Key Points
OxLDL enhances GPVI-driven platelet procoagulant activity ex vivo.
P2YR antagonists and tyrosine kinase inhibitors may reduce oxLDL-enhanced platelet procoagulant activity more so than aspirin.
Abstract
Low-density lipoprotein (LDL) contributes to atherogenesis and cardiovascular disease through interactions with peripheral blood cells, especially platelets. However, mechanisms by which LDL affects platelet activation and atherothrombosis, and how to best therapeutically target and safely prevent such responses remain unclear. Here, we investigate how oxidized low-density lipoprotein (oxLDL) enhances glycoprotein VI (GPVI)-mediated platelet hemostatic and procoagulant responses, and how traditional and emerging antiplatelet therapies affect oxLDL-enhanced platelet procoagulant activity ex vivo. Human platelets were treated with oxLDL and the GPVI-specific agonist, crosslinked collagen-related peptide, and assayed for hemostatic and procoagulant responses in the presence of inhibitors of purinergic receptors (P2YR), cyclooxygenase (COX), and tyrosine kinases. Ex vivo, oxLDL enhanced GPVI-mediated platelet dense granule secretion, α-granule secretion, integrin activation, thromboxane generation and aggregation, as well as procoagulant phosphatidylserine exposure and fibrin generation. Studies of washed human platelets, as well as platelets from mouse and nonhuman primate models of hyperlipidemia, further determined that P2YR antagonists (eg, ticagrelor) and Bruton tyrosine kinase inhibitors (eg, ibrutinib) reduced oxLDL-mediated platelet responses and procoagulant activity, whereas COX inhibitors (eg, aspirin) had no significant effect. Together, our results demonstrate that oxLDL enhances GPVI-mediated platelet procoagulant activity in a manner that may be more effectively reduced by P2YR antagonists and tyrosine kinase inhibitors compared with COX inhibitors.
Introduction
Dyslipidemia and lipoprotein accumulation drive atherogenesis and cardiovascular disease, a leading preventable cause of mortality worldwide.1-3 Lipoprotein levels are established risk factors for atherosclerosis and clinically significant atherothrombotic events such as myocardial infarction and stroke. In these contexts, atherosclerotic plaques and features of a diseased vessel wall initiate and promote thrombosis through interactions with platelets.4 Oxidized low-density lipoprotein (oxLDL) at sites of inflammation and plaque rupture contributes to macrophage infiltration. OxLDL is also recognized to lower platelet activation threshold ex vivo;5-7 however, the mechanisms by which oxLDL interacts with platelets and potentiates platelet activation, and how to best therapeutically target such interactions to safely prevent atherothrombosis in the context of cardiovascular disease remain to be elucidated.
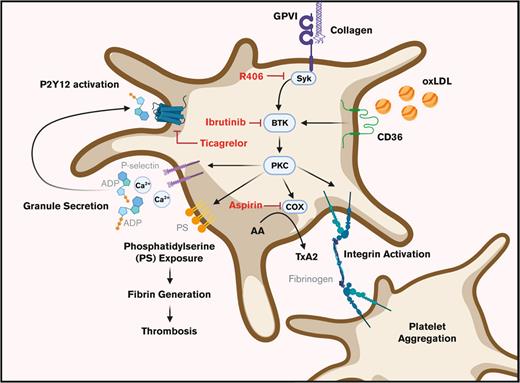
Circulating lipids and lipoproteins accumulate and are readily oxidized at sites of vascular inflammation, where oxidized phospholipids may bind to and activate platelets via CD36, a glycoprotein and scavenger receptor highly expressed on the platelet surface.8,9 After interactions with oxLDL, CD36 is posited to lower platelet activation thresholds by other agonists.6,10-12 Similarly, collagen-activated glycoprotein VI (GPVI) receptor associates with Fc receptor γ-chain and becomes activated by Src family kinases (SFKs) on intracellular immunoreceptor tyrosine-based activation motifs (ITAMs) to phosphorylate downstream substrates, such as Bruton tyrosine kinase (BTK), that drive thrombo-inflammatory and procoagulant platelet responses.13,14 Once activated, platelets externalize phosphatidylserine (PS) on their extracellular surface, supporting thrombin generation, fibrin formation, and coagulation.15,16 However, biochemical and functional connections between CD36 and GPVI and their combined roles in platelet procoagulant activity remain largely unknown and mechanistically unspecified.
Current antiplatelet therapies are effective at reducing specific platelet responses. For instance, dual antiplatelet therapy (DAPT), a combination of cyclooxygenase (COX) inhibitor aspirin and a purinergic receptor (P2Y12) antagonist, typically ticagrelor, clopidogrel, or prasugrel, is typically used after coronary intervention procedures.17 However, these antiplatelet agents also carry risks of bleeding and are further ineffective at preventing reoccurring thrombotic events.17 Furthermore, the efficacy of aspirin vs P2Y12 inhibitors in personalized aspects of cardiovascular disease is not well established and more novel antiplatelet agents targeting platelet signaling to limit platelet activation have yet to be optimized.18-20
We previously noted increased oxLDL-related proinflammatory and prothrombotic activities in nonhuman primate models of diet-induced obesity,21,22 where immunoreceptor ITAM signaling responses downstream of GPVI, CD36, and Fc-γ receptor pathways involving tyrosine kinases such as BTK offer new therapeutic targets.22-26 Here, we investigate how oxLDL affects GPVI/ITAM-driven platelet procoagulant activities, and how traditional and more novel antiplatelet therapies affect these platelet responses ex vivo. Our findings provide insight into how oxLDL enhances platelet GPVI/ITAM responses and informs how clinically relevant antiplatelet agents (eg, aspirin, ticagrelor), as well as therapeutic tyrosine kinase inhibitors (TKIs) (eg, ibrutinib) may effectively limit platelet activity in dyslipidemia. Altogether, we elucidate mechanisms by which oxLDL potentiates platelet activation downstream of GPVI and assess the potential of pharmacologic agents to reduce platelet activation, and the procoagulant and thrombotic characteristics induced by oxLDL in the setting of hyperlipidemia, atherosclerosis, and thrombosis.
Materials and methods
Citrate-anticoagulated blood and washed platelets were prepared from healthy human donors in accordance with an Oregon Health & Science University institutional review board–approved protocol for aggregometry, flow cytometry, and other ex vivo experiments as previously described.27,28 LDL reagents for this study were purchased from Kalen Biomedical (Montgomery Village, MD) and were analyzed for protein and lipid content by mass spectrometry (supplemental Figures 1 and 2). Data from all experiments were analyzed using GraphPad Prism 9 software (San Diego, CA) and represented as the mean ± standard error of the mean. Refer to the supplemental Materials for additional “Materials and methods.”
