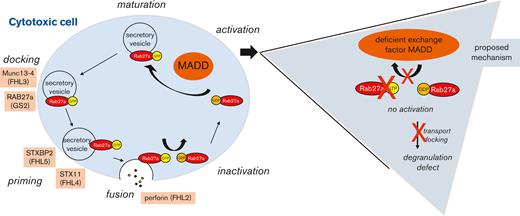
Key Points
MADD-deficient cytotoxic cells display defect of degranulation, most likely due to impaired activation of RAB27A.
Patients with MADD deficiency may have a predisposition to develop HLH.
Abstract
Most hereditary forms of hemophagocytic lymphohistiocytosis (HLH) are caused by defects of cytotoxicity, including the vesicle trafficking disorder Griscelli syndrome type 2 (GS2, RAB27A deficiency). Deficiency of the mitogen-activated protein kinase activating death domain protein (MADD) results in a protean syndrome with neurological and endocrinological involvement. MADD acts as a guanine nucleotide exchange factor for small guanosine triphosphatases, including RAB27A. A homozygous splice site mutation in MADD was identified in a female infant with syndromic features, secretory diarrhea, and features of HLH. Aberrant splicing caused by this mutation leads to an in-frame deletion of 30 base pairs and favors other aberrant variants. Patient natural killer (NK) cells and cytotoxic T cells showed a severe degranulation defect leading to absent perforin-mediated cytotoxicity. Platelets displayed defective adenosine triphosphate secretion, similar to that in GS2. To prove causality, we introduced a CRISPR/Cas9-based MADD knockout in the NK cell line NK-92mi. MADD-deficient NK-92mi cells showed a degranulation defect and impaired cytotoxicity similar to that of the patient. The defect of cytotoxicity was confirmed in another patient with MADD deficiency. In conclusion, RAB27A-interacting MADD is involved in vesicle release by cytotoxic cells and platelets. MADD deficiency causes a degranulation defect and represents a novel disease predisposing to an HLH phenotype.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome characterized by uncontrolled immune activation. In most hereditary forms of HLH, impaired killing of antigen-presenting cells during immune responses results in uncontrolled T-cell and macrophage activation and a consecutive cytokine storm. The key genetic defects either affect perforin (FHL2) or proteins involved in exocytosis of perforin-containing vesicles, that is, degranulation defects, such as familial HLH type 3 to 5, Griscelli syndrome type 2 (GS2, RAB27Aa deficiency), and Chédiak-Higashi syndrome.1,2
Mitogen-activated protein kinase activating death domain protein (MADD) is located on chromosome 11 and consists of 36 exons. MADD pre-messenger RNA undergoes alternative splicing that results in at least 7 different isoforms with different protein functions.3,4 The N-terminal region of the MADD protein contains a differentially expressed normal vs neoplastic domain that is known to interact with members of the Rab family of small guanosine triphosphatases.5 The interaction of Rab3 and MADD in synaptic vesicle transport plays an important role in neurotransmission.6,7 In addition, MADD (Rab3GEP) functions as a guanosine diphosphate/guanosine triphosphate exchange factor for RAB27A, which has been studied in melanocytes.8,9 RAB27A is important for regulated exocytosis in cytotoxic cells, platelets, and melanocytes.10-12 However, the role of MADD activity, its interaction with RAB27A in lymphocytes, and the effects on exocytosis of lytic granules remain unclear.
When MADD deficiency was diagnosed in a patient with features of HLH, we hypothesized a vesicle trafficking defect, which was eventually confirmed in the cytotoxic cells and platelets in this patient and in a second patient. We reproduced these findings by MADD knockout in a natural killer (NK) cell line, proving the causal relationship between MADD deficiency and the predisposition to develop HLH.
Methods
All assays with fresh human peripheral blood mononuclear cell were performed with previously published protocols.13 The cytotoxicity of patient T cells against L1210 target cells was determined using a standard 4-hour chromium-51 release assay. Effector cells (T-cell blasts stimulated with phytohemagglutinin [PHA] and interleukin 2 [IL-2] and gamma-irradiated feeder cells for 12 days) were loaded with anti-CD3 (clone UCHT3, BD) for 1 hour before incubation with different target cell ratios.
