Key Points
Third-line CAR T-cell therapy is unlikely to be cost-effective relative to SOC therapies in unselected patients with R/R FL.
The benefits of CAR T-cell therapy in adults with FL could be clarified with randomized clinical trials and longer term clinical follow-up.
Abstract
Follicular lymphoma (FL) is traditionally considered treatable but incurable. In March 2021, the US Food and Drug Administration approved the use of chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed or refractory (R/R) FL after ≥2 lines of therapy. Priced at $373 000, CAR T-cell therapy is potentially curative, and its cost-effectiveness compared with other modern R/R FL treatment strategies is unknown. We developed a Markov model to assess the cost-effectiveness of third-line CAR T-cell vs standard of care (SOC) therapies in adults with R/R FL. We estimated progression rates for patients receiving CAR T-cell and SOC therapies from the ZUMA-5 trial and the LEO CReWE study, respectively. We calculated costs, discounted life years, quality-adjusted life years (QALYs), and the incremental cost-effectiveness ratio (ICER) of CAR T-cell vs SOC therapies with a willingness-to-pay threshold of $150 000 per QALY. Our analysis was conducted from a US payer’s perspective over a lifetime horizon. In our base-case model, the cost of the CAR T-cell strategy was $731 682 compared with $458 490 for SOC therapies. However, CAR T-cell therapy was associated with incremental clinical benefit of 1.50 QALYs, resulting in an ICER of $182 127 per QALY. Our model was most sensitive to the utilities associated with CAR T-cell therapy remission and third-line SOC therapies and to the total upfront CAR T-cell therapy cost. Under current pricing, CAR T-cell therapy is unlikely to be cost-effective in unselected patients with FL in the third-line setting. Both randomized clinical trials and longer term clinical follow-up can help clarify the benefits of CAR T-cell therapy and optimal sequencing in patients with FL.
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL), accounting for 20% to 30% of NHL cases.1 It is most commonly diagnosed in older adults; in the United States, the median age of diagnosis of FL is 63 years.2 Traditionally FL has been a treatable but incurable disease.1 Chemoimmunotherapy combining an anti-CD20 antibody with cytotoxic chemotherapy is first-line treatment for most patients and produces responses lasting years in many of these patients.3
However, it is very common for patients with FL to relapse and remit over the course of their lives. In fact, ∼20% of patients experience early recurrence of FL after first-line chemoimmunotherapy, which is associated with poor survival.1 Options for patients who relapse include second-generation anti-CD20 antibodies, immunomodulator imide drugs, phosphatidylinositol 3-kinase (PI3K) inhibitors, and enhancer of zeste homolog 2 (EZH2) inhibitors.3-5 These therapies are often administered over lengthy intervals or indefinitely without curative intent and can impose a high cost burden and cumulative toxicities.4
Recently, there has been increased interest in the use of chimeric antigen receptor (CAR) T-cell therapy in patients with relapsed or refractory (R/R) FL.6 In March 2021, the US Food and Drug Administration (FDA) approved axicabtagene ciloleucel (axi-cel; Gilead Sciences, Foster City, CA), an anti-CD19 CAR T-cell therapy, for use in patients with R/R FL after ≥2 lines of therapy.7 This accelerated approval was based on a single-arm phase 2 study that found high response rates in indolent NHL, including 79% complete response in patients with FL.8 Of these patients, 74% remained in remission in an updated analysis 3 years after the start of the study. Despite encouraging efficacy, axi-cel entails a significant upfront cost, with the drug alone priced at $373 000, and its cost-effectiveness relative to other modern R/R FL strategies is currently unknown.9 In this study, we set out to assess the cost-effectiveness of a third-line CAR T-cell therapy strategy compared with standard of care (SOC) therapies in adults with R/R FL in the United States.
Methods
Patients and intervention
Our model was constructed to mirror the cohort of patients with FL enrolled in ZUMA-5, an international, phase 2, single-arm trial evaluating the efficacy of axi-cel in R/R indolent NHL.8 The patients in the FL cohort in ZUMA-5 had a median age of 60 years. Ninety-nine percent had received previous anti-CD20 monoclonal antibody and alkylating agent, and 63% had ≥3 previous lines of therapy.
Model construction
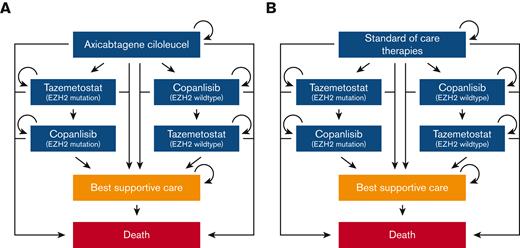
We utilized a Markov model in this analysis (Figure 1; supplemental Appendix A; supplemental Figures 1 and 2). Individuals entered the model requiring third-line therapy for R/R FL and received either CAR T-cell therapy or a SOC therapy. SOC therapies were based on 4 common therapies received in third-line by patients with R/R FL in the large multicenter cohort LEO CReWE study, including immunochemotherapy, hematopoietic stem cell transplantation (HSCT), lenalidomide with anti-CD20 monoclonal antibody, and anti-CD20 monoclonal antibody.10 Individuals who progressed after receipt of third-line CAR T-cell or SOC therapy could proceed to fourth-line targeted therapy (EZH2 inhibitor or PI3K inhibitor based on EZH2 mutation status). Individuals who progressed after receipt of a fourth-line targeted therapy could then start a fifth-line alternative class of targeted therapy. For example, if an individual with an EZH2 mutation progressed while on an EZH2 inhibitor, the individual could then start a PI3K inhibitor.
Simplified diagram of Markov model structure. (A) Treatment sequence for patients who receive third-line CAR T-cell therapy (axi-cel). (B) Treatment sequence for patients who receive third-line SOC therapies (immunochemotherapy, HSCT, lenalidomide with anti-CD20 monoclonal antibody, and anti-CD20 monoclonal antibody).
