Key Points
Eltrombopag combined with standard IST increased the complete response rate in treatment-naïve children with severe aplastic anemia.
Eltrombopag combined with IST increased the overall response rate in pediatric patients with SAA but not in those with vSAA.
Abstract
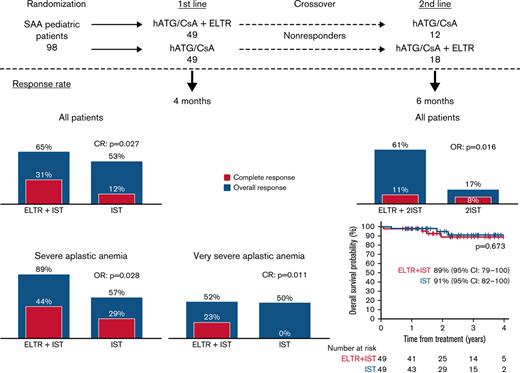
We compared the efficacy and safety of eltrombopag (ELTR) combined with immunosuppressive therapy (IST) and IST alone in treatment-naïve children with severe (SAA) and very severe (vSAA) aplastic anemia. Ninety-eight pediatric patients were randomized to receive horse antithymocyte globulin (hATG) and cyclosporin A (CsA) with (n = 49) or without (n = 49) ELTR. The primary endpoint was the overall response rate (ORR) at 4 months. After 4 months, nonresponders were crossed over to the alternative group. In all patients, the ORR in ELTR + IST and IST groups was similar (65% vs 53%; P = .218); however, the complete response (CR) rate was significantly higher in the ELTR + IST group (31% vs 12%; P = .027). In severity subgroups, the ORR was 89% vs 57% (P = .028) in favor of IST + ELTR in SAA, but it did not differ in patients with vSAA (52% vs 50%; P = .902). At 6 months after the crossover, 61% of initial ELTR(−) patients achieved a response compared with 17% of initial ELTR(+) patients (P = .016). No significant difference in ELTR + IST and IST groups was observed in the 3-year overall survival (OS) (89% vs 91%; P = .673) or the 3-year event-free survival (EFS) (53% vs 41%; P = .326). There was no unexpected toxicity related to ELTR. Adding ELTR to standard IST was well tolerated and increased the CR rate. The greatest benefit from ELTR combined with IST was observed in patients with SAA but not in those with vSAA. The second course of IST resulted in a high ORR in initial ELTR(−) patients who added ELTR and had limited efficacy among patients who received ELTR upfront. This trial was registered at Clinicaltrials.gov as #NCT03413306.
Introduction
Acquired aplastic anemia (AA) in children is a rare, potentially fatal disease that results from immune-mediated destruction of hematopoietic stem cells and progenitor cells and is characterized by pancytopenia and hypocellular bone marrow (BM).1,2 Severe AA in children can be treated effectively with allogeneic hematopoietic stem cell transplantation (HSCT) from a matched sibling donor (MSD) or immunosuppressive therapy (IST) with horse antithymocyte globulin (hATG) and cyclosporine (CsA), which achieve comparable long-term survival rates of approximately 90%.3,4 However, event-free survival (EFS), as assessed based on lack of hematological improvement, relapse, or malignant transformation, is significantly inferior among patients receiving IST compared with those undergoing HSCT from MSDs: approximately one-third of the patients treated with hATG and CsA do not respond, approximately 30% of responders develop relapse, and in approximately 8% to 18% of patients, clonal evolution occurs.5,6 The efficacy of IST is limited by the reduced number of residual stem cells and the ability of IST to suppress autoreactive T cells permanently. Thus, complementary approaches are needed to overcome these limitations. One of the options to improve the quality of the hematologic response is to influence stem cell function. Previous attempts to improve the response by adding various hematopoietic growth factors to IST have failed.7 Thrombopoietin (TPO) is the principal endogenous regulator of platelet production. In vitro and animal models have shown that eltrombopag (ELTR), an oral synthetic small molecule TPO agonist, interacts selectively with TPO receptor without competing with endogenous TPO, leading to increased proliferation and differentiation of human BM stem cells and multilinear progenitor cells into megakaryocytes and increased platelet production.8 ELTR has been shown to induce hematologic responses in adult patients with refractory severe AA (SAA).9,10 Moreover, the addition of ELTR to IST in first-line treatment increased the overall response rate (ORR) of newly diagnosed adult and pediatric patients with SAA by more than 20% compared with that of a historical cohort.11
To directly evaluate the role of ELTR as an addition to standard IST with hATG and CsA among children with SAA, we initiated a randomized trial and here report the principal results.
Methods
Study design
This is an open-label, investigator-initiated, randomized prospective multicenter trial. The study was approved by the Institutional Review Board and ethics committee of the D. Rogachev Center, conducted in accordance with the Declaration of Helsinki, and registered at clinicaltrials.gov under trial #NCT03413306. All patients or their parents/legal guardians provided written informed consent.
Patients
Patients aged 2 to 18 years with previously untreated SAA without matched sibling donors were eligible. A total of 177 patients were screened, and 100 patients were enrolled in the study. The reasons for exclusion were: not meeting inclusion criteria (n = 68), declined to participate (n = 5), and alternative study (n = 4). The main reasons for not meeting inclusion criteria were front-line HSCT from MSD (n = 38), nonsevere AA (n = 14), and others (n = 16) (supplemental Figure 1). Enrolled patients with newly diagnosed SAA were randomized at a 1:1 ratio between December 2016 and October 2020. The median time from SAA diagnosis to enrollment was 8 (1 to 59) days. History and clinical signs evaluation contributed to screening for classical inherited BM failure syndromes (IBMFS). The diepoxybutane test was applied to rule out Fanconi anemia. Telomere length was estimated using flow–fluorescence in situ hybridization (FISH) technique to exclude dyskeratosis congenita.12 Paroxysmal nocturnal hemoglobinuria (PNH) clones were assessed by flow cytometry.13 The low limit of detection (LOD) was established as 20 definitive glycosylphosphatidylinositol (GPI)-negative events to report a detectable population of PNH erythrocytes, neutrophils, and monocytes.14 Standard cytogenetic and FISH for monosomy 7 were used to exclude clonal abnormalities.
