Key Points
Factors XII and XI drive thrombosis in ECMO. FXII appears to promote organ damage in ECMO independent of thrombin generation.
Thrombin-mediated activation of FXI may overshadow the role of FXII in ECMO when tissue factor levels are high.
Abstract
Previous studies suggested that contact pathway factors drive thrombosis in mechanical circulation. We used a rabbit model of veno-arterial extracorporeal circulation (VA-ECMO) to evaluate the role of factors XI and XII in ECMO-associated thrombosis and organ damage. Factors XI and XII (FXI, FXII) were depleted using established antisense oligonucleotides before placement on a blood-primed VA-ECMO circuit. Decreasing FXII or FXI to < 5% of baseline activity significantly prolonged ECMO circuit lifespan, limited the development of coagulopathy, and prevented fibrinogen consumption. Histological analysis suggested that FXII depletion mitigated interstitial pulmonary edema and hemorrhage whereas heparin and FXI depletion did not. Neither FXI nor FXII depletion was associated with significant hemorrhage in other organs. In vitro analysis showed that membrane oxygenator fibers (MOFs) alone are capable of driving significant thrombin generation in a FXII- and FXI-dependent manner. MOFs also augment thrombin generation triggered by low (1 pM) or high (5 pM) tissue factor concentrations. However, only FXI elimination completely prevented the increase in thrombin generation driven by MOFs, suggesting MOFs augment thrombin-mediated FXI activation. Together, these results suggest that therapies targeting FXII or FXI limit thromboembolic complications associated with ECMO. Further studies are needed to determine the contexts wherein targeting FXI and FXII, either alone or in combination, would be most beneficial in ECMO. Moreover, studies are also needed to determine the potential mechanisms coupling FXII to end-organ damage in ECMO.
Introduction
Extracorporeal membrane oxygenation (ECMO) offers lifesaving support for patients with cardiac and/or respiratory failure, but hemorrhagic and thrombotic complications are a major cause of morbidity and mortality.1-3 Despite the widespread use of aggressive anticoagulation, circuit changes secondary to thrombi are required in up to 30% of cases and many patients suffer thrombotic complications.4 The incidence of ECMO-associated intracranial hemorrhage is as high as 10% to 20% in some studies.4-6 Bleeding and thrombotic complications are associated with a 30% to 40% decrease in survival.6 Clearly, novel strategies to limit thrombotic complications in ECMO are critically needed.
Despite advances in ECMO circuit design, the approach to anticoagulation has changed little since the inception of ECMO and remains primarily dependent on unfractionated heparin.7 Heparin is an effective anticoagulant with multiple benefits, including low cost, short half-life, and reversibility. However, the fact that ECMO carries significant thrombotic complications despite aggressive heparin anticoagulation with accompanying hemorrhagic risks speaks to the shortcomings of this approach. There has been recent interest in short-acting direct thrombin inhibitors (eg, bivalirudin) in ECMO. Although the pharmacokinetics of drugs like bivalirudin may be superior to heparin, substantial bleeding risk will accompany any agent that targets thrombin or fundamentally impairs thrombin generation.8
Multiple lines of evidence suggest that contact pathway factors (ie, factors XI and XII) play a significant role in pathological thrombosis but a more limited role in hemostasis.9-12 Factors XI and XII (FXI, FXII) have been suggested as targets to limit thromboembolic complications in multiple settings, including ECMO.10-14 However, the data supporting this approach in the context of a complete ECMO circuit are limited. FXII was targeted in 2 previous animal studies using a complete ECMO circuit.13,15 FXI was evaluated in a limited animal study of ECMO.14 Moreover, there are no direct comparisons of FXI and FXII as potential targets in ECMO. Here, we used a rabbit ECMO model to better define the potential of targeting FXI and FXII to limit ECMO-associated thrombosis. We show that targeting either FXI or FXII extends ECMO circuit life and limits fibrinogen consumption. Targeting FXII prevents ECMO-associated organ damage. We also show in vitro data suggesting that targeting FXI may be an effective anticoagulation strategy when contact activation of hemostatic proteases is combined with tissue factor (TF)-mediated initiation of coagulation.
Materials and methods
Rabbit ECMO model
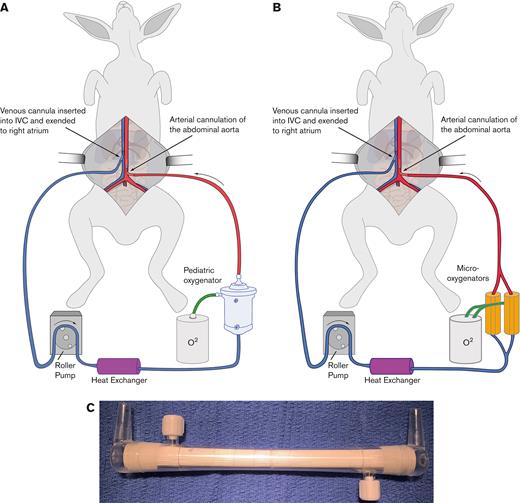
New Zealand white rabbits were placed on veno-arterial ECMO via cannulation of the abdominal aorta and vena cava. The ECMO circuits were created with either a Medos Hilite LT Infant 800 oxygenator (Xenios AG) for the heparin/protamine cohort or by parallel application of 2 Micro-1 oxygenators (Dongguan Kewei Medical Instrument Co) for the citrate/calcium cohort (Figure 1). The circuit was primed with either heparinized or citrated blood collected from 2 donor rabbits. Once the circuit was running for ∼5 minutes, protamine was administered to reverse the effects of the heparin in the donor blood. Alternatively, calcium was administered to normalize plasma calcium, reversing citrate anticoagulation. These groups of animals are referred to as the heparin/protamine cohort or the citrate/calcium cohort, respectively.
Schematic of the ECMO circuit. Shown is a schematic of the rabbit VA-ECMO circuit used in the protamine/heparin cohort (A) and the citrate/calcium cohort (B). Note that the Medos Hilite LT Infant 800 oxygenator used in the protamine/heparin cohort was replaced with 2 Micro-1 oxygenators (C) in the citrate/calcium cohort.
