualize the overall burden of CMV viral reactivation, we next assessed the pattern of CMV kinetics across the 3 cohorts. We created spline curves fitted over the average daily CMV viral load across all patients in each group during the first year after transplantation (Figure 2). These curves help visualize the time to initial viral reactivation, resolution of viremia, and any recurrences. Of note, the average viral load also included those with no viral reactivation or viral load of 0 IU/mL. As shown in Figure 2, early posttransplant CMV viral load was highest in the PTCy group, followed by MMF and MTX. However, at later time points the average CMV viral load was lowest in the PTCy group. Similar patterns of CMV viral kinetics were observed when considering patients with CMV-seropositive or -seronegative donors (data not shown).
CMV viral kinetics according to GVHD prophylaxis after HLA-matched PBSC transplantation. A spline curve fitted over the average CMV viral load in log10 scale stratified by GVHD prophylaxis shows early and late CMV viral kinetics within the first year posttransplant.
CMV viral kinetics according to GVHD prophylaxis after HLA-matched PBSC transplantation. A spline curve fitted over the average CMV viral load in log10 scale stratified by GVHD prophylaxis shows early and late CMV viral kinetics within the first year posttransplant.
To quantify the data visualized by the spline curves, we compared the AUC of CMV viral load within the first year posttransplant across cohorts. The unadjusted normalized AUC in the MMF was 0.0125 units higher than that in the MTX group in the first year posttransplant (95% CI, 0.061-0.189; P < .001); the unadjusted normalized AUC in the PTCy group was 0.016 units higher than that in the MTX group (95% CI, −0.126 to 0.158; P = .827) (Table 3). Further summarized in Table 3 is the unadjusted relationship of other factors with mean normalized AUC. By multivariate analysis, all factors after adjustment were moved closer to the null than the unadjusted results (Table 3).
Univariate and multivariate linear regression analysis of factors affecting AUC of CMV viral load in the first year posttransplant
| Univariate . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | AUC mean difference∗ (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 0.125 (0.061-0.189) | <.001 | |
| PTCy | 0.016 (−0.126 to 0.158) | .827 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 0.148 (0.085-0.211) | <.001 | |
| Donor CMV serostatus | − | 1 | |
| + | −0.069 (−0.132 to −0.007) | .03 | |
| Conditioning regimen | Nonmyeloablative | 1 | |
| Myeloablative | −0.123 (−0.188 to −0.059) | <.001 | |
| RIC | −0.018 (−0.145 to 0.109) | .782 | |
| Recipient age at transplant | As continuous | 0.003 (0.001-0.006) | .003 |
| Donor age at transplant | As continuous | −0.003 (−0.005 to −0.001) | .001 |
| Univariate . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | AUC mean difference∗ (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 0.125 (0.061-0.189) | <.001 | |
| PTCy | 0.016 (−0.126 to 0.158) | .827 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 0.148 (0.085-0.211) | <.001 | |
| Donor CMV serostatus | − | 1 | |
| + | −0.069 (−0.132 to −0.007) | .03 | |
| Conditioning regimen | Nonmyeloablative | 1 | |
| Myeloablative | −0.123 (−0.188 to −0.059) | <.001 | |
| RIC | −0.018 (−0.145 to 0.109) | .782 | |
| Recipient age at transplant | As continuous | 0.003 (0.001-0.006) | .003 |
| Donor age at transplant | As continuous | −0.003 (−0.005 to −0.001) | .001 |