Results
oxLDL levels associate with enhanced platelet GPVI reactivity
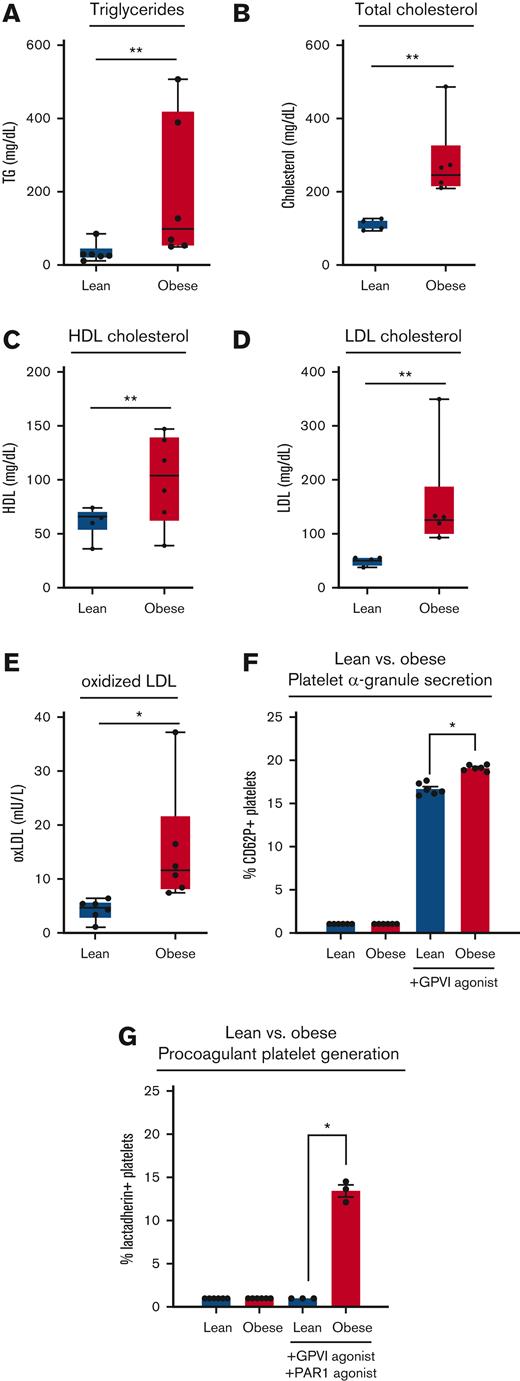
First, we examined the relationship between GPVI/ITAM-driven platelet activity and oxLDL levels in blood samples collected from a cohort of rhesus macaque nonhuman primates fed a high-fat diet for >2 years. Relative to lean controls, animals with diet-induced obesity had significantly higher levels of serum triglycerides (Figure 1A), total cholesterol (Figure 1B), high-density lipoprotein cholesterol (Figure 1C), and LDL cholesterol (Figure 1D) as well as oxLDL immunoreactivity (Figure 1E). Flow cytometry analysis of platelets in whole blood from obese animals found enhanced α-granule secretion in response to ex vivo stimulation with the GPVI-specific agonist, CRP-XL (Figure 1F). Relative to lean animals, platelets from obese animals also had enhanced procoagulant PS exposure after GPVI and PAR1 activation (Figure 1G). Together, these results support a model where increased lipoprotein oxidation in vivo contributes to proinflammatory and procoagulant states in vascular disease.9
Elevated oxLDL and enhanced GPVI-mediated procoagulant platelet generation in obese nonhuman primates. Blood was collected from a cohort of lean (n = 6) and diet-induced obese (n = 6) rhesus macaques to prepare serum for analysis of triglycerides (A), total cholesterol (B), high-density lipoprotein cholesterol (C), LDL cholesterol (D), and oxLDL immunoreactivity (E). Citrate-anticoagulated whole blood from lean and obese animals was stimulated with GPVI agonist (0.1 μg/mL CRP-XL) or a combination of GPVI and PAR1 agonist (1 μg/mL CRP-XL, 20 μg/mL TRAP-6) before flow cytometry analysis of platelet α-granule secretion (F) and platelet PS exposure (G). Statistical analysis was performed using a one-way analysis of variance (ANOVA) test and the corresponding P values of significance are as indicated: ∗P < .01, ∗∗P < .001, and ∗∗∗P < .0001. CRP-XL, crosslinked collagen-related peptide.
Elevated oxLDL and enhanced GPVI-mediated procoagulant platelet generation in obese nonhuman primates. Blood was collected from a cohort of lean (n = 6) and diet-induced obese (n = 6) rhesus macaques to prepare serum for analysis of triglycerides (A), total cholesterol (B), high-density lipoprotein cholesterol (C), LDL cholesterol (D), and oxLDL immunoreactivity (E). Citrate-anticoagulated whole blood from lean and obese animals was stimulated with GPVI agonist (0.1 μg/mL CRP-XL) or a combination of GPVI and PAR1 agonist (1 μg/mL CRP-XL, 20 μg/mL TRAP-6) before flow cytometry analysis of platelet α-granule secretion (F) and platelet PS exposure (G). Statistical analysis was performed using a one-way analysis of variance (ANOVA) test and the corresponding P values of significance are as indicated: ∗P < .01, ∗∗P < .001, and ∗∗∗P < .0001. CRP-XL, crosslinked collagen-related peptide.
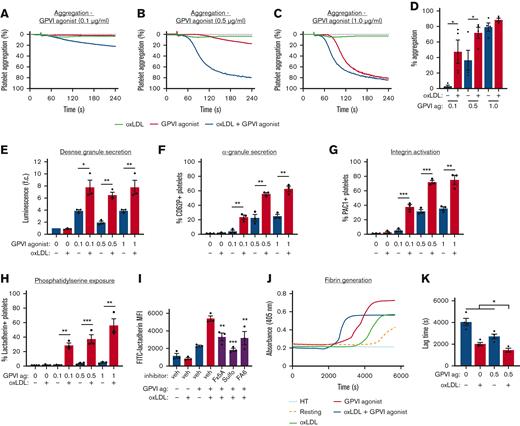
We sought to specify the effects of oxLDL on GPVI-mediated platelet activation. Washed platelets were prepared from healthy human donors and pretreated with oxLDL (20 μg/mL) alone or in combination with the GPVI-specific agonist, CRP-XL (Figure 2A-C). Treatment of platelets with oxLDL (20 μg/mL) or CRP-XL alone (0.5 μg/mL) did not cause significant platelet aggregation, (2.6% ± 0.4% and 5% ± 3%, respectively). However, pretreatment of platelets with oxLDL before CRP-XL stimulation enhanced platelet aggregation to 81.8% (Figure 2B). No significant effects of oxLDL were noted after stimulation of platelets with 1 μg/mL CRP-XL, which maximally aggregated platelets in the absence of oxLDL (Figure 2C-D). These ex vivo studies of washed human platelets and others, as discussed below, were carried out without the addition of calcium to limit amplification of platelet responses by store-operated calcium entry.29 Flow cytometry experiments with fluorescently labeled oxLDL (dil-oxLDL) confirmed that washed human platelets bind to oxLDL in a concentration dependent manner that is readily blocked by inhibitors of the putative oxLDL receptor CD36 (supplemental Figure 3).
