Key Points
sCD25 appears to be a valuable adverse prognostic parameter in adult patients with HLH.
The outcome of adult patients with HLH with underlying malignancies is poor.
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare but often fatal hyperinflammatory syndrome caused by an inborn or acquired error of immunity. In adults, the underlying immunodeficiency generally arises alongside severe infections, malignancies, autoimmune diseases, and immunosuppressive treatment. To analyze risk factors and outcome in adults, we conducted a multicenter retrospective study. A total of 62 adult (age ≥18 years) patients met at least one of the following inclusion criteria: (1) ≥5 of 8 HLH-2004 criteria, (2) HScore ≥ 200 plus 4 HLH-2004 criteria, or (3) mutation compatible with an HLH diagnosis. Most patients (65%) were male, and the median age at diagnosis was 53.5 years (range, 19-81 years). All patients were assigned to 4 etiologic subgroups based on their most likely HLH trigger. The survival probability of the 4 etiologic subgroups differed significantly (P = .004, log-rank test), with patients with an underlying malignancy having the worst clinical outcome (1-year survival probability of 21%). The parameters older age, malignant trigger, elevated serum levels of aspartate transferase, creatinine, international normalized ratio, lactate dehydrogenase, sCD25, and a low albumin level and platelet count at treatment initiation were significantly (P < .1) associated with worse overall survival in the univariate Cox regression model. In multivariate analysis, sCD25 remained the only significant prognostic factor (P = .005). Our results suggest that sCD25 could be a useful marker for the prognosis of patients with HLH that might help to stratify therapeutic interventions.
Introduction
The term hemophagocytic lymphohistiocytosis (HLH) describes a rare but often fatal hyperinflammatory syndrome caused by an inborn or acquired error of immunity.1 Most cases in newborns and young children can be traced back to genetic causes impairing the function of natural killer (NK) and cytotoxic T cells. Therefore, certain autosomal-recessive mutations associated with granule-dependent lymphocyte toxicity are subsumed and referred to as familial hemophagocytic lymphohistiocytosis (FHL).2 In contrast, acquired forms of HLH are typically triggered by malignancies, infections, or autoimmune diseases2; HLH appearing alongside autoimmune diseases is regularly denoted as macrophage activation syndrome (MAS/MAS-HLH).3 However, appearances of HLH caused by genetic abnormalities can manifest at an older age as well4,5; therefore, making a clear clinical separation between the 2 entities is not always possible. Rather, a model of continuous risk or threshold of developing HLH, because of genetic and secondary triggers alike, has been proposed within the past few years.6,7
The first published case series in 1939 described solely adult patients with fever, lymphadenopathy, hepatosplenomegaly, and findings of generalized erythrophagocytosis,8 but most of the subsequent studies, spearheaded by Farquhar and Claireaux’s publication Familial haemophagocytic reticulosis9 in 1952, comprised children. Accordingly, to date, diagnostic and therapeutic recommendations are primarily based on the Histiocyte Society’s prospective pediatric HLH-94 and HLH-2004 trials.10,11 The most commonly applied diagnostic HLH-2004 criteria consist of the following 8 items: (1) fever; (2) splenomegaly; (3) cytopenia of at least 2 lineages; (4) hypertriglyceridemia or hypofibrinogenemia; (5) hyperferritinemia; (6) hemophagocytosis in bone marrow, spleen, or lymph nodes; (7) low/absent NK cell activity; and (8) elevated levels of sCD25 (ie, alpha chain of the soluble interleukin-2 receptor, sIL-2Rα).11 At least 5 of these criteria or a disease-causing mutation in HLH-associated genes must be present to verify an HLH diagnosis. Of note, a further helpful diagnostic score, the so-called HScore, which predicts the probability of an HLH diagnosis in adults, was published in 2014 and can be assessed online.12 The recommended first-line therapy follows the immunosuppressive and cytotoxic HLH-94/2004 protocol with its main agents being dexamethasone, etoposide, and ciclosporin.13,14 However, because of differences with regard to diverging etiologies, tolerance to cytotoxic therapies, and laboratory findings,15 the mere adoption of pediatric guidelines is often problematic or even impossible when treating adults.16 For example, functional assays such as NK cell activity are of lesser relevance in the broad adult population with acquired HLH,16,17 in whom alterations on the cellular level rather resemble states of hyperinflammation such as sepsis, than intrinsic cytotoxic defects as seen in genetic HLH.18
In the past few decades, there has been a steady growth in the number of publications and increasing awareness of HLH.17,19,20 However, given the general rarity of the syndrome, with estimated incidences of ∼1 to 2 per 1 000 000 per year,21-24 most of the available data are derived from retrospective studies.20
The aim of this study was to investigate the epidemiologic data, underlying triggers, diagnostic criteria, performed therapies, outcome, and possible prognostic factors in adult HLH.
Therefore, we conducted a multicenter retrospective analysis of adult patients with HLH in Munich. Through the cooperation of 4 hematology/oncology clinics, most cases within the metropolitan area of Munich have been included.
Patients and methods
The research was approved by the ethics committee of the medical faculty of LMU Munich (project number 20-0932). Written informed consent was waived because of the retrospective and anonymous data collection.
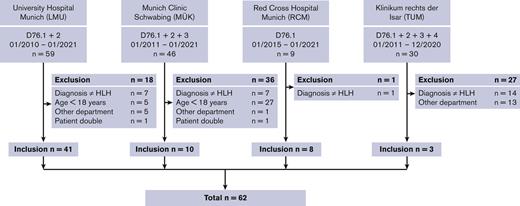
Inclusion criteria were as follows: for this multicenter retrospective study, patients with HLH-associated ICD-10 codes (D76.-) were searched by the medical statistics departments of the participating hospitals in their respective digital patient documentation systems. The patient files of all suspected HLH cases were reviewed manually, and the data of interest were extracted. Sixty-one adults (≥18 years) who met at least 5 of the HLH-2004 criteria11 or had a HScore12 > 200 and 4 positive HLH-2004 criteria were included for further analysis. In addition, in accordance with the HLH-2004 criteria, 1 male patient with a mutation in the XIAP gene was included as well, despite only fulfilling 4 of the diagnostic criteria and reaching 107 points in the HScore. A CONSORT (Consolidated Standards of Reporting Trials) diagram of the included patients per clinic is shown in Figure 1.
CONSORT diagram of patient inclusion. The search-related ICD-10 codes and time spans, numbers, and reasons for exclusions, and the total of included patients per hospital is shown.
CONSORT diagram of patient inclusion. The search-related ICD-10 codes and time spans, numbers, and reasons for exclusions, and the total of included patients per hospital is shown.
