Key Points
Currently, patients receive a single dose of tisagenlecleucel across a wide dose range.
Clinicians could choose to give a higher dose of tisagenlecleucel upfront, within the approved dose range, to optimize response.
Abstract
Remarkable complete response rates have been shown with tisagenlecleucel, a chimeric antigen receptor (CAR) T-cell therapy targeting CD19, in patients up to age 26 years with refractory/relapsed B-cell acute lymphoblastic leukemia; it is US Food and Drug Administration approved for this indication. Currently, patients receive a single dose of tisagenlecleucel across a wide dose range of 0.2 to 5.0 × 106 and 0.1 to 2.5 × 108 CAR T cells per kg for patients ≤50 and >50 kg, respectively. The effect of cell dose on survival and remission is not yet well established. Our primary goal was to determine if CAR T-cell dose affects overall survival (OS), event-free survival (EFS), or relapse-free-survival (RFS) in tisagenlecleucel recipients. Retrospective data were collected from Pediatric Real World CAR Consortium member institutions and included 185 patients infused with commercial tisagenlecleucel. The median dose of viable transduced CAR T cells was 1.7 × 106 CAR T cells per kg. To assess the impact of cell dose, we divided responders into dose quartiles: 0.134 to 1.300 × 106 (n = 48 [27%]), 1.301 to 1.700 × 106 (n = 46 [26%]), 1.701 to 2.400 × 106 (n = 43 [24%]), and 2.401 to 5.100 × 106 (n = 43 [24%]). OS, EFS, and RFS were improved in patients who received higher doses of tisagenlecleucel (P = .031, .0079, and .0045, respectively). Higher doses of tisagenlecleucel were not associated with increased toxicity. Because the current tisagenlecleucel package insert dose range remains broad, this work has implications in regard to targeting higher cell doses, within the approved dose range, to optimize patients’ potential for long-standing remission.
Introduction
Remarkable complete response rates of 70% to 90% have been shown with chimeric antigen receptor (CAR) T-cell therapy in patients with refractory B-cell acute lymphoblastic leukemia (B-ALL).1,2 Tisagenlecleucel (Kymriah) is a CD19-directed CAR T-cell product and was approved by the US Food and Drug Administration for treatment of relapsed/refractory B- ALL in children and young adults.
In the pivotal ELIANA trial,2 which led to commercial approval, there was a target dose of transduced cells of 2.0 to 5.0 × 106 CAR-transduced viable T cells per kg of body weight (for patients ≤50 kg) and 1.0 to 2.5 × 108 T cells (for patients >50 kg) as a single infusion. However, patients could be infused according to the following dose range if safety release criteria were met: 0.2 to 5.0 × 106 T cells per kg of body weight (for patients ≤50 kg) and 0.1 to 2.5 × 108 T cells (for patients >50 kg). These latter doses are the current dose ranges used for commercial tisagenlecleucel infusion. To date, it is unclear whether cell dose influences CAR T-cell outcomes. It is of great interest to determine CAR T-cell properties optimized for efficacy and toxicity mitigation. The goal of this study was to use data from the Pediatric Real World CAR Consortium (PRWCC) to analyze the effect of infused CAR T-cell dose on efficacy and toxicity.
Methods
Study design
We used data extracted from the PRWCC across 15 pediatric oncology centers delivering commercial tisagenlecleucel. Centers obtained institutional review board approval, and deidentified data were captured using a Health Insurance Portability and Accountability Act–compliant REDCap data capture tool. The study was conducted according to the Declaration of Helsinki. Patients with relapsed/refractory ALL who underwent leukapheresis shipment to Novartis (Hanover, NJ) for commercial tisagenlecleucel manufacturing from 30 August 2017 to 6 March 2020 were included. Infused cell dose was recorded by institution in accordance with the Novartis certificate of analysis. For patients >50 kg, where flat dosing was used, dose was divided by patient kg of body weight to allow cross-cohort comparative analysis. Additional exploratory analysis was preformed, restricted to patients ≤50 kg to eliminate effect of dose capping in patients >50 kg.
Statistical analysis
All infused patients with dose information available were included (n = 180 of 185 infused). Of note, there were no out-of-specification products because of inadequate cell dose in this cohort. Four dose groups were defined using dose quartiles. Clinical and demographic characteristics were summarized by dose group using median and interquartile range for continuous variables and frequency and percentage for categorical variables. For comparisons of baseline characteristics by dose, dose group was treated as ordinal. For continuous variables, association with dose was tested with linear regression, with dose groups assigned scores of 1 to 4. For ordinal and binary variables, association with dose was tested with a χ2 test of linear-by-linear association.3 Association between dose and race/ethnicity was tested with a χ2 test with dose treated as ordinal and race/ethnicity as nominal. Tests of linear-by-linear association were also used to assess the relationship between dose group and cytokine release syndrome (CRS) and neurotoxicity (any grade and grade ≥3) and day-28 response.