oxLDL potentiates GPVI-mediated platelet aggregation, platelet PS exposure, and platelet-driven fibrin formation. Replicate samples of washed human platelets (2 × 108/mL, in modified HEPES/Tyrode buffer without additional calcium) were incubated with the GPVI-specific agonist CRP-XL) alone (0.1, 0.5, 1 μg/mL), oxLDL alone (20 μg/mL), and oxLDL in combination with CRP-XL. Platelet samples were monitored at 37°C under continuous stirring at 1200 rpm and changes in light transmission were measured (A-D). (E) Washed platelets (2 × 108/mL) were incubated with oxLDL (0, 20 μg/mL) in combination with CRP-XL (0, 0.1, 0.5, 1 μg/mL) and monitored using luciferase enzyme activity (CHRONO-LUME) to detect ATP released as a measure of platelet dense granule secretion. (F) Platelets were stained with APC-CD62P to monitor for platelet surface expression of P-selectin as a marker of α-granule secretion and (G) stained with FITC-PAC-1 to monitor for platelet surface integrin activation. (H) Platelets were incubated with oxLDL (0, 20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0, 0.1, 0.5, 1 μg/mL) before fixation and staining with FITC-lactadherin to monitor for platelet surface PS exposure. (I) Platelets were similarly assessed with FITC-lactadherin staining in response to oxLDL and CRP-XL stimulation after a 10-minute preincubation with CD36 inhibitors Fx-5A (250 μM), sulfo-N-succinimidyl oleate (“sulfo,” 50 μM), or FA6-152 (5 μg/mL). (J) Platelets were incubated with CaCl2 (8.3 mM) and citrated PPP (33% final). Fibrin formation was measured as a change in turbidity at an absorbance of 405 nm. (K) Representative traces are shown and the lag time was calculated. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. ATP, adenosine triphosphate; CaCl2, calcium dichloride; FITC, fluorescein isothiocyanate; HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; PPP, platelet-poor plasma.
oxLDL potentiates GPVI-mediated platelet aggregation, platelet PS exposure, and platelet-driven fibrin formation. Replicate samples of washed human platelets (2 × 108/mL, in modified HEPES/Tyrode buffer without additional calcium) were incubated with the GPVI-specific agonist CRP-XL) alone (0.1, 0.5, 1 μg/mL), oxLDL alone (20 μg/mL), and oxLDL in combination with CRP-XL. Platelet samples were monitored at 37°C under continuous stirring at 1200 rpm and changes in light transmission were measured (A-D). (E) Washed platelets (2 × 108/mL) were incubated with oxLDL (0, 20 μg/mL) in combination with CRP-XL (0, 0.1, 0.5, 1 μg/mL) and monitored using luciferase enzyme activity (CHRONO-LUME) to detect ATP released as a measure of platelet dense granule secretion. (F) Platelets were stained with APC-CD62P to monitor for platelet surface expression of P-selectin as a marker of α-granule secretion and (G) stained with FITC-PAC-1 to monitor for platelet surface integrin activation. (H) Platelets were incubated with oxLDL (0, 20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0, 0.1, 0.5, 1 μg/mL) before fixation and staining with FITC-lactadherin to monitor for platelet surface PS exposure. (I) Platelets were similarly assessed with FITC-lactadherin staining in response to oxLDL and CRP-XL stimulation after a 10-minute preincubation with CD36 inhibitors Fx-5A (250 μM), sulfo-N-succinimidyl oleate (“sulfo,” 50 μM), or FA6-152 (5 μg/mL). (J) Platelets were incubated with CaCl2 (8.3 mM) and citrated PPP (33% final). Fibrin formation was measured as a change in turbidity at an absorbance of 405 nm. (K) Representative traces are shown and the lag time was calculated. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. ATP, adenosine triphosphate; CaCl2, calcium dichloride; FITC, fluorescein isothiocyanate; HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; PPP, platelet-poor plasma.
Next, we examined the effects of oxLDL on steps progressing GPVI-mediated platelet activation, including dense granule secretion, α-granule secretion, and “inside-out” integrin activation. Platelets were treated with oxLDL alone or in combination with CRP-XL before incubation with CHRONO-LUME reagent to follow adenosine 5′-diphosphate (ADP) secretion as a marker of dense granule secretion. In parallel, platelet samples were also stained with APC-CD62P and FITC-PAC-1 to assess for α-granule secretion and integrin activation, respectively, using flow cytometry. Treatment of platelets with CRP-XL increased platelet dense granule secretion, α-granule secretion, and integrin activation (supplemental Figure 4C,E,G) in a dose-dependent manner, whereas oxLDL alone had no significant effect on these responses over a concentration range of 0 to 50 μg/mL (supplemental Figure 4D,F,H). Stimulation of platelets with oxLDL (20 μg/mL) in combination with a range of concentrations of CRP-XL significantly enhanced dense granule secretion (Figure 2E), α-granule secretion (Figure 2F), and integrin activation (Figure 2G). For instance, when platelets were pretreated with oxLDL (20 μg/mL) in combination with CRP-XL (0.5 μg/mL), the measured luminescence was 6.5-fold greater than that of unstimulated platelets (Figure 2E). When platelets were pretreated with oxLDL (20 μg/mL) in combination with CRP-XL (0.5 μg/mL), CD62P+ platelets increased to 55.7% (±1.9%) (Figure 2F) and the percentage of PAC-1+ platelets increased to 71.0% (±2.4%) (Figure 2G). Notably, these GPVI-mediated platelet activation parameters were enhanced in a manner that correlated to degree of LDL oxidation state, because native (non-oxLDL), low-, and medium-oxLDL preparations had less pronounced effects relative to highly oxidized LDL (supplemental Figure 5).
Next, we evaluated GPVI/ITAM-driven platelet procoagulant responses to oxLDL with flow cytometry, first measuring FITC-lactadherin binding of platelets as a marker of PS exposure. Neither oxLDL nor CRP-XL treatment alone significantly increased platelet PS exposure, (supplemental Figure 4A-B). Treatment of platelets with oxLDL in combination with CRP-XL significantly increased platelet PS exposure (Figure 2H). For instance, 3.3% (±0.8%) of platelets were positive for FITC-lactadherin binding when stimulated with CRP-XL alone (0.5 μg/mL); however, when platelets were pretreated with oxLDL in combination with CRP-XL, the percentage of lactadherin-positive platelets increased ∼10-fold to 37.2% (± 0.8%) (Figure 2H). To examine the role of the putative oxLDL receptor CD36 in these procoagulant platelet responses, we pretreated platelets with 3 distinct inhibitors of CD36 before combined oxLDL and CRP-XL stimulation and flow cytometry analysis of PS exposure (Figure 2I). Pretreatment of platelets with Fx-5A,30 sulfo-N-succinimidyl oleate,31 or FA6-152,32 each significantly inhibited platelet PS exposure in response to oxLDL and CRP-XL stimulation (Figure 2I).