For the calculations of the diagnostic criteria, the maximum/minimum values of the corresponding laboratory findings up to a maximum of 14 days before treatment initiation were used. When no data were available for this period, the first value after treatment initiation was used. For patients who had more than 1 HLH-associated hospital stays, only the data from the first stay were included. Exceptions are genetic and NK cell activity tests, which were incorporated independent of the time of diagnosis. Five patients had been reported to the German HLH register up to July 2017, and part of their data were published by Birndt et al.25
Statistics
All statistical analyses were performed with R statistical software (version 4.0.3; The R Foundation for Statistical Computing, Vienna, Austria). Kaplan-Meier curves and the log-rank test were used to compare overall survival by etiologies. Univariate and multivariate Cox regression models were calculated to detect possible prognostic factors on overall survival. All parameters of the univariate Cox regression with a P value < .1 were included in the multivariate analysis. The assumption of proportional hazards was checked for all covariates; only platelet count did not fulfill the proportional hazard assumption in the univariate analysis. Because the prognostic value of low platelet counts has been described in previous publications,25-27 and to represent all 3 blood lineages, these were included in the analysis. The laboratory values measured on the day of the beginning of a specific HLH-directed therapy were used for the calculations of the Cox regression models. When no data were available, primarily the first value up to 72 hours before or secondarily 72 hours after treatment initiation was noted. If the same laboratory parameter was conducted more than once per day, only the first available value, usually from the morning’s blood sample, was taken into account for further calculations. Laboratory data that were not determined exactly above or below a specific cutoff point were transformed by adding or subtracting 1 from the last decimal number (eg, for sCD25 values >7500 U/mL, the value 7501 U/mL was used). The time span from the beginning of an HLH-specific therapy to death by any cause or date of last follow-up until 31 January 2021 was used for calculations on overall survival.
Results
Patient overview and etiologies
Of the 62 individuals included, 40 were male (65%) and the median age at diagnosis was 53.5 years (range, 19-81 years). The increasing awareness of adult HLH over the years becomes apparent because 17 cases (17 of 62, 27%) were treated in 2020 alone (supplemental Figure 1). All patients were assigned to 4 different HLH subgroups because of their underlying disease or most likely trigger of the HLH episode: malignancies or infections were present in 22 patients each (35%), 10 (16%) had an autoimmune or systemic disease, and no clear trigger could be found in the remaining 8 individuals (13%). Two patients had undergone stem cell transplantation (SCT) because of underlying malignancies (autologous SCT in late recurrence of Hodgkin lymphoma and allogeneic SCT in high-risk pre–T-ALL) before a specific viral trigger (cytomegalovirus pneumonia and Epstein-Barr virus [EBV] reactivation, respectively) for their HLH episode was found. Therefore, they were included in the infectious subgroup with reference to Lehmberg et al.28 An overview of the exact etiologies is shown in Table 1. In 7 patients with malignant triggers (7 of 22, 32%), HLH was diagnosed before the underlying neoplasm, with a median time span between diagnoses of 5 days (range, 2-45 days). The median follow-up of the surviving patients was 288 days (range, 3-2611 days).
Etiologies
| Etiology . | n . | (%) . | Comment . |
|---|---|---|---|
| Malignancies | 22 | (35) | |
| Myeloid | 2 | (3) | |
| AML | 1 | (2) | |
| MDS | 1 | (2) | |
| B-lymphoid | 11 | (18) | |
| Hodgkin lymphoma | 4 | (6) | EBV association n = 4 |
| DLBCL | 3 | (5) | EBV association n = 2 |
| B-cell lymphoma | 1 | (2) | |
| Primarily cerebral B-cell lymphoma | 1 | (2) | |
| Multiple myeloma | 2 | (3) | After autologous SCT n = 2 |
| T/NK-lymphoid | 8 | (13) | |
| T/NK-cell lymphoma | 2 | (3) | |
| PTCL-NOS | 2 | (3) | |
| Angioimmunoblastic T-cell lymphoma | 1 | (2) | EBV association |
| Mycosis fungoides | 1 | (2) | |
| NK-cell leukemia | 1 | (2) | EBV association |
| T-cell neoplasia (not further classified) | 1 | (2) | |
| Clinical suspicion of lymphoma | 1 | (2) | |
| Infections | 22 | (35) | |
| Viral | 16 | (26) | |
| EBV | 12 | (19) | After SCT n = 1 |
| CMV | 3 | (5) | After SCT n = 1 |
| HHV-6 | 1 | (2) | |
| Bacteria | 3 | (5) | |
| Escherichia coli | 1 | (2) | |
| Streptococcus agalactiae | 1 | (2) | |
| Streptococcus pyogenes | 1 | (2) | |
| Fungal | 2 | (3) | |
| Aspergillus fumigatus | 1 | (2) | Viral coinfection suspected |
| Candida glabrata | 1 | (2) | After urosepsis (Klebsiella spp.) |
| Unknown/multiple | 1 | (2) | |
| Autoimmune/systemic | 10 | (16) | |
| AOSD | 5 | (8) | |
| Antisynthetase syndrome | 2 | (3) | |
| Hypereosinophilic syndrome | 1 | (2) | |
| Immune complex vasculitis | 1 | (2) | |
| Sweet syndrome | 1 | (2) | |
| Idiopathic | 8 | (13) |
| Etiology . | n . | (%) . | Comment . |
|---|---|---|---|
| Malignancies | 22 | (35) | |
| Myeloid | 2 | (3) | |
| AML | 1 | (2) | |
| MDS | 1 | (2) | |
| B-lymphoid | 11 | (18) | |
| Hodgkin lymphoma | 4 | (6) | EBV association n = 4 |
| DLBCL | 3 | (5) | EBV association n = 2 |
| B-cell lymphoma | 1 | (2) | |
| Primarily cerebral B-cell lymphoma | 1 | (2) | |
| Multiple myeloma | 2 | (3) | After autologous SCT n = 2 |
| T/NK-lymphoid | 8 | (13) | |
| T/NK-cell lymphoma | 2 | (3) | |
| PTCL-NOS | 2 | (3) | |
| Angioimmunoblastic T-cell lymphoma | 1 | (2) | EBV association |
| Mycosis fungoides | 1 | (2) | |
| NK-cell leukemia | 1 | (2) | EBV association |
| T-cell neoplasia (not further classified) | 1 | (2) | |
| Clinical suspicion of lymphoma | 1 | (2) | |
| Infections | 22 | (35) | |
| Viral | 16 | (26) | |
| EBV | 12 | (19) | After SCT n = 1 |
| CMV | 3 | (5) | After SCT n = 1 |
| HHV-6 | 1 | (2) | |
| Bacteria | 3 | (5) | |
| Escherichia coli | 1 | (2) | |
| Streptococcus agalactiae | 1 | (2) | |
| Streptococcus pyogenes | 1 | (2) | |
| Fungal | 2 | (3) | |
| Aspergillus fumigatus | 1 | (2) | Viral coinfection suspected |
| Candida glabrata | 1 | (2) | After urosepsis (Klebsiella spp.) |
| Unknown/multiple | 1 | (2) | |
| Autoimmune/systemic | 10 | (16) | |
| AOSD | 5 | (8) | |
| Antisynthetase syndrome | 2 | (3) | |
| Hypereosinophilic syndrome | 1 | (2) | |
| Immune complex vasculitis | 1 | (2) | |
| Sweet syndrome | 1 | (2) | |
| Idiopathic | 8 | (13) |
AML, acute myelogenous leukemia; AOSD, adult-onset Still’s disease; CMV, cytomegalovirus; DLBCL, diffuse large B-cell lymphoma; HHV-6, human herpesvirus 6; MDS, myelodysplastic syndrome; PTCL-NOS, peripheral T-cell lymphoma not otherwise specified.