Schematic of the ECMO circuit. Shown is a schematic of the rabbit VA-ECMO circuit used in the protamine/heparin cohort (A) and the citrate/calcium cohort (B). Note that the Medos Hilite LT Infant 800 oxygenator used in the protamine/heparin cohort was replaced with 2 Micro-1 oxygenators (C) in the citrate/calcium cohort.
Refer to the supplemental Material for additional details regarding the ECMO model and methods.
Results
Depleting FXII prolongs ECMO circuit lifespan
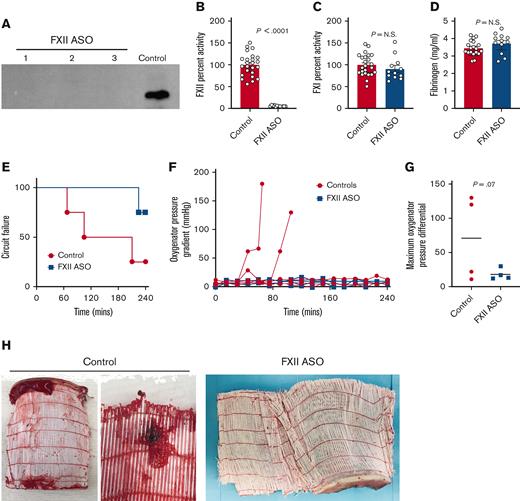
To determine the potential of targeting FXII as a means to limit ECMO-associated thromboembolism, we used an established, specific antisense oligonucleotide (ASO) to limit FXII production.16,17 Rabbits were treated with FXII ASO (20 mg/kg) beginning 4 weeks before ECMO to allow time for decay of circulating FXII protein. For each rabbit placed on ECMO, 2 additional rabbits that served as blood donors were treated with FXII ASO in parallel. FXII ASO treatment significantly diminished circulating FXII. Plasma FXII protein levels were below the detection limit of western blot analyses (Figure 2A). Some residual FXII activity (∼5% of normal) was detectable using a one-stage aPTT-based clotting assay (Figure 2B). Consistent with the established specificity of the FXII ASO, the activity of FXI and fibrinogen were unaffected (Figure 2C-D).
Targeting FXII limits thrombotic complications and prolongs ECMO circuit life (heparin/protamine cohort). (A) Shown is a western blot of plasma FXII in 3 rabbits after 4 weeks of FXII ASO treatment (20 mg/kg) and in control rabbit plasma. Note that the plasma levels of FXII protein in these animals were all below the detection limit of this assay. (B) FXII ASO treatment resulted in a significant decrease in plasma FXII activity (∼5% of controls) but had no effect on FXI activity (C), or fibrinogen levels (D). Note that plasma from FXII ASO-treated rabbits was analyzed before (control) and after ASO treatment (FXII ASO) for factor activities and fibrinogen. Data obtained before treatment were included in the control cohort. (E-H) Shown are the results of experiments where donor rabbit blood was anticoagulated with heparin, followed by protamine reversal. (E) FXII-depletion prolonged the time to circuit failure (P = .07, Kaplan-Meier analysis). (F) Shown are the pressures across the oxygenator as a function of time for each animal. (G) The maximal pressures across the oxygenator were higher in control animals relative to animals with depleted FXII (P = .07, Mann-Whitney U test.) (H) Shown are examples of large clots observed in 2 of the oxygenators used in control animals. In contrast, none of the oxygenators used in FXII ASO-treated rabbits had gross evidence of clots. P values were generated using a Mann-Whitney U test; the data represent the mean and standard error of the mean (SEM). N.S., not significant.
Targeting FXII limits thrombotic complications and prolongs ECMO circuit life (heparin/protamine cohort). (A) Shown is a western blot of plasma FXII in 3 rabbits after 4 weeks of FXII ASO treatment (20 mg/kg) and in control rabbit plasma. Note that the plasma levels of FXII protein in these animals were all below the detection limit of this assay. (B) FXII ASO treatment resulted in a significant decrease in plasma FXII activity (∼5% of controls) but had no effect on FXI activity (C), or fibrinogen levels (D). Note that plasma from FXII ASO-treated rabbits was analyzed before (control) and after ASO treatment (FXII ASO) for factor activities and fibrinogen. Data obtained before treatment were included in the control cohort. (E-H) Shown are the results of experiments where donor rabbit blood was anticoagulated with heparin, followed by protamine reversal. (E) FXII-depletion prolonged the time to circuit failure (P = .07, Kaplan-Meier analysis). (F) Shown are the pressures across the oxygenator as a function of time for each animal. (G) The maximal pressures across the oxygenator were higher in control animals relative to animals with depleted FXII (P = .07, Mann-Whitney U test.) (H) Shown are examples of large clots observed in 2 of the oxygenators used in control animals. In contrast, none of the oxygenators used in FXII ASO-treated rabbits had gross evidence of clots. P values were generated using a Mann-Whitney U test; the data represent the mean and standard error of the mean (SEM). N.S., not significant.
A total of 8 rabbits (4 pretreated with FXII ASO and 4 saline carrier–treated controls) were placed on ECMO using a simplified, uncoated ECMO circuit with a Medos Hilite LT Infant 800 oxygenator (Figure 1A). The circuits were primed with donor blood from 2 rabbits pretreated with FXII ASO or saline over the same time frame. Here, rabbit donor blood was heparinized to prevent thrombosis within the circuit before ECMO initiation. Once the rabbits were cannulated and the circuit was flowing well, heparin was reversed with protamine. This group is referred to as the “heparin/protamine” cohort. Analysis of plasma anti-Xa levels after protamine reversal confirmed that there was no detectable heparin in any of the 8 rabbits analyzed. According to a preestablished protocol, the rabbits were monitored closely until the circuit failed or until a total of 240 minutes of ECMO time was achieved. Consistent with studies showing that FXII plays no role in hemostasis,10-13,18 surgical bleeding was minimal and indistinguishable between the 2 cohorts.