To further assess the effects of oxLDL + CRP-XL-driven PS exposure on platelet procoagulant activity, we next evaluated fibrin generation with light absorbance turbidity assays over the course of 2 hours (Figure 2J). Washed platelets were pretreated with oxLDL (20 μg/mL) alone and in combination with CRP-XL (0.5 μg/mL). Citrated PPP and CaCl2, (25 mM) were sequentially added to each platelet mixture in equal proportions. Fibrin generation began at a lag time of 2000 seconds (±140 seconds) when stimulated with CRP-XL alone and at 2700 seconds (±215 seconds) when stimulated with oxLDL alone (Figure 2J). When platelets were pretreated with oxLDL in combination with CRP-XL, fibrin generation occurred at 1450 seconds (±175 seconds) after stimulation (Figure 2J). The time to half of maximum fibrin generation was reached at 2320 seconds (±190 seconds) after stimulation, vs. at 4160 seconds (±180 seconds) and at 3450 seconds (±235 seconds) when stimulated with oxLDL or CRP-XL alone, respectively (Figure 2K). Altogether, these results demonstrate that oxLDL enhances GPVI-driven platelet dense granule secretion, α-granule secretion, integrin activation in a manner related to the potentiation of platelet aggregation, and procoagulant responses, including platelet PS exposure and fibrin generation.
Effects of purinergic receptor (P2YR) and COX inhibitors on oxLDL-mediated platelet responses
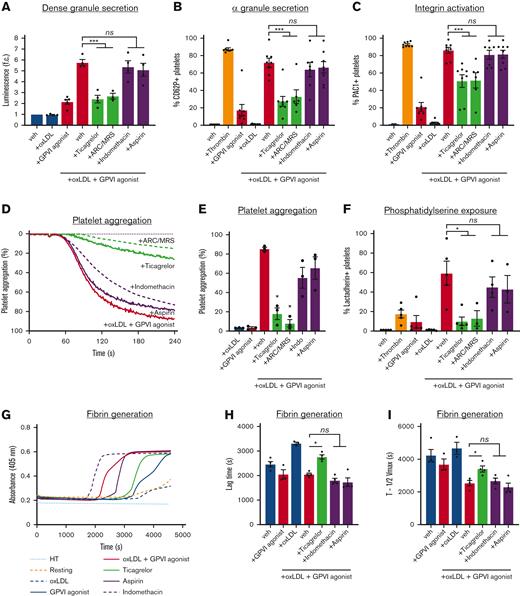
Pharmacologic inhibitors of P2YRs (eg, clopidogrel and ticagrelor) and COXs (eg, aspirin) are routinely used as antiplatelet agents against procoagulant activities in atherosclerotic cardiovascular diseases. We subsequently sought to assess the effects P2YR and COX inhibitors on GPVI-driven platelet responses enhanced by oxLDL. To investigate the effects of P2YRs and their inhibition on oxLDL-mediated GPVI-mediated platelet function, washed human platelets were pretreated with P2Y12 receptor inhibitor ticagrelor (0.25, 0.50, 0.75, 1, 5, and 10 μM), as well as a combination of P2Y12 antagonist AR-C 66096 (ARC; 10 μM) and P2Y1 antagonist MRS2179 (MRS; 10 μM). To investigate the effects of COX inhibition, we also treated platelets with low-dose aspirin (acetylsalicylic acid; 0.01, 0.02 mM) and high-dose aspirin (acetylsalicylic acid; 1.0, 2.0 mM), as well as indomethacin (10 μM). Platelets were then stimulated with oxLDL (20 μg/mL) and CRP-XL (0.5 μg/mL). Pretreatment of platelets with ticagrelor reduced platelet dense granule secretion (Figure 3A), α-granule secretion (Figure 3B), and integrin activation (Figure 3C) in a dose-dependent manner (supplemental Figure 6). In contrast, treatment of platelets with aspirin before combined oxLDL and CRP-XL stimulation had no significant effect on platelet dense granule secretion (Figure 3A), α-granule secretion (Figure 3B), and integrin activation (Figure 3C). Similarly, small-molecule inhibitors of P2YRs (AR-C 66096 and MRS2179; 10 μM) but not COX (indomethacin, 10 μM) reduced the effect of oxLDL on GPVI-mediated platelet dense granule secretion (Figure 3A), α-granule secretion (Figure 3B), and integrin activation (Figure 3C) to a similar extent as ticagrelor.
P2YR antagonists but not COX inhibitors reduce oxLDL potentiation of platelet GPVI responses. (A) Replicate samples (n ≥ 3) of washed human platelets (2 × 108/mL; in modified HEPES/Tyrode buffer without addition of calcium) were treated with P2YR inhibitors ticagrelor (750 nM) and ARC/MRS (10 μM), and COX inhibitors aspirin (1 mM) and indomethacin (10 μM) for 10 minutes and stimulated with oxLDL (20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0.5 μg/mL). Platelet samples were monitored using the luciferase enzyme CHRONO-LUME to detect the luminescence of ATP released as a measure of platelet dense granule secretion. (B) Platelets were stained with APC-CD62P to monitor for platelet surface expression of P-selectin and (C) stained with FITC-PAC-1 to monitor for platelet surface integrin activation. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗∗∗P < .001. (D) Replicate samples (n = 4) of washed human platelets (2 × 108/mL) were treated with P2YR inhibitors ticagrelor (750 nM) and ARC/MRS (10 μM), and COX inhibitors aspirin (1 mM) and indomethacin (10 μM) for 10 minutes and stimulated with oxLDL (20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0.5 μg/mL). Platelet samples were monitored at 37°C under continuous stirring at 1200 rpm and the changes in light transmission were measured. (E) Representative traces of platelet samples treated with each select inhibitor are shown and quantified. (F) Platelets were stained with FITC-lactadherin to monitor for platelet surface PS exposure. (G) Platelet samples were incubated with CaCl2 (8.3 mM) and citrated PPP (33% final). Fibrin formation was measured as a change in turbidity at an absorbance of 405 nm. (H-I) Representative traces are shown and the lag time and time to half the maximum absorbance were calculated. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .05; ns, not significant.
P2YR antagonists but not COX inhibitors reduce oxLDL potentiation of platelet GPVI responses. (A) Replicate samples (n ≥ 3) of washed human platelets (2 × 108/mL; in modified HEPES/Tyrode buffer without addition of calcium) were treated with P2YR inhibitors ticagrelor (750 nM) and ARC/MRS (10 μM), and COX inhibitors aspirin (1 mM) and indomethacin (10 μM) for 10 minutes and stimulated with oxLDL (20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0.5 μg/mL). Platelet samples were monitored using the luciferase enzyme CHRONO-LUME to detect the luminescence of ATP released as a measure of platelet dense granule secretion. (B) Platelets were stained with APC-CD62P to monitor for platelet surface expression of P-selectin and (C) stained with FITC-PAC-1 to monitor for platelet surface integrin activation. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗∗∗P < .001. (D) Replicate samples (n = 4) of washed human platelets (2 × 108/mL) were treated with P2YR inhibitors ticagrelor (750 nM) and ARC/MRS (10 μM), and COX inhibitors aspirin (1 mM) and indomethacin (10 μM) for 10 minutes and stimulated with oxLDL (20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0.5 μg/mL). Platelet samples were monitored at 37°C under continuous stirring at 1200 rpm and the changes in light transmission were measured. (E) Representative traces of platelet samples treated with each select inhibitor are shown and quantified. (F) Platelets were stained with FITC-lactadherin to monitor for platelet surface PS exposure. (G) Platelet samples were incubated with CaCl2 (8.3 mM) and citrated PPP (33% final). Fibrin formation was measured as a change in turbidity at an absorbance of 405 nm. (H-I) Representative traces are shown and the lag time and time to half the maximum absorbance were calculated. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .05; ns, not significant.