| Multivariate . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | AUC mean difference∗ (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 0.087 (−0.019 to 0.193) | .106 | |
| PTCy | −0.005 (−0.146 to 0.137) | .947 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 0.103 (0.014-0.192) | .023 | |
| Donor CMV serostatus | − | 1 | |
| + | −0.027 (−0.091 to 0.037) | .406 | |
| Conditioning regimen | Nonmyeloablative | 1 | |
| Myeloablative | −0.026 (−0.135 to 0.082) | .636 | |
| RIC | −0.006 (−0.138 to 0.127) | .933 | |
| Donor age at transplant | As continuous | −0.002 (−0.005 to 0.001) | .236 |
| Recipient age at transplant | As continuous | 0.002 (−0.001 to 0.005) | .136 |
| Multivariate . | . | . | . |
|---|---|---|---|
| Covariate . | Category . | AUC mean difference∗ (95% CI) . | P . |
| GVHD prevention method | MTX | 1 | |
| MMF | 0.087 (−0.019 to 0.193) | .106 | |
| PTCy | −0.005 (−0.146 to 0.137) | .947 | |
| Donor relationship | Sibling | 1 | |
| Unrelated | 0.103 (0.014-0.192) | .023 | |
| Donor CMV serostatus | − | 1 | |
| + | −0.027 (−0.091 to 0.037) | .406 | |
| Conditioning regimen | Nonmyeloablative | 1 | |
| Myeloablative | −0.026 (−0.135 to 0.082) | .636 | |
| RIC | −0.006 (−0.138 to 0.127) | .933 | |
| Donor age at transplant | As continuous | −0.002 (−0.005 to 0.001) | .236 |
| Recipient age at transplant | As continuous | 0.002 (−0.001 to 0.005) | .136 |
Positive value for mean difference indicates absolute higher viral load AUC compared with the reference (MTX) group, whereas a negative value indicates absolute lower viral load AUC.
Impact of sirolimus on CMV reactivation among patients receiving MMF and PTCy
The inhibition of mTOR by sirolimus may have suppressive effects on viral amplification as CMV upregulates the mTOR pathway during replication.11 In addition, mTOR inhibition improves memory T-cell and antibody responses to several viral pathogens.12-14 After allogeneic hematopoietic cell transplantation (HCT), sirolimus exposure has been inversely associated with the incidence of CMV viremia necessitating preemptive therapy.15 We, therefore, looked at the impact of sirolimus use in the GVHD prophylaxis regimen on CMV reactivation. As expected, with our institutional practice and research protocols enrolling patients during the study period, the concurrent use of sirolimus for GVHD prophylaxis was only seen in the MMF and PTCy groups.
On univariate analysis, sirolimus was associated with significant reduction in the risk of first CMV reactivation at any level by day 100 posttransplant within the MMF group (Table 4). The risk reduction was most pronounced for higher levels of viral reactivation (HR, 0.25; 95% CI, 0.08-0.8; P = .019 for reactivation ≥1000 IU/mL). The mean normalized AUC in the first year posttransplant in the patients who received MMF and sirolimus was 0.113 units lower than those who did not receive sirolimus (95% CI, −0.241 to 0.015; P = .085). For multivariate analysis, the variables of conditioning intensity and sirolimus use were combined because of collinearity. Within the MMF cohort, myeloablative and nonmyeloablative conditioning without sirolimus were associated with significantly higher risk of CMV reactivation at >0, ≥250, and ≥1000 IU/mL than nonmyeloablative conditioning with sirolimus, after adjusting for other factors (supplemental Table 3A-C). A similar pattern was seen for multivariate analysis looking at 1-year viral AUC among patients who received MMF (supplemental Table 3D). In contrast, no significant effect of sirolimus was seen among PTCy recipients (Table 4). Multivariate analysis could not be performed in the PTCy cohort because of the limited sample size.