Overall survival (OS) was defined as time from infusion to death resulting from any cause. Event-free survival (EFS) was defined as time from infusion to the earliest of nonresponse, relapse, or death. Patients who did not respond were considered to have had an EFS event on day 28. OS and EFS were censored at last follow-up. Relapse-free survival (RFS) was defined only in responders (n = 154) and was defined as time from day 28 to relapse. Patients who died or underwent hematopoietic stem cell transplantation (HSCT) before relapse were censored at time of death/HSCT, and all other relapse-free patients were censored at last follow-up. OS, EFS, and RFS were summarized by dose group using Kaplan-Meier curves and compared using the log-rank test. Cox regression models were used to investigate associations between dose group and other clinical and demographic characteristics and OS, EFS, and RFS.
Results
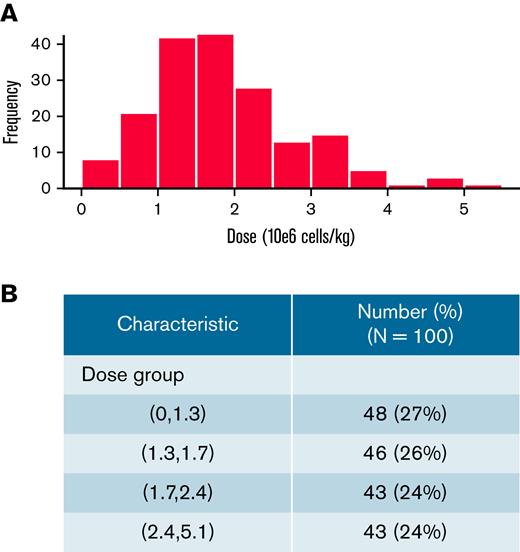
We conducted a retrospective multi-institutional analysis across PRWCC centers (n = 15). All patients who were infused and evaluable with tisagenlecleucel and had a recorded cell dose (n = 180) were included. Distribution and frequency of cell doses are shown in Figure 1A. As shown, a majority of patients received a cell dose <2 × 106 cells per kg. The median cell dose of this cohort was 1.7 × 106 cells per kg.
Dose distribution of tisagenlecleucel in 184 infused and evaluable patients. Frequency of dosing (A) and patient breakdown into quartiles (B).
Dose distribution of tisagenlecleucel in 184 infused and evaluable patients. Frequency of dosing (A) and patient breakdown into quartiles (B).
To examine associations between cell dose, demographic characteristics, and outcome, we divided all infused patients into 4 groups based on dose quartiles (Figure 1B): 0.1341.300 × 106 (D1; n = 48 [27%]), 1.301 to 1.700 × 106 (D2; n = 46 [26%]), 1.701 to 2.400 × 106 (D3; n = 43 [24%]), and 2.401 to 5.100 × 106 (D4; n = 43 [24%]). Clinical and demographic characteristics are summarized across dose groups in Table 1. We found no significant difference between dose quartiles with regard to sex, race/ethnicity, number of prior relapses, indication of CAR T-cell infusion (refractory or relapse), number of lines of therapy before CAR T cells, prior CD19-directed therapy, prior SCT, preinfusion disease burden, or months from initial diagnosis to CAR T-cell infusion. Interestingly, patients who were younger both at initial ALL diagnosis and at CAR T-cell infusion had higher cell doses infused than older patients (P < .001 for both). More favorable baseline cytogenetic risk was associated with higher infused doses (5% of D1 had favorable cytogenetic risk vs 33% in D4; P = .002 for trend; 43 patients were missing initial cytogenetic risk). Higher infused dose was also associated with higher CAR T-cell viability (median viability was 86%, 88%, 88%, and 90% for lowest to highest dose group, respectively; P = .020 for trend) and higher percentage of CAR T cell+ viable cells (median percentage of CAR T cell+ viable cells was 11.6%, 14.1%, 16.0%, and 15.7% for lowest to highest dose group, respectively; P = .017 for trend).