Next, platelets were pretreated P2YR inhibitors ticagrelor (750 nM), ARC/MRS (10 μM), COX inhibitors aspirin (1 mM), indomethacin (10 μM), stimulated with oxLDL (20 μg/mL) in combination with CRP-XL (0.5 μg/mL), to assess platelet aggregation (Figure 3D-E). P2YR inhibitors significantly decreased platelet aggregation in response to oxLDL and CRP-XL, whereas COX inhibitors had only minor effects (Figure 3D-E). P2YR antagonists also significantly reduced the percentage of lactadherin-positive platelets generated by combined oxLDL and CRP-XL stimulation, whereas COX inhibitors, aspirin and indomethacin had no significant effect (Figure 3F). For instance, when platelets were stimulated with CRP-XL (0.5 μg/mL) alone, 3.0% (±0.7%) platelets were lactadherin-positive, whereas 68.7% (±10.4%) of platelets were lactadherin-positive following when treated with oxLDL (20 μg/mL) in combination with CRP-XL (0.5 μg/mL). When platelets were pretreated with P2YR inhibitors ticagrelor or ARC/MRS, the percentage of lactadherin-positive platelets was reduced to 5.7% (±1.1%) and 12.9% (±8.0%) after stimulation with oxLDL and CRP-XL, respectively.
To evaluate the effect of P2YR and COX inhibition on fibrin generation, platelets were pretreated with ticagrelor, aspirin, or indomethacin, and then stimulated with oxLDL (20 μg/mL) in combination with CRP-XL (0.5 μg/mL). Citrated PPP and CaCl2 (25 mM) were sequentially added to each platelet mixture in equal proportions, and fibrin formation was measured as a change in turbidity at an absorbance of 405 nm over the course of 2 hours (Figure 3G). Evaluating for the lag time (Figure 3H) and time to half of maximum fibrin generation (Figure 3I), we found that fibrin generation began at 2050 seconds (±50 seconds) and reached half to maximum at 2535 seconds (±160 seconds), respectively. P2YR inhibitor ticagrelor increased the average lag time to 2760 seconds (±80 seconds) and time to half maximum to 3400 seconds (±180 seconds). In contrast, aspirin did not significantly change the lag time or the time to half maximum (Figure 3H-I). Overall, these results demonstrate that P2YR inhibition limits the potentiation of ex vivo platelet aggregation and procoagulant activity enhanced by oxLDL, when COX inhibition had no effect.
oxLDL potentiates platelet ITAM signaling
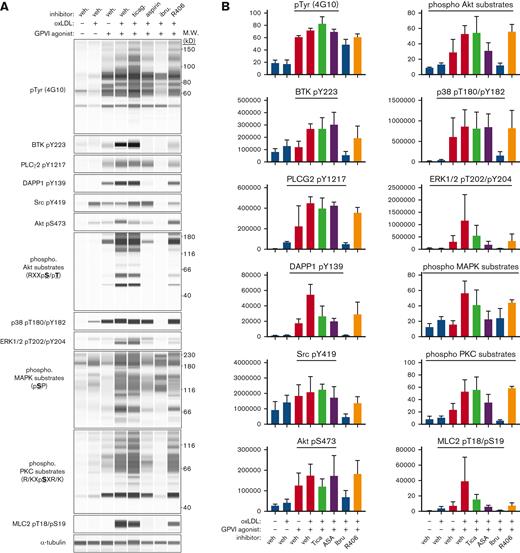
As a ligand of CD36, oxLDL has been detailed to contribute to platelet activation through NOX2-mediated reactive oxygen species generation,11 ERK5 signaling, and caspase-3 activation;7 however, the roles for intracellular signaling (eg, ITAM effectors) and secondary mediators (eg, ADP secretion and thromboxane generation) remain to be incorporated into models of oxLDL-enhanced platelet activation. To elucidate how oxLDL potentiates biochemical activities to enhance GPVI-mediated platelet procoagulant responses, we surveyed platelet protein kinase activities through analysis of protein phosphorylation using quantitative capillary electrophoresis–based immunoassays (ProteinSimple Jess).10,33,34 Lysates were prepared in sample buffer from washed human platelets under resting and oxLDL- and CRP-XL-stimulated conditions. Immunoassay analysis quantified minimal phosphorylation of GPVI/ITAM signaling events in resting platelets as well as in platelets stimulated with oxLDL alone (20 μg/mL) (Figure 4) after stimulation with CRP-XL (0.5 μg/mL), including tyrosine kinase substrates, protein kinase C substrates, Akt substrates, mitogen activated protein kinase (MAPK) substrates, or other targets in platelet GPVI/ITAM signaling pathways (Figure 4). When platelets were simultaneously stimulated with oxLDL and CRP-XL, the phosphorylation of all kinase substrates examined were upregulated relative to effects of each agonist alone. Pretreatment of platelets with ibrutinib effectively inhibited platelet GPVI/ITAM signaling in response to oxLDL and CRP-XL stimulation. Ticagrelor and aspirin more selectively inhibited platelet ITAM signaling responses. Together, these results suggest that BTK signaling through PI3K/Akt has a central role in mediating the potentiated signal from CD36 activation and GPVI activation from oxLDL and CRP-XL, respectively.
Antiplatelet agents and TKIs differentially reduce oxLDL potentiation of platelet GPVI/ITAM signaling. Replicate samples (n = 3) of washed human platelets (1 × 109/mL; in modified HEPES/Tyrode buffer without addition of calcium) were pretreated with P2YR inhibitor ticagrelor, BTK inhibitor ibrutinib (10 μM), or Syk inhibitor R406 (10 μM) for 10 minutes and then stimulated with oxLDL (20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0.5 μg/mL) for 5 minutes at 37°C. After collection into sample buffer, platelet lysates were separated by capillary electrophoresis and stained with indicated antisera for quantitative immunoblot analysis on a ProteinSimple Jess western blotting system. (A) Representative immunoblot analysis and (B) quantitation of signal intensities with indicated antisera; α-tubulin serves as a loading control for total protein levels. Positions of molecular weight (kD) markers are indicated.