Knockout of MADD in NK92 cells was performed by lentiviral transduction of the NK cell line NK-92 with a vector encoding a Cas9 and a guide RNA (GCGCAGCAATCGATAGATCC) targeting exon 4 of MADD.14 Knockout was confirmed by polymerase chain reaction amplification and sequencing of exon 4 of resulting clones.15 The resulting cells were subcloned into bulks of 10 individual clones, and the knockout efficiency of Cas9 was confirmed by polymerase chain reaction amplification and sequencing of exon 4. Degranulation assay of cell lines: NK-92mi cells were incubated with K562 cells (ratio 1:1) or PMA/Ionomycin or medium for 4 h at 37°C. Cells were washed and stained with anti-CD56 and anti-CD107a and measured by flow cytometry. Means were compared using a multiple t test (GraphPad Prism 9). Cytotoxicity assay of cell lines: eFlour647 prestained K562 target cells were incubated with NK-92mi cells at different E:T ratios for 4 hours at 37°C. Cells were washed and stained with propidium iodide before flow cytometry measurement. Dead target cells were defined as the proportion of allophycocyanin positive/propidium iodide positive (APC+/PI+). Patient platelet secretion assay: adenosine triphosphate secretion by platelets in citrate anticoagulated blood was stimulated by thrombin (1 U/mL) and collagen (2 μg/mL) and visualized by a Luciferin-Luciferase assay at a Chronolog Lumiaggregometer.16
The project received ethical approval by the Hamburg Chamber of Physicians, and parents gave their consent for the research performed.
Results and discussion
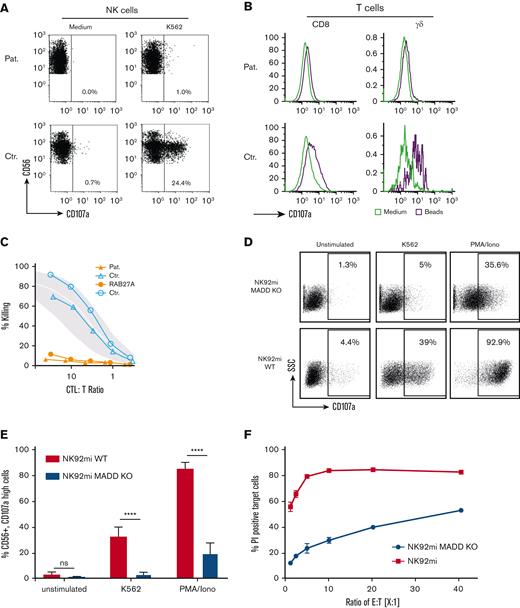
A 2-month-old female infant born to consanguineous parents was admitted with complex syndromic features of dystrophy, endocrinological dysfunction, and developmental delay. A brother with similar symptoms had died at 2 years of life. When she displayed partial features of HLH (Table 1) with a postnatally acquired cytomegalovirus infection (CMV, 19 000 copies/mL blood) and a persistent secretory enteropathy, similar to patients with familial HLH type 5,17 broad immunological workup was performed (supplemental Tables 1 and 2). Serum levels of immunoglobulins and B-cell counts were normal. T-cell differentiation studies showed a normal proportion of naive CD4 T cells but with an expansion of cytotoxic effectors likely related to CMV infection. T-cell proliferation and upregulation of CD25/69 activation markers were regular. A severe degranulation defect of resting and IL-2–stimulated NK and cytotoxic T lymphocytes (CTLs) was detected (Figure 1A-B). However, on targeted sequencing, no mutations were found in the pertinent genes associated with cytotoxicity defects.