SAA was defined as BM cellularity <25% and at least 2 of the following: absolute neutrophil count (ANC) <0.5 × 109/L, platelet count <20 × 109/L, or reticulocyte count <20 × 109/L, according to the Camitta criteria.15 Very SAA (vSAA) was defined using the same criteria as were used for SAA with the following modification: neutrophils <0.2 × 109/L. Patients were either treatment-naïve or previously treated with cyclosporine or granulocyte-stimulating factor (G-CSF) for <1 month.
Treatment regimens
In the standard IST group, the patients received 40 mg/kg per day hATG (ATGAM, Pfizer) for 4 days and 5 mg/kg oral CsA (Novartis) daily starting at day 1 to maintain whole blood trough concentrations of 150 to 300 ng/mL. In addition, 1 mg/kg per day of methylprednisolone was IV-administered for days 1 to 4, followed by administration of 1 mg/kg per day of oral prednisolone for 10 days without tapering. Administration of filgrastim (leukostim, BIOCAD, Russia) 10 μg/kg per day subcutaneously from day 5 was allowed per institutional discretion. CsA was continued for ≥18 months after hematological response plateau achievement, followed by tapering by 5% to 10% of the daily dose per month.
In the ELTR + IST group, the patients additionally received ELTR (Novartis) at a dose of 2 mg/kg per day (the dose was rounded up to match a tablet [25 mg or 50 mg]) starting on day 1 of hATG. The initial dosage of ELTR was reduced by 50% in 2 Asian patients due to differences in pharmacokinetics.16 The duration of ELTR treatment was ≥120 days: in the absence of at least a partial response (PR) at 4 months, ELTR was discontinued, and the patients received second-line treatment; if at least a stable PR was achieved (blood counts correspond to PR and remain stable for 8 weeks), the dose of ELTR was tapered until withdrawal over 8 weeks. The study design is presented in supplemental Figure 2. If ELTR-related grade ≥3 hepatic toxicity as assessed with the National Cancer Institute Common Terminology Criteria for Adverse Events occurred, drug administration was suspended until the toxicity resolved and then resumed at 50% of the initial dose and escalated if tolerated. The patients received antifungal prophylaxis with either voriconazole or posaconazole until the ANC was >0.5 × 109/L. Laboratory monitoring is reported in the supplemental protocol.
Endpoints
The primary endpoint of this study was the ORR at 4 months from treatment initiation, defined as the proportion of patients who achieved either complete response (CR) or PR. The key secondary endpoints included platelet count, neutrophil count, and hemoglobin level at 4 months; cumulative incidence of ORR and CR; time to response; tolerability and toxicities of the treatment; overall survival (OS); EFS; cumulative incidence of relapse; clonal evolution (defined as the appearance of new MDS-associated clonal cytogenetic abnormalities or the development of secondary acute myeloid leukemia); and evolution into clinical PNH.
Response assessment criteria and nonresponders
The hematological response was defined according to the protocol as follows: CR was defined as peripheral blood ANC ≥1.0 × 109/L, hemoglobin (Hb) ≥100 g/L, and platelet count ≥100 × 109/L; PR was defined as transfusion independence with ANC ≥0.5 × 109/L, Hb ≥85 g/dL, and platelet count ≥30 × 109/L. Overall response (OR) was defined as PR or CR.
The hematological response was assessed at 4 months from the first day of hATG and monthly thereafter to document the best response. Per protocol, nonresponders were considered patients who did not achieve at least a PR by 4 months. For patients who did not achieve at least a neutrophil response (ANC ≥0.5 × 109/L) by 4 months and thus had a high risk of severe infection, HSCT from an alternative donor was allowed as early as the second-line setting. According to the protocol crossover principle, nonresponders received a second course of hATG with ELTR if the initial course was standard IST or hATG without ELTR if the initial course was ELTR + IST. The hematological response to the second course of treatment was evaluated at 6 months. EFS was measured from day 1 of the first treatment course until the date of death, relapse, second-line treatment, or clonal evolution. OS was measured from study enrollment until death from any cause or the date of lost follow-up.
Statistical analysis
The cut-off for clinical data was 1 July 2021. Response rates were compared between groups using Pearson’s χ-squared test. Kaplan-Meier curves and log-rank test were used to compare OS and EFS. For cumulative incidence curves, we used Gray's test. Adjusted odds ratios of OR and CR were estimated in multivariate logistic regression. The significance level was set at 0.05. The sample size was calculated based on the assumption of a 20% advantage in ORR at 4 months in the ELTR + IST group compared with standard IST. Detailed information about the statistical analyses is presented in supplemental File 2.
Results
Patients
One hundred eligible pediatric patients from 12 centers were randomized at a 1:1 ratio between December 2016 and October 2020. The full analysis set (FAS) included all randomized patients except 2 patients excluded due to the lack of data after randomization, and ninety-eight patients were eligible for modified intention-to-treat (mITT) analysis: 49 patients who received standard IST and 49 patients who received ELTR + IST (supplemental Figure 1). Thus, our FAS is an mITT population. As there were no protocol violations among FAS, it was the same as the per-protocol set. The demographic and baseline characteristics of the patients were similar across the 2 groups (Table 1). The median ELTR starting dose was 1.9 (range, 0.9-2.4) mg/kg. The median duration of ELTR treatment was 173 (range, 14-613) days. G-CSF was administered to a similar number of patients in both groups (45/49 [92%] patients in the ELTR + IST group and 44/49 [90%] patients in the IST group), and the median duration of G-CSF administration was also similar (36 [range, 1-150] days vs 46 [range, 1-151] days; P = .415). The actual dose of G-CSF received by the patients was not evaluated as this was not the subject of this study. The median follow-up was 2.19 (range, 0.08-4.54) years for all patients and 2.28 (range, 0.71-4.54) years for surviving patients.