Univariate analysis of impact of sirolimus on CMV reactivation among MMF and PTCy recipients before day 100
| MMF . | . | . | . | . | . |
|---|---|---|---|---|---|
| CMV reactivation before d 100 . | Number of patients . | Number of patients with reactivation . | Sirolimus . | HR (95% CI) . | P . |
| >0 IU/mL | 351 | 261 | No | 1 | |
| 63 | 39 | Yes | 0.65 (0.47-0.89) | .007 | |
| ≥250 IU/mL | 351 | 146 | No | 1 | |
| 63 | 15 | Yes | 0.51 (0.30-0.87) | .013 | |
| ≥1000 IU/mL | 351 | 61 | No | 1 | |
| 63 | 3 | Yes | 0.25 (0.08-0.80) | .019 |
| MMF . | . | . | . | . | . |
|---|---|---|---|---|---|
| CMV reactivation before d 100 . | Number of patients . | Number of patients with reactivation . | Sirolimus . | HR (95% CI) . | P . |
| >0 IU/mL | 351 | 261 | No | 1 | |
| 63 | 39 | Yes | 0.65 (0.47-0.89) | .007 | |
| ≥250 IU/mL | 351 | 146 | No | 1 | |
| 63 | 15 | Yes | 0.51 (0.30-0.87) | .013 | |
| ≥1000 IU/mL | 351 | 61 | No | 1 | |
| 63 | 3 | Yes | 0.25 (0.08-0.80) | .019 |
| PTCy . | . | . | . | . | . |
|---|---|---|---|---|---|
| CMV reactivation before d 100 . | Number of patients . | Number of patients with reactivation . | Sirolimus . | HR (95% CI) . | P . |
| >0 IU/mL | 36 | 25 | No | 1 | |
| 8 | 6 | Yes | 1.22 (0.51-2.91) | .651 | |
| ≥250 IU/mL | 36 | 17 | No | 1 | |
| 8 | 5 | Yes | 1.31 (0.53-3.22) | .556 |
| PTCy . | . | . | . | . | . |
|---|---|---|---|---|---|
| CMV reactivation before d 100 . | Number of patients . | Number of patients with reactivation . | Sirolimus . | HR (95% CI) . | P . |
| >0 IU/mL | 36 | 25 | No | 1 | |
| 8 | 6 | Yes | 1.22 (0.51-2.91) | .651 | |
| ≥250 IU/mL | 36 | 17 | No | 1 | |
| 8 | 5 | Yes | 1.31 (0.53-3.22) | .556 |
Incidence of CMV disease
We next compared the risk of CMV disease across the 3 cohorts. Overall, 33 (4%) patients developed CMV disease in the first 100 days posttransplant, and 51 patients (7%) developed CMV disease within the first year posttransplant. Compared with MTX, the risk of CMV disease by day 100 and 1 year posttransplant was not significantly different for PTCy or MMF (Table 5). Given the differences seen in early vs late viral load, we also looked for any differences in risk of CMV disease after day 100 among survivors at day 100, and the results were qualitatively similar (supplemental Table 4). In univariate analysis, donor CMV seropositivity vs seronegativity yielded an HR of 0.4 (95% CI, 0.19-0.87; P = .02) for CMV disease by day 100 (Table 5); this result remained significant in an adjusted model that included CMV donor serostatus and PTCy vs non-PTCy GVHD prophylaxis (Table 5).