Antiplatelet agents and TKIs differentially reduce oxLDL potentiation of platelet GPVI/ITAM signaling. Replicate samples (n = 3) of washed human platelets (1 × 109/mL; in modified HEPES/Tyrode buffer without addition of calcium) were pretreated with P2YR inhibitor ticagrelor, BTK inhibitor ibrutinib (10 μM), or Syk inhibitor R406 (10 μM) for 10 minutes and then stimulated with oxLDL (20 μg/mL) in combination with GPVI-specific agonist CRP-XL (0.5 μg/mL) for 5 minutes at 37°C. After collection into sample buffer, platelet lysates were separated by capillary electrophoresis and stained with indicated antisera for quantitative immunoblot analysis on a ProteinSimple Jess western blotting system. (A) Representative immunoblot analysis and (B) quantitation of signal intensities with indicated antisera; α-tubulin serves as a loading control for total protein levels. Positions of molecular weight (kD) markers are indicated.
Ibrutinib and fostamatinib reduce oxLDL-driven platelet procoagulant activity
Given the enhanced phosphorylation of ITAM signaling effectors in platelets stimulated with oxLDL and CRP-XL (Figure 4), and emerging interests in TKIs as antiplatelet agents,25 we sought to determine the effects of Syk and BTK inhibition on GPVI-mediated platelet responses enhanced by oxLDL. Washed human platelets were pretreated with BTK inhibitor ibrutinib (10 μM) or Syk inhibitor fostamatinib (R406, 10 μM) before stimulation with oxLDL (20 μg/mL) in combination with CRP-XL (0.5 μg/mL). In light transmission aggregometry assays, preincubation of platelets with ibrutinib before combined oxLDL and CRP-XL stimulation eliminated platelet aggregation, whereas the Syk inhibitor R406 did not significantly change platelet aggregation in response to oxLDL and CRP-XL (Figure 5A). Flow cytometry assessment found that ibrutinib abrogated platelet PS exposure in response to oxLDL and CRP-XL, whereas R406 also significantly, although less completely, inhibited PS exposure (Figure 5B). Likewise, under control conditions, fibrin generation began at 1730 seconds (±180 seconds) and reached half to maximum at 2670 seconds (±140 seconds), respectively (Figure 5C). When pretreated with ibrutinib, the lag time and time to half maximum increased to 4450 seconds (±460 seconds) and 5700 seconds (±740 seconds), respectively. In contrast, R406 did not significantly change the lag time or the time to half maximum, at 2110 seconds (±100 seconds) and 3240 seconds (±580 seconds), respectively (Figure 5C).
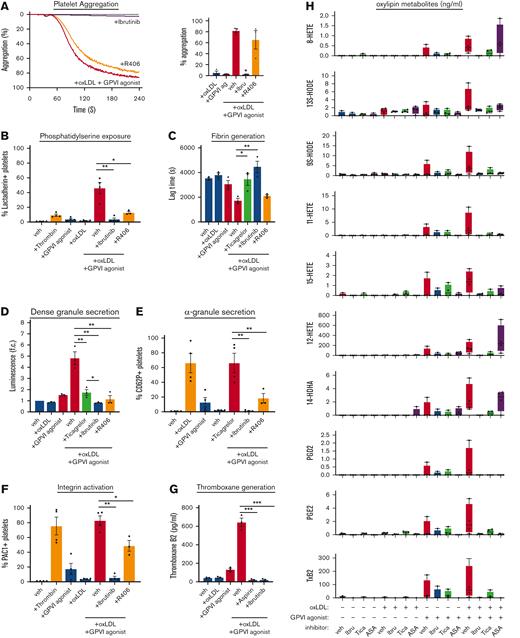
BTK and Syk inhibitors reduce oxLDL potentiation of platelet hemostatic and procoagulant activity. (A) Replicate samples (n = 4) of washed human platelets (2 × 108/mL; in modified HEPES/Tyrode buffer without addition of calcium) were treated with BTK inhibitor ibrutinib (10 μM) or Syk inhibitor R406 (10 μM) for 10 minutes and stimulated with GPVI-specific agonist CRP-XL (0.5 μg/mL) in combination with oxLDL (20 μg/mL). Platelets were monitored at 37°C under continuous stirring at 1200 rpm and the changes in light transmission were measured. Representative platelet aggregation traces of platelet samples treated with each select inhibitor are shown. (B) Platelets were stained with FITC-lactadherin to monitor for platelet surface PS exposure. Platelet samples were incubated with CaCl2 (8.3 mM) and citrated PPP (33% final). Fibrin formation was measured as a change in turbidity at an absorbance of 405 nm and the lag time (C) was quantified. (D) Platelets were monitored using the luciferase enzyme CHRONO-LUME to detect the luminescence of ATP released as a measure of platelet dense granule secretion, stained with APC-CD62P to monitor for platelet surface expression of P-selectin as a marker of α-granule secretion (E), and stained with FITC-PAC-1 to monitor for platelet surface integrin activation (F). Following the platelet aggregation assays shown in panel A, platelet supernatants were extracted and analyzed for thromboxane generation by ELISA (G). Additional platelet supernatant samples were similarly prepared from platelets treated with oxLDL, CRP-XL, and inhibitors of interest to measure levels of indicated oxylipin metabolite levels with mass spectrometry (H). Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. ELISA, enzyme-linked immunosorbent assay.
BTK and Syk inhibitors reduce oxLDL potentiation of platelet hemostatic and procoagulant activity. (A) Replicate samples (n = 4) of washed human platelets (2 × 108/mL; in modified HEPES/Tyrode buffer without addition of calcium) were treated with BTK inhibitor ibrutinib (10 μM) or Syk inhibitor R406 (10 μM) for 10 minutes and stimulated with GPVI-specific agonist CRP-XL (0.5 μg/mL) in combination with oxLDL (20 μg/mL). Platelets were monitored at 37°C under continuous stirring at 1200 rpm and the changes in light transmission were measured. Representative platelet aggregation traces of platelet samples treated with each select inhibitor are shown. (B) Platelets were stained with FITC-lactadherin to monitor for platelet surface PS exposure. Platelet samples were incubated with CaCl2 (8.3 mM) and citrated PPP (33% final). Fibrin formation was measured as a change in turbidity at an absorbance of 405 nm and the lag time (C) was quantified. (D) Platelets were monitored using the luciferase enzyme CHRONO-LUME to detect the luminescence of ATP released as a measure of platelet dense granule secretion, stained with APC-CD62P to monitor for platelet surface expression of P-selectin as a marker of α-granule secretion (E), and stained with FITC-PAC-1 to monitor for platelet surface integrin activation (F). Following the platelet aggregation assays shown in panel A, platelet supernatants were extracted and analyzed for thromboxane generation by ELISA (G). Additional platelet supernatant samples were similarly prepared from platelets treated with oxLDL, CRP-XL, and inhibitors of interest to measure levels of indicated oxylipin metabolite levels with mass spectrometry (H). Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001. ELISA, enzyme-linked immunosorbent assay.