Baseline characteristics of the patients by treatment group
| Characteristics . | ELTR + IST (n = 49), n (%) . | IST (n = 49), n (%) . | P value∗ . |
|---|---|---|---|
| Age (yr), median (range) | 10.5 (2-17.7) | 8.7 (2.1-16.8) | .285 |
| Sex | |||
| Male | 35 (71.4) | 30 (61.2) | .285 |
| Female | 14 (28.6) | 19 (38.8) | — |
| Ethnicity | |||
| White | 47 (96) | 46 (94) | .646 |
| Asian | 2 (4.0) | 3 (6.0) | — |
| Type of AA | |||
| Idiopathic | 45 (91.8) | 42 (85.7) | .337 |
| Hepatitis-associated | 4 (8.2) | 7 (14.3) | — |
| Severity of AA | |||
| Severe | 18 (36.7) | 21 (42.9) | .536 |
| Very severe | 31 (63.3) | 28 (57.1) | — |
| Absolute neutrophil count, per μL, median (range) | 150 (0-960) | 170 (0-1170) | .803 |
| Reticulocyte count, per μL, median (range) | 12 320 (0-70 140) | 14 750 (0-75 900) | .561 |
| Platelet count, per μL, median (range) | 15 (2-36) | 12 (1-37) | .094 |
| PNH | |||
| Negative | 35 (71.4) | 30 (61.2) | .285 |
| Positive | 14 (28.6) | 19 (38.8) | — |
| GPI-deficient neutrophils (PNH+) | |||
| <1% | 10 (20.4) | 12 (24.5) | — |
| ≥1% | 4 (8.2) | 7 (14.3) | — |
| Cytogenetics | |||
| Normal karyotype | 19 (38.8) | 21 (42.9) | .681 |
| Insufficient metaphases | 30 (61.2) | 28 (57.1) | — |
| Telomere length | |||
| Normal | 38 (86.4) | 34 (70.8) | .071 |
| Below normal | 6 (13.6) | 14 (29.2) | — |
| ND | 5 | 1 | — |
| Characteristics . | ELTR + IST (n = 49), n (%) . | IST (n = 49), n (%) . | P value∗ . |
|---|---|---|---|
| Age (yr), median (range) | 10.5 (2-17.7) | 8.7 (2.1-16.8) | .285 |
| Sex | |||
| Male | 35 (71.4) | 30 (61.2) | .285 |
| Female | 14 (28.6) | 19 (38.8) | — |
| Ethnicity | |||
| White | 47 (96) | 46 (94) | .646 |
| Asian | 2 (4.0) | 3 (6.0) | — |
| Type of AA | |||
| Idiopathic | 45 (91.8) | 42 (85.7) | .337 |
| Hepatitis-associated | 4 (8.2) | 7 (14.3) | — |
| Severity of AA | |||
| Severe | 18 (36.7) | 21 (42.9) | .536 |
| Very severe | 31 (63.3) | 28 (57.1) | — |
| Absolute neutrophil count, per μL, median (range) | 150 (0-960) | 170 (0-1170) | .803 |
| Reticulocyte count, per μL, median (range) | 12 320 (0-70 140) | 14 750 (0-75 900) | .561 |
| Platelet count, per μL, median (range) | 15 (2-36) | 12 (1-37) | .094 |
| PNH | |||
| Negative | 35 (71.4) | 30 (61.2) | .285 |
| Positive | 14 (28.6) | 19 (38.8) | — |
| GPI-deficient neutrophils (PNH+) | |||
| <1% | 10 (20.4) | 12 (24.5) | — |
| ≥1% | 4 (8.2) | 7 (14.3) | — |
| Cytogenetics | |||
| Normal karyotype | 19 (38.8) | 21 (42.9) | .681 |
| Insufficient metaphases | 30 (61.2) | 28 (57.1) | — |
| Telomere length | |||
| Normal | 38 (86.4) | 34 (70.8) | .071 |
| Below normal | 6 (13.6) | 14 (29.2) | — |
| ND | 5 | 1 | — |
ND, no data.
The demographics and baseline characteristics of the patients were similar across the 2 groups. The median values of continuous variables were compared between randomization groups by the Mann-Whitney test, and the frequencies of categorical variables were compared using Pearson’s χ-squared test. The P values of these tests are shown in the last column.
Hematological response
In the IST group, 26/49 patients responded, and the ORR was 53.1%, with 6 (12.2%) CRs and 20 (40.8%) PRs. In the ELTR + IST group, 32/49 patients responded, and the ORR was 65.3%, with 15 CRs (30.6%) and 17 PRs (34.7%). The difference in ORR at 4 months between the 2 groups was not significant (P = .218). However, the difference in the CR rate at 4 months between the 2 groups was significant (P = .027) in favor of the ELTR + IST group (Table 2).
Hematological response to first-line treatment by treatment group
| . | ELTR + IST (n = 49), n (%) . | IST (n = 49), n (%) . | Difference in % (95% CI)∗ . | P value . |
|---|---|---|---|---|
| 4 mo | ||||
| OR | 32 (65) | 26 (53) | 12 (−7 to 32) | .218 |
| CR | 15 (31) | 6 (12) | 18 (3 to 34) | .027 |
| PR | 17 (35) | 20 (41) | — | — |
| NR | 16 (33) | 23 (47) | −14 (−33 to 5) | .149 |
| Death | 1 (2) | 0 (0) | — | — |
| 6 mo | ||||
| OR | 27 (55) | 25 (51) | 4 (−16 to 24) | .686 |
| CR | 17 (35) | 9 (18) | 16 (−1 to 34) | .067 |
| PR | 10 (20) | 16 (33) | — | — |
| Relapse | 5 (10) | 1 (2) | — | — |
| NR | 16 (33) | 22 (45) | — | — |
| Second-line therapy | 12 (24) | 14 (29) | — | — |
| HSCT | 2 (4) | 2 (4) | — | — |
| Death | 1 (2) | 1 (2) | — | — |
| 1 y | ||||
| OR | 25 (51) | 22 (45) | 6 (−14 to 26) | .544 |
| CR | 16 (33) | 16 (33) | 0 (−19 to 19) | 1.0 |
| PR | 9 (18) | 6 (12) | — | — |
| Relapse | 7 (14) | 4 (8) | — | — |
| NR | 14 (29) | 21 (43) | — | — |
| Second-line therapy | 8 (16) | 14 (29) | — | — |
| HSCT | 2 (4) | 3 (6) | — | — |
| Second-line therapy + HSCT | 4 (8) | 4 (8) | — | — |
| Death | 1 (2) | 1 (2) | — | — |
| Not reached | 2 (4) | 1 (2) | — | — |
| . | ELTR + IST (n = 49), n (%) . | IST (n = 49), n (%) . | Difference in % (95% CI)∗ . | P value . |
|---|---|---|---|---|
| 4 mo | ||||
| OR | 32 (65) | 26 (53) | 12 (−7 to 32) | .218 |
| CR | 15 (31) | 6 (12) | 18 (3 to 34) | .027 |
| PR | 17 (35) | 20 (41) | — | — |
| NR | 16 (33) | 23 (47) | −14 (−33 to 5) | .149 |
| Death | 1 (2) | 0 (0) | — | — |
| 6 mo | ||||
| OR | 27 (55) | 25 (51) | 4 (−16 to 24) | .686 |
| CR | 17 (35) | 9 (18) | 16 (−1 to 34) | .067 |
| PR | 10 (20) | 16 (33) | — | — |
| Relapse | 5 (10) | 1 (2) | — | — |
| NR | 16 (33) | 22 (45) | — | — |
| Second-line therapy | 12 (24) | 14 (29) | — | — |
| HSCT | 2 (4) | 2 (4) | — | — |
| Death | 1 (2) | 1 (2) | — | — |
| 1 y | ||||
| OR | 25 (51) | 22 (45) | 6 (−14 to 26) | .544 |
| CR | 16 (33) | 16 (33) | 0 (−19 to 19) | 1.0 |
| PR | 9 (18) | 6 (12) | — | — |
| Relapse | 7 (14) | 4 (8) | — | — |
| NR | 14 (29) | 21 (43) | — | — |
| Second-line therapy | 8 (16) | 14 (29) | — | — |
| HSCT | 2 (4) | 3 (6) | — | — |
| Second-line therapy + HSCT | 4 (8) | 4 (8) | — | — |
| Death | 1 (2) | 1 (2) | — | — |
| Not reached | 2 (4) | 1 (2) | — | — |
NR, no response; CI, confidence interval.