Univariate and bivariate analysis of factors associated with CMV disease
| Univariate . | . | . | . | . | . |
|---|---|---|---|---|---|
| Covariate . | Number of patients . | Number of events . | Category . | HR (95% CI) . | P . |
| CMV disease by d 100 | |||||
| GVHD prophylaxis | 322 | 11 | MTX | 1 | |
| 414 | 18 | MMF | 1.28 (0.60-2.70) | .525 | |
| 44 | 4 | PTCy | 2.70 (0.87-8.38) | .086 | |
| Donor relationship | 320 | 10 | Sibling | 1 | |
| 460 | 23 | Unrelated | 1.62 (0.77-3.40) | .2 | |
| Donor CMV serostatus | 408 | 24 | − | 1 | |
| 372 | 9 | + | 0.40 (0.19-0.87) | .02 | |
| Conditioning regimen | 379 | 16 | Nonmyeloablative/RIC | 1 | |
| 401 | 17 | Myeloablative | 1.01 (0.51-1.99) | .988 | |
| CMV disease by 1 y | |||||
| GVHD prophylaxis | 322 | 16 | MTX | 1 | |
| 414 | 31 | MMF | 1.52 (0.83-2.78) | .175 | |
| 44 | 4 | PTCy | 1.88 (0.63-5.66) | .261 | |
| Donor relationship | 320 | 15 | Sibling | 1 | |
| 460 | 36 | Unrelated | 1.70 (0.93-3.10) | .083 | |
| Donor CMV serostatus | 408 | 33 | − | 1 | |
| 372 | 18 | + | 0.58 (0.33-1.03) | .065 | |
| Conditioning regimen | 379 | 26 | Nonmyeloablative/RIC | 1 | |
| 401 | 25 | Myeloablative | 0.91 (0.53-1.57) | .733 | |
| Univariate . | . | . | . | . | . |
|---|---|---|---|---|---|
| Covariate . | Number of patients . | Number of events . | Category . | HR (95% CI) . | P . |
| CMV disease by d 100 | |||||
| GVHD prophylaxis | 322 | 11 | MTX | 1 | |
| 414 | 18 | MMF | 1.28 (0.60-2.70) | .525 | |
| 44 | 4 | PTCy | 2.70 (0.87-8.38) | .086 | |
| Donor relationship | 320 | 10 | Sibling | 1 | |
| 460 | 23 | Unrelated | 1.62 (0.77-3.40) | .2 | |
| Donor CMV serostatus | 408 | 24 | − | 1 | |
| 372 | 9 | + | 0.40 (0.19-0.87) | .02 | |
| Conditioning regimen | 379 | 16 | Nonmyeloablative/RIC | 1 | |
| 401 | 17 | Myeloablative | 1.01 (0.51-1.99) | .988 | |
| CMV disease by 1 y | |||||
| GVHD prophylaxis | 322 | 16 | MTX | 1 | |
| 414 | 31 | MMF | 1.52 (0.83-2.78) | .175 | |
| 44 | 4 | PTCy | 1.88 (0.63-5.66) | .261 | |
| Donor relationship | 320 | 15 | Sibling | 1 | |
| 460 | 36 | Unrelated | 1.70 (0.93-3.10) | .083 | |
| Donor CMV serostatus | 408 | 33 | − | 1 | |
| 372 | 18 | + | 0.58 (0.33-1.03) | .065 | |
| Conditioning regimen | 379 | 26 | Nonmyeloablative/RIC | 1 | |
| 401 | 25 | Myeloablative | 0.91 (0.53-1.57) | .733 | |
| Bivariate . | . | . | . |
|---|---|---|---|
| Covariate . | Categories . | HR (95% CI) . | P . |
| CMV disease by d 100 (total events = 33) | |||
| Donor CMV serostatus | − | 1 | |
| + | 0.40 (0.19-0.86) | .019 | |
| GVHD prophylaxis | MMF/MTX | 1 | |
| PTCy | 2.39 (0.86-6.67) | .096 | |
| CMV disease by 1 y (total events = 51) | |||
| Donor CMV serostatus | − | 1 | |
| + | 0.58 (0.33-1.03) | .064 | |
| GVHD prophylaxis | MMF/MTX | 1 | |
| PTCy | 1.48 (0.53-4.13) | .452 | |
| Bivariate . | . | . | . |
|---|---|---|---|
| Covariate . | Categories . | HR (95% CI) . | P . |
| CMV disease by d 100 (total events = 33) | |||
| Donor CMV serostatus | − | 1 | |
| + | 0.40 (0.19-0.86) | .019 | |
| GVHD prophylaxis | MMF/MTX | 1 | |
| PTCy | 2.39 (0.86-6.67) | .096 | |
| CMV disease by 1 y (total events = 51) | |||
| Donor CMV serostatus | − | 1 | |
| + | 0.58 (0.33-1.03) | .064 | |
| GVHD prophylaxis | MMF/MTX | 1 | |
| PTCy | 1.48 (0.53-4.13) | .452 | |
CMV infection and chronic GVHD