Patient characteristics across dose cohorts
| Characteristic . | Dose, × 106 CAR T cells per kg . | P∗ . | |||
|---|---|---|---|---|---|
| 0-1.3 n = 48 . | 1.3-1.7 n = 46 . | 1.7-2.4 n = 43 . | 2.4-5.1 n = 43 . | ||
| Sex | .7 | ||||
| Female | 19 (40) | 15 (33) | 22 (51) | 16 (37) | |
| Male | 29 (60) | 31 (67) | 21 (49) | 27 (63) | |
| Age at diagnosis, y | 13 (8-16) | 8 (3-16) | 6 (2-12) | 5 (3-8) | <.001 |
| Age at infusion, y | 17 (13-20) | 13 (9-18) | 11 (6-16) | 10 (7-13) | <.001 |
| Race/ethnicity | .3 | ||||
| Asian | 2 (4.3) | 0 (0) | 3 (7.1) | 2 (4.8) | |
| Black | 3 (6.5) | 3 (6.8) | 0 (0) | 1 (2.4) | |
| Hispanic | 18 (39) | 21 (48) | 16 (38) | 12 (29) | |
| >1 race | 1 (2.2) | 1 (2.3) | 2 (4.8) | 0 (0) | |
| Non-Hispanic White | 22 (48) | 19 (43) | 21 (50) | 27 (64) | |
| Unknown | 2 | 2 | 1 | 1 | |
| Initial cytogenetic risk | .002 | ||||
| Favorable | 2 (5.0) | 4 (12) | 7 (21) | 10 (33) | |
| Intermediate | 15 (38) | 12 (35) | 12 (36) | 10 (33) | |
| Unfavorable | 23 (57) | 18 (53) | 14 (42) | 10 (33) | |
| Unknown | 8 | 12 | 10 | 13 | |
| N of relapses pre-CAR | 1.50 (1.00-2.00) | 1.00 (1.00-2.00) | 1.00 (1.00-2.00) | 1.00 (1.00-2.00) | >.9 |
| Relapsed or refractory indication | .6 | ||||
| Refractory | 8 (17) | 10 (22) | 7 (16) | 6 (14) | |
| Relapsed | 40 (83) | 36 (78) | 36 (84) | 37 (86) | |
| N of additional lines of therapy pre-CAR | 2.00 (2.00-3.00) | 3.00 (1.00-3.75) | 2.00 (1.00-3.00) | 2.00 (1.50-3.00) | .3 |
| Prior CD19-directed therapy | 11 (23) | 9 (20) | 11 (26) | 6 (14) | .4 |
| Pre-CAR SCT | 13 (27) | 14 (30) | 9 (21) | 9 (21) | .3 |
| Preinfusion disease burden overall | .9 | ||||
| High | 25 (54) | 23 (51) | 21 (50) | 21 (49) | |
| Low | 9 (20) | 8 (18) | 10 (24) | 14 (33) | |
| No detectable disease | 12 (26) | 14 (31) | 11 (26) | 8 (19) | |
| Unknown | 2 | 1 | 1 | 0 | |
| CAR viability, % | 86 (83-92) | 88 (83-93) | 88 (85-92) | 90 (86-94) | .020 |
| Unknown | 0 | 0 | 0 | 1 | |
| Determination of CAR expression (% CAR+viable cells) | 11.6 (8.6-15.4) | 14.1 (11.1-17.7) | 16.0 (12.0-20.9) | 15.7 (9.9-19.1) | .017 |
| Unknown | 1 | 0 | 1 | 2 | |
| Time from diagnosis to CAR T-cell infusion, mo | 28 (17-58) | 34 (12-54) | 30 (12-56) | 39 (12-64) | .8 |
| Characteristic . | Dose, × 106 CAR T cells per kg . | P∗ . | |||
|---|---|---|---|---|---|
| 0-1.3 n = 48 . | 1.3-1.7 n = 46 . | 1.7-2.4 n = 43 . | 2.4-5.1 n = 43 . | ||
| Sex | .7 | ||||
| Female | 19 (40) | 15 (33) | 22 (51) | 16 (37) | |
| Male | 29 (60) | 31 (67) | 21 (49) | 27 (63) | |
| Age at diagnosis, y | 13 (8-16) | 8 (3-16) | 6 (2-12) | 5 (3-8) | <.001 |
| Age at infusion, y | 17 (13-20) | 13 (9-18) | 11 (6-16) | 10 (7-13) | <.001 |
| Race/ethnicity | .3 | ||||
| Asian | 2 (4.3) | 0 (0) | 3 (7.1) | 2 (4.8) | |
| Black | 3 (6.5) | 3 (6.8) | 0 (0) | 1 (2.4) | |
| Hispanic | 18 (39) | 21 (48) | 16 (38) | 12 (29) | |
| >1 race | 1 (2.2) | 1 (2.3) | 2 (4.8) | 0 (0) | |
| Non-Hispanic White | 22 (48) | 19 (43) | 21 (50) | 27 (64) | |
| Unknown | 2 | 2 | 1 | 1 | |
| Initial cytogenetic risk | .002 | ||||
| Favorable | 2 (5.0) | 4 (12) | 7 (21) | 10 (33) | |
| Intermediate | 15 (38) | 12 (35) | 12 (36) | 10 (33) | |
| Unfavorable | 23 (57) | 18 (53) | 14 (42) | 10 (33) | |