After the primary response assessment, another 2 nonresponders in the ELTR + IST group achieved PR by days 252 and 216, respectively, which improved to CR.
The subgroup analysis based on the severity of AA showed that the ORR at 4 months was significantly higher in the ELTR + IST group than in the IST group in patients with SAA (ANC ≥0.2 × 109/L; 89% vs 57%; P = .028) and was not different in patients with vSAA (ANC <0.2 × 109/L; 52% vs 50%; P = .902) (Table 3). The cumulative incidence of response is shown in Figure 1.
Four-month hematological response to first-line treatment depending on the severity of AA
| . | SAA (ANC ≥0.2 × 109/L) (n = 39) . | vSAA (ANC <0.2 × 109/L) (n = 59) . | ||||||
|---|---|---|---|---|---|---|---|---|
| ELTR + IST (n = 18), n (%) . | IST (n = 21), n (%) . | Difference in % (95% CI) . | P value . | ELTR + IST (n = 31), n (%) . | IST (n = 28), n (%) . | Difference in % (95% CI) . | P value . | |
| OR | 16 (89) | 12 (57) | 32 (6 to 57) | .028 | 16 (52) | 14 (50) | 2 (−24 to 27) | .902 |
| CR | 8 (44) | 6 (29) | 16 (−14 to 46) | .303 | 7 (23) | 0 (0) | 23 (8 to 37) | .011 |
| PR | 8 (44) | 6 (29) | — | — | 9 (29) | 14 (50) | — | — |
| NR | 2 (11) | 9 (43) | −32 (−57 to −6) | .028 | 14 (45) | 14 (50) | −5 (−30 to 21) | .710 |
| Death | 0 (0) | 0 (0) | — | — | 1 (3) | 0 (0) | — | — |
| . | SAA (ANC ≥0.2 × 109/L) (n = 39) . | vSAA (ANC <0.2 × 109/L) (n = 59) . | ||||||
|---|---|---|---|---|---|---|---|---|
| ELTR + IST (n = 18), n (%) . | IST (n = 21), n (%) . | Difference in % (95% CI) . | P value . | ELTR + IST (n = 31), n (%) . | IST (n = 28), n (%) . | Difference in % (95% CI) . | P value . | |
| OR | 16 (89) | 12 (57) | 32 (6 to 57) | .028 | 16 (52) | 14 (50) | 2 (−24 to 27) | .902 |
| CR | 8 (44) | 6 (29) | 16 (−14 to 46) | .303 | 7 (23) | 0 (0) | 23 (8 to 37) | .011 |
| PR | 8 (44) | 6 (29) | — | — | 9 (29) | 14 (50) | — | — |
| NR | 2 (11) | 9 (43) | −32 (−57 to −6) | .028 | 14 (45) | 14 (50) | −5 (−30 to 21) | .710 |
| Death | 0 (0) | 0 (0) | — | — | 1 (3) | 0 (0) | — | — |
Cumulative incidence of hematologic response by treatment group and depending on the severity of AA. (A) Cumulative incidence of OR by treatment group. (B) Cumulative incidence of CR by treatment group. (C) Cumulative incidence of OR in SAA (ANC ≥0.2 × 109/L) patients by treatment group. (D) Cumulative incidence of OR in vSAA (ANC <0.2 × 109/L) patients by treatment group. Shaded areas are 95% CIs. Competing events included death. The start of the second-line treatment with hATG/CsA ± ELTR and HSCT was considered censoring. P values are given for the 4-month point. The cumulative incidence of the overall response at 4 months was 65% (95% CI, 52-79) in the ELTR + IST group and 54% (95% CI, 40-69) in the IST group (A). The cumulative incidences for complete response at 4 months were 31% (95% CI, 18-44) and 13% (95% CI, 3-22), respectively (B). The cumulative incidence of the overall response at 4 months in patients with SAA (ANC ≥0.2 × 109/L) was 89% (95% CI, 89-100) in the ELTR + IST group and 57% (95% CI, 35-79) in the IST group (C). The cumulative incidence of the overall response at 4 months in patients with vSAA (ANC <0.2 × 109/L) was 52% (95% CI, 34-70) and 52% (95% CI, 33-72) (D).