Circuit failure occurred more rapidly in control rabbits compared with FXII ASO-treated rabbits (Figure 2E). Only 1 of the 4 control rabbits reached the predetermined completion time of 240 minutes, whereas 3 of the 4 FXII ASO-pretreated rabbits completed the 240-minute run time. This did not reach statistical significance (P = .07, chi square test). Circuit failure, characterized by rapidly rising pressure gradients across the oxygenator (Figure 2F-G) with gross thrombi visible in the oxygenator (Figure 2H) was experience by 2 of the 4 control rabbits. Consistent with this, the maximum pressure across the oxygenator was overall higher in the control rabbits than in the FXII ASO-treated animals (Figure 2G). Of the 2 remaining control rabbits, 1 rabbit experienced circuit failure because of a large thrombus in the venous cannula, and there were no obvious thrombi in the circuit of the 1 control rabbit that reached 240 minutes. ECMO had to be terminated at ∼220 minutes in 1 of the 4 FXII ASO-treated rabbits owing to insufficient venous drainage. The reason for this was unclear. No thrombi were seen in the circuit and pressures across the oxygenator remained low throughout this ECMO run. The other 3 FXII ASO-treated rabbits completed the 240-minute experiment without complications or evidence of thrombi in the circuit (Figure 2H).
Factors XII and XI promote thrombosis in ECMO
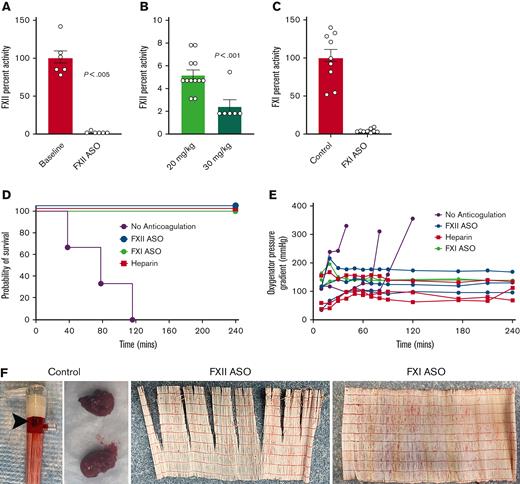
To define the thrombotic potential of FXII in ECMO in a separate experiment, we changed the experimental protocol to remove confounding factors. We changed the donor blood anticoagulant from heparin to citrate, allowing restoration of hemostatic function by normalizing plasma calcium rather than administering protamine. Protamine can cause hypotension, endothelial dysfunction, and complement activation, making it a potentially confounding factor.19-22 Because the large pediatric oxygenator can function despite significant clot burden, we changed the circuit to use 2 small animal micro-oxygenators attached in parallel (Micro-1, Dongguan Kewei Medical Instruments Co) (Figure 1B). Lastly, we increased the FXII ASO dose to 30 mg/kg because residual FXII activity could trigger thrombin generation. The increased FXII ASO dose resulted in better reduction of FXII activity to ∼2% of normal (Figure 3A-B).
Targeting FXI or FXII limits thrombotic complications and prolongs ECMO circuit life (citrate/calcium cohort). (A) A higher dose of FXII ASO (30 mg/kg) decreased circulating FXII activity to ∼2% of normal, a level significantly lower than that achieved with 20 mg/kg (B). (C) A separate cohort of rabbits was treated with an FXI-specific ASO resulting in FXI activity levels < 5% of baseline at the time of ECMO initiation. (D-F) Shown are the results of experiments where donor rabbit blood was anticoagulated with citrate, followed by normalization of plasma calcium. (D) Depletion of either FXI or FXII significantly prolonged the life of a revised ECMO circuit using 2 micro-oxygenators placed in parallel. (E) Shown are the pressures across the oxygenators as a function of time for each animal. Note that all 3 control rabbits experienced significant pressure spikes across the oxygenators just before circuit failure, whereas oxygenator pressure remained relatively stable in the heparin-treated animals, as well as the FXII- and FXI-depleted animals. (F) Thrombi were readily evident on all the oxygenators used for the control animals, whereas none of the oxygenators used in heparin, FXI ASO-, or FXII ASO-treated rabbits had evidence of visible thrombi.
Targeting FXI or FXII limits thrombotic complications and prolongs ECMO circuit life (citrate/calcium cohort). (A) A higher dose of FXII ASO (30 mg/kg) decreased circulating FXII activity to ∼2% of normal, a level significantly lower than that achieved with 20 mg/kg (B). (C) A separate cohort of rabbits was treated with an FXI-specific ASO resulting in FXI activity levels < 5% of baseline at the time of ECMO initiation. (D-F) Shown are the results of experiments where donor rabbit blood was anticoagulated with citrate, followed by normalization of plasma calcium. (D) Depletion of either FXI or FXII significantly prolonged the life of a revised ECMO circuit using 2 micro-oxygenators placed in parallel. (E) Shown are the pressures across the oxygenators as a function of time for each animal. Note that all 3 control rabbits experienced significant pressure spikes across the oxygenators just before circuit failure, whereas oxygenator pressure remained relatively stable in the heparin-treated animals, as well as the FXII- and FXI-depleted animals. (F) Thrombi were readily evident on all the oxygenators used for the control animals, whereas none of the oxygenators used in heparin, FXI ASO-, or FXII ASO-treated rabbits had evidence of visible thrombi.