| Unknown | 8 | 12 | 10 | 13 | |
| N of relapses pre-CAR | 1.50 (1.00-2.00) | 1.00 (1.00-2.00) | 1.00 (1.00-2.00) | 1.00 (1.00-2.00) | >.9 |
| Relapsed or refractory indication | .6 | ||||
| Refractory | 8 (17) | 10 (22) | 7 (16) | 6 (14) | |
| Relapsed | 40 (83) | 36 (78) | 36 (84) | 37 (86) | |
| N of additional lines of therapy pre-CAR | 2.00 (2.00-3.00) | 3.00 (1.00-3.75) | 2.00 (1.00-3.00) | 2.00 (1.50-3.00) | .3 |
| Prior CD19-directed therapy | 11 (23) | 9 (20) | 11 (26) | 6 (14) | .4 |
| Pre-CAR SCT | 13 (27) | 14 (30) | 9 (21) | 9 (21) | .3 |
| Preinfusion disease burden overall | .9 | ||||
| High | 25 (54) | 23 (51) | 21 (50) | 21 (49) | |
| Low | 9 (20) | 8 (18) | 10 (24) | 14 (33) | |
| No detectable disease | 12 (26) | 14 (31) | 11 (26) | 8 (19) | |
| Unknown | 2 | 1 | 1 | 0 | |
| CAR viability, % | 86 (83-92) | 88 (83-93) | 88 (85-92) | 90 (86-94) | .020 |
| Unknown | 0 | 0 | 0 | 1 | |
| Determination of CAR expression (% CAR+viable cells) | 11.6 (8.6-15.4) | 14.1 (11.1-17.7) | 16.0 (12.0-20.9) | 15.7 (9.9-19.1) | .017 |
| Unknown | 1 | 0 | 1 | 2 | |
| Time from diagnosis to CAR T-cell infusion, mo | 28 (17-58) | 34 (12-54) | 30 (12-56) | 39 (12-64) | .8 |
Data are presented as n (%) or median (interquartile range). Association with dose was tested with linear regression, with dose groups assigned scores of 1-4. Association between dose and ordinal variables was tested with χ2 test of linear-by-linear association. Association between dose and race/ethnicity was tested with χ2 test, with dose treated as ordinal and race/ethnicity as nominal.
Pearson χ2 test; χ2 test of linear-by-linear association; linear regression; χ2 test of ordinal association.
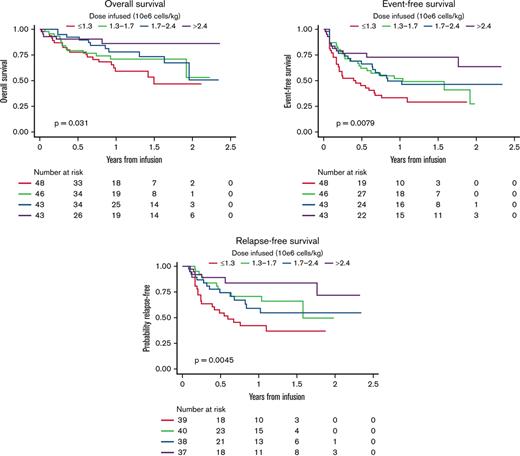
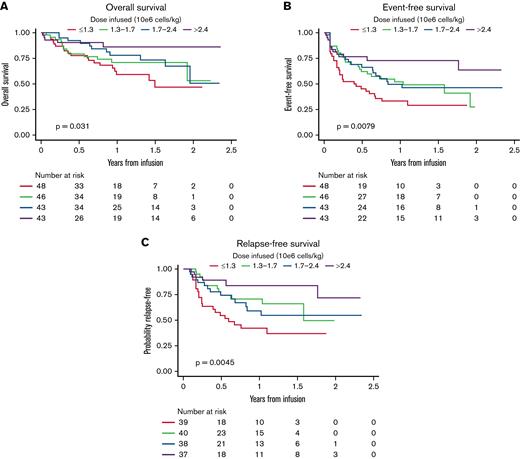
OS
Analyses for OS included 180 participants; OS at 3 years was 58.9% (95% CI, 47.6-72.9). Among 132 patients who were alive at last follow-up, the median follow-up was 402.5 days (range, 33-863). OS by dose group is shown in Figure 2. Dosing was significantly associated with survival (log-rank P = .031). In univariable Cox models for OS (supplemental Table 1), higher dose was associated with improved survival (P = .026), and the magnitude of the effect increased across increasing dose groups (hazard ratio [HR], 0.68; 95% CI, 0.33-0.1.38 for D2 vs D1; HR, 0.53; 95% CI, 0.25-1.11 for D3 vs D1; and HR, 0.26; 95% CI, 0.10-0.69 for D4 vs D1). Interestingly, male patients had better OS compared with female patients (HR, 0.51; 95% CI, 0.29-0.91 for male vs female patients; P = .021). Another characteristic that was significantly associated with OS was the number of additional lines of therapy before CAR T-cell infusion, where more lines were associated with inferior outcomes (HR, 1.17; 95% CI, 1.01-1.35 for each additional line; P = .032), and patients with no detectable disease or low disease burden had improved outcomes compared with patients with high disease burden (HR, 0.18; 95% CI, 0.07-0.52 and HR, 0.30; 95% CI, 0.12-0.77, respectively; P < .001). In a multivariable Cox model, dose group and preinfusion disease burden remained significantly associated with OS (Table 2). Patients receiving higher CAR T-cell dosing had improved