Cumulative incidence of hematologic response by treatment group and depending on the severity of AA. (A) Cumulative incidence of OR by treatment group. (B) Cumulative incidence of CR by treatment group. (C) Cumulative incidence of OR in SAA (ANC ≥0.2 × 109/L) patients by treatment group. (D) Cumulative incidence of OR in vSAA (ANC <0.2 × 109/L) patients by treatment group. Shaded areas are 95% CIs. Competing events included death. The start of the second-line treatment with hATG/CsA ± ELTR and HSCT was considered censoring. P values are given for the 4-month point. The cumulative incidence of the overall response at 4 months was 65% (95% CI, 52-79) in the ELTR + IST group and 54% (95% CI, 40-69) in the IST group (A). The cumulative incidences for complete response at 4 months were 31% (95% CI, 18-44) and 13% (95% CI, 3-22), respectively (B). The cumulative incidence of the overall response at 4 months in patients with SAA (ANC ≥0.2 × 109/L) was 89% (95% CI, 89-100) in the ELTR + IST group and 57% (95% CI, 35-79) in the IST group (C). The cumulative incidence of the overall response at 4 months in patients with vSAA (ANC <0.2 × 109/L) was 52% (95% CI, 34-70) and 52% (95% CI, 33-72) (D).
The quality of hematologic response at 2 months was significantly better in the experimental group, based on a higher ANC (median, 1.0 × 109/L vs 0.75 × 109/L; P = .038), platelet count (median, 49 × 109/L vs 26 × 109/L; P = .004) and proportion of patients with transfusion independence (55.1% vs 34.7%; P = .042). However, at 4 months, all these values except ANC were similar in both groups. The ANC was significantly higher in the ELTR + IST group than in the IST group at 2, 3, and 4 months from the start of treatment (supplemental Figure 3).
There was a trend toward a faster response, measured as median time to PR, in the ELTR + IST group than in the IST group (78 [16-258] vs 123 [13-124] days; P = .096). The median time to CR was similar in both groups (384 [26-823] days in the ELTR + IST group vs 371 [44-551] days in the IST group; P = .345).
Of the patients with OR who had no events (relapse or death), 7/25 (28%) in the IST group and 10/32 (31%) in the ELTR + IST group discontinued CsA. The median duration of CsA treatment for them was 2.7 years (range, 2.2-3.6) and 2.8 years (range, 0.7-3.5), respectively.
Predictors of response
The adjusted odds ratios of response evaluated in multivariate logistic regressions separately for OR and CR are shown in supplemental Figure 4. Only a higher initial ANC and PNH-negative status were significantly related to a higher ORR. For CR, the addition of ELTR to standard IST, higher initial ANC, and younger (2 to 5 years) and older (13 to 18 years) age of children were significantly correlated with the CR.
Adverse events (AEs)
Overall, both treatment groups had acceptable toxicities (Table 4). The most frequent AE considered to be related to ELTR and observed with greater frequency and severity in ELTR + IST group were liver test abnormalities (grade 1 to 2, 29/49 [59%] in the ELTR + IST group vs 17/49 [35%] in the IST group; P = .009; grade 3 to 4, 30/49 [61%] vs 16/49 [33%]; P = .005) and skin hyperpigmentation (6/49 [12%] vs 0%; P = .027). In the ELTR + IST group, there were more instances of any-grade hyperbilirubinemia (22/49 [45%] in the ELTR + IST group vs 9/49 [18%] in the IST group; P = .005), increased alanine aminotransferase levels (22/49 [45%] vs 13/49 [27%]; P = .058), and increased aspartate aminotransferase levels (15/49 [31%] vs 11/49 [22%]; P = .360).
AEs by treatment group
| AE . | IST group (n = 49), patients with AE . | ELTR + IST group (n = 49), patients with AE . | ||||
|---|---|---|---|---|---|---|
| NCI CTCAE grade | 1-2 | 3-4 | 5 | 1-2 | 3-4 | 5 |
| Febrile neutropenia | 17 | 2 | 0 | 12 | 6 | 0 |
| Infection | 5 | 15 | 0 | 2 | 16 | 1 |
| Nausea | 0 | 1 | 0 | 3 | 0 | 0 |
| Vomiting | 0 | 1 | 0 | 2 | 0 | 0 |
| Diarrhea | 2 | 1 | 0 | 3 | 0 | 0 |
| ALT increase | 7 | 6 | 0 | 12 | 10 | 0 |
| AST increase | 5 | 6 | 0 | 7 | 8 | 0 |
| Bilirubin increase | 5 | 4 | 0 | 10 | 12 | 0 |
| Rash | 2 | 3 | 0 | 1 | 5 | 0 |
| Myalgia | 1 | 2 | 0 | 0 | 6 | 0 |
| Joint pain | 0 | 6 | 0 | 1 | 7 | 0 |
| Renal failure | 2 | 2 | 0 | 2 | 3 | 0 |
| Hypertension | 6 | 1 | 0 | 6 | 2 | 0 |
| Skin hyperpigmentation | 0 | 0 | 0 | 6 | 0 | 0 |
| Prolonged QTc interval | 4 | 0 | 0 | 7 | 1 | 0 |
| Nephritis | 2 | 0 | 0 | 0 | 0 | 0 |
| Tremor | 1 | 0 | 0 | 1 | 0 | 0 |
| Psychosis | 0 | 0 | 0 | 0 | 1 | 0 |
| Blurred vision | 0 | 0 | 0 | 0 | 1 | 0 |
| Ileus | 0 | 1 | 0 | 0 | 1 | 0 |
| Neoplasm benign∗ | 0 | 0 | 0 | 0 | 1 | 0 |
| AE . | IST group (n = 49), patients with AE . | ELTR + IST group (n = 49), patients with AE . | ||||
|---|---|---|---|---|---|---|
| NCI CTCAE grade | 1-2 | 3-4 | 5 | 1-2 | 3-4 | 5 |
| Febrile neutropenia | 17 | 2 | 0 | 12 | 6 | 0 |
| Infection | 5 | 15 | 0 | 2 | 16 | 1 |
| Nausea | 0 | 1 | 0 | 3 | 0 | 0 |
| Vomiting | 0 | 1 | 0 | 2 | 0 | 0 |
| Diarrhea | 2 | 1 | 0 | 3 | 0 | 0 |
| ALT increase | 7 | 6 | 0 | 12 | 10 | 0 |
| AST increase | 5 | 6 | 0 | 7 | 8 | 0 |
| Bilirubin increase | 5 | 4 | 0 | 10 | 12 | 0 |
| Rash | 2 | 3 | 0 | 1 | 5 | 0 |
| Myalgia | 1 | 2 | 0 | 0 | 6 | 0 |
| Joint pain | 0 | 6 | 0 | 1 | 7 | 0 |
| Renal failure | 2 | 2 | 0 | 2 | 3 | 0 |
| Hypertension | 6 | 1 | 0 | 6 | 2 | 0 |
| Skin hyperpigmentation | 0 | 0 | 0 | 6 | 0 | 0 |
| Prolonged QTc interval | 4 | 0 | 0 | 7 | 1 | 0 |
| Nephritis | 2 | 0 | 0 | 0 | 0 | 0 |
| Tremor | 1 | 0 | 0 | 1 | 0 | 0 |
| Psychosis | 0 | 0 | 0 | 0 | 1 | 0 |
| Blurred vision | 0 | 0 | 0 | 0 | 1 | 0 |
| Ileus | 0 | 1 | 0 | 0 | 1 | 0 |
| Neoplasm benign∗ | 0 | 0 | 0 | 0 | 1 | 0 |
n, number of patients with the AE; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
A patient with multiple occurrences of AEs of the same grade was counted once.