FXII can activate FXI, ultimately leading to thrombin generation. FXII also activates prekallikrein to the active protease kallikrein, which has been linked to complement activation and cleavage of bradykinin from high molecular weight kininogen.18 Recent studies also indicate that kallikrein can activate FXI, providing an additional link between FXII activation and thrombin generation.23 Therefore, FXII could promote thrombosis in ECMO through FXI-dependent and -independent mechanisms. To specifically define the role of FXI in ECMO, we treated a total of 9 rabbits with an established FXI ASO at 40 mg/kg per dose, weekly for 3 weeks.17 Three of these animals were placed on ECMO using our revised circuit and the remainder were used as blood donors. As expected, this dosing regimen decreased FXI levels to <5% of baseline (Figure 3C). All the activated partial thromboplastin times (aPTTs) in the FXI-depleted rabbits were essentially not clottable (>300 seconds; data not shown). Previous studies have demonstrated the specificity of the FXI and FXII ASOs used here with neither affecting the prothrombin time or other hemostatic parameters in treated rabbits.17
A total of 12 rabbits were enrolled using this revised protocol; 3 control rabbits receiving no anticoagulation, 3 FXII ASO-treated rabbits, 3 FXI ASO-treated rabbits, and 3 rabbits anticoagulated with heparin throughout the experiment enrolled to ensure the revised circuit functioned as expected. Refer to the supplemental Material for details regarding heparin dosing in these animals. Note that the FXII ASO- and FXI ASO-treated rabbits received no heparin. Baseline platelet counts were similar between the 4 groups (supplemental Figure 1). The ECMO circuit was primed with donor blood from 2 rabbits pretreated with FXII ASO, FXI ASO, or blood from control rabbits treated with saline over the same time frame. After the rabbits were cannulated and the circuit was flowing at 100 mL/kg per minute, calcium was normalized, reversing the citrate. These animals are referred to as the “citrate/calcium cohort.” The 3 rabbits anticoagulated with heparin completed the 240-minute experiment without complications or evidence of circuit thrombi, demonstrating that the micro-oxygenators functioned as expected (Figure 3D). The 3 control (nonanticoagulated) rabbits all experienced circuit failure within 120 minutes of ECMO initiation (Figure 3D). In contrast, the FXII- and FXI-depleted rabbits all remained on ECMO for the entire 240-minute experiment without evidence of complications or thrombosis (Figure 3D). All 3 control rabbits experienced spikes in pressure across the oxygenators just before circuit failure, whereas the pressure gradient remained stable in the heparin-treated, as well as the FXII- and FXI-depleted animals (Figure 3E). Note that the baseline pressure gradient across these oxygenators was higher because of the smaller-sized inlet connections compared with the Medos Hilite LT Infant 800 oxygenator used in the protamine/heparin cohort. Gross thrombi were found in all the oxygenators used with control rabbits. None of the oxygenators used with heparin-treated, FXII-depleted, or FXI-depleted rabbits exhibited thrombi (Figure 3F).
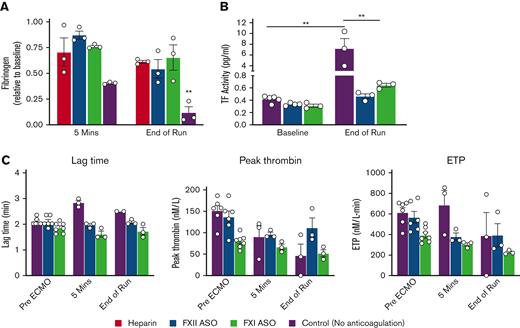
We measured plasma fibrinogen in all 12 rabbits before ECMO initiation, ∼5 minutes after normalization of calcium, and at the end of the ECMO run. Consistent with the fact that fibrinogen quickly coats ECMO circuit components,7,24 there was a modest decrease in plasma fibrinogen in the heparin-treated as well as the FXII- and FXI-depleted rabbits ∼5 minutes after calcium normalization (Figure 4A). However, this initial decrease was more substantial in the control rabbits (Figure 4A). At the end of the ECMO run, fibrinogen levels were similarly decreased to ∼60% of baseline in the heparin-treated and FXII- and FXI-depleted cohorts but were substantially depleted in the control group (Figure 4A). Next, we measured circulating TF activity in plasma collected pre-ECMO (donors and recipients) and at the end of the ECMO run. Rabbits anticoagulated with heparin were not included in these analyses because heparin interferes with this assay. TF activity was similarly low in all of the pre-ECMO samples (Figure 4B). TF activity was significantly increased in the control animals at the end of the ECMO run relative to pre-ECMO samples. There was a trend toward an increase in TF activity in the FXI ASO-treated rabbits, but this was not statistically significant given the low number of replicates (Figure 4B). We also measured TF-initiated thrombin generation in plasma from the control, FXII-, and FXI-depleted rabbits using a thrombin generation assay (TGA). TGAs were not performed in the heparin group as heparin strongly inhibits this assay. Consistent with the fact that FXII plays no role in physiological hemostasis, there was no significant difference in the lag time, peak thrombin, or endogenous thrombin potential between the control and FXII-depleted rabbits pre-ECMO (Figure 4C). Moreover, as expected, FXI depletion resulted in a decrease in peak thrombin and endogenous thrombin potential at baseline.25,26 Shortly after ECMO initiation, plasma from control rabbits showed an increased lag time and diminished peak thrombin, suggesting a consumptive coagulopathy. In fact, 1 of the control rabbits had no detectable thrombin generation at the end of the ECMO run. In contrast, thrombin generation remained relatively stable in the FXII- and FXI-depleted rabbits over the ECMO course (Figure 4C).
Targeting FXI or FXII limits fibrinogen consumption, coagulopathy, and circulating TF in ECMO. (A) Shown are plasma fibrinogen levels at baseline, 5 minutes after citrate reversal, and at the end of the ECMO run. Note that depletion of FXI or FXII maintained plasma fibrinogen levels compared with heparin treatment. (B) Shown are measurements of plasma TF activity pre- and post-ECMO. (C) Shown are results of TGAs initiated with 5 pM TF in control (purple), FXII-depleted (blue), and FXI-depleted (green) rabbits pre-ECMO, 5 minutes after citrate reversal, and at the end of the ECMO run. ECMO resulted in a significant increase in the lag time and a decrease in peak thrombin in control rabbits, whereas thrombin generation remained relatively stable in the FXII ASO-treated rabbits. As expected, FXI depletion resulted in a decrease in thrombin generation at baseline, but overall thrombin generation remained stable throughout the ECMO run. Note that 1 of the control rabbits had no measurable thrombin generation at the end of the ECMO run. ∗∗P < .01, 2-way analysis of variance; data represent the mean and SEM.