Diagnostic criteria
An overview of all applied diagnostic HLH-2004 criteria and parameters of the HScore are shown in Table 2.
Diagnostic criteria
| Criterium . | Total . | Malignancy . | Infection . | Autoimmune . | Idiopathic . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | Total . | (%) . | N . | Total . | (%) . | N . | Total . | (%) . | N . | Total . | (%) . | N . | Total . | (%) . | |
| Ferritin | |||||||||||||||
| Ferritin ≥ 500 μg/L | 62 | 62 | (100) | 22 | 22 | (100) | 22 | 22 | (100) | 10 | 10 | (100) | 8 | 8 | (100) |
| Ferritin ≥ 2000 μg/L | 60 | 62 | (97) | 22 | 22 | (100) | 22 | 22 | (100) | 8 | 10 | (80) | 8 | 8 | (100) |
| Ferritin > 6000 μg/L | 45 | 62 | (73) | 17 | 22 | (77) | 16 | 22 | (73) | 7 | 10 | (70) | 5 | 8 | (63) |
| Temperature | |||||||||||||||
| Fever | 60 | 62 | (97) | 22 | 22 | (100) | 21 | 22 | (95) | 10 | 10 | (100) | 7 | 8 | (88) |
| Temperature ≥ 38.4°C | 56 | 60 | (93) | 20 | 21 | (95) | 19 | 21 | (90) | 10 | 10 | (100) | 7 | 8 | (88) |
| Temperature > 39.4°C | 33 | 60 | (55) | 10 | 21 | (48) | 13 | 21 | (62) | 5 | 10 | (50) | 5 | 8 | (63) |
| Organomegaly | |||||||||||||||
| Splenomegaly | 54 | 60 | (90) | 19 | 20 | (95) | 18 | 22 | (82) | 10 | 10 | (100) | 7 | 8 | (88) |
| Hepatomegaly | 38 | 61 | (62) | 11 | 21 | (52) | 15 | 22 | (68) | 7 | 10 | (70) | 5 | 8 | (63) |
| Hepatosplenomegaly | 38 | 61 | (62) | 11 | 21 | (52) | 15 | 22 | (68) | 7 | 10 | (70) | 5 | 8 | (63) |
| Hemoglobin | |||||||||||||||
| Hemoglobin < 9.0 g/dL | 44 | 62 | (71) | 17 | 22 | (77) | 14 | 22 | (64) | 8 | 10 | (80) | 5 | 8 | (63) |
| Hemoglobin ≤ 9.2 g/dL | 47 | 62 | (76) | 20 | 22 | (91) | 14 | 22 | (64) | 8 | 10 | (80) | 5 | 8 | (63) |
| Platelets | |||||||||||||||
| Platelets < 100/nL | 49 | 62 | (79) | 19 | 22 | (86) | 19 | 22 | (86) | 4 | 10 | (40) | 7 | 8 | (88) |
| Platelets ≤ 110/nL | 52 | 62 | (84) | 20 | 22 | (91) | 19 | 22 | (86) | 6 | 10 | (60) | 7 | 8 | (88) |
| White blood cells | |||||||||||||||
| Neutrophils < 1.0/nL | 26 | 61 | (43) | 10 | 22 | (45) | 8 | 21 | (38) | 5 | 10 | (50) | 3 | 8 | (38) |
| Leukocytes ≤ 5.0/nL | 50 | 62 | (81) | 19 | 22 | (86) | 17 | 22 | (77) | 9 | 10 | (90) | 5 | 8 | (63) |
| Cytopenia | |||||||||||||||
| Bicytopenia (HLH-04) | 43 | 62 | (69) | 17 | 22 | (77) | 14 | 22 | (64) | 5 | 10 | (50) | 7 | 8 | (88) |
| Bicytopenia (HScore) | 55 | 62 | (89) | 21 | 22 | (95) | 18 | 22 | (82) | 9 | 10 | (90) | 7 | 8 | (88) |
| Pancytopenia (HLH-04) | 18 | 61 | (30) | 8 | 22 | (36) | 7 | 21 | (33) | 3 | 10 | (30) | 0 | 8 | (0) |
| Pancytopenia (HScore) | 33 | 62 | (53) | 17 | 22 | (77) | 10 | 22 | (45) | 4 | 10 | (40) | 2 | 8 | (25) |
| sCD25 ≥ 2400 U/mL | 53 | 60 | (88) | 20 | 21 | (95) | 20 | 21 | (95) | 8 | 10 | (80) | 5 | 8 | (63) |
| Histopath. hemophagocytosis | 33 | 57 | (58) | 7 | 19 | (37) | 12 | 20 | (60) | 8 | 10 | (80) | 6 | 8 | (75) |
| Triglycerides | |||||||||||||||
| TGs ≥ 132.7 mg/dL | 55 | 61 | (90) | 20 | 22 | (91) | 20 | 22 | (91) | 9 | 10 | (90) | 6 | 7 | (86) |
| TGs ≥ 265 mg/dL | 32 | 61 | (52) | 15 | 22 | (68) | 11 | 22 | (50) | 3 | 10 | (30) | 3 | 7 | (43) |
| TGs > 354 mg/dL | 20 | 61 | (33) | 8 | 22 | (36) | 8 | 22 | (36) | 3 | 10 | (30) | 1 | 7 | (14) |
| Fibrinogen | |||||||||||||||
| Fibrinogen ≤ 1.5 g/L | 29 | 61 | (48) | 13 | 21 | (62) | 9 | 22 | (41) | 3 | 10 | (30) | 4 | 8 | (50) |
| Fibrinogen ≤ 2.5 g/L | 43 | 61 | (70) | 15 | 21 | (71) | 17 | 22 | (77) | 6 | 10 | (60) | 5 | 8 | (63) |
| TGs ≥ 265 mg/dL or fibrinogen < 1.5 g/L | 43 | 61 | (70) | 18 | 21 | (86) | 15 | 22 | (68) | 5 | 10 | (50) | 5 | 8 | (63) |
| Low/absent NK cell activity | 3 | 13 | (23) | 0 | 2 | (0) | 2 | 7 | (29) | 1 | 2 | (50) | 0 | 2 | (0) |
| AST ≥ 30 U/L | 56 | 62 | (90) | 19 | 22 | (86) | 21 | 22 | (95) | 8 | 10 | (80) | 8 | 8 | (100) |
| Previous immunosuppression | 20 | 62 | (32) | 10 | 22 | (45) | 5 | 22 | (23) | 2 | 10 | (20) | 3 | 8 | (38) |
| Criterium . | Total . | Malignancy . | Infection . | Autoimmune . | Idiopathic . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N . | Total . | (%) . | N . | Total . | (%) . | N . | Total . | (%) . | N . | Total . | (%) . | N . | Total . | (%) . | |