Inflammatory myofibroblastic tumor considered unrelated to study treatment.
No severe cutaneous eruptions, thrombotic events, or cataracts were noted, as there were no cases of QTc prolongation >0.5 seconds in the ELTR + IST group considered to be related to ELTR.
Infectious complications and febrile neutropenia occurred in similar proportions of patients in the IST and ELTR + IST groups (39/49 [80%] patients and 36/49 [73%] patients, respectively; P = .475).
Two serious AEs in the ELTR + IST group considered unrelated to ELTR led to early discontinuation of the drug: grade 4 nonfatal ileus required surgery with histological verification of inflammatory myofibroblastic tumor of the colon after resection (patient number 3) and grade 5 bacterial sepsis in a 17-year-old adolescent (patient number 65) in the ELTR + IST group; the patient died on day 30. The 4-month mortality in the study was, therefore, 1%.
The only AEs related to ELTR that led to interruptions or discontinuation of ELTR treatment were liver test abnormalities. Overall, liver test abnormalities were observed in 29/49 (59%) patients in the ELTR + IST group. Among these 29 patients, 11/49 (22%) had transient, reversible grade 1 to 2 elevations of blood bilirubin or liver enzyme levels and did not require drug interruption or dose reduction. In 8/49 (16%) patients, ELTR administration was interrupted for a median of 10 (5 to 18) days due to grade 2 to 3 elevations of liver function tests and resumed at a 50% dose after event resolution. In 10/49 (20%) patients, ELTR was prematurely discontinued due to grade 3 to 4 liver function test abnormalities that were unresolved for >21 days after drug discontinuation. However, the feasibility of treatment per protocol in the ELTR + IST group was relatively high: the median cumulative dose of ELTR for 120 days per patient was 204 (121 to 240) mg/kg (85% of the planned 240 mg/kg), and the median duration of ELTR treatment over the 120 days was 99 (14 to 120) days (83% of the planned 120 days) (supplemental Table 1).
Long-term outcomes
Overall survival
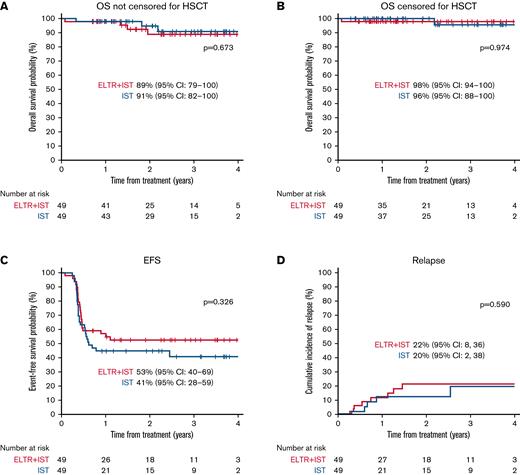
There were 4 deaths in the ELTR + IST group: 1 patient died on day 30 from sepsis while on treatment, and 3 nonresponders died due to complications after HSCT. Three patients died in the IST group: 1 nonresponder died due to fatal sepsis, and 2 nonresponders died due to complications after HSCT. The estimated 3-year OS was comparable in both treatment groups: 91% in the IST group vs 89% in the ELTR + IST group (P = .673) (Figure 2A).
OS, EFS, and cumulative incidence of relapse of patients with SAA by treatment group.
OS, EFS, and cumulative incidence of relapse of patients with SAA by treatment group.
Relapse
Among the 34 responders in the ELTR + IST group, 8 (24%) patients relapsed, compared with 5/26 (19%) patients in the IST group (P = .689). The cumulative incidence of relapse was comparable in both treatment groups: 20% in the IST group vs 22% in the ELTR + IST group (P = .590) (Figure 2D). The median time from response to relapse was 5.5 (1.1-11.05) months in the ELTR + IST group and 4.6 (1.3-25.9) months in the IST group. Of note, 4/8 relapses in the ELTR + IST group occurred in patients who quickly achieved a response (the median time to PR was 57.5 [46-78] days) but had to discontinue ELTR before 4 months due to permanent hepatotoxicity. One relapsed patient showed improvement of hematopoiesis after CsA dose correction, 1 underwent HSCT, while 6 children received a second course of hATG, and only 2 resumed ELTR. The hematologic response was achieved in 4/6 relapsed patients (2 CR, 2 PR); 2 patients failed to regain a response and underwent HSCT.
In the IST group, all 5 relapsed patients received second-course hATG/CsA with the addition of ELTR; 4 of 5 achieved a CR, and 1 underwent HSCT.
EFS
The EFS data for all patients are presented in Figure 2C. A total of 23 events were noted in the ELTR + IST group (1 death, 14 NR, 8 relapses), and 28 events were noted in the IST group (22 NR, 1 clonal evolution/NR, and 5 relapses). The estimated 3-year EFS was not significantly different between the 2 treatment groups: 41% in the IST group vs 53% in the ELTR + IST group (P = .326).
Nonresponders and second-line crossover treatment
There was a greater number of nonresponders in the IST group than in the ELTR + IST group (23/49 [47%] vs 16/49 [33%]; P = .149).