Simplified diagram of Markov model structure. (A) Treatment sequence for patients who receive third-line CAR T-cell therapy (axi-cel). (B) Treatment sequence for patients who receive third-line SOC therapies (immunochemotherapy, HSCT, lenalidomide with anti-CD20 monoclonal antibody, and anti-CD20 monoclonal antibody).
Treatment dosing and administration for CAR T-cell therapy was based on the ZUMA-5 trial.8 For SOC and targeted therapies, these pharmacological parameters were based on pivotal clinical trials.11-13 For the targeted therapies, these were the trials that led to the approval of tazemetostat and copanlisib.14,15 Copanlisib was selected as the PI3K inhibitor in our model in light of the recent voluntary withdrawal of umbralisib, duvelisib, and idelalisib from the market. Patients were allowed to enter a best supportive care (BSC) health state after relapsing on third- and later-line therapies before proceeding to death.
Our analysis was conducted from a US payer’s perspective with a 1-month Markov cycle.16 A lifetime horizon was utilized as has been done in several other CAR T-cell therapy cost-effectiveness analyses in the literature.16-19 Costs and utilities were discounted by 3% annually.20,21 Model outputs were used to calculate an incremental cost-effectiveness ratio (ICER) for the third-line CAR T-cell strategy compared with the SOC strategy. The ICER reflects the cost in 2021 US dollars for each additional quality-adjusted life year (QALY) gained because of the CAR T-cell strategy. We used a willingness-to-pay threshold (WTP) of $150 000 per QALY gained.16,22-24 TreeAge Pro Healthcare (TreeAge Software, Williamstown, MA) was used to develop our Markov model. Additional statistical analyses were performed using R (www.R-project.org) and STATA (StataCorp, College Station, TX). Our model was validated in accordance with the International Society for Pharmacoeconomics and Outcomes Research guidelines (supplemental Appendix B).25
Transition probabilities
Transition probability base-case estimates are listed in Table 1. Standard extrapolation techniques were utilized to derive progression rates for each therapy from the respective clinical trial.26,27 Briefly, we recreated individual patient-level data (IPLD) from published progression-free survival (PFS) curves and at-risk tables for each trial. IPLD were fit with standard parametric models (exponential, generalized γ, Weibull, Gompertz, log-normal, and log-logistic). Statistical goodness-of-fit based on the Akaike information criterion and Bayesian information criterion along with clinical experience were utilized to select the most appropriate parametric function to model these IPLD in our Markov model (supplemental Appendix A; supplemental Figures 3-9).
Model clinical parameters
| Parameter . | Base-case estimate . | Range . | Study or data source . |
|---|---|---|---|
| PFS for CAR T-cell therapy (axi-cel) | Log-normal: μ = 3.7836, σ = 1.8214 | — | Jacobson et al, 20228 |
| PFS for SOC therapies | Gompertz: λ = 0.0514, γ = −0.0289 | — | Casulo et al, 202210 |
| PFS for PI3K inhibitor (copanlisib) | Weibull: λ = 0.0442, κ = 1.0770 | — | Dreyling et al, 202015 |
| PFS for EZH2 inhibitor (tazemetostat), wild-type | Log-normal: μ = 2.1452, σ = 1.3242 | — | Morschhauser et al, 202014 |
| PFS for EZH2 inhibitor (tazemetostat), mutation | Log-normal: μ = 2.2847, σ = 1.0814 | — | Morschhauser et al, 202014 |
| Time from progression to start of next therapy, mo | 6 | 3-12 | Nastoupil et al, 201428 |
| Median starting age of cohort, y | 60 | 58-62 | Jacobson et al, 20228 |
| Probability of background death, % | — | — | Arias and Xu, 202029 |
| Discount rate, % | 3 | 1.5-6.0 | Sanders et al, 201620; Huntington et al, 201821 |
| Probability of EZH2 mutation, % | 20 | 16-24 | Morschhauser et al, 202014 |
| Recipients of CAR T-cell therapy receiving 12 mo of IVIG, % | 27 | 21.6-32.4 | Jacobson et al, 20228 |
| Probability of dose reduction of PI3K inhibitor, monthly, % | 4.34 | 3.47-5.21 | Dreyling et al, 202015 |
| Probability of dose reduction of EZH2 inhibitor, monthly, % | 0.41 | 0.32-0.49 | Morschhauser et al, 202014 |
| Reduced dose (PI3K inhibitor and EZH2 inhibitor), % | 75 | 60-90 | Expert opinion; FDA package inserts30 |
| Probability of discontinuation of PI3K inhibitor, monthly, % | 4.12 | 3.30-4.94 | Dreyling et al, 202015 |
| Probability of discontinuation of EZH2 inhibitor, monthly, % | 0.23 | 0.18-0.28 | Morschhauser et al, 202014 |
| Probability of CAR T-cell therapy–related mortality (first mo), % | 0.68 | 0.54-0.81 | Jacobson et al, 20228 |