| Ferritin | |||||||||||||||
| Ferritin ≥ 500 μg/L | 62 | 62 | (100) | 22 | 22 | (100) | 22 | 22 | (100) | 10 | 10 | (100) | 8 | 8 | (100) |
| Ferritin ≥ 2000 μg/L | 60 | 62 | (97) | 22 | 22 | (100) | 22 | 22 | (100) | 8 | 10 | (80) | 8 | 8 | (100) |
| Ferritin > 6000 μg/L | 45 | 62 | (73) | 17 | 22 | (77) | 16 | 22 | (73) | 7 | 10 | (70) | 5 | 8 | (63) |
| Temperature | |||||||||||||||
| Fever | 60 | 62 | (97) | 22 | 22 | (100) | 21 | 22 | (95) | 10 | 10 | (100) | 7 | 8 | (88) |
| Temperature ≥ 38.4°C | 56 | 60 | (93) | 20 | 21 | (95) | 19 | 21 | (90) | 10 | 10 | (100) | 7 | 8 | (88) |
| Temperature > 39.4°C | 33 | 60 | (55) | 10 | 21 | (48) | 13 | 21 | (62) | 5 | 10 | (50) | 5 | 8 | (63) |
| Organomegaly | |||||||||||||||
| Splenomegaly | 54 | 60 | (90) | 19 | 20 | (95) | 18 | 22 | (82) | 10 | 10 | (100) | 7 | 8 | (88) |
| Hepatomegaly | 38 | 61 | (62) | 11 | 21 | (52) | 15 | 22 | (68) | 7 | 10 | (70) | 5 | 8 | (63) |
| Hepatosplenomegaly | 38 | 61 | (62) | 11 | 21 | (52) | 15 | 22 | (68) | 7 | 10 | (70) | 5 | 8 | (63) |
| Hemoglobin | |||||||||||||||
| Hemoglobin < 9.0 g/dL | 44 | 62 | (71) | 17 | 22 | (77) | 14 | 22 | (64) | 8 | 10 | (80) | 5 | 8 | (63) |
| Hemoglobin ≤ 9.2 g/dL | 47 | 62 | (76) | 20 | 22 | (91) | 14 | 22 | (64) | 8 | 10 | (80) | 5 | 8 | (63) |
| Platelets | |||||||||||||||
| Platelets < 100/nL | 49 | 62 | (79) | 19 | 22 | (86) | 19 | 22 | (86) | 4 | 10 | (40) | 7 | 8 | (88) |
| Platelets ≤ 110/nL | 52 | 62 | (84) | 20 | 22 | (91) | 19 | 22 | (86) | 6 | 10 | (60) | 7 | 8 | (88) |
| White blood cells | |||||||||||||||
| Neutrophils < 1.0/nL | 26 | 61 | (43) | 10 | 22 | (45) | 8 | 21 | (38) | 5 | 10 | (50) | 3 | 8 | (38) |
| Leukocytes ≤ 5.0/nL | 50 | 62 | (81) | 19 | 22 | (86) | 17 | 22 | (77) | 9 | 10 | (90) | 5 | 8 | (63) |
| Cytopenia | |||||||||||||||
| Bicytopenia (HLH-04) | 43 | 62 | (69) | 17 | 22 | (77) | 14 | 22 | (64) | 5 | 10 | (50) | 7 | 8 | (88) |
| Bicytopenia (HScore) | 55 | 62 | (89) | 21 | 22 | (95) | 18 | 22 | (82) | 9 | 10 | (90) | 7 | 8 | (88) |
| Pancytopenia (HLH-04) | 18 | 61 | (30) | 8 | 22 | (36) | 7 | 21 | (33) | 3 | 10 | (30) | 0 | 8 | (0) |
| Pancytopenia (HScore) | 33 | 62 | (53) | 17 | 22 | (77) | 10 | 22 | (45) | 4 | 10 | (40) | 2 | 8 | (25) |
| sCD25 ≥ 2400 U/mL | 53 | 60 | (88) | 20 | 21 | (95) | 20 | 21 | (95) | 8 | 10 | (80) | 5 | 8 | (63) |
| Histopath. hemophagocytosis | 33 | 57 | (58) | 7 | 19 | (37) | 12 | 20 | (60) | 8 | 10 | (80) | 6 | 8 | (75) |
| Triglycerides | |||||||||||||||
| TGs ≥ 132.7 mg/dL | 55 | 61 | (90) | 20 | 22 | (91) | 20 | 22 | (91) | 9 | 10 | (90) | 6 | 7 | (86) |
| TGs ≥ 265 mg/dL | 32 | 61 | (52) | 15 | 22 | (68) | 11 | 22 | (50) | 3 | 10 | (30) | 3 | 7 | (43) |
| TGs > 354 mg/dL | 20 | 61 | (33) | 8 | 22 | (36) | 8 | 22 | (36) | 3 | 10 | (30) | 1 | 7 | (14) |
| Fibrinogen | |||||||||||||||
| Fibrinogen ≤ 1.5 g/L | 29 | 61 | (48) | 13 | 21 | (62) | 9 | 22 | (41) | 3 | 10 | (30) | 4 | 8 | (50) |
| Fibrinogen ≤ 2.5 g/L | 43 | 61 | (70) | 15 | 21 | (71) | 17 | 22 | (77) | 6 | 10 | (60) | 5 | 8 | (63) |
| TGs ≥ 265 mg/dL or fibrinogen < 1.5 g/L | 43 | 61 | (70) | 18 | 21 | (86) | 15 | 22 | (68) | 5 | 10 | (50) | 5 | 8 | (63) |
| Low/absent NK cell activity | 3 | 13 | (23) | 0 | 2 | (0) | 2 | 7 | (29) | 1 | 2 | (50) | 0 | 2 | (0) |
| AST ≥ 30 U/L | 56 | 62 | (90) | 19 | 22 | (86) | 21 | 22 | (95) | 8 | 10 | (80) | 8 | 8 | (100) |
| Previous immunosuppression | 20 | 62 | (32) | 10 | 22 | (45) | 5 | 22 | (23) | 2 | 10 | (20) | 3 | 8 | (38) |
The fulfilled diagnostic criteria of the HLH-2004 criteria and the HScore of the total population and within the etiologic subgroups is shown.
AST, aspartate transferase; Histopath., histopathological; TG, triglyceride.
Ferritin
All patients (62 of 62, 100%) had elevated serum ferritin values >500 μg/L. Most patients also surpassed the 2 considerably higher cutoff points of the HScore at 2000 μg/L (60 of 62, 97%) and 6000 μg/L (45 of 62, 73%). The median ferritin level of the population was 11 832 μg/L (range, 701-232 000 μg/L).