Finally, we investigated the possible association between GVHD prophylaxis regimen, CMV reactivation, and chronic GVHD given the recent intriguing finding that CMV infection may abrogate the protective effects of PTCy against chronic GVHD.6 For our analysis of the chronic GVHD outcome, we included patients who developed National Institutes of Health consensus–defined chronic GVHD10 requiring systemic immunosuppressive treatment, and those who developed late acute GVHD treated with systemic immunosuppression, given that most of these patients will develop chronic GVHD. As expected, patients in the PTCy group had a lower probability of chronic GVHD/late acute GVHD at 1 year (32%) compared with patients in the MTX (47%) or MMF (53%; overall Gray model P = .0225) groups. Next, CMV reactivation at any level, ≥250 and ≥1000 IU/mL was evaluated as a time-dependent covariate for the outcome of chronic GVHD as previously defined. By univariate and multivariate Cox regression analyses, there was no significant impact of CMV reactivation on chronic GVHD by 1 year posttransplant (HR, 1.12; 95% CI, 0.90-1.40; P = .31 and P = .298 for univariate and multivariate analyses, respectively). Importantly, testing the interaction between CMV infection and GVHD prophylaxis for the outcome of chronic GVHD suggested no significant interaction between these 2 variables (P = .95); thus, a subset analysis by GVHD prophylaxis was not performed.
Discussion
Our study shows that GVHD prophylaxis with PTCy is independently associated with higher risk of early CMV reactivation among patients receiving HLA-matched related and unrelated PBSC transplantation. However, late CMV reactivation and overall viral burden in the first year posttransplant were similar in patients receiving PTCy and those receiving MTX. MMF was associated with higher risk of early CMV reactivation at higher thresholds (≥1000 IU/mL), late CMV reactivation, and 1-year viral AUC in univariate analyses. The protective effect of sirolimus on CMV reactivation appeared to be reduced in PTCy recipients. The incidence of early or late CMV disease was not significantly different between GVHD prophylaxis regimens.
Recently, an analysis of Center for International Blood and Marrow Transplant Research (CIBMTR) data demonstrated a two-fold increase in cumulative incidence of CMV viremia and non-CMV herpes virus infections by day 180 posttransplant in recipients of haplo and HLA-matched sibling bone marrow or PBSCs receiving PTCy when compared with HLA-matched sibling transplantation using calcineurin inhibitor–based GVHD prophylaxis.6,16 Although our results are consistent with this analysis, our study extends these findings for a cohort including recipients of HLA-matched unrelated donor transplants, which was not included in the CIBMTR analysis. Moreover, given the detailed quantitative CMV viral load and disease data available, our study described not only the time to initial viremia but the response to preemptive therapy and the incidence of recurrent infections, capturing the overall burden of CMV infection and disease in the first year posttransplant.
Higher CMV viral levels in the early posttransplant period following PTCy suggest an initial period of T-cell compromise associated with reduced viral immunity. Although PTCy spares the regulatory T-cell compartment and enhances B-cell reconstitution, the broad early impairment of donor conventional T cells by PTCy may affect early immunity against CMV.17-20 Alternatively, early cytokine dysregulation or early effects on innate and humoral immunity may predispose patients to high level viremia in the setting of PTCy. In contrast, the overall lower burden of CMV viral reactivation in the entire first year after transplant may reflect the ability of PTCy to indirectly promote adaptive antiviral immunity by decreasing the rates of severe acute and chronic GVHD and associated immunosuppressive treatment. The relatively early institution of preemptive antiviral therapy9,21 practiced at our site may be another reason for the effective control of CMV replication.