| Probability of receipt of bridging therapy (first mo), % | 4.65 | 3.78-5.58 | Jacobson et al, 20228 |
| Probability of procession to BSC after progression, % | 11.6 | 9.28-13.9 | Barnes et al, 201831 |
| Probability of death from BSC state, monthly, % | 55 | 25-60 | Odejide et al, 201632; expert opinion |
| Parameter . | Base-case estimate . | Range . | Study or data source . |
|---|---|---|---|
| PFS for CAR T-cell therapy (axi-cel) | Log-normal: μ = 3.7836, σ = 1.8214 | — | Jacobson et al, 20228 |
| PFS for SOC therapies | Gompertz: λ = 0.0514, γ = −0.0289 | — | Casulo et al, 202210 |
| PFS for PI3K inhibitor (copanlisib) | Weibull: λ = 0.0442, κ = 1.0770 | — | Dreyling et al, 202015 |
| PFS for EZH2 inhibitor (tazemetostat), wild-type | Log-normal: μ = 2.1452, σ = 1.3242 | — | Morschhauser et al, 202014 |
| PFS for EZH2 inhibitor (tazemetostat), mutation | Log-normal: μ = 2.2847, σ = 1.0814 | — | Morschhauser et al, 202014 |
| Time from progression to start of next therapy, mo | 6 | 3-12 | Nastoupil et al, 201428 |
| Median starting age of cohort, y | 60 | 58-62 | Jacobson et al, 20228 |
| Probability of background death, % | — | — | Arias and Xu, 202029 |
| Discount rate, % | 3 | 1.5-6.0 | Sanders et al, 201620; Huntington et al, 201821 |
| Probability of EZH2 mutation, % | 20 | 16-24 | Morschhauser et al, 202014 |
| Recipients of CAR T-cell therapy receiving 12 mo of IVIG, % | 27 | 21.6-32.4 | Jacobson et al, 20228 |
| Probability of dose reduction of PI3K inhibitor, monthly, % | 4.34 | 3.47-5.21 | Dreyling et al, 202015 |
| Probability of dose reduction of EZH2 inhibitor, monthly, % | 0.41 | 0.32-0.49 | Morschhauser et al, 202014 |
| Reduced dose (PI3K inhibitor and EZH2 inhibitor), % | 75 | 60-90 | Expert opinion; FDA package inserts30 |
| Probability of discontinuation of PI3K inhibitor, monthly, % | 4.12 | 3.30-4.94 | Dreyling et al, 202015 |
| Probability of discontinuation of EZH2 inhibitor, monthly, % | 0.23 | 0.18-0.28 | Morschhauser et al, 202014 |
| Probability of CAR T-cell therapy–related mortality (first mo), % | 0.68 | 0.54-0.81 | Jacobson et al, 20228 |
| Probability of receipt of bridging therapy (first mo), % | 4.65 | 3.78-5.58 | Jacobson et al, 20228 |
| Probability of procession to BSC after progression, % | 11.6 | 9.28-13.9 | Barnes et al, 201831 |
| Probability of death from BSC state, monthly, % | 55 | 25-60 | Odejide et al, 201632; expert opinion |
Our model considers that a FL progression event in a clinical trial may not necessarily lead to immediate initiation of next-line therapy.33 We therefore included time between treatment states in our model as the average time from progression while on CAR T-cell or SOC therapies to the next-line of therapy.28 In addition, we included dose reduction and discontinuation states for patients on targeted therapies. The background probability of death during each line of therapy was derived from the US life tables.29 Prior work and expert opinion were utilized to determine the probability of transitioning from a BSC state to death to calibrate with trial overall survival (supplemental Appendix A; supplemental Figure 10).32
Costs
The costs included in our model are summarized in Table 2. The cost of axi-cel, including leukapheresis and dose preparation, was obtained from the 2021 Centers for Medicare & Medicaid Services (CMS) Hospital Outpatient Prospective Payment System.34 The costs of IV medications, including copanlisib, immunochemotherapy, and bridging and conditioning therapies, were obtained from the July 2021 CMS average sales price (ASP) files, listed at 106% of the ASP.35 We assumed a mean weight of 70 kg and total body surface area of 1.7 m2. Drug wastage was accounted for in our model by rounding up to the next full single vial for each dose administered. Administration costs for CAR T-cell therapy and bridging and conditioning therapies were based on the 2021 CMS Physician Fee Schedule.36 US FDA package inserts were used to determine the length of infusion for each drug.30 We assumed that patients were admitted to the hospital on the day of CAR T-cell infusion and remained in the hospital for 7 additional days after infusion per the ZUMA-5 trial; the cost of daily inpatient hospitalization was derived from literature.8,37
Model costs and utilities
| Cost . | Base-case estimate, $ . | Range, $ . | Study or data source . |
|---|---|---|---|
| CAR T-cell therapy, total upfront∗ | 443 118 | 373 000-711 884 | (Component sources detailed below) |
| Axi-cel (includes leukapharesis and dose preparation) | 395 380 | 316 304-474 465 | HCPCS Q204138 |