Targeting FXI or FXII limits fibrinogen consumption, coagulopathy, and circulating TF in ECMO. (A) Shown are plasma fibrinogen levels at baseline, 5 minutes after citrate reversal, and at the end of the ECMO run. Note that depletion of FXI or FXII maintained plasma fibrinogen levels compared with heparin treatment. (B) Shown are measurements of plasma TF activity pre- and post-ECMO. (C) Shown are results of TGAs initiated with 5 pM TF in control (purple), FXII-depleted (blue), and FXI-depleted (green) rabbits pre-ECMO, 5 minutes after citrate reversal, and at the end of the ECMO run. ECMO resulted in a significant increase in the lag time and a decrease in peak thrombin in control rabbits, whereas thrombin generation remained relatively stable in the FXII ASO-treated rabbits. As expected, FXI depletion resulted in a decrease in thrombin generation at baseline, but overall thrombin generation remained stable throughout the ECMO run. Note that 1 of the control rabbits had no measurable thrombin generation at the end of the ECMO run. ∗∗P < .01, 2-way analysis of variance; data represent the mean and SEM.
FXII depletion limits ECMO-associated lung damage
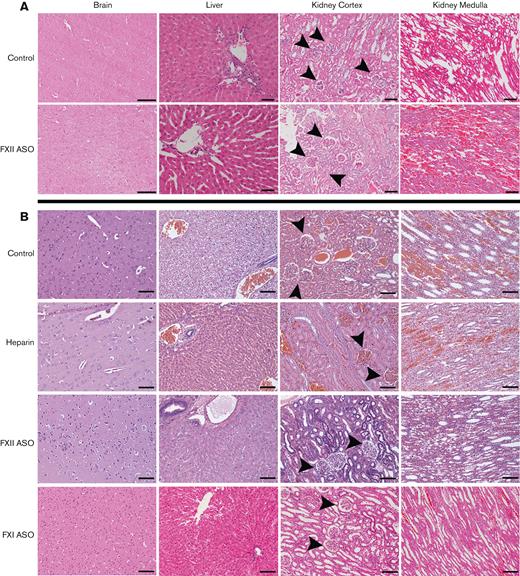
Immediately after ECMO, the rabbits were euthanized, and the brain, lungs, liver, and kidneys harvested for histology. Tissue sections were reviewed by a pathologist blinded to treatment. The brain sections analyzed from the rabbits in the heparin/protamine and citrate/calcium cohorts showed mild perivascular edema with intact brain parenchyma and no evidence of hemorrhage, ischemia, or necrosis, regardless of treatment (Figure 5). Liver and kidney tissue from the protamine/heparin and citrate/calcium cohorts revealed various degrees of congestion regardless of treatment, likely reflecting systemic venous congestion (Figure 5). There was no evidence of liver necrosis, hemorrhage, or significant hepatocyte damage in any of the cohorts (Figure 5). Petechial hemorrhages were noted in the renal medulla; these were more frequent in 1 animal in each cohort, but there were no clear treatment-dependent differences. There was no evidence of significant glomerular damage or necrosis in any of the other kidney tissues.
Histological analysis of organs. Shown are representative H&E-stained sections of organ tissue harvested from rabbits in the heparin/protamine cohort (A) and the citrate/calcium cohort (B). No significant necrosis, hemorrhage, or parenchymal damage was identified in any of the brain or liver tissues in either cohort, regardless of treatment. There was uniform evidence of renal and liver venous congestion in the control and heparin-treated animals, regardless of treatment. Glomeruli (arrowheads) were generally intact without evidence of necrosis. Scale bars, 100 μm; original magnification ×100.
Histological analysis of organs. Shown are representative H&E-stained sections of organ tissue harvested from rabbits in the heparin/protamine cohort (A) and the citrate/calcium cohort (B). No significant necrosis, hemorrhage, or parenchymal damage was identified in any of the brain or liver tissues in either cohort, regardless of treatment. There was uniform evidence of renal and liver venous congestion in the control and heparin-treated animals, regardless of treatment. Glomeruli (arrowheads) were generally intact without evidence of necrosis. Scale bars, 100 μm; original magnification ×100.
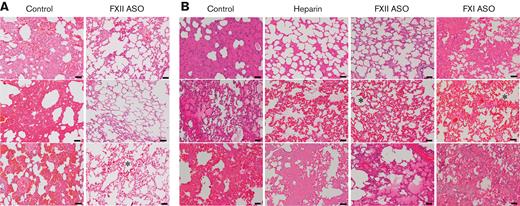
Analyses of pulmonary tissue revealed more striking differences between the FXII-depleted rabbits and the other cohorts. All the lung tissue demonstrated venous congestion and emphysematous air space dilation, regardless of treatment or method of initial anticoagulation. Several lungs in each cohort had evidence of capillary congestion, a common finding in ECMO.27 Lung tissue was scored based on the total area of tissue with evidence of edema and/or hemorrhage: 0 = <10%, 1 = 10% to 25%, 2 = 25% to 50%, 3 = >50% area involved. All the lung tissue from control rabbits (both heparin/protamine and citrate/calcium cohorts) had significant interstitial edema and/or hemorrhage defined as a score of 2 or 3, P = .005 compared with controls, Fisher exact test (Figure 6A-B and supplemental Table 1). Only 1 of the 7 FXII-depleted rabbits (citrate/calcium cohort) had evidence of significant edema/hemorrhage (Figure 6B and supplemental Table 1). Although statistically meaningful comparisons with the heparin and FXI ASO-treated cohort were not possible given the relatively small cohort sizes, neither heparin treatment nor FXI depletion appeared to confer the same degree of protection from edema/hemorrhage as FXII depletion (supplemental Table 1). Occasional neutrophils were seen in areas of edema/hemorrhage, but there was no evidence of pneumonitis. Refer to supplemental Figure 2 for higher powered views. To better characterize this pathology, we performed a Movat pentachrome stain on tissue sections from the citrate/calcium cohort (supplemental Figure 3). Although not absolutely specific, this methodology stains fibrin(ogen) red. The areas of edema/hemorrhage seen on hematoxylin and eosin (H&E) staining, stained red in the pentachrome stain, suggested the presence of fibrin(ogen). It is unclear whether this pathology represents edema or hemorrhage, but it is notable that extravasated red cells were not seen on H&E or pentachrome stained sections, suggesting the pathology is primarily related to loss of vascular integrity and not frank hemorrhage.