Our study provides novel insight into the effects of MMF on CMV reactivation in patients receiving HCT. MMF has previously been associated with higher risk of CMV infections after solid organ transplantation,22 but detailed quantitative analysis of CMV reactivation and disease after HCT has not been previously reported. Because MMF inhibits both T- and B-lymphocyte proliferation, intensified immunosuppression from impairment of both cellular and humoral immunity against CMV is a potential mechanism for this effect.7 In addition, compared with MTX, MMF has been associated with a significantly increased risk of severe acute GVHD in myeloablative HCT.23 This may translate into increased use of immunosuppressive treatments, which may negatively affect viral immunity. The fact that the risk of CMV reactivation persists after MMF has been withdrawn is consistent with this concept.
A particular novel finding in our study was that, although the well-established association between sirolimus and reduction in CMV reactivation24,25 was seen in our MMF cohort, a similar protective effect was not observed with PTCy. This intriguing finding will need to be verified in a larger cohort. One prior study26 comparing sirolimus vs cyclosporine in haplo transplantation using PTCy and MMF showed no difference in CMV reactivation or disease, implying also that sirolimus did not offer protection against CMV reactivation when combined with PTCy. One hypothesis to explain this finding is that the immune cells necessary to carry out the antiviral effects of sirolimus are either functionally or quantitatively impaired by exposure to PTCy. Laboratory investigations are needed to study the mechanism of this interaction.
The availability of longitudinal quantitative CMV virologic assessments and CMV disease data in a cohort of patients uniformly treated under a single institution’s standard practice of monitoring and preemptive antiviral treatment is a major strength of our study. Furthermore, because the study period preceded implementation of letermovir prophylaxis, similar data will not be available without the confounding factor of routine use of prophylaxis. One limitation of this study is the relatively low number of patients in the PTCy group. We did specifically investigate the possible association between CMV reactivation, GVHD prophylaxis, and chronic GVHD given the recent intriguing finding reported in the CIBMTR analysis6 suggesting that CMV infection may abrogate the protective effect of PTCy against chronic GVHD, but we found no significant interaction between GVHD prophylaxis and CMV infection on chronic GVHD.
Overall, we conclude that early posttransplant CMV antiviral immunity may be compromised by PTCy, but that later, PTCy-mediated GVHD protection spares the use of immunosuppressive treatment and may thereby provide an advantage in restoring viral immunity, resulting in the effective prevention of progression to higher viral load and CMV disease. Comparison of viral kinetics of other double-stranded DNA viruses, such as human herpesvirus 6, BK virus, Epstein-Barr virus, and adenovirus, between methods of GVHD prophylaxis, correlative studies of functional CMV-specific immunity, and studies examining the effect of sirolimus among PTCy recipients will be of significant interest in future prospective studies.
Acknowledgments
The authors thank Chris Davis for assistance with data retrieval and Rachel Blazevic, Brenda Akoto, Larry Mose, Marta Levkova, Thi Banh, Jordan Sheldon, and Erika Lovas for assistance with chart review. The authors thank the Fred Hutchinson Cancer Center Infectious Disease Quality Assurance Program for funding to perform chart review.
This work was supported by funding from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, the Career Development Award (K23AI163343) (D.Z.)
Authorship
Contribution: M.U.O. designed the study, interpreted the data, and wrote the manuscript; H.X. and T.A.G. conducted the statistical analysis; M.B.M. interpreted the data; and all authors reviewed the data and provided critical review of the manuscript.
Conflict-of-interest disclosure: M.B.M. received research funding from Merck and served as a consultant for Allovir, Helocyte, Evrys Bio, and Symbio. G.R.H. has consulted for Generon Corporation, NapaJen Pharma, iTeos Therapeutics, and Neoleukin Therapeutics and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, Serplus Technology, Heat Biologics, Laevoroc Oncology, Genentech, and iTeos Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Masumi Ueda Oshima, 1100 Fairview Ave N, D1-100, Seattle, WA 98109; e-mail: mueda@fredhutch.org.
References
Author notes
Data are available on request from the corresponding author, Masumi Ueda Oshima (mueda@fredhutch.org).
The full-text version of this article contains a data supplement.