| Rituximab, 10 mg (bridging; SOC therapy) | 87.78 | 70.20-105.30 | HCPCS J931235 |
| Bendamustine, 1 mg (bridging; SOC therapy) | 21.26 | 17.01-25.51 | HCPCS J903635 |
| Obinutuzumab, 10 mg (SOC therapy) | 64.42 | 51.54-77.30 | HCPCS J930135 |
| Ifosfamide, 1 g (bridging) | 24.54 | 19.63-29.45 | HCPCS J920835 |
| Mesna, 200 mg (bridging) | 1.89 | 1.51-2.27 | HCPCS J920935 |
| Etoposide, 10 mg (bridging) | 0.71 | 0.57-0.85 | HCPCS J918135 |
| Mitoxantrone, 5 mg (bridging) | 29.19 | 23.35-35.03 | HCPCS J929335 |
| Cyclophosphamide, 25 mg (conditioning) | 1.14 | 0.91-1.37 | HCPCS J853035 |
| Fludarabine, 50 mg (conditioning) | 94.50 | 75.60-113.40 | HCPCS J918535 |
| Tocilizumab, 1 mg | 5.53 | 4.42-6.64 | HCPCS J326235 |
| Methylprednisolone, 80 mg | 10.41 | 8.33-12.49 | HCPCS J104035 |
| IVIG, 500 mg | 43.18 | 34.54-51.82 | HCPCS J145935 |
| Infusion of CAR T-cell therapy on d 0 | 310.75 | 248.60-372.90 | HCPCS 0540T38 |
| Inpatient hospitalization (/d) | 2 761.04 | 2 208.83-3 313.25 | Kaiser Family Foundation37 |
| Total cost of AE treatment, CAR T-cell therapy | 18 335.05 | 14 668.04-22 002.06 | Medicare IPPS39 |
| Allogeneic HSCT | 215 865 | 172 692-259 038 | Lin et al, 201916 |
| Autologous HSCT | 77 922 | 62 338-93 506 | Lin et al, 201916 |
| Lenalidomide, 20 mg | 800.98 | 640.78-961.18 | Medicare Part D plan finder40 |
| Tazemetostat, 200 mg | 68.09 | 54.47-81.71 | Medicare Part D plan finder40 |
| Copanlisib, 1 mg | 80.96 | 64.77-97.15 | HCPCS J905738 |
| Total cost of AE treatment, allogeneic HSCT | 8 595.39 | 6876.31-10 314.47 | Laport et al, 201612 |
| Total cost of AE treatment, autologous HSCT | 18 543.27 | 14 834.62-22 251.92 | Jurinovic et al, 201841 |
| Monthly AE treatment, obinutuzumab + bendamustine | 354.20 | 283.36-425.04 | Sehn et al, 201642 |
| Monthly AE treatment, lenalidomide + rituximab | 468.17 | 374.54-561.80 | Leonard et al, 201913 |
| Monthly AE treatment, rituximab monotherapy | 151.54 | 121.23-181.85 | Leonard et al, 201913 |
| Monthly AE treatment, EZH2 inhibitor | 54.51 | 43.61-65.41 | Medicare IPPS39 |
| Monthly AE treatment, PI3K inhibitor | 505.89 | 404.71-607.07 | Medicare IPPS37,39 |
| EZH2 full genetic sequence testing | 282.88 | 226.30-339.46 | HCPCS 8123643 |
| Positron emission tomography–computed tomography | 1 480.34 | 1 184.27-1 776.41 | HCPCS 7881638 |
| Diagnostic computed tomography (neck, chest, abdomen, pelvis) | 725.22 | 580.18-870.26 | HCPCS 70492, 71270, 7417738 |
| Chemotherapy IV infusion, first h | 148.30 | 118.64-177.96 | HCPCS 9641336 |
| Chemotherapy IV infusion, additional h | 31.40 | 25.12-37.68 | HCPCS 9641536 |
| Chemotherapy IV infusion, additional sequence | 71.88 | 57.50-86.26 | HCPCS 9641736 |
| Preinfusion medication | 13.29 | 10.63-15.95 | Barnes et al, 201831 |
| Routine office visit | 147.95 | 118.36-177.54 | HCPCS 9921536 |
| Complete blood count with differential | 7.77 | 137.46-199.03 | HCPCS 8502543 |
| Comprehensive metabolic panel | 10.56 | 8.45-12.67 | HCPCS 8005343 |
| Lactate dehydrogenase | 6.04 | 4.83-7.25 | HCPCS 8361543 |
| Uric acid | 4.52 | 3.62-5.42 | HCPCS 8455043 |
| BSC, monthly | 258.56 | 206.85-310.27 | Barnes et al, 201831 |
| Lymphoma end-of-life care | 60 525 | 48 420.00-72 630.00 | Campbell et al, 200444; Huntington et al, 201821 |
| Nonlymphoma end-of-life care | 48 341 | 38 672.80-58 009.20 | Hogan et al, 200145; Huntington et al, 201821 |
| Cost . | Base-case estimate, $ . | Range, $ . | Study or data source . |
|---|---|---|---|
| CAR T-cell therapy, total upfront∗ | 443 118 | 373 000-711 884 | (Component sources detailed below) |
| Axi-cel (includes leukapharesis and dose preparation) | 395 380 | 316 304-474 465 | HCPCS Q204138 |
| Rituximab, 10 mg (bridging; SOC therapy) | 87.78 | 70.20-105.30 | HCPCS J931235 |
| Bendamustine, 1 mg (bridging; SOC therapy) | 21.26 | 17.01-25.51 | HCPCS J903635 |
| Obinutuzumab, 10 mg (SOC therapy) | 64.42 | 51.54-77.30 | HCPCS J930135 |
| Ifosfamide, 1 g (bridging) | 24.54 | 19.63-29.45 | HCPCS J920835 |
| Mesna, 200 mg (bridging) | 1.89 | 1.51-2.27 | HCPCS J920935 |
| Etoposide, 10 mg (bridging) | 0.71 | 0.57-0.85 | HCPCS J918135 |
| Mitoxantrone, 5 mg (bridging) | 29.19 | 23.35-35.03 | HCPCS J929335 |
| Cyclophosphamide, 25 mg (conditioning) | 1.14 | 0.91-1.37 | HCPCS J853035 |
| Fludarabine, 50 mg (conditioning) | 94.50 | 75.60-113.40 | HCPCS J918535 |
| Tocilizumab, 1 mg | 5.53 | 4.42-6.64 | HCPCS J326235 |
| Methylprednisolone, 80 mg | 10.41 | 8.33-12.49 | HCPCS J104035 |
| IVIG, 500 mg | 43.18 | 34.54-51.82 | HCPCS J145935 |