Pretreatment of platelets with ibrutinib before oxLDL and CRP-XL stimulation reduced platelet dense granule secretion (Figure 5D), α-granule secretion (Figure 5E), and integrin activation (Figure 5F) to levels similar to resting platelets. Syk inhibitor R406 similarly reduced platelet dense granule secretion (Figure 5D) and also significantly inhibited α-granule secretion (Figure 5E) and integrin activation, but to a lesser extent (Figure 5F). In addition to luminescence and flow cytometry assays, we also collected platelet releasate after aggregometry for ELISA of thromboxane B2 (TxB2) as a marker of thromboxane A2 generation. Platelets stimulated with oxLDL alone (20 μg/mL) and CRP-XL alone (0.5 μg/mL) generated 46.7 pg/mL (±6.3 pg/mL) and 132.2 pg/mL (±15.3 pg/mL) TxB2, respectively (Figure 5G). When platelets were stimulated with both agonists simultaneously, TxB2 concentration was enhanced to 642.0 pg/mL (±46.5 pg/mL) (Figure 5G). Pretreatment of platelets with ibrutinib significantly inhibited TxB2 generation to levels similar to that of aspirin (Figure 5G). In addition to ELISA measurements of platelet TBX2, additional mass spectrometry analyses of platelet oxylipin metabolites likewise noted enhanced TxB2 generation in platelets stimulated with CRP-XL in combination with oxLDL (Figure 5H).
Ibrutinib inhibits platelet procoagulant activity in animal models of hyperlipidemia
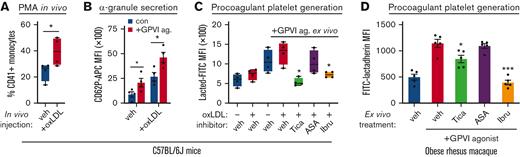
To examine the effects of conventional antiplatelet agents (eg, aspirin and ticagrelor) as well as TKIs (eg, ibrutinib and fostamatinib) on oxLDL-enhanced platelet procoagulant activities in physiological context, we carried out experiments with mouse as well as nonhuman primate models of hyperlipidemia and cardiovascular disease. First, to specify the effects of oxLDL on procoagulant platelet activities, we injected healthy, wild-type C57BL/6J mice (median age 6 weeks) with vehicle alone (phosphate-buffered saline) or oxLDL. At 10 minutes after intravenous oxLDL or vehicle injection, whole blood was collected from mice for flow cytometry analysis of platelet activity (Figure 6). In agreement with previous studies,35 intravenous oxLDL injection increased circulating platelet-monocyte aggregates in mouse whole blood (Figure 6A). Relative to vehicle alone, intravenous oxLDL injection also upregulated P-selectin levels on circulating platelets and enhanced platelet α-granule secretion in response to GPVI stimulation with CRP-XL ex vivo (Figure 6B). Flow cytometry analysis of whole blood from mice treated with inhibitors of interest 10 minutes before oxLDL injection also found increased procoagulant platelet PS exposure in response to ex vivo CRP-XL stimulation in a manner significantly inhibited by intraperitoneal injection of ticagrelor and ibrutinib but not aspirin (Figure 6C). In addition to studies of mice injected with oxLDL and inhibitors of interest, we also examined the effects of ibrutinib, ticagrelor, and aspirin as inhibitors of platelet procoagulant activity in the context of hyperlipidemia and increased oxLDL in nonhuman primates. Citrate-anticoagulated blood was collected from obese rhesus macaques as detailed above (Figure 1). Pretreatment of whole blood from obese animals with ticagrelor or ibrutinib significantly inhibited PS exposure in response to CRP-XL stimulation, whereas aspirin had minimal effect (Figure 6D). These experiments in mice and nonhuman primates support a model whereby oxLDL enhances ITAM GPVI platelet procoagulant responses in a manner inhibited by ticagrelor and ibrutinib but not aspirin.
oxLDL enhances platelet procoagulant activity in vivo. (A) Wild-type C57BL/6J mice (median age, 6 weeks) were intravenously injected with vehicle (phosphate-buffered saline) alone or oxLDL 10 minutes before blood collection into citrate (n = 5 per condition). Whole blood samples were analyzed for platelet-monocyte aggregate formation as previously described.35,49 (B) Whole blood samples from vehicle- and oxLDL-injected animals were subsequently stimulated with GPVI agonist (0.1 μg/mL CRP-XL) before flow cytometry analysis of CD62P as a marker of platelet α-granule secretion. (C) Sets of mice (n = 5 each) were administered by vehicle (dimethyl sulfoxide), ticagrelor (100 mg/kg), aspirin (30 mg/kg), or ibrutinib (10 mg/kg) by intraperitoneal injection before oxLDL injection, blood collection, and flow cytometry analysis of FITC-lactadherin to follow platelet PS exposure. (D) Aliquots of citrate-anticoagulated whole blood from obese rhesus macaques (detailed in Figure 1, in previous section) were pretreated with vehicle (dimethyl sulfoxide), ticagrelor, aspirin, or ibrutinib before stimulation with GPVI agonist (0.1 μg/mL CRP-XL), lactadherin-FITC staining, and flow cytometry assessment of platelet PS exposure. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .01.
oxLDL enhances platelet procoagulant activity in vivo. (A) Wild-type C57BL/6J mice (median age, 6 weeks) were intravenously injected with vehicle (phosphate-buffered saline) alone or oxLDL 10 minutes before blood collection into citrate (n = 5 per condition). Whole blood samples were analyzed for platelet-monocyte aggregate formation as previously described.35,49 (B) Whole blood samples from vehicle- and oxLDL-injected animals were subsequently stimulated with GPVI agonist (0.1 μg/mL CRP-XL) before flow cytometry analysis of CD62P as a marker of platelet α-granule secretion. (C) Sets of mice (n = 5 each) were administered by vehicle (dimethyl sulfoxide), ticagrelor (100 mg/kg), aspirin (30 mg/kg), or ibrutinib (10 mg/kg) by intraperitoneal injection before oxLDL injection, blood collection, and flow cytometry analysis of FITC-lactadherin to follow platelet PS exposure. (D) Aliquots of citrate-anticoagulated whole blood from obese rhesus macaques (detailed in Figure 1, in previous section) were pretreated with vehicle (dimethyl sulfoxide), ticagrelor, aspirin, or ibrutinib before stimulation with GPVI agonist (0.1 μg/mL CRP-XL), lactadherin-FITC staining, and flow cytometry assessment of platelet PS exposure. Statistical analysis was performed using a one-way ANOVA test and the corresponding P values of significance are as indicated: ∗P < .01.