Overview of patient HLH criteria
| . | Patient . | Norm . | HLH criteria . |
|---|---|---|---|
| Fever | Yes | <38.5°C | Yes |
| Splenomegaly | Yes | — | Yes |
| Hemoglobin, g/L | 80∗ | 92-150 | <90 |
| Platelets × 109/L | 140∗ | 150-500 | <150 |
| Neutrophil count × 109/L | 1.5 | 1.3-7.9 | <1 |
| Ferritin, μg/L | 1465 | 7-140 | >500 |
| Triglycerides, mg/dL | 205∗ | 70-180 | >265 |
| Soluble Il2 receptor, U/mL | 7119 | <623 | >2400 |
| Fibrinogen, g/L | 1.9 | 1.5-3.5 | <1.5 |
| D dimers, mg/L | 1.8 | <0.5 | — |
| Bone marrow aspirate | Not done | — | Hemophagocytosis |
| HLA-DR expression of T cells†, % | 25 | 2-8 | — |
| . | Patient . | Norm . | HLH criteria . |
|---|---|---|---|
| Fever | Yes | <38.5°C | Yes |
| Splenomegaly | Yes | — | Yes |
| Hemoglobin, g/L | 80∗ | 92-150 | <90 |
| Platelets × 109/L | 140∗ | 150-500 | <150 |
| Neutrophil count × 109/L | 1.5 | 1.3-7.9 | <1 |
| Ferritin, μg/L | 1465 | 7-140 | >500 |
| Triglycerides, mg/dL | 205∗ | 70-180 | >265 |
| Soluble Il2 receptor, U/mL | 7119 | <623 | >2400 |
| Fibrinogen, g/L | 1.9 | 1.5-3.5 | <1.5 |
| D dimers, mg/L | 1.8 | <0.5 | — |
| Bone marrow aspirate | Not done | — | Hemophagocytosis |
| HLA-DR expression of T cells†, % | 25 | 2-8 | — |
The patient displayed moderate immune dysregulation in the context of a cytomegalovirus infection, meeting 4 (bold print) out of 8 HLH criteria.
HLA-DR, Human Leukocyte Antigen–DR isotype.
Parameters were outside the norm not formally fulfilling the cutoff of the respective criterion.
T cells were substantially activated (elevated HLA-DR expression and soluble IL2 receptor), exceeding appropriate antiviral response.
Reduced degranulation and cytotoxicity of MADD-deficient patient cells and knockout cells. (A-C) Patient cells compared with healthy control cells. (A-B) Degranulation of fresh NK cells after stimulation with K562 cells (A) and of d3 PHA/IL-2–activated CD8 T cells, and γδ T cells stimulated with anti-CD3/CD28 beads (B) as detected by upregulation of CD107a. (C) Defective cytotoxicity of patient d12 PHA/IL-2 blasts from the patient, a patient with RAB27A deficiency and 2 healthy donors on L1210 targets in a 51Cr redirected lysis assay. The gray area indicates mean ± SD of a cohort of 25 healthy donors. Assays with patient cells were repeated twice with similar result. (D-F) NK-92mi MADD knockout cells vs NK-92mi wild-type cells. (D) Representative plot of reduced degranulation after stimulation with K562 or PMA/ionomycin. (E) Significantly lower proportion of degranulated NK-92mi MADD knockout cells after stimulation than that of wild-type cells (3 test series with triplicates each, P < .0001). (F) Defective cytotoxicity in a flow cytometric assay, reported as the proportion of propidium iodide (PI)–positive cells.
Reduced degranulation and cytotoxicity of MADD-deficient patient cells and knockout cells. (A-C) Patient cells compared with healthy control cells. (A-B) Degranulation of fresh NK cells after stimulation with K562 cells (A) and of d3 PHA/IL-2–activated CD8 T cells, and γδ T cells stimulated with anti-CD3/CD28 beads (B) as detected by upregulation of CD107a. (C) Defective cytotoxicity of patient d12 PHA/IL-2 blasts from the patient, a patient with RAB27A deficiency and 2 healthy donors on L1210 targets in a 51Cr redirected lysis assay. The gray area indicates mean ± SD of a cohort of 25 healthy donors. Assays with patient cells were repeated twice with similar result. (D-F) NK-92mi MADD knockout cells vs NK-92mi wild-type cells. (D) Representative plot of reduced degranulation after stimulation with K562 or PMA/ionomycin. (E) Significantly lower proportion of degranulated NK-92mi MADD knockout cells after stimulation than that of wild-type cells (3 test series with triplicates each, P < .0001). (F) Defective cytotoxicity in a flow cytometric assay, reported as the proportion of propidium iodide (PI)–positive cells.
Eventually, whole-exome sequencing revealed a homozygous point mutation (c.963+1G>A) in the donor splice site of intron 4 of MADD. Details on the nonimmunological phenotype and the effects of the mutation on splicing can be found in the recent first clinical description of MADD deficiency (Schneeberger et al,18 patient 4). In brief, this mutation leads to an in-frame deletion of 30 base pairs in exon 4 in the vast majority of transcripts and other splice variants with loss of function reducing the amount of MADD protein.