Among all nonresponders, 6 patients (2 in the ELTR + IST group and 4 in the IST group) who did not achieve at least a neutrophil response at 4 months underwent HSCT from an alternative donor as a second line of treatment. One nonresponder in the IST group did not receive ELTR in addition to hATG/CsA in the second course due to persistent transaminases elevation and achieved a PR. Twelve of 16 (75%) nonresponders in the ELTR + IST group received the second course of hATG/CsA without ELTR, and 18 of 23 (78%) patients in the IST group received the second course of hATG/CsA with ELTR. At the response assessment point 6 months after second-line hATG, 11 of 18 (61%) initial ELTR(−) patients achieved a response (2 CRs and 9 PRs) compared with only 2 of 12 (17%) patients (1 CR and 1 PR) in the initial ELTR(+) group (P = .016) (Table 5). Accordingly, the cumulative incidence of response was different at 6 months (66% vs 17% in the 2hATG + ELTR and 2hATG crossover groups; P = .029) (supplemental Figure 5). Fifteen nonresponders (9 in ELTR + IST, 6 in IST) underwent HSCT from an alternative donor as the third line of treatment.
Six-month hematological response to second-line crossover treatment
| Second-line crossover treatment (NR only) . | IST crossover group (n = 12), n (%) . | IST + ELTR crossover group (n = 18), n (%) . | Difference in % (95% CI) . | P value . |
|---|---|---|---|---|
| OR | 2 (17) | 11 (61) | 44 (14 to 75) | .016 |
| CR | 1 (8) | 2 (11) | 3 (−19 to 24) | .999 |
| PR | 1 (8) | 9 (50) | — | — |
| Median time to overall response, days (range) | Not reached (55 to 70) | 118 (26 to 182) | — | .029 |
| Second-line crossover treatment (NR only) . | IST crossover group (n = 12), n (%) . | IST + ELTR crossover group (n = 18), n (%) . | Difference in % (95% CI) . | P value . |
|---|---|---|---|---|
| OR | 2 (17) | 11 (61) | 44 (14 to 75) | .016 |
| CR | 1 (8) | 2 (11) | 3 (−19 to 24) | .999 |
| PR | 1 (8) | 9 (50) | — | — |
| Median time to overall response, days (range) | Not reached (55 to 70) | 118 (26 to 182) | — | .029 |
The clinical courses of all patients, including the doses and durations of ELTR, are presented in supplemental Figures 6 and 7.
Clonal evolution
No myelodysplastic syndrome or acute myeloid leukemia cases were reported after a median follow-up of 2.28 (0.71-4.54) years. There were 3 cases of cytogenetic abnormality: 2 patients in the IST group and 1 patient in the ELTR + IST group (supplemental Table 2). In 1 of these 3 patients, the abnormality was accompanied by refractoriness to standard IST treatment at 4 months and was considered a clinically significant event. The other 2 patients achieved a PR at 4 months and developed transient abnormalities (monosomy 7 and abnormalities of chromosome 9), which were not detected during further follow-up. The duration of follow-up of these 2 patients was 2.9 and 3.5 years, respectively, and both achieved a CR.
No cases of clinical hemolytic PNH occurred in any treatment group. Follow-up evaluations for PNH status at 4 and 12 months after the start of the treatment are presented in supplemental Table 3.
Discussion
This is the first randomized study to compare the efficacy and safety of ELTR in combination with hATG and CsA with those of standard IST in children with SAA.
The first prospective study by Townsley and colleagues showed an encouraging improvement in the hematologic response in the adult cohort, and the ORR at 6 months was approximately 90% among patients who received ELTR plus hATG and CsA. Notably, the highest OR and CR rates were observed in patients who received ELTR with hATG starting on day 1 and up to 6 months in cohort 3 (92% and 54% at 6 months, respectively). Additionally, the vast majority of responses were achieved within 3 months.11 Thus, the primary endpoint in our study was the ORR at 4 months, which was chosen to enable comparisons of early responses.
At the start of the protocol in December 2016, the recommended dose of ELTR in children with SAA was undetermined, and a dose of 2 mg/kg was chosen via extrapolation of the dose used in adult patients with refractory AA.9,10
hATG is a standard for IST in pediatric patients with SAA in Russia based on previous trials.17-19
G-CSF administration was allowed per protocol to keep the ANC ≥0.5 × 109/L since it has not been shown to increase the response rates and survival in previous studies20,21 and, thus, was considered neutral in relation to response achievement. G-CSF was administered to equal proportions of patients and with a similar duration in the ELTR + IST and IST groups.
In our protocol, CsA was continued for ≥18 months after sustained hematological response achievement followed by slow dose tapering with thorough control of blood counts to identify early signs of relapse, although some reports suggest that prolonged CsA administration after hATG delays but does not prevent relapse in SAA.22 We adhered to the concept of prolonged treatment of CsA based on reports that relapse risk is significantly associated with rapid CsA discontinuation: 60% compared with 7.6% in the slow tapering group (P = .001).23
Our study failed to reach a target advantage of 20% in ORR at 4 months in favor of ELTR + IST. A recently published prospective nonrandomized trials in newly diagnosed adult24 and pediatric25 patients with SAA also did not demonstrate an increased ORR with the addition of ELTR. Assi and colleagues showed a similar ORR at 6 months: 76% in the ELTR + IST arm compared with 71% in the IST arm.24 Groarke and colleagues showed no difference in either the ORR or CR at 6 months between the ELTR + IST group and historical IST group in pediatric patients (ORR: 70% vs 72%; CR: 30% vs 23%, respectively).25 The most recent large randomized multicenter trial indicated that the addition of ELTR to IST significantly increased ORR and CR at 3 and 6 months in patients with newly diagnosed SAA, but it should be noted that the vast majority of enrolled patients were adults except for 9 adolescents aged 15 to 18 years.26 We found that the CR rate in our study was significantly higher in the ELTR + IST group than in the IST group (31% vs 12%; P = .027). This significant CR improvement with ELTR is the first randomized evidence of a benefit of ELTR addition to IST in treatment-naïve SAA children.
The analysis of the patient subgroups based on the severity of AA showed the significant ORR improvement with ELTR combined with IST compared with IST alone in children with SAA who have ANC ≥0.2 × 109/L (89% vs 57%; P = .028), but we did not find a significant difference in patients with vSAA (52% vs 50%; P = .902). These findings are undergoing validation in our extension trial. This study may be considered as a basis for other trials appropriately sized to detect clinically relevant differences.