Fever, hepatosplenomegaly, and cytopenia
Considering the parameters of the so-called clinical HLH triad consisting of fever, splenomegaly, and cytopenia of at least 2 lineages,16 60 individuals (60 of 62, 97%) presented with fever, and splenomegaly was detected in 54 of 60 patients (90%) who had undergone adequate imaging. Hepatomegaly was described in 38 patients (38 of 61, 62%). Concerning cytopenia, differences between the HLH-2004 criteria and the HScore must be considered because in the latter, leukocytes are used instead of neutrophil granulocytes, and the cutoffs for anemia and thrombocytopenia are set slightly higher. Of the 62 patients, 55 (89%) presented with at least a bicytopenia in the HScore, opposed to 43 individuals (69%) when the HLH-2004 criteria were applied.
sCD25
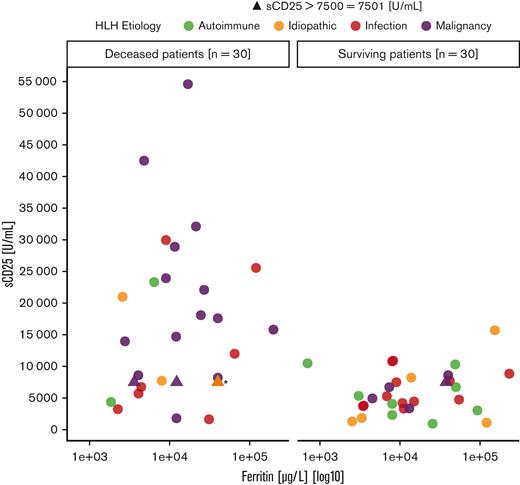
sCD25 was measured in 60 patients, with 53 (88%) surpassing the cutoff of 2400 U/mL and therefore fulfilling the corresponding HLH-2004 criterion. The median sCD25 level of the population was 7501 U/mL (range, 939-54 589 U/mL). Of 60 patients, 21 (35%) had highly elevated values of >10 000 U/mL. An overview of the sCD25 and ferritin levels of the population, separated by outcome, is shown in Figure 2.
sCD25 and ferritin levels by outcome. The 4 etiologic subgroups are depicted by different colors. ∗Overlap of 3 patients (etiologies: 2 × infection, 1 × idiopathic) with sCD25 levels of 7501 U/mL and ferritin levels of 40 001 μg/L.
sCD25 and ferritin levels by outcome. The 4 etiologic subgroups are depicted by different colors. ∗Overlap of 3 patients (etiologies: 2 × infection, 1 × idiopathic) with sCD25 levels of 7501 U/mL and ferritin levels of 40 001 μg/L.
Hemophagocytosis
During the first HLH-associated hospital stay, of the 62 patients, 57 (92%) underwent at least 1 bone marrow examination. Hemophagocytosis was found in 33 of these 57 patients (58%). In 30 patients, hemophagocytosis was detected in the initial examination, whereas a second analysis was necessary for its detection in 2 individuals. In 1 patient, proof of hemophagocytosis was only found in the postmortem obduction. Of the 57 patients, 39 (68%) received both a bone marrow biopsy and aspiration, and 9 patients each received either an aspiration (16%) or biopsy (16%) only. In 23 patients with verified hemophagocytosis who received both a bone marrow aspiration and biopsy, hemophagocytosis was detected in the aspiration only in 10 patients (43%), in the biopsy only in 8 patients (35%), and in both the aspiration and biopsy in 5 patients (22%). Additional proof of hemophagocytosis in splenic tissue was found in 3 patients (2 in postmortem obduction, 1 after splenectomy).
Triglycerides and fibrinogen
Of 61 patients, 43 (70%) had either triglycerides level of ≥265 mg/dL (32 of 61, 52%) or fibrinogen level of <1.50 g/L (29 of 61, 48%) and therefore fulfilled the accompanying HLH-2004 criterion.
NK cell activity
Specific lymphocyte degranulation tests were performed in 16 of 62 patients (26%). However, because of NK cell depletion in 3 samples, its activity could only be measured in 13 cases. Reduced NK cell activity was verified in 3 individuals; 2 presented with decreased spontaneous and interleukin-2 (IL-2)–stimulated NK cell degranulation. One of them was diagnosed with heterozygous mutations in 2 familial HLH-related genes afterward (see “Genetics”). The other patient showed no mutations in HLH-associated genes, but a T-cell large granular lymphocyte leukemia (T-LGL) was detected 4 months after the HLH episode and may possibly have caused the pathological results. In the third case, decreased NK cell activity was only described within the medical reports, and result-specific documents were not available for further examinations.
Genetics
HLH-specific genetic testing was performed in 6 of 62 patients (10%). Homozygous mutations in the PRF1 gene (FHL2)29 were found in 2 individuals: one was a 51-year-old man with idiopathic HLH (homozygous c.272C>T; p.Ala91Val), and the other one was a 35-year-old woman from consanguine parents with an EBV-triggered HLH episode (homozygous c.208G>T; p.Asp70Tyr). Heterozygous variants of unknown significance in the UNC13D (FHL3)30 (c.175G>A; p.Ala59Thr) and STXP2 genes (FHL5)31,32 (c.1298C>T; p.Ala433Val) were detected in a 42-year-old man from consanguine parents with an EBV-triggered HLH episode. In addition, 5 male patients were tested for mutations in the XIAP gene.33-35 In 2 of these 5 male patients, aged 19 and 42 years, a hemizygous variant (c.978_1099del; p.Cys327Ter, and c.1141C>T; p.Arg381∗, respectively) was detected.
Immunosuppression
Immunosuppression before developing HLH was present in 20 of 62 patients (32%): a chemotherapy cycle was administered to 6 patients up to 90 days before developing HLH, 5 previously received immunosuppressive drugs, 4 had undergone SCT, 4 were on prednisolone therapy, and 1 patient had undergone renal transplantation with subsequent ciclosporin therapy.
Metabolic imaging
To assess the findings of metabolic imaging in patients with active HLH, the respective imaging results were analyzed. Positron emission tomography–computed tomography scan was performed in 25 patients (25 of 62, 40%) and 1 patient received full-body scintigraphy (1 of 62, 2%). Of 25 patients, 19 (76%) either showed an increased standardized uptake value in lymph nodes or had generalized lymphadenopathy. In 17 individuals (17 of 26, 65%), a generalized increased standardized uptake value in the skeleton/bone marrow was described, and hypermetabolic splenomegaly was found in 10 patients (10 of 25, 40%).
Initial treatment
An overview of the agents used for initial HLH treatment is shown in Table 3. The exact therapy combination for each patient is shown in supplemental Figure 2.