OS (HR, 0.70; 95% CI, 0.34-1.46 for D2 vs D1; HR, 0.40; 95% CI, 0.18-0.87 for D3 vs D1; and HR, 0.22; 95% CI, 0.08-0.61 for D4 vs D1; P = .008). Independent of cell dose, patients with no detectable disease or low disease burden also had improved OS (HR, 0.18; 95% CI, 0.06-0.5 and HR, 0.38; 95% CI, 0.15-0.99; P < .001). Exploratory analysis of OS across dose quartiles in a limited sample restricted to patients ≤50 kg, without dose capping, recapitulated dose effect on OS (P = .029; supplemental Figure 1).
OS, EFS, and RFS across CAR T-cell dose quartiles. Higher doses of tisagenlecleucel resulted in improved OS (A), EFS (B), and RFS (C).
OS, EFS, and RFS across CAR T-cell dose quartiles. Higher doses of tisagenlecleucel resulted in improved OS (A), EFS (B), and RFS (C).
Multivariable analysis
| Characteristic . | OS . | EFS . | RFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Dose group, × 106CAR T cells per kg | .008 | <.001 | .002 | ||||||
| 0-1.3 | — | — | — | — | — | — | |||
| 1.3-1.7 | 0.7 | 0.34-1.46 | 0.51 | 0.29-0.88 | 0.4 | 0.19-0.82 | |||
| 1.7-2.4 | 0.4 | 0.18-0.87 | 0.4 | 0.22-0.73 | 0.43 | 0.2-0.89 | |||
| 2.4-5.1 | 0.22 | 0.08-0.61 | 0.24 | 0.12-0.49 | 0.18 | 0.07-0.49 | |||
| Age at infusion, y | 0.98 | 0.94-1.03 | .4 | 0.97 | 0.94-1.00 | .085 | 0.98 | 0.94-1.03 | .4 |
| Preinfusion disease burden overall | <.001 | <.001 | .004 | ||||||
| High | — | — | — | — | — | — | |||
| Low | 0.38 | 0.15-0.99 | 0.34 | 0.18-0.65 | 0.38 | 0.17-0.88 | |||
| No detectable disease | 0.18 | 0.06-0.50 | 0.29 | 0.16-0.52 | 0.38 | 0.19-0.74 | |||
| Sex | |||||||||
| Female | — | — | — | — | — | — | |||
| Male | 0.56 | 0.31-1.02 | .057 | 0.75 | 0.48-1.15 | .2 | 0.69 | 0.4-1.21 | .2 |
| Characteristic . | OS . | EFS . | RFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| Dose group, × 106CAR T cells per kg | .008 | <.001 | .002 | ||||||
| 0-1.3 | — | — | — | — | — | — | |||
| 1.3-1.7 | 0.7 | 0.34-1.46 | 0.51 | 0.29-0.88 | 0.4 | 0.19-0.82 | |||
| 1.7-2.4 | 0.4 | 0.18-0.87 | 0.4 | 0.22-0.73 | 0.43 | 0.2-0.89 | |||
| 2.4-5.1 | 0.22 | 0.08-0.61 | 0.24 | 0.12-0.49 | 0.18 | 0.07-0.49 | |||
| Age at infusion, y | 0.98 | 0.94-1.03 | .4 | 0.97 | 0.94-1.00 | .085 | 0.98 | 0.94-1.03 | .4 |
| Preinfusion disease burden overall | <.001 | <.001 | .004 | ||||||
| High | — | — | — | — | — | — | |||
| Low | 0.38 | 0.15-0.99 | 0.34 | 0.18-0.65 | 0.38 | 0.17-0.88 | |||
| No detectable disease | 0.18 | 0.06-0.50 | 0.29 | 0.16-0.52 | 0.38 | 0.19-0.74 | |||
| Sex | |||||||||
| Female | — | — | — | — | — | — | |||
| Male | 0.56 | 0.31-1.02 | .057 | 0.75 | 0.48-1.15 | .2 | 0.69 | 0.4-1.21 | .2 |
EFS
Across 180 patients analyzed for EFS, 88 experienced an EFS event. Among those who were alive and event free at last follow-up, the median follow-up was 402.5 days (range, 33-856). EFS by dose group is shown in Figure 2. Dose group was significantly associated with EFS (log-rank P = .0079). In the univariable analysis for EFS (supplemental Table 2), patients receiving the highest dose range had better EFS (HR, 0.33; 95% CI, 0.17-0.65 for highest vs lowest dose group; P = .009). Interestingly, male patients also had better EFS compared with female patients (HR, 0.66; 95% CI, 0.43-1.00; P = .049). Patients with no detectable disease or low disease burden had improved outcomes compared with those with high disease burden (HR, 0.34; 95% CI, 0.19-0.60 and HR, 0.31; 95% CI, 0.16-0.58, respectively; P < .001). Multivariable analysis of EFS demonstrated that patients infused with the highest dose range had improved EFS (HR, 0.24; 95% CI, 0.12-0.49 for D4 vs D1; P < .001). Younger age at infusion was also associated with improved EFS, although the effect was not statistically significant (HR, 0.97; 95% CI, 0.94-1.00 for each 1-year increase in age; P = .085). Independent of dose, patients with no detectable disease or low disease burden also had improved EFS (HR, 0.29; 95% CI, 0.16-0.52 and HR, 0.34; 95% CI, 0.18-0.65, respectively, compared with those with high disease burden; P < .001). Exploratory analysis of EFS across dose quartiles in a limited sample restricted to patients ≤50 kg, without dose capping, recapitulated dose effect on EFS (P = .0014; supplemental Figure 1).
RFS
Post-CAR HSCT was distributed without significant difference across dose quartiles (supplemental Table 3). RFS was analyzed across 154 responders, 54 of whom experienced post-CAR relapse without interval HSCT (supplemental Table 3). Among those who were relapse free at last follow-up, the median follow-up was 300 days (range, 33-856). RFS by dose group is shown in Figure 2. Dose group was significantly associated with RFS with or without censoring for transplantation (log-rank P = .0045 and .0019, respectively; supplemental Figure 2). Moreover, when patients were not censored for transplantation, RFS was also improved in those who received higher doses. In the univariable analysis for RFS (supplemental Table 4), patients receiving the highest dose range had improved RFS (HR, 0.24; 95% CI, 0.10-0.59; P = .0506). Male patients had improved RFS compared with female patients, although the difference was not statistically significant (HR, 0.60; 95% CI, 0.35-1.03; P = .066). Patients with no detectable disease or low disease burden had improved RFS compared with those with high disease burden (HR, 0.44; 95% CI, 0.23-0.85 and HR, 0.33; 95% CI, 0.15-0.75, respectively; P = .003). Multivariable analysis of RFS demonstrated that patients infused with the highest T-cell dose had improved RFS (HR, 0.18; 95% CI, 0.07-0.49; P = .002). RFS was additionally improved in patients with no detectable disease or low disease burden (HR, 0.38; 95% CI, 0.17-0.88 and HR, 0.43; 95% CI, 0.19-0.74, respectively; P = .004). Exploratory analysis of RFS across dose quartiles in a limited sample restricted to patients ≤50 kg, without dose capping, recapitulated dose effect on RFS (P = .0015; supplemental Figure 1).
Response rates and toxicity
We assessed the impact of cell dose on response rate at day 28 after CAR T-cell infusion. Response was defined as no evidence of morphologic leukemia in the bone marrow. As shown in Table 3, dose was not associated with initial response after CAR T-cell therapy (response rate was 83%, 89%, 88%, and 92% for the dose groups from lowest to highest, respectively; P = .2) and was not associated with the initial response rate in the <50 kg cohort (supplemental Table 5).