Importantly, ELTR added to IST allowed for a more rapid neutrophil recovery and significantly increased the ANC at 2, 3, and 4 months after the start of treatment compared with IST alone, while G-CSF was administered to similar proportions of patients in both groups.
The only significant predictors for better ORR in the multivariate logistic regression were initial ANC, as previously reported, and PNH-negative status. The latter seems paradoxical as PNH-positive status has been shown to be a predictor of a better response to IST.27,28 However, a predictive role of the PNH clone was shown before the introduction of ELTR into frontline therapy for SAA.
There were no significant differences in OS between the 2 groups, which is in line with the above-mentioned studies.22-26 The EFS and relapse rates were also similar in both treatment groups.
Per this protocol, after 4 months, a crossover to the second course of hATG/CsA with or without ELTR was conducted for nonresponders, depending on the initial randomization. We found significant differences in ORR between the 2 crossover groups 6 months after second-line therapy. This result suggests that the ELTR + IST combination as second-line therapy in pediatric patients may provide a higher response rate than ELTR alone, as shown in previous studies in adults with refractory SAA.9,10 Note that although molecular testing to exclude IBMFS was not carried out at screening due to its too long processing and analysis time, testing for germline variants applying “BM insufficiency NGS panel” (227 genes) was performed in the vast majority of nonresponders at 4 months, and IBMFS was not confirmed in any assessed refractory patient.
The safety profile in both groups was acceptable. The only AEs related to ELTR that led to interruption or discontinuation of ELTR treatment were liver test abnormalities, as expected. New safety issues related to ELTR were not identified.
No cases of clonal evolution to myelodysplastic syndrome, acute myeloid leukemia, or hemolytic PNH occurred in our study at a median follow-up of 2.3 years. One patient in the IST group (2%) and no patients in the ELTR + IST group developed clinically significant cytogenetic abnormalities, compared with 9% and 13% of patients, respectively, in the pediatric cohort of the study conducted by Groarke and colleagues.25 However, in a recent study of ELTR in combination with rabbit ATG/CsA in treatment-naïve patients with AA by Imada and colleagues, clinically significant clonal evolution or cytogenetic abnormalities were also not observed during a long follow-up.29 De Latour and colleagues showed that the prevalence of somatic mutations was not higher in the ELTR group than in the IST group.26 Therefore, the role of adding ELTR upfront in boosting clonal evolution is still unclear, and a much longer follow-up is needed to shed light on this issue.
In conclusion, the results of this randomized study support the addition of ELTR to IST in the first-line treatment of pediatric patients with SAA. This addition accelerates and improves the quality of the hematological response by significantly increasing the ANC and CR rates. The greatest benefit from the ELTR and IST combination was observed in patients with SAA and ANC ≥0.2 × 109/L, providing a cumulative ORR of about 90%, perhaps due to a larger number of residual stem and progenitor cells in this cohort compared with vSAA which will lead to more chance that ELTR + IST will protect residual stem cells and result in hematopoietic recovery. The addition of ELTR to upfront IST in pediatric patients with vSAA is also attractive not only because it contributes to a CR and faster ANC recovery in responders but rapidly identifies early nonresponders with profound residual stem cell deficiency who urgently require HSCT. However, these findings do not change the current algorithm of SAA treatment in children where HSCT from MSD remains the first-line option, and the use of matched unrelated donor HSCT upfront is supported based on the ability to provide the graft within 3 to 4 months of diagnosis.30 ELTR in combination with IST is effective as a second-line treatment in pediatric patients failing to respond to the first-line IST.
Acknowledgments
The authors thank all department, laboratory, and other hospital staff of Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology, and Immunology, Moscow, Russia. The authors thank all investigators and laboratory staff who are not mentioned in the author list. Their names and affiliations are: Svetlana Plyasunova, Dmitry Konovalov, Alexander Karachunskiy, Oleg Bydanov, Yulia Rumyantseva, Natalia Timofeeva, Alexandra Semchenkova, Dmitry Stefanov, Vitaly Dyomkin, and Natalia Steshina (Dmitry Rogachev National Medical Center of Pediatric Hematology, Oncology, and Immunology); Elena Skorobogatova and Ekaterina Pristanskova (Pirogov Russian Clinical Children’s Hospital); Svetlana Chernova and Andrey Egorov (Almazov National Medical Research Center); Irina Garbuzova, Yulia Fedyukova, and Maria Prudnikova (St. Petersburg Children’s City Hospital No 1); Larisa Fechina and Maria Butusova (Ekaterinburg Regional Clinical Children’s Hospital); Ekaterina Orlova (Voronezh Regional Clinical Children’s Hospital); Olga Nikonova (Perm Regional Clinical Hospital); Elena Ryabtseva (Piotrovich Regional Clinical Children’s Hospital); Svetlana Farafonova (Regional Clinical Children’s Hospital, Nizhniy Novgorod), Marina Borisova and Tatiana Kadricheva (Krasnoyarsk Regional Clinical Hospital). The authors thank the Gift of Life and Science for Children charitable foundations for continued study support.
Authorship
Contribution: O.G. collected, analyzed, and interpreted the data, and prepared the manuscript; T.S., I.K., D.B., U.P., K.A., M.S., E.S., D.E., V.M., D.V., L.K., D.L., A. Pshonkin, G.O., N.K., and D.G. participated in treatment at the D. Rogachev Center; Y.O. led the cytogenetic analyses; A. Popov led the flow cytometry analyses; E.R. led the molecular genetic analyses; O.M. and K.V. contributed to the statistical analysis; M.M. led the HSCT program; B.P., E.B., Y.D., E.G., D.S., E.K., S.M., O.S., N.Y., O.P., and E.E. participated in treatment at regional study sites; G.N., M.M., and A.M. generated the idea, formulated the treatment protocol, and edited the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: A.M. received lecturer’s fees from Novartis. M.M. received lecturer’s fees from Miltenyi Biotec. The remaining authors declare no competing financial interests.
Correspondence: Olga Goronkova, Department of Pediatric Hematology and Oncology, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology, and Immunology, Samory Mashela Str, 1, 117997, Moscow, Russia; e-mail: goronkova@yandex.ru.
References
Author notes
For original data, please contact the corresponding author, Olga Goronkova (goronkova@yandex.ru). The study protocol is included as a data supplement available with the online version of this article. Individual participant data will not be shared.
The online version of this article contains a data supplement.