Initial treatment
| Agent . | Total n (%) . | Malignancy . | Infection . | Autoimmune . | Idiopathic . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoids | 61 | (98) | 22 | (100) | 21 | (95) | 10 | (100) | 8 | (100) |
| Dexamethasone | 45 | (74) | 18 | (82) | 17 | (81) | 3 | (30) | 7 | (88) |
| Prednisolone∗ | 16 | (26) | 4 | (18) | 4 | (19) | 7 | (70) | 1 | (13) |
| Etoposide | 27 | (44) | 11 | (50) | 9 | (41) | 2 | (20) | 5 | (63) |
| Cyclosporine A | 14 | (23) | 4 | (18) | 5 | (23) | 2 | (20) | 3 | (38) |
| Rituximab | 16 | (26) | 6 | (27) | 10 | (45) | 0 | (0) | 0 | (0) |
| Intravenous immunoglobulins | 19 | (31) | 5 | (23) | 10 | (45) | 0 | (0) | 4 | (50) |
| Cyclophosphamide | 13 | (21) | 8 | (36) | 2 | (9) | 3 | (30) | 0 | (0) |
| Anakinra | 9 | (15) | 1 | (5) | 3 | (14) | 5 | (50) | 0 | (0) |
| Tocilizumab | 2 | (3) | 1 | (5) | 0 | (0) | 1 | (10) | 0 | (0) |
| Alemtuzumab | 1 | (2) | 0 | (0) | 1 | (5) | 0 | (0) | 0 | (0) |
| Ruxolitinib | 1 | (2) | 0 | (0) | 1 | (5) | 0 | (0) | 0 | (0) |
| Cytokine adsorption | 9 | (15) | 4 | (18) | 4 | (18) | 0 | (0) | 1 | (13) |
| Total | 62 | 22 | 22 | 10 | 8 | |||||
| Agent . | Total n (%) . | Malignancy . | Infection . | Autoimmune . | Idiopathic . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoids | 61 | (98) | 22 | (100) | 21 | (95) | 10 | (100) | 8 | (100) |
| Dexamethasone | 45 | (74) | 18 | (82) | 17 | (81) | 3 | (30) | 7 | (88) |
| Prednisolone∗ | 16 | (26) | 4 | (18) | 4 | (19) | 7 | (70) | 1 | (13) |
| Etoposide | 27 | (44) | 11 | (50) | 9 | (41) | 2 | (20) | 5 | (63) |
| Cyclosporine A | 14 | (23) | 4 | (18) | 5 | (23) | 2 | (20) | 3 | (38) |
| Rituximab | 16 | (26) | 6 | (27) | 10 | (45) | 0 | (0) | 0 | (0) |
| Intravenous immunoglobulins | 19 | (31) | 5 | (23) | 10 | (45) | 0 | (0) | 4 | (50) |
| Cyclophosphamide | 13 | (21) | 8 | (36) | 2 | (9) | 3 | (30) | 0 | (0) |
| Anakinra | 9 | (15) | 1 | (5) | 3 | (14) | 5 | (50) | 0 | (0) |
| Tocilizumab | 2 | (3) | 1 | (5) | 0 | (0) | 1 | (10) | 0 | (0) |
| Alemtuzumab | 1 | (2) | 0 | (0) | 1 | (5) | 0 | (0) | 0 | (0) |
| Ruxolitinib | 1 | (2) | 0 | (0) | 1 | (5) | 0 | (0) | 0 | (0) |
| Cytokine adsorption | 9 | (15) | 4 | (18) | 4 | (18) | 0 | (0) | 1 | (13) |
| Total | 62 | 22 | 22 | 10 | 8 | |||||
Only patients who did not receive additional dexamethasone.
All but 1 patient received immunosuppressive therapy with glucocorticoids: dexamethasone was used in 45 patients (45 of 61, 73%), and 16 patients (16 of 61, 26%) were primarily treated with (methyl-) prednisolone.
Twenty-seven patients (44%) received etoposide and 14 patients (23%) cyclosporine A. Rituximab was applied to 16 patients (26%), in particular because of EBV-associated HLH: 10 had an active EBV infection, 5 had EBV-associated malignancies, and 1 patient had B-cell non-Hodgkin lymphoma. Eighteen patients (29%) received infusions of intravenous immunoglobulins. Furthermore, immunosuppressive agents such as cyclophosphamide, anakinra, and tocilizumab were applied to 13 (21%), 9 (15%), and 2 (3%) patients, respectively. One patient received adjunctive treatment with ruxolitinib and later alemtuzumab. In 6 patients (10%), some of the aforementioned substances were administered as part of standardized chemotherapy protocols: 3 patients (etiologies: EBV-positive Hodgkin lymphoma, B-cell lymphoma, polymorph EBV-associated lymphoproliferation, respectively) received R-CHOP (rituximab–cyclophosphamide, Hydroxydaunorubicin [ie, doxorubicin], Oncovin [ie, vincristine], prednisolone), and 1 patient each was treated with R-CHOEP (R-CHOP + etoposide) (EBV-positive NK cell leukemia), CHOEP-14 (T-cell lymphoma), and BEACOPP (bleomycin, etoposide, Adriamycin [ie, doxorubicin], cyclophosphamide, Oncovin [ie, vincristine], procarbazine, prednisolone) (EBV-positive Hodgkin lymphoma), respectively.
Allogeneic SCT was performed in a 19-year-old male patient with a mutation in the XIAP gene and an HLH recurrence 4 months after the first EBV-triggered episode. He remained in clinical remission until the end of the study period (95 days).
A cytokine adsorber was used in 9 patients (15%) with an established external blood circuit.
Outcome
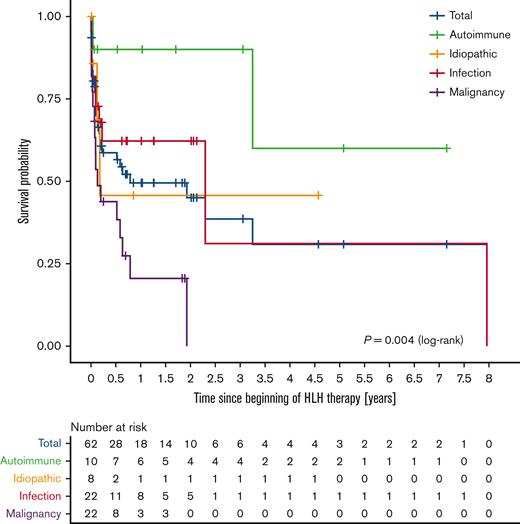
Of the 62 patients, 32 (52%) died within the study period. Median survival of the whole population was 288 days. The log-rank test revealed a significant difference (P = .004) in the survival probability of the 4 etiologic subgroups (Figure 3). Patients with underlying malignancies had the worst clinical outcome with a 1-year survival probability of 21%.
Kaplan-Meier curves by HLH etiology. The survival probability among the 4 etiologic subgroups differed significantly (P = .004, log-rank).
Kaplan-Meier curves by HLH etiology. The survival probability among the 4 etiologic subgroups differed significantly (P = .004, log-rank).
Prognostic factors
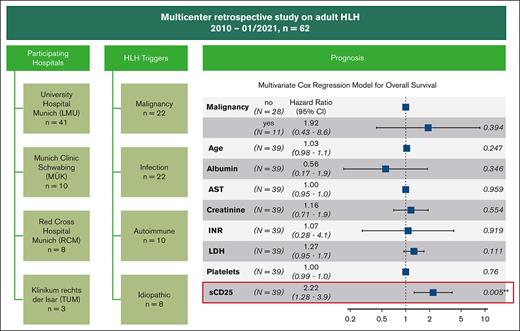
By univariate analysis, older age, malignant triggers, elevated serum levels of creatinine, lactate dehydrogenase, AST, international normalized ratio, sCD25, as well as a low platelet count and albumin level at treatment initiation were associated with worse overall survival. An elevated level of sCD25 was the only parameter that remained statistically significant (P = .005) in the multivariate analysis (Table 4).