Discussion
In this study, we investigated how oxLDL augments platelet GPVI/ITAM signaling, secondary feedback activation mechanisms, and platelet procoagulant activity. We found that ex vivo, oxLDL enhances platelet activation in responses to GPVI agonists, including ITAM signaling, dense granule secretion, α-granule secretion, integrin activation, platelet aggregation, and thromboxane generation. Likewise, oxLDL also upregulates platelet procoagulant activity, increasing PS exposure on the platelet surface and accelerating fibrin generation following GPVI stimulation. We also assessed the effects of antiplatelet agents, including P2YR inhibitor ticagrelor and COX inhibitor aspirin, as well as inhibitors targeting Syk (fostamatinib/R406) and BTK (ibrutinib) on these oxLDL-mediated platelet responses. We found that ex vivo, P2YR blockade as well as Syk and BTK inhibition reduced oxLDL-mediated platelet responses and procoagulant activity, whereas COX inhibition with aspirin had no significant effects. Overall, our results demonstrate that oxLDL potentiates GPVI-mediated platelet responses and procoagulant activity in a manner limited by P2YR antagonists and BTK inhibitors.
Mechanistic studies have established that oxLDL activates the scavenger receptor CD36 on platelets to upregulate signaling pathways that enhance platelet responses, including reactive oxygen species generation and caspase activation,10,36 and transduce intracellular signaling through associations among CD36, Syk, and SFKs.8,11,12,37 Studies have also shown that MAPK JNK2 is phosphorylated in platelets exposed to oxLDL mediated by SFKs, which is essential for downstream signaling events and upregulation of ERK5 and MAPK signaling pathways.10,12,38 However, it has remained generally unknown how oxLDL activation in combination with other platelet agonists affect protein activity along GPVI/ITAM signaling cascades. Our study further demonstrates that CD36 signaling overlaps with the GPVI signaling pathway, in such that the phosphorylation of proteins along the Syk-BTK-PI3K/Akt axis is increased even at subthreshold levels of CD36 and GPVI stimulation. We found in combination with GPVI-specific agonist CRP-XL, oxLDL enhanced the phosphorylation of generalize tyrosine kinase substrates, protein kinase C substrates, Akt substrates, and MAPK substrates, as well as DAPP1, PLCγ2, Akt, GSK3, ERK1/2, and other specific effectors of platelet activation (Figure 4). In addition, we also demonstrated that inhibition of central upstream kinases, in particular Syk and BTK, within this signaling cascade reduces the potentiation of protein phosphorylation induced by oxLDL (Figure 4). Notably, the BTK inhibitor ibrutinib inhibited platelet signaling as well as functional activity to a greater extent than fostamatinib, suggesting that BTK has a more central role in the convergent signaling cascade from CD36 and GPVI. Future systems biology as well as clinical studies will more precisely and comprehensively define these signaling events, with ex vivo and in vivo assays.39,40
Although TKIs such as fostamatinib and ibrutinib may offer promise as antithrombotic agents in specific contexts,26,41 they are not yet used therapeutically to inhibit platelet activation in cardiovascular disease. At present, the efficacy of aspirin as an antiplatelet agent for secondary prevention of cardiovascular disease is well established, but the clinical benefit of aspirin as the primary prevention remains unclear.42 Moreover, the specific effects of aspirin on oxLDL-mediated platelet activation mechanisms have remained largely unaddressed. Likewise, roles for other widely used antiplatelet agents, including P2Y12 antagonists, as inhibitors of oxLDL-enhanced platelet activity have not yet been explored.18,19,43 In this study, we found that platelet P2Y12 inhibition with ticagrelor significantly reduces the potentiation of platelet signaling, aggregation, and platelet procoagulant activity enhanced by oxLDL, whereas COX inhibition with aspirin had no significant effect. Ticagrelor treatment also significantly inhibited oxLDL-augmented dense granule secretion, α-granule secretion, and integrin activation. These results suggest that oxLDL enhances platelet activities by means of the secondary feedback mechanisms of ADP secretion and P2YR activation, where P2Y12 inhibitors (ticagrelor) more potently inhibit activation relative to aspirin. Notably, these effects were evident in whole blood samples from mice and nonhuman primates that contained physiologic levels of calcium, as well as in washed platelet preparations in modified HEPES/Tyrode buffer without additional calcium.
Aspirin and P2YR antagonists are used together as dual DAPT, following procedures such as percutaneous coronary intervention to prevent thrombosis and cardiovascular events. However, bleeding complications still represent a limitation of DAPT. To this end, small-molecule TKIs, that are now effective therapeutics in a range of hematologic malignancies as well as immunologic and inflammatory disorders, also offer a means to inhibit specific aspects of platelet activation.44-46 Our group and others have assessed roles for TKIs in GPVI-mediated platelet responses, including agents targeting Src, Syk, BTK, Jak, and other tyrosine kinases.23,24,39,47,48 In this study, we investigated how Syk/BTK may also reduce procoagulant platelet responses induced by oxLDL. We found that both the BTK inhibitor ibrutinib and Syk inhibitor fostamatinib reduced platelet granule secretion, integrin activation, and procoagulant activity, decreasing PS exposure on the platelet surface and decreasing the rate of fibrin generation. Interestingly, ibrutinib was more potent than fostamatinib in reducing oxLDL-mediated platelet responses, suggesting that BTK inhibitors may be more effective than Syk inhibitors in reducing the synergistic effects of CD36 and GPVI on platelet procoagulant activity. Overall, our results suggest that tyrosine kinase signaling via BTK plays a central role in oxLDL-mediated platelet activation in a manner that may be therapeutically targeted to reduce platelet procoagulant activity in cardiovascular disease.
Acknowledgments
This work was supported by the Oregon Health & Science University Faculty Initiative Pool Fund, the Medical Research Foundation of Oregon, a Scholar Award from the American Society of Hematology (J.E.A.), and the National Institutes of Health (F30HL158079 [T.J.Z.], R01HL132985 [N.P.], R01HL101972 [O.J.T.M.], and R01HL146549 [J.E.A.]). Mass spectrometry studies were supported in part by National Institutes of Health grants to Oregon State University (S10RR022589 and S10OD026922) and Oregon Health & Science University (P30EY010572, P30CA069533, and S10OD-012246).
Authorship
Contribution: T.J.Z., P.A.M., J.F.S., and J.E.A. conceived and designed the research; T.J.Z., T.C.L.K., P.A.M., A.R.M., L.Y., J.C., K.D.Z., S.E.R., I.P.-I, J.P., A.P.R., J.F.S., and J.E.A. performed experiments; T.J.Z. and J.E.A. analyzed data; T.J.Z. and J.E.A. interpreted results of the experiments, prepared figures, and drafted the manuscript; T.J.Z., L.Y., J.C., M.K.L., C.D.W., J.J.S., J.F.S., M.T.H., O.J.T.M., and J.E.A. edited and revised the manuscript; T.J.Z., D.O.S., N.P., P.K., A.T.R., J.F.S., O.J.T.M., and J.E.A. provided funding and other resources for this study; and all authors approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph E. Aslan, Knight Cardiovascular Institute, Oregon Health & Science University, 3303 S Bond Ave, Mail code CH13B, Portland, OR 97239; e-mail: aslanj@ohsu.edu.
References
Author notes
Full material/reagents list and reproducible methods are provided in supplemental Methods.
Data are available on request from the corresponding author, Joseph E. Aslan (aslanj@ohsu.edu).
The full-text version of this article contains a data supplement.