Response rates by dose group
| Characteristic . | Dose, × 106 CAR T cells per kg . | P∗ . | |||
|---|---|---|---|---|---|
| 0-1.3 n = 48 . | 1.3-1.7 n = 46 . | 1.7-2.4 n = 43 . | 2.4-5.1 n = 43 . | ||
| Responded | 39 (83) | 40 (89) | 38 (88) | 37 (92) | .2 |
| Unknown | 1 | 1 | 0 | 3 | |
| Characteristic . | Dose, × 106 CAR T cells per kg . | P∗ . | |||
|---|---|---|---|---|---|
| 0-1.3 n = 48 . | 1.3-1.7 n = 46 . | 1.7-2.4 n = 43 . | 2.4-5.1 n = 43 . | ||
| Responded | 39 (83) | 40 (89) | 38 (88) | 37 (92) | .2 |
| Unknown | 1 | 1 | 0 | 3 | |
Data are given as n (%).
χ2 test of linear-by-linear association.
We additionally assessed the impact of CAR T-cell dose on the rate of CRS or neurologic toxicity. In this cohort, higher CAR T-cell dosing was not associated with an increased risk of developing CRS, neurotoxicity, or grade ≥3 adverse events (Table 4). Moreover, there was no difference in treatment with steroids or tocilizumab across quartiles.
Toxicity across dose quartiles
| Characteristic . | Dose, × 106 CAR T cells per kg . | P∗ . | |||
|---|---|---|---|---|---|
| 0-1.3 n = 48 . | 1.3-1.7 n = 46 . | 1.7-2.4 n = 43 . | 2.4-5.1 n = 43 . | ||
| Did patient develop CRS? | 25 (52) | 28 (61) | 26 (60) | 28 (65) | .2 |
| Did patient experience neurotoxicity? | 11 (23) | 10 (22) | 7 (16) | 11 (26) | >.9 |
| Unknown | 1 | 1 | 0 | 1 | |
| Grade ≥3 CRS | 13 (27) | 10 (22) | 8 (19) | 6 (14) | .11 |
| Unknown | 0 | 1 | 0 | 0 | |
| Grade ≥3 neurotoxicity | 2 (4.3) | 4 (9.1) | 1 (2.3) | 4 (9.5) | .6 |
| Unknown | 1 | 2 | 0 | 1 | |
| Treatment of neurotoxicity (choice, steroids [systemic]) | .2 | ||||
| Checked | 2 (4.2) | 3 (6.5) | 1 (2.3) | 6 (14) | |
| Unchecked | 46 (96) | 43 (93) | 42 (98) | 37 (86) | |
| Treatment of neurotoxicity (choice, IT steroids) | |||||
| Unchecked | 48 (100) | 46 (100) | 43 (100) | 43 (100) | |
| Treatment of neurotoxicity (choice, tocilizumab) | .6 | ||||
| Checked | 2 (4.2) | 2 (4.3) | 0 (0) | 2 (4.7) | |
| Unchecked | 46 (96) | 44 (96) | 43 (100) | 41 (95) | |
| Characteristic . | Dose, × 106 CAR T cells per kg . | P∗ . | |||
|---|---|---|---|---|---|
| 0-1.3 n = 48 . | 1.3-1.7 n = 46 . | 1.7-2.4 n = 43 . | 2.4-5.1 n = 43 . | ||
| Did patient develop CRS? | 25 (52) | 28 (61) | 26 (60) | 28 (65) | .2 |
| Did patient experience neurotoxicity? | 11 (23) | 10 (22) | 7 (16) | 11 (26) | >.9 |
| Unknown | 1 | 1 | 0 | 1 | |
| Grade ≥3 CRS | 13 (27) | 10 (22) | 8 (19) | 6 (14) | .11 |
| Unknown | 0 | 1 | 0 | 0 | |
| Grade ≥3 neurotoxicity | 2 (4.3) | 4 (9.1) | 1 (2.3) | 4 (9.5) | .6 |
| Unknown | 1 | 2 | 0 | 1 | |
| Treatment of neurotoxicity (choice, steroids [systemic]) | .2 | ||||
| Checked | 2 (4.2) | 3 (6.5) | 1 (2.3) | 6 (14) | |
| Unchecked | 46 (96) | 43 (93) | 42 (98) | 37 (86) | |
| Treatment of neurotoxicity (choice, IT steroids) | |||||
| Unchecked | 48 (100) | 46 (100) | 43 (100) | 43 (100) | |
| Treatment of neurotoxicity (choice, tocilizumab) | .6 | ||||
| Checked | 2 (4.2) | 2 (4.3) | 0 (0) | 2 (4.7) | |
| Unchecked | 46 (96) | 44 (96) | 43 (100) | 41 (95) | |
Data are given as n (%).