To further characterize the degree of the cytotoxic defect, we performed a cytotoxicity assay with T-cell blasts from the patient, in comparison with a patient with a RAB27A null mutation. The degree of impairment in the T cells of the patient with MADD deficiency was similar to that in the T cells without RAB27A, supporting the hypothesis that the defective activating interaction between MADD and RAB27A is pathophysiologically related to the functional deficit (Figure 1C). To verify that malfunction of MADD causes the defect, we generated a MADD knockout in the NK cell line NK-92mi using CRISPR/Cas9 technology. We directed the nuclease to exon 4 of the MADD locus because this exon is present in all splice variants and is affected in the patient. NK-92mi knockout cells also showed a marked loss of degranulation ability and cytotoxicity (Figure 1D-F). Other immunologic cellular functions were not affected (supplemental Figure 1).
Patients with GS2 and other trafficking disorders (eg, FHL3, FHL5) display a platelet secretion defect19-21. In the patient with MADD deficiency, adenosine triphosphate secretion by platelets was defective after stimulation with thrombin (4 nmol/109 platelets; norm 13-40) and collagen (0.4 nmol/109 platelets; norm >7). However, as in most patients with GS2, FHL3, and FHL5, no bleeding disorder was clinically apparent. A recent publication identified MADD as the GEF for RAB27A and Rab3 in primary human endothelial cells, concluding that MADD acts as a master regulator of von Willebrand factor secretion.22
Although studies have shown that MADD dysfunction leads to abnormal melanosome distribution via impaired RAB27A activity in melanocytes,23 no hair pigmentation defect was detectable macroscopically or microscopically, in contrast to most patients with GS2.24 Until 4 years of age, the girl experienced several episodes of elevated temperature, without the full picture of HLH. She remains severely disabled by the syndromic features of the condition.
Another severely affected patient (patient 2 in Schneeberger et al18) with a compound heterozygous defect in MADD (c.914G>T, p.Gly305Val and intragenic deletion of exons 11-23) displayed reduced degranulation of cytotoxic cells, more pronounced in CTLs than in NK cells, a reduction of killing capacity comparable to the first patient (supplemental Figure 2), and a prolonged closure time in the platelet function analyzer.
In summary, MADD plays an important immunological role in the exocytosis of cytotoxic granules by lymphocytes. MADD deficiency results in a vesicle trafficking disorder affecting various cell types and may predispose to features of HLH.
Acknowledgments
The authors thank Maja Hempel and Kerstin Kutsche for genetic analyses.
The project was funded by Playmobil-Stiftung and Deutsche Kinderkrebsstiftung DKS 2018.11. The experimental workup was supported by Advanced Diagnostics Unit, Institute for Immunodeficiency and FREEZE-Biobank, Center for Biobanking, Medical Center, and Faculty of Medicine, University of Freiburg, Freiburg, Germany.
Authorship
Contribution: K.S., M.G., and S.S. designed and performed experiments; H.L., K.L., K.C., G.J., K.W., and S.L. took care of the patients and collected patient data; K.L., K.C., S.E., and I.M. supervised the experimental workup; K.S. and K.L. wrote the manuscript; and all authors revised and approved of the manuscript.
Conflict-of-interest disclosure: K.S. was supported by Fördergemeinschaft Kinderkrebs-Zentrum Hamburg eV. M.G. and S.E. were supported by Deutsche Forschungsgemeinschaft SFB1160 (TPA01). The remaining authors declare no competing financial interests.
Correspondence: Kai Lehmberg, Division of Pediatric Stem Cell Transplantation and Immunology, Department of Pediatric Hematology and Oncology, University Medical Center Eppendorf, Hamburg, Germany; e-mail: k.lehmberg@uke.de.
References
Author notes
∗K.S. and M.G. contributed equally to this work.
Original data are available on request from the corresponding author, Kai Lehmberg (klehmberg@uke.de).
The human genetic aspects and clinical phenotype of patients 1 and 2 were reviewed in the article by Schneeberger et al.18
The full-text version of this article contains a data supplement.