FXII depletion appears to limit ECMO-related lung pathology. Shown are representative H&E-stained sections of lung tissue harvested immediately after termination of ECMO. (A) Lung tissue from 3 of the 4 control rabbits (the remaining animal was not evaluated because of problems related to tissue processing) in the heparin/protamine cohort had evidence of significant interstitial hemorrhage and edema. In contrast, lung tissue harvested from the 4 FXII ASO-treated animals was relatively intact. (B) Lung tissue from control rabbits in the citrate/calcium cohort paralleled findings in the heparin/protamine cohort with significant interstitial edema and hemorrhage. Lung tissue from the FXI-depleted and heparin-treated animals in this cohort had more moderate evidence of interstitial edema/hemorrhage. In contrast, only 1 of the 4 FXII ASO-treated rabbits in this cohort had evidence of edema (bottom panel). Several of the lungs had evidence of capillary congestion (indicated by an asterisk), a common finding in ECMO. Scale bars, 50 μm; original magnification ×100.
FXII depletion appears to limit ECMO-related lung pathology. Shown are representative H&E-stained sections of lung tissue harvested immediately after termination of ECMO. (A) Lung tissue from 3 of the 4 control rabbits (the remaining animal was not evaluated because of problems related to tissue processing) in the heparin/protamine cohort had evidence of significant interstitial hemorrhage and edema. In contrast, lung tissue harvested from the 4 FXII ASO-treated animals was relatively intact. (B) Lung tissue from control rabbits in the citrate/calcium cohort paralleled findings in the heparin/protamine cohort with significant interstitial edema and hemorrhage. Lung tissue from the FXI-depleted and heparin-treated animals in this cohort had more moderate evidence of interstitial edema/hemorrhage. In contrast, only 1 of the 4 FXII ASO-treated rabbits in this cohort had evidence of edema (bottom panel). Several of the lungs had evidence of capillary congestion (indicated by an asterisk), a common finding in ECMO. Scale bars, 50 μm; original magnification ×100.
MOFs drive thrombin generation
Our data support the conclusion that targeting either FXI or FXII limits thrombosis in ECMO. However, these studies were performed in healthy animals, whereas ECMO is generally used to treat patients that are critically ill, suffering from infection, with severe inflammatory diseases, and/or having postsurgical complications. These clinical states would result in high levels of circulating TF. Robust TF-mediated thrombin generation can lead to thrombin-mediated activation of FXI, resulting in further thrombin generation. To begin addressing this issue, we compared the ability of membrane oxygenator fibers (MOFs) to generate thrombin alone or in the presence of TF, in normal plasma from a healthy donor (control) as well as commercially available FXII-depleted (FXIIO) and FXI-depleted (FXIO) human plasmas using a TGA. Note that both the FXIIO and FXIO plasmas have <0.01 U/mL FXI or FXII activity.
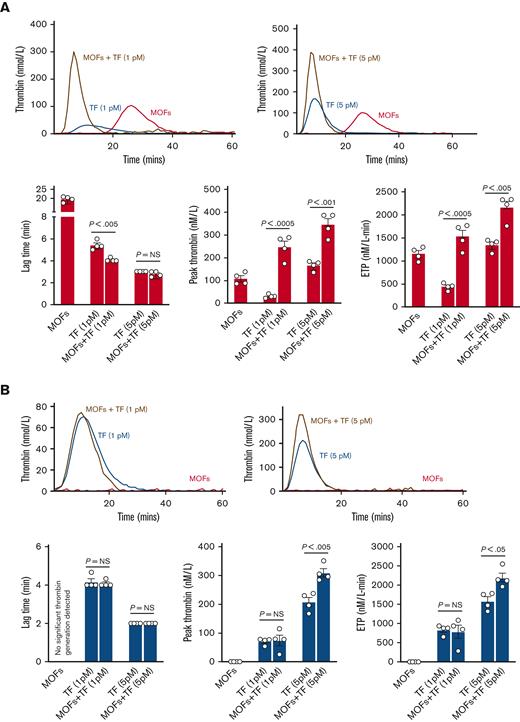
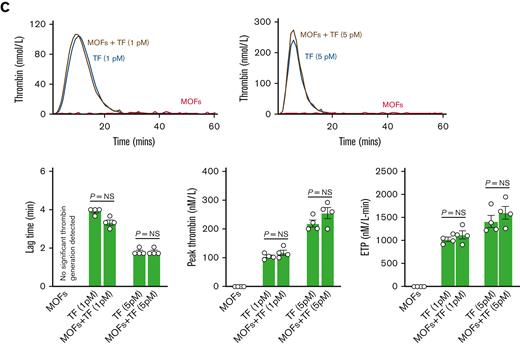
MOFs alone were capable of driving significant thrombin generation in control plasma, although with a longer lag time than TF-driven thrombin generation (Figure 7A). When combined with a low concentration of TF (1 pM), the addition of MOFs decreased the lag time and significantly augmented thrombin generation relative to 1 pM TF alone (Figure 7A). MOFs had no impact on the already short lag time when combined with a higher concentration of TF (5 pM) but still significantly augmented the total thrombin generated (Figure 7A). These data indicate that MOFs are capable of driving thrombin generation in the absence of TF and that MOFs augment thrombin generation when combined with TF.
MOF-driven thrombin generation is dependent on factors XII and XI. (A) Shown are representative TGA curves and quantitation of the lag time, peak thrombin, and endogenous thrombin potential when MOFs are added to normal human plasma alone or in combination with TF. Note that MOFs alone are capable of significant thrombin generation in the absence of TF. MOFs also augment TF-mediated thrombin generation when used in combination with either a low (1 pM) or high (5 pM) concentration of TF. (B) Shown are the results of TGA assays where MOFs were added to FXII-depleted human plasma (FXIIO) with or without TF. Note that depletion of FXII completely abrogated thrombin generation driven by MOFs alone. FXII elimination also abrogated the increase in thrombin generation seen when MOFs were added to a low concentration of TF. However, MOFs still significantly increased thrombin generation when added together with a higher concentration of TF. (C) Shown are the results of TGA assays where MOFs were added to FXI-depleted human plasma (FXIO) with or without TF. FXI depletion also eliminated thrombin generation triggered by MOFs alone. Moreover, FXI depletion also completely mitigated the increase in thrombin generation seen when MOFs were added together with either low or high concentrations of TF. P values were generated using a Student t test; data represent the mean and SEM.