| Infusion of CAR T-cell therapy on d 0 | 310.75 | 248.60-372.90 | HCPCS 0540T38 |
| Inpatient hospitalization (/d) | 2 761.04 | 2 208.83-3 313.25 | Kaiser Family Foundation37 |
| Total cost of AE treatment, CAR T-cell therapy | 18 335.05 | 14 668.04-22 002.06 | Medicare IPPS39 |
| Allogeneic HSCT | 215 865 | 172 692-259 038 | Lin et al, 201916 |
| Autologous HSCT | 77 922 | 62 338-93 506 | Lin et al, 201916 |
| Lenalidomide, 20 mg | 800.98 | 640.78-961.18 | Medicare Part D plan finder40 |
| Tazemetostat, 200 mg | 68.09 | 54.47-81.71 | Medicare Part D plan finder40 |
| Copanlisib, 1 mg | 80.96 | 64.77-97.15 | HCPCS J905738 |
| Total cost of AE treatment, allogeneic HSCT | 8 595.39 | 6876.31-10 314.47 | Laport et al, 201612 |
| Total cost of AE treatment, autologous HSCT | 18 543.27 | 14 834.62-22 251.92 | Jurinovic et al, 201841 |
| Monthly AE treatment, obinutuzumab + bendamustine | 354.20 | 283.36-425.04 | Sehn et al, 201642 |
| Monthly AE treatment, lenalidomide + rituximab | 468.17 | 374.54-561.80 | Leonard et al, 201913 |
| Monthly AE treatment, rituximab monotherapy | 151.54 | 121.23-181.85 | Leonard et al, 201913 |
| Monthly AE treatment, EZH2 inhibitor | 54.51 | 43.61-65.41 | Medicare IPPS39 |
| Monthly AE treatment, PI3K inhibitor | 505.89 | 404.71-607.07 | Medicare IPPS37,39 |
| EZH2 full genetic sequence testing | 282.88 | 226.30-339.46 | HCPCS 8123643 |
| Positron emission tomography–computed tomography | 1 480.34 | 1 184.27-1 776.41 | HCPCS 7881638 |
| Diagnostic computed tomography (neck, chest, abdomen, pelvis) | 725.22 | 580.18-870.26 | HCPCS 70492, 71270, 7417738 |
| Chemotherapy IV infusion, first h | 148.30 | 118.64-177.96 | HCPCS 9641336 |
| Chemotherapy IV infusion, additional h | 31.40 | 25.12-37.68 | HCPCS 9641536 |
| Chemotherapy IV infusion, additional sequence | 71.88 | 57.50-86.26 | HCPCS 9641736 |
| Preinfusion medication | 13.29 | 10.63-15.95 | Barnes et al, 201831 |
| Routine office visit | 147.95 | 118.36-177.54 | HCPCS 9921536 |
| Complete blood count with differential | 7.77 | 137.46-199.03 | HCPCS 8502543 |
| Comprehensive metabolic panel | 10.56 | 8.45-12.67 | HCPCS 8005343 |
| Lactate dehydrogenase | 6.04 | 4.83-7.25 | HCPCS 8361543 |
| Uric acid | 4.52 | 3.62-5.42 | HCPCS 8455043 |
| BSC, monthly | 258.56 | 206.85-310.27 | Barnes et al, 201831 |
| Lymphoma end-of-life care | 60 525 | 48 420.00-72 630.00 | Campbell et al, 200444; Huntington et al, 201821 |
| Nonlymphoma end-of-life care | 48 341 | 38 672.80-58 009.20 | Hogan et al, 200145; Huntington et al, 201821 |
| Utility . | Base-case estimate (QALY) . | Range . | Study or data source . |
|---|---|---|---|
| Axi-cel, mo 1 and 2 | 0.646 | 0.517-0.775 | NICE46; Lin et al, 201916; expert opinion |
| HSCT (autologous and allogeneic), mo 1 and 2 | 0.646 | 0.517-0.775 | NICE46; Lin et al, 201916; expert opinion |
| Third-line, without progression | 0.846 | 0.677-1.00 | NICE46; expert opinion |
| Fourth- and fifth-line targeted therapy | 0.785 | 0.628-0.942 | NICE46; expert opinion |
| Progression, not on therapy | 0.450 | 0.360-0.540 | Lin et al, 201916 |
| BSC | 0.450 | 0.360-0.540 | Lin et al, 201916 |
| Utility . | Base-case estimate (QALY) . | Range . | Study or data source . |
|---|---|---|---|
| Axi-cel, mo 1 and 2 | 0.646 | 0.517-0.775 | NICE46; Lin et al, 201916; expert opinion |
| HSCT (autologous and allogeneic), mo 1 and 2 | 0.646 | 0.517-0.775 | NICE46; Lin et al, 201916; expert opinion |
| Third-line, without progression | 0.846 | 0.677-1.00 | NICE46; expert opinion |
| Fourth- and fifth-line targeted therapy | 0.785 | 0.628-0.942 | NICE46; expert opinion |
| Progression, not on therapy | 0.450 | 0.360-0.540 | Lin et al, 201916 |
| BSC | 0.450 | 0.360-0.540 | Lin et al, 201916 |
IPPS, Inpatient Prospective Payment System; NICE, National Institute for Health and Care Excellence.
Inclusive of cost of axi-cel, leukapheresis, dose preparation, bridging/conditioning therapies, inpatient hospitalization, and AE management.
The cost for the oral drug tazemetostat was obtained from the publicly available Medicare Plan Finder tool based on methods utilized by the Memorial Sloan Kettering Drug Pricing Lab.40,47 Given that recent studies have suggested that pharmaceutical payer assistance programs cover a significant portion of patient cost sharing for oral cancer drugs, along with the fact that we have taken a US payer’s perspective in this analysis, our oral drug cost calculations did not include patient out-of-pocket costs.48-50 Instead, the oral drug costs in our model represent the amounts reimbursed by Part D prescription plans and Medicare reinsurance.