Results of the Cox regression models
| Parameter . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | |
| Age | 1.02 | 1.00-1.04 | .06 | 1.03 | 0.98-1.08 | .25 |
| Male sex | 1.89 | 0.81-4.40 | .14 | — | — | — |
| Malignancy | 3.40 | 1.61-7.19 | .001 | 1.92 | 0.43-8.64 | .39 |
| Albumin | 0.54 | 0.28-1.04 | .07 | 0.56 | 0.17-1.88 | .35 |
| AST∗ | 1.02 | 1.00-1.05 | .07 | 1.00 | 0.95-1.05 | .96 |
| Creatinine | 1.32 | 1.04-1.68 | .02 | 1.16 | 0.71-1.92 | .55 |
| Ferritin† | 1.01 | 0.91-1.11 | .93 | — | — | — |
| Fibrinogen | 1.00 | 1.00-1.00 | .24 | — | — | — |
| Hemoglobin | 0.93 | 0.77-1.13 | .48 | — | — | — |
| INR | 3.24 | 1.50-7.00 | .003 | 1.07 | 0.28-4.06 | .92 |
| LDH† | 1.15 | 1.02-1.29 | .02 | 1.27 | 0.95-1.70 | .11 |
| Leukocytes | 1.01 | 0.97-1.05 | .65 | — | — | — |
| Neutrophils | 1.02 | 0.97-1.07 | .55 | — | — | — |
| Platelets | 0.99 | 0.98-1.00 | .005 | 1.00 | 0.99-1.01 | .76 |
| sCD25‡ | 2.40 | 1.67-3.44 | <.0001 | 2.22 | 1.28-3.87 | .005 |
| Triglycerides | 1.00 | 1.00-1.00 | .78 | — | — | — |
| Parameter . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio . | 95% CI . | P . | Hazard ratio . | 95% CI . | P . | |
| Age | 1.02 | 1.00-1.04 | .06 | 1.03 | 0.98-1.08 | .25 |
| Male sex | 1.89 | 0.81-4.40 | .14 | — | — | — |
| Malignancy | 3.40 | 1.61-7.19 | .001 | 1.92 | 0.43-8.64 | .39 |
| Albumin | 0.54 | 0.28-1.04 | .07 | 0.56 | 0.17-1.88 | .35 |
| AST∗ | 1.02 | 1.00-1.05 | .07 | 1.00 | 0.95-1.05 | .96 |
| Creatinine | 1.32 | 1.04-1.68 | .02 | 1.16 | 0.71-1.92 | .55 |
| Ferritin† | 1.01 | 0.91-1.11 | .93 | — | — | — |
| Fibrinogen | 1.00 | 1.00-1.00 | .24 | — | — | — |
| Hemoglobin | 0.93 | 0.77-1.13 | .48 | — | — | — |
| INR | 3.24 | 1.50-7.00 | .003 | 1.07 | 0.28-4.06 | .92 |
| LDH† | 1.15 | 1.02-1.29 | .02 | 1.27 | 0.95-1.70 | .11 |
| Leukocytes | 1.01 | 0.97-1.05 | .65 | — | — | — |
| Neutrophils | 1.02 | 0.97-1.07 | .55 | — | — | — |
| Platelets | 0.99 | 0.98-1.00 | .005 | 1.00 | 0.99-1.01 | .76 |
| sCD25‡ | 2.40 | 1.67-3.44 | <.0001 | 2.22 | 1.28-3.87 | .005 |
| Triglycerides | 1.00 | 1.00-1.00 | .78 | — | — | — |
AST, aspartate transaminase; CI, confidence interval; INR, international normalized ratio; LDH, lactate dehydrogenase; sCD25, alpha chain of the soluble interleukin-2 receptor.
Absolute laboratory values divided by 100 before entering to the Cox regression model.
Absolute laboratory values divided by 1000 before entering to the Cox regression model.
Absolute laboratory values divided by 10 000 before entering to the Cox regression model.
Discussion
Patient overview
The epidemiologic and clinical data concerning age, sex ratio, clinical findings, and underlying triggers within the population are in accordance with previous publications.25,36,37 Likewise, differences in outcome in relation to etiology, foremost the dismal prognosis of patients with accompanying malignancies, especially in the presence of T-cell/NK-cell lymphoma,22,38-42 have been described before.24,26,43-45 Although T-cell– and NK cell–associated malignancies generally have a poor prognosis, even worse in combination with HLH,46 one would expect a better overall survival in patients with hematologic malignancies for which effective chemotherapy protocols are available. However, given the toxicity of these protocols, they often cannot be applied right away and without dose reductions because of compromised organ functions and poor clinical condition during florid HLH disease. Of note, 4 of the 5 patients with hematologic neoplasms who were treated with standard chemotherapy protocols died within the study period (3 within 100 days). Interestingly, among 8 patients with EBV-associated malignancies, 4 were diagnosed with Hodgkin lymphoma–triggered HLH. Thus, EBV, alongside being the most frequent infectious pathogen,36 seems to play a role in malignancy-triggered HLH as well.40
In stark contrast to HLH caused by malignancies, the subgroup with autoimmune triggers had the best overall survival. This finding is as expected because autoimmune diseases have a better prognosis in general. Moreover, there is significant overlap concerning the immunosuppressive therapies for the underlying trigger and HLH.47 Especially in patients with systemic juvenile idiopathic arthritis (sJIA)/AOSD, in which HLH can reach a prevalence of 10% to 15%,48-50 HLH is thought to represent an extreme form of AOSD disease activity rather than a separate hyperinflammatory syndrome on top of the underlying disease.51-54 Therefore, therapy response to glucocorticoids in MAS-HLH is generally good, resulting in a lesser need of etoposide-containing chemotherapies,17 compared with HLH triggered by EBV and malignancies.28,55 Accordingly, only 2 of 10 patients (20%) within the autoimmune subgroup received etoposide.
Effects of therapy
With respect to the nonrandomized retrospective study design, we did not compare the effects of the initial treatment protocols on overall survival. More severe and refractory cases are likely to have received an intensified treatment scheme, for example with etoposide, leading to a bias toward worse outcomes. Note that no patient was treated with the monoclonal interferon-γ antibody emapalumab and only 1 with Janus kinase inhibitor ruxolitinib. Emapalumab has proven effective in patients <14 years of age with genetic HLH,56 and ruxolitinib showed possible benefit in the treatment of adult and pediatric patients with HLH.57-59 Therefore, improvements in outcomes owing to the increased use of these novel substances might be achieved in the future.