MOF-driven thrombin generation is dependent on factors XII and XI. (A) Shown are representative TGA curves and quantitation of the lag time, peak thrombin, and endogenous thrombin potential when MOFs are added to normal human plasma alone or in combination with TF. Note that MOFs alone are capable of significant thrombin generation in the absence of TF. MOFs also augment TF-mediated thrombin generation when used in combination with either a low (1 pM) or high (5 pM) concentration of TF. (B) Shown are the results of TGA assays where MOFs were added to FXII-depleted human plasma (FXIIO) with or without TF. Note that depletion of FXII completely abrogated thrombin generation driven by MOFs alone. FXII elimination also abrogated the increase in thrombin generation seen when MOFs were added to a low concentration of TF. However, MOFs still significantly increased thrombin generation when added together with a higher concentration of TF. (C) Shown are the results of TGA assays where MOFs were added to FXI-depleted human plasma (FXIO) with or without TF. FXI depletion also eliminated thrombin generation triggered by MOFs alone. Moreover, FXI depletion also completely mitigated the increase in thrombin generation seen when MOFs were added together with either low or high concentrations of TF. P values were generated using a Student t test; data represent the mean and SEM.
MOFs alone were incapable of thrombin generation in FXIIO or FXIO plasma, indicating that FXII and FXI are critical for MOF-driven thrombin generation (Figure 7B-C). Similarly, MOFs failed to augment thrombin generation when added together with a low concentration of TF (1 pM) in either FXIIO or FXIO plasmas (Figure 7B-C). Conversely, when MOFs were combined with a higher concentration of TF (5 pM), they significantly augmented thrombin generation in FXIIO plasma (Figure 7B). However, FXIO plasma abrogated the ability of MOFs to augment thrombin generation even at this higher (5 pM) concentration of TF (Figure 7C). These results suggest that (1) MOFs can initiate thrombin generation in the absence of TF by activating FXII and (2) MOFs are also capable of augmenting thrombin-mediated activation of FXI. Note that the control, FXIIO, and FXIO plasmas were not derived from the same donor(s), therefore direct comparisons cannot be made across plasmas.
Discussion
The data presented here indicate that targeting FXI or FXII limits thrombosis and extends circuit life in ECMO, suggesting that FXII-mediated activation of FXI is a major driver of thrombosis in mechanical circulation. FXII depletion appeared to limit ECMO-associated lung pathology, but the mechanisms driving this effect remain to be determined. FXII-dependent inflammatory pathways independent of thrombin generation may play a role. Our in vitro TGA data showed that in contexts where MOFs are the primary initiator of thrombin generation (ie, there is no TF present), eliminating either FXII or FXI eliminates thrombin generation and subsequent clot formation. Our TGA data also suggested that MOFs can augment thrombin generation in the presence of TF, and that in contexts where there are higher concentrations of TF combined with MOFs, eliminating FXII alone does not limit thrombin generation as efficiently as eliminating FXI. These data suggest that MOFs are capable of promoting thrombin-mediated activation of FXI, bypassing FXII-mediated activation of FXI. This hypothesis parallels what has been observed previously with biological macromolecules such as DNA and polyphosphates.28-30
FXII undergoes autoactivation when bound to negatively charged surfaces.11,12,31 Once activated, FXII activates FXI, ultimately leading to thrombin generation.11,12,31 Although FXII plays no role in surgical hemostasis,11,32-35 it appears to drive thrombosis in multiple nonmechanical circulation experimental settings.9,17,33-41 The data presented here are consistent with the view that FXII-mediated activation of FXI plays a major role in ECMO-associated thrombosis and are consistent with previous studies suggesting that targeting FXII or FXI limits ECMO-associated thrombosis.13-15 Our data and previous studies indicate that FXII can be targeted without bleeding risk. We saw evidence of edema and/or hemorrhage in the lungs from the FXI-depleted rabbits comparable with what was seen with heparin. We did not see evidence of significant hemorrhage in any of the other organs evaluated from the FXI ASO-treated animals or at the surgical site. Data from previous animal studies have suggested that targeting FXI in ECMO limits bleeding risk relative to heparin anticoagulation14; Pollack et al used a small-molecule inhibitor of FXIa in a canine ECMO model targeting an aPTT of ∼2.3× baseline.14 In our study, residual FXI activity was <5% of baseline and aPTTs were unclottable. Humans with mild to moderate FXI deficiency have mild bleeding symptoms relative to other factor deficiencies.11 Moreover, a clinical trial of a human FXI ASO, analogous to the rabbit FXI ASO used in this study, showed that FXI depletion to levels of ∼20% of normal before total knee replacement surgery (ie, patients underwent knee replacement with low FXI activity) significantly decreased postoperative thrombosis relative to postoperative enoxaparin treatment, without any increase in intraoperative bleeding.16 Taken together, the available data suggest that FXI could be a beneficial target in ECMO. However, additional studies are needed. The optimal intensity of anti-FXI therapy in ECMO is unclear and will likely be context dependent. Patients with FXI deficiency generally do not experience severe bleeding, however injury to tissues high in fibrinolytic activity (eg, oral mucosa, urinary tract) tends to result in more significant bleeding in patients with FXI deficiency.42 Injury to these tissues can be a concern in patients requiring ECMO. Moreover, it has been proposed that a major function of FXI is to improve clot stability, and ECMO tends to create a hyperfibrinolytic state. The ideal drug targeting FXI in ECMO would be one with a short half-life that could be readily titrated to a desired effect based on perceived bleeding risks.