The dose and frequency of targeted therapies and lymphodepleting chemotherapy were based on the respective clinical trial.8,14,15 Costs for SOC therapies were based on the proportion of patients receiving immunochemotherapy, HSCT (both autologous and allogeneic), lenalidomide with anti-CD20 monoclonal antibody, or anti-CD20 monoclonal antibody in the LEO CReWE study.10 Specifically, costs for immunochemotherapy were based on an obinutuzumab plus bendamustine regimen, and anti-CD20 monoclonal antibody costs were based on rituximab. Drug dosage and frequency for these SOC therapies were based on pivotal clinical trials.11-13
Patients in our model received routine follow-up consisting of physician office visits, labs, and imaging per the respective clinical trial. Costs for these follow-up components were derived from the 2021 CMS Physician Fee Schedule and 2021 Q3 Medicare Clinical Laboratory Fee Schedule.43,36 Costs of severe adverse events (AEs), including cytokine release syndrome and neurologic toxicities, were also incorporated into our model (supplemental Appendix A; supplemental Table 1). We assumed that grade 3+ AEs resulted in inpatient hospitalization with costs derived from 2021 Medicare diagnosis–related group payments.39 Cytokine release syndrome management was also assumed to include tocilizumab and methylprednisolone administration with costs derived from the July 2021 CMS ASP file.35 Prior work informed the costs of BSC and end-of-life care.31,51 All costs were converted to 2021 US dollars using the medical care component of the Consumer Price Index.52
Utilities
Utilities in our model were informed by prior work and expert opinion and are summarized in Table 2.16,46 In our base case, individuals receiving CAR T-cell therapy had lower utility during the first 2 months with subsequent utility depending on ongoing remission. The utility for patients on SOC therapies was a weighted value based on the proportion of patients receiving immunochemotherapy, HSCT, lenalidomide with anti-CD20 monoclonal antibody, or anti-CD20 monoclonal antibody in the LEO CReWE study.10 As was done for CAR T-cell therapy, we assumed that patients receiving HSCT would have a lower utility in the first 2 months with higher utility thereafter. These utility values, representing a spectrum from death (0) to perfect health (1), were used to calculate QALYs.
Sensitivity analyses
To examine uncertainty in our model, we performed several sensitivity analyses. During 1-way sensitivity analyses, individual parameters were varied across a range to determine their impact on the ICER. These ranges are detailed in Tables 1 and 2. Most parameters were varied by 20% above and below their base-case value. The total upfront cost of CAR T-cell therapy, inclusive of the price of axi-cel, leukapheresis, dose preparation, bridging/conditioning therapies, inpatient hospitalization, and AE management, was varied across a larger range from $373 000 to $711 884 to account for differences in public vs private payer reimbursement for this key parameter.53,54 During probabilistic sensitivity analysis (PSA), we performed 10 000 Monte Carlo simulations, each time randomly sampling from the distribution of model inputs. Costs were described by γ distributions, and probabilities and utilities were represented by beta distributions. PSA distributions were constructed to cover the ranges used in 1-way sensitivity analyses. The difference method was utilized for ordered utilities.55
We also performed 8 scenario analyses (supplemental Appendix A; supplemental Table 2). In the first scenario, we modeled a best-case PFS scenario for CAR T-cell therapy recipients in which patients do not progress after the ZUMA-5 observation period. In this scenario, PFS remained at 61% from month 27 onward. In the second scenario, we fitted an exponential distribution to IPLD generated from ZUMA-5, resulting in a 5-year PFS of 32%. These scenarios were included to reflect the lack of long-term follow-up data for patients with R/R FL receiving CAR T-cell therapy, modeling scenarios in which 5-year PFS is improved or reduced relative to our base case, respectively. In our base case, modeled with a log-normal distribution, 5-year and 10-year PFS was 43% and 29%, respectively.
In the third scenario, we assumed that all patients who received CAR T-cell therapy received prophylactic IV immunoglobulin (IVIG) for the first 5 years following CAR T-cell treatment. This assumption differed from our base case, in which only the percentage of patients who received IVIG in ZUMA-5 were assumed to receive IVIG for the first year after receiving CAR T-cell therapy. In our fourth scenario, we pooled together the IPLD generated for patients with and without an EZH2 mutation from Morschhauser et al to fit a parametric distribution to the combined data.14 This differed from our base case in which separate distributions were used to fit the IPLD for patients with an EZH2 mutation and the IPLD for those without an EZH2 mutation as stratified in the trial.
In our fifth scenario, we conducted our analysis over a 10-year time horizon, different from the lifetime horizon utilized in our base-case analysis. In our sixth scenario, we assumed that patients were hospitalized for a total of 14 days after receipt of CAR T-cell treatment based on real-world data.56 In our seventh scenario analysis, we approximated the comparison of CAR T-cell therapy to later-lines of therapy, with the comparator arm only including EZH2 and PI3K inhibitors. Finally, in an eighth scenario analysis, we adopted the efficacy of CAR T-cell therapy (PFS; hazard ratio, 0.3) reported from a propensity score–matched analysis using patient-level data from ZUMA-5 and the SCHOLAR-5 cohort, an international retrospective cohort of individuals with R/R FL.57
Results
Base-case analysis
In our base-case model, the cost of the third-line CAR T-cell arm was $731 682 compared with $458 490 for use of SOC therapies. However, the CAR T-cell arm was associated with an improvement of 1.65 discounted life years and 1.50 QALYs relative to the SOC arm (8.65 vs 7.00 life years and 7.04 vs 5.54 QALYs, respectively). This resulted in an ICER of $182 127 per QALY (Table 3).