IT, intrathecal.
χ2 test of linear-by-linear association; Fisher exact test.
Discussion
The goal of this study was to explore the impact of cell dose on outcome after tisagenlecleucel. Current dosing of tisagenlecleucel according to the manufacturer’s package insert dictates that a single dose of tisagenlecleucel contains 0.2 to 5.0 × 106 CAR+ viable T cells per kg of body weight for patients ≤50 kg or 0.1 to 2.5 × 108 CAR+ viable T cells for patients >50 kg, suspended in 1 to 3 patient-specific infusion bags for IV infusion. Therefore, there exists a wide dose range. Tisagenlecleucel products are occasionally manufactured in dosing aliquots per bag that would permit infusion of 1, 2, or more bags, while remaining within the recommended dose range. To interrogate tisagenlecleucel dose effect, we divided patients into dose quartiles using actual per-kg doses administered to patients receiving commercial tisagenlecleucel and demonstrated that higher CAR T-cell dosing was associated with improved OS, EFS, and RFS in multivariable models (all P < .05).
Preclinical experiments by Milone et al6 established that there is a threshold of CAR T cells required to achieve an antileukemic effect. In their model, T cells expressing CD19ζ or CD19-BB-ζ CAR T cells were administered to immune-deficient NOD-SCID-γ−/− mice with B-ALL. They found a dose-dependent antileukemic effect in the mice treated with 5 or 20 × 106 CAR T cells. In animals that received only 1 × 106 CAR T cells, there was only a partial effect on leukemia clearance, supporting that a CAR T-cell dosing threshold is required to achieve leukemia eradication.
In the pivotal ELIANA study, patients received a median dose of 3.1 × 106 CAR T cells per kg (range, 0.2-5.4 × 106).2 The median dose in this study across recipients of commercial tisagenlecleucel was 1.7 × 106 CAR T cells per kg, which is substantially lower than the dosing described in the clinical trial; 76% of patients received ≤2.4 × 106 cells per kg in our study. Lower numbers of CAR T cells were also observed by Pasquini et al7; a median dose of 2 × 106 cells per kg was observed in their study for patients ≤50 kg with ALL. Numerous possibilities may account for this discrepant dosing, including manufacturing changes and scalability challenges with commercialization, types of patients receiving tisagenlecleucel, and/or additional unknown factors. Given these differences, we felt it was important to investigate posttisagenlecleucel outcomes based on cell dose.
In an evaluation of 79 patients with B-ALL from both the ENSIGN and ELIANA trials, clinical responses were seen in all evaluated dose ranges (patients ≤50 kg, 0.2-5.0 × 106/kg; patients >50 kg, 0.1 to 2.5 × 108 CAR+ viable T cells).8 Patients were also evaluated at certain time points for CAR T-cell expansion and persistence. In responding patients, they identified a median peak expansion of 31.6% CD3+ CAR+ cells (range, 1.10-84.9). In nonresponding patients, there was rapid clearance of the CAR+ T cells. Because of the retrospective nature of this study, CAR expression and CAR T-cell expansion were not evaluated. However, one could envision that higher doses of CAR T cells may result in greater persistence and better outcomes compared with lower doses, as shown in this study. This should be considered in future analyses of CAR-mediated dose response.
A recent study by Ayuk et al9 examined doses of axicabtagene ciloleucel prospectively in 21 patients who were being treated for aggressive B-cell lymphoma using polymerase chain reaction to determine peak CAR T-cell count in vivo. In this study of real-world patients, the median peak CAR T-cell count was 16.14 CAR T cells per μL. Patients with ≥16.14 cells per μL peak CAR T cells were considered strong expanders and were found to have improved day-30 objective responses compared with those who were not strong expanders (91% vs 40%; P = .02).
In a recent phase 1 study of KTE-X19 for adult patients with ALL, a dose of 1 × 106 CAR T cells per kg dose had the most favorable risk/benefit ratio without compromising activity when combined with appropriate adverse event management.10 KTE-X19 is a CD28 CAR T-cell therapy containing a CD3z and CD28 costimulatory domain,11,12 whereas tisagenlecleucel has a 4-1BB domain, which, in addition to the patient population, could account for the differences. Kymriah is approved for patients up to age 26 years, and the median age in the phase 1 study was 46 years (range, 18-77).
One concern with increasing CAR T-cell dosing is the possibility of increased rates of CRS and/or neurotoxicity. In the pivotal ELIANA trial,2 77% of patients had CRS. Neurologic toxicity was observed in 40% of patients. Data from multiple