The ASOs used in the this study provided a proof-of-principle for evaluating the roles of FXII and FXI in ECMO-associated thrombosis and organ damage. Although the available evidence indicates that the ASO approach used here is highly efficient, specific, and clinically safe,16,17 these modalities would obviously not be clinically suitable for urgent mechanical circulation needs such as ECMO. However, they could be used clinically for elective indications, such as cardiopulmonary bypass or ventricular assist devices. Of course, additional studies are needed to determine the therapeutic potential of targeting FXII and/or FXI in these contexts, and also to ensure that this approach has no unanticipated effects in the context of mechanical circulation.
Although targeting FXII showed benefit in our preclinical models of ECMO, it remains to be determined how effective targeting FXII would be in limiting ECMO-associated thrombosis in “real-world” settings where factors in addition to the ECMO circuit promote thrombosis.43 The shear forces associated with ECMO result in unfolding of von Willebrand factor, platelet activation, and rapid platelet deposition on the surfaces of the circuit.7,44,45 ECMO in patients that are critically ill is also associated with increased circulating TF derived from inflammatory and endothelial cell activation.46-48 Our data demonstrate that MOFs promote significant thrombin generation in vitro in the absence of TF, and MOF-driven thrombin generation is critically dependent on factors XI and XII. Moreover, MOFs were capable of augmenting TF-driven thrombin generation, but the roles of FXI and FXII in this process appear to be TF concentration dependent. At lower concentrations of TF (ie, 1 pM) elimination of FXI and FXII prevented any MOF-induced increase in thrombin generation relative to TF alone. In contrast, at a higher concentration of TF (ie, 5 pM), only elimination of FXI prevented an MOF-induced increase in thrombin generation. These data suggest that in contexts where there is sufficient TF to generate a significant initial burst of thrombin, MOF-mediated promotion of thrombin-mediated FXI activation becomes more important than FXII-mediated FXI activation. This conclusion parallels previous observations showing that certain biological macromolecules (ie, polyphosphates, DNA) enhance thrombin-mediated activation of FXI.28-30 Together, these studies suggest that targeting FXII alone may not provide sufficient thromboprophylaxis in certain contexts. FXI may be a better therapeutic target in ECMO in contexts where circulating TF is high. Future studies are needed to better define contexts where targeting FXII may be inadequate in ECMO, and to determine what degree of FXI activity carries the best risk-to-benefit ratio in terms of limiting thrombosis without significantly increasing bleeding risk.
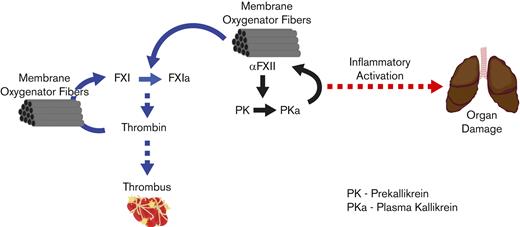
Our data suggest a role for FXII in ECMO-associated lung damage. This was most evident in comparisons of FXII ASO-treated rabbits vs controls that received no anticoagulation. Indeed, the significantly elevated circulating TF levels seen in control rabbits post-ECMO may have reflected organ damage. FXII lies at a critical nexus between the hemostatic and inflammatory systems. In addition to FXI, FXII activates prekallikrein to the active protease kallikrein49,50 (refer to Visual Abstract). FXII and kallikrein are multifunctional proteases capable of promoting key inflammatory and hemostatic events. FXII and kallikrein can activate complement components31,51,52 but the importance of this in vivo remains to be defined. FXII can directly activate neutrophils and promote release of neutrophil extracellular traps by binding to urokinase plasminogen activator receptor.53 Kallikrein cleaves high molecular weight kininogen, resulting in release of bradykinin, a key mediator of inflammatory processes and vascular leak.10,12,54 Kallikrein-mediated bradykinin generation has been linked to signaling pathways that decrease circulating prostacyclin, resulting in increased endothelial TF expression,55,56 suggesting that kallikrein can promote thrombin generation independent of FXI. Previous studies also showed that kallikrein can activate FIX in the absence of FXI.23,57,58 ECMO creates a profoundly proinflammatory state, characterized by significant systemic increases in inflammatory cytokines, myeloid cell activation, endothelial damage/leak, and complement activation.59,60 It remains to be determined whether the relative sparing of lung pathology observed in the FXII-depleted rabbits in this study is, at least in part, connected to these FXII-dependent inflammatory pathways. Further studies are needed to better define the role of FXII and prekallikrein in mechanical circulation–associated inflammation. We may learn that in mechanical circulation contexts where there is concern for both thrombosis and inflammation, combinations of therapies targeting FXI and/or FXII and prekallikrein may be more beneficial than targeting either alone. This approach would limit the acceleration of thrombin generation driven by FXI, and inflammatory pathways driven by the FXII/kallikrein axis.
Acknowledgments
The authors thank Andy Watt for in vitro screening of rabbit FXI and FXII ASOs and Chris Woods for his graphical artistic assistance.
It is with deep sadness that the authors report James S. Tweddell passed away during the preparation of this manuscript. He was a leader in the field of pediatric cardiothoracic surgery whose clinical and scientific work improved the lives of countless children. He was also a wonderful colleague and friend and will be sorely missed.
Authorship
Contribution: J.S.T. and J.S.P. designed the study, performed research, analyzed data, and wrote the manuscript; M.K., J.A.R, D.G.L., A.P.O., and F.Z. designed the study, performed research, helped with data analysis, and edited the manuscript; K.W.R., J.K.M., B.G., and L.R. performed research; and B.P.M. and A.R. provided critical reagents and assisted with research design.
Conflict-of-interest disclosure: J.S.T., J.S.P., and F.Z. received research funding from Ionis Pharmaceuticals for work unrelated to that presented here. The remaining authors declare no competing financial interests.
Correspondence: Joseph S. Palumbo, Cancer and Blood Diseases Institute, MLC 7015, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: joe.palumbo@cchmc.org.
References
Author notes
Data are available on request from the corresponding author, Joseph S. Palumbo (Joe.Palumbo@cchmc.org).
The full-text version of this article contains a data supplement.