Base-case cost-effectiveness analysis
| Base-case model . | PSA model . | |||||
|---|---|---|---|---|---|---|
| Strategy . | Cost, $ . | Incremental cost, $ . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | ICER ($/QALY) . | ICER 95% confidence interval ($/QALY) . |
| Third-line CAR T-cell therapy | 731 682 | 273 191 | 7.04 | 1.50 | 182 127 | 147 469-229 576 |
| Third-line SOC therapies | 458 490 | — | 5.54 | — | — | — |
| Base-case model . | PSA model . | |||||
|---|---|---|---|---|---|---|
| Strategy . | Cost, $ . | Incremental cost, $ . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | ICER ($/QALY) . | ICER 95% confidence interval ($/QALY) . |
| Third-line CAR T-cell therapy | 731 682 | 273 191 | 7.04 | 1.50 | 182 127 | 147 469-229 576 |
| Third-line SOC therapies | 458 490 | — | 5.54 | — | — | — |
Sensitivity analyses
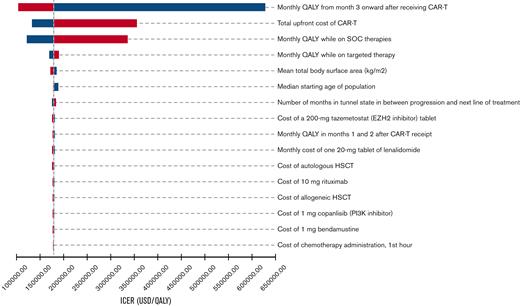
During 1-way sensitivity analysis, our model was most sensitive to the utility associated with CAR T-cell therapy remission, the total upfront cost of CAR T-cell therapy, and the utility associated with SOC therapies (Figure 2). In particular, an increase in the total upfront cost of CAR T-cell therapy from $443 118 to $711 884 resulted in an increase of the ICER to $355 164 per QALY. Decreasing the cost of CAR T-cell therapy from $443 118 to $373 000 resulted in a decrease of the ICER to $131 661 per QALY. Threshold analysis demonstrated that the total upfront cost of CAR T-cell therapy would need to be decreased by ∼10% to be cost-effective at a WTP threshold of $150 000 per QALY. Other model parameters that had a significant effect on our ICER estimate included the cost of HSCT, mean total body surface area, and utility associated with targeted therapy. During PSA, the median ICER was $182 119 per QALY (95% confidence interval, $147 469/QALY to $229 476/QALY), and 96% of iterations produced ICERs above the WTP threshold of $150 000 per QALY (Figure 3).
One-way sensitivity analyses tornado diagram. Ranges utilized in analyses are detailed in Tables 1 and 2. Parameters that produced ≥$1500 per QALY change in ICER when varied across the full range were included in the tornado diagram. Blue denotes ICER changes associated with lower values in the range, and red denotes ICER changes associated with higher values in the range. USD, US dollars.
One-way sensitivity analyses tornado diagram. Ranges utilized in analyses are detailed in Tables 1 and 2. Parameters that produced ≥$1500 per QALY change in ICER when varied across the full range were included in the tornado diagram. Blue denotes ICER changes associated with lower values in the range, and red denotes ICER changes associated with higher values in the range. USD, US dollars.
Cost-effectiveness acceptability curve. These results reflect 10 000 iterations of the Markov model utilized in this study during PSA.
Cost-effectiveness acceptability curve. These results reflect 10 000 iterations of the Markov model utilized in this study during PSA.
In our first scenario analysis, in which we modeled a best-case PFS scenario for CAR T-cell recipients, the ICER decreased to $44 020 per QALY. In our second scenario analysis, in which we utilized an exponential distribution allowing for higher rates of progression after CAR T-cell receipt, the CAR T-cell strategy was absolutely dominated by the SOC therapies strategy; the costs were $812 214 and $461 581, respectively, and the QALYs were 5.06 and 5.53, respectively. In the third scenario, in which all patients who received CAR T-cell therapy received prophylactic IVIG for 5 years, the ICER increased to $244 917 per QALY. In the fourth scenario, in which IPLD for EZH2 was pooled, the ICER increased to $195 013 per QALY. In our fifth scenario, in which we employed a 10-year time horizon, the ICER increased to $305 755 per QALY. In our sixth scenario, in which patients were hospitalized for 14 days after receiving CAR T-cell therapy, the ICER increased to $189 012 per QALY. In our seventh scenario analysis approximating CAR T-cell therapy compared with beyond the third-line setting (ie, EZH2/PI3K inhibitors only), the ICER decreased to $63 542 per QALY. Lastly, in our eighth scenario in which the efficacy of CAR T-cell therapy compared with SOC was based on the ZUMA-5–matched SCHOLAR-5 cohort, the ICER decreased to $60 502 per QALY.
Discussion
In the ZUMA-5 trial, axi-cel (CAR T-cell therapy) administered to patients with R/R indolent NHL after ≥2 lines of systemic therapy demonstrated promising clinical benefit and safety profile.8 Although it carries a considerable price tag, axi-cel is administered once and is potentially curative. In this study, we developed a Markov model utilizing inputs from the ZUMA-5 trial and pivotal trials assessing SOC and targeted therapies in indolent NHL to determine the cost-effectiveness of third-line CAR T-cell therapy relative to SOC therapies in patients with R/R FL in the United States. We did not find CAR T-cell therapy to be cost-effective in this setting in our base-case model at a WTP threshold of $150 000 per QALY, a finding supported by the majority of iterations in our PSA. Our scenario analyses also demonstrated that parameters including CAR T-cell therapy cost, utilities for CAR T-cell therapy remission and third-line SOC therapies, 5-year CAR T-cell therapy PFS, and time horizon of analysis play an important role in influencing the cost-effectiveness of CAR T-cell therapy in the R/R FL setting.