Role of sCD25
With regard to the Cox regression models, the significant results concerning sCD25 are of special interest. The high sensitivity of sCD25 in diagnosing HLH in children60 and adults61-63 and its prognostic value in the pediatric setting64,65 have been described before. In adults, Yoon et al63 reported that sCD25 had the highest diagnostic value of all 8 HLH-2004 criteria, with a sensitivity of more than 90% and a specificity of 80%, and Hayden et al62 showed that the area under the curve of sCD25 in diagnosing HLH reached 0.90. However, data addressing the prognostic significance of sCD25 in adult patients with HLH are scarce. The main reason for this can be attributed to limited laboratory availability in previous studies.20,27,36,43,60,66,67 Likewise, Damoiseaux68 reported a high diagnostic value of measuring sCD25 in patients with HLH, without addressing any further prognostic benefit to it. Nevertheless, in 1 publication with 35 adult patients with HLH, individuals with underlying EBV infections and malignancies presented with significantly higher sCD25 values and worse overall survival.69 In another study, where sCD25 values were available in 19 of 31 adult patients with HLH of different etiologies, the extent of the maximum sCD25 value during the HLH episode was significantly associated with a higher mortality.41
Because of the lacking widespread availability, sCD25, alongside NK cell activity, was not included in the HScore.12 However, 2 scoping reviews stated that sCD25 measurement is an inexpensive and valuable test for diagnosing HLH and assessing disease activity.20,60 The primacy of sCD25 as a marker for HLH disease activity was also mentioned by Jordan et al.70 Because sCD25 represents T-cell activity,68,71 and HLH is thought to be mainly driven by excessive activation of T cells,72,73 this could explain the fatal outcome of patients with highly elevated sCD25 values (no patient with a maximum sCD25 level > 15 701 U/mL survived within our population, supplemental Figure 3). Therefore, the question arises whether selected patients could benefit from immediate anti–T-cell treatment. In addition to etoposide, which proved to primarily affect activated T cells in the mouse model74 and lead to better short-time survival when applicated within the first 2 weeks after HLH diagnosis,26 second line–targeted substances such as alemtuzumab75 or antithymocyte globulin76 might be favorable as well.73 In line with that, a retrospective study on EBV-triggered pediatric and adult patients with HLH showed that elevated sCD25 levels were associated with an increased need for etoposide-containing chemotherapy treatment.77 Thus, we strongly recommend measuring sCD25 as soon as possible when the suspicion of HLH arises; even more so because it often takes several days until the results are available, especially when shipping of blood samples to external laboratories is necessary. Of note, in addition to the wider measuring range, the chemiluminescent immunoassay is superior to the enzyme-linked immunosorbent assay in terms of a shorter turnaround time, making it the preferred choice for sCD25 measurement in patients with HLH.68 Whether sCD25 levels primarily reflect T-cell activation or also play a role in HLH pathogenesis is uncertain. Given the not yet fully understood and probably serum level–dependent function of sCD25 as an immune system regulating substance,68 a pathophysiological involvement in HLH seems conceivable. Hence, whether the use of monoclonal sCD25 antibodies might be an additional treatment option in selected patients outside single-case descriptions78,79 should be addressed in future studies.
Role of ferritin
In contrast to some previous studies,80,81 the extent of elevated serum ferritin levels around treatment initiation did not show significant results in the univariate Cox regression model (P = .93). In the clinical setting, elevated ferritin values often raise first suspicion toward a possible HLH.82 With consideration of its characteristic as a marker for macrophage activation in HLH,83,84 it appears tempting to also address HLH disease activity and a prognostic value to it. After all, the broad availability and cost-effectiveness of ferritin85 predisposes this parameter for regular measurements to assess therapy response and the course of HLH activity.83,86 Accordingly, 2 studies regarding the decline in the rate of ferritin after treatment initiation showed significant results concerning patients’ outcome.67,87 One further study, investigating prognostic factors of 30- and 100-day short-time survival in 124 adult patients with HLH with different etiologies, showed that the change in ferritin levels was the most significant risk factor in multivariate analyses, whereas initial and peak ferritin levels did not correlate with 30-day mortality in patients with malignancies (n = 43).88 Similarly, in a study with 102 adult patients with HLH, ferritin values at treatment initiation did not differ significantly between the remission and nonremission groups.89 Concerning long-term survival in the aforementioned study, ferritin values within the first 2 weeks of treatment did not show significant results on overall survival, but values at weeks 3 and 4 after initial therapy did.89 Taken together, the regular measurement of ferritin in patients with HLH is recommended for evaluating treatment response, but isolated single values should not lead to hasty assumptions on prognosis.
Combination of sCD25 and ferritin: the optimized HLH inflammatory index
Another interesting and promising use of ferritin and sCD25 in HLH and hematologic malignancies alike was introduced in a recent study by Zoref-Lorenz et al.90 Through the combination of these parameters, they developed the optimized HLH inflammatory index consisting of sCD25 > 3900 U/mL and ferritin > 1000 μg/L, which proved to be of diagnostic and prognostic value in patients with hematologic malignancies with and without accompanying HLH alike.90 Likewise, a prognostic value of sCD25 had also been described for patients with lymphomas of B- and T-cell origin in the absence of HLH in preceding studies.91-93 Our data underline the possible prognostic value of sCD25 in adult patients with HLH independent of the underlying trigger.
Limitations
The major limitation of this study is its retrospective approach and therefore limited availability of data. Thus, multivariate analysis could only be performed for 39 (etiologies: autoimmune, n = 6; idiopathic, n = 6; infection, n = 16; malignancy, n = 11) patients. Furthermore, we could only include patients who received an HLH-associated ICD-10 code. Therefore, a selection bias toward patients with refractory disease and fatal outcomes seems possible. Moreover, through the inclusion of 4 hematology/oncology clinics, a bias toward patients with malignancy-associated HLH was inevitable. However, a strength of our study was the broad availability of sCD25 values in most patients. Given the lack of sufficient prognostic markers for adult patients with HLH,67 our results suggest that further studies on the role of sCD25 should be performed. Fortunately, its widespread laboratory availability has been expanding within the past decade.60,68 Because of the rarity and heterogeneity of HLH, prospective studies are hard to carry out, but may increasingly come up in the future alongside the steadily growing awareness of the disease.
Acknowledgments
The authors thank Andreas Humpe and Doris Schmidbauer of the Department of Transfusion Medicine, Cell Therapeutics and Hemostaseology at University Hospital LMU Munich for their help in providing selected data for the study (not part of this publication). T.W. is a doctoral student at Ludwig Maximilian University of Munich (LMU). This manuscript is submitted in fulfillment of the requirement for his medical doctoral thesis.
Authorship
Contribution: K.S. and R.M. initiated and designed the research; T.W. collected and analyzed the data, made the figures, and wrote the paper; K.S. directed and supervised the research; H.-J.S. and F.H. contributed to the study design, gave advice on the collection and analysis of the data, and provided data; H.S.-K., S.-S.S., M.S., C.-M.W., P.B., M.H., K.E.N., K.S.G., F.B., and M.v.B.-B. provided data from their institutions; and all authors reviewed the manuscript critically.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karsten Spiekermann, Department of Medicine III, University Hospital LMU Munich, Marchioninistraße 15, 81377 Munich, Germany; e-mail: karsten.spiekermann@med.uni-muenchen.de.
References
Author notes
For data sharing, please contact the corresponding author, Karsten Spiekermann (karsten.spiekermann@med.uni-muenchen.de).
The full-text version of this article contains a data supplement.