To our knowledge, this is the first cost-effectiveness analysis to examine the use of CAR T-cell therapy compared with SOC therapies in the third-line setting in patients with R/R FL. Prior studies have examined the cost-effectiveness of CAR T-cell therapy in the setting of other forms of R/R NHL. For instance, in their study utilizing data from the ZUMA-1 and JULIET trials, Lin et al found CAR T- cell therapy to potentially be cost-effective in the setting of multiply R/R adult diffuse large B-cell lymphoma at a WTP threshold of $150 000 per QALY in an optimistic scenario but not in other scenarios, dependent on long-term outcomes.16 Notably, the comparator strategy in their analysis comprised salvage chemoimmunotherapy and stem cell transplantation, consistent with the SOC in R/R diffuse large B-cell lymphoma and similar to the third-line comparator strategy in our model. In another study utilizing data from ZUMA-2, Simons et al also found CAR T-cell therapy to potentially be cost-effective at a WTP threshold of $150 000 in the setting of R/R mantle cell lymphoma with a comparator arm comprising salvage chemoimmunotherapy and targeted therapies.17
Our model has several strengths. First, our model includes the latest data from recent trials reflective of advancements in the treatment of R/R FL. In the comparator arm, this included SOC therapies informed by the recently published LEO CReWE study as well as later-line EZH2 inhibitors and PI3K inhibitors.3,10,14,15 Second, we account for AEs in our model, including associated costs as well as dose reduction and discontinuation in the case of targeted therapies. Third, we include time between treatment states as well as a BSC state to appropriately calibrate our model.
Our model also has limitations. First, CAR T-cell therapy approval for FL was based on a single-arm phase 2 trial, which introduces an element of uncertainty when comparing treatments in the setting of a cost-effectiveness analysis. Although we utilized parametric curve fitting to extrapolate long-term outcomes in our base-case model and explored other possible rates of progression in our scenario analyses, both randomized trials and long-term follow-up data for patients with R/R FL receiving CAR T-cell therapy will be important. Second, R/R FL is a very heterogenous disease, and both treatment selection and sequencing in the third- and later-lines of therapy are not well defined. New treatments for R/R FL are also emerging, such as bispecific T-cell engagers. Our model attempted to accommodate the diverse third-line FL treatment options by drawing from the real-world LEO CReWE study.10 Our model also incorporated PI3K and EZH2 inhibitors aligned with current consensus treatment recommendations.3 Third, the LEO CReWE study includes third-line R/R FL therapy data gathered over an extended period (2002-2018), and thus, the proportion of treatments used as third-line may not be equivalent to current practice. Although another recent retrospective cohort analysis (SCHOLAR-5) provides a more contemporary look at routine treatment for R/R FL, a significant proportion of patients were in fourth-line or greater and received experimental therapies, limiting its generalizability and ability to inform our intended third-line FL SOC arm. Importantly, the proportion of patients in LEO CReWE who had received certain newer treatment modalities such as lenalidomide with or without anti-CD20 monoclonal antibody were comparable to that in SCHOLAR-5.
Fourth, specific utility data for each FL treatment scenario are not currently available, requiring us to draw upon utility data pertaining to patients with FL treated with lenalidomide and rituximab along with utilities from prior CAR T-cell studies and expert opinion of the hematologist-oncologists on our team.16,46 Fifth, we took a US payer’s perspective in our analysis. As CAR T-cell therapy is approved for use in patients with R/R FL in other countries, additional analyses will be required to determine the cost-effectiveness of CAR T-cell use in different health care systems around the world. Finally, although we chose a third-line CAR T-cell therapy comparison based on the current FDA indication of ≥2 prior lines of therapy in R/R FL and currently available comparator arm data, patients are not limited to receiving CAR T-cell therapy as a third-line treatment and can receive it in a fourth or later line. Thus, our base-case results are limited to the setting in which patients with R/R FL receive CAR T-cell therapy in third-line and is unable to directly compare the ICER of third-line CAR T-cell therapy with later-line CAR T-cell therapy use. However, in our scenario analysis comparing CAR T-cell therapy to only 2 lines of available treatment (PI3K/EZH2 inhibitors), approximating CAR T-cell therapy against a later-line SOC, CAR T-cell therapy was cost-effective with an ICER of $63 542 per QALY.
In conclusion, CAR T-cell therapy is unlikely to be a cost-effective treatment modality over a lifetime horizon compared with SOC therapies as a third-line treatment for unselected patients with R/R FL. However, for select patients at high risk and those relapsing after third-line therapy, CAR T-cell therapy may be cost-effective compared with current standard therapies. Both randomized clinical trials and longer term clinical follow-up are needed to clarify the benefits and optimal sequencing of CAR T-cell therapy in patients with FL.
Acknowledgment
This work was supported by the American Society of Hematology Physician-Scientist Career Development Award (K.C.P.).
Authorship
Contribution: K.C.P. and S.F.H. had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis, were responsible for the concept and design of the study, and for acquisition, analysis, and interpretation of the data, and drafting the manuscript; K.C.P. performed the statistical analysis and obtained the funding; S.F.H. supervised the work; and all authors critically revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: I.I. was a consultant for Celgene/Jazz, Kite, Epizyme, Beam Therapeutics, and ADC Therapeutics. L.G. was a consultant for and received honoraria from Bristol Myers Squibb/Celgene. S.E.S. was a consultant for Carrum Health. F.M.F. was a consultant for Seattle Genetics, Mallinckrodt, Acrotech Biopharma, and Secura Bio; and received research funding from Miragen, Kura Oncology, Kyowa Kirin, Daiichi Sankyo, and Astex Pharmaceuticals. S.F.H. was a consultant for Janssen, Bayer, Genentech, AbbVie, Flatiron Health, Novartis, BeiGene, AstraZeneca, ADC Therapeutics, Epizyme, Merck, Seattle Genetics, TG Therapeutics, and Tyme; received honoraria from Pharmacyclics, AstraZeneca, and Seattle Genetics; and received research funding from Celgene, DTRM Biopharm, TG Therapeutics, Debiopharm Group, and Agios. The remaining authors declare no competing financial interests.
Correspondence: Scott Huntington, Division of Hematology, Department of Internal Medicine, Yale School of Medicine, 333 Cedar St, PO Box 208028, New Haven, CT 06520; e-mail: scott.huntington@yale.edu.
References
Author notes
Data are available on request from the corresponding author, Scott F. Huntington (scott.huntington@yale.edu).
The full-text version of this article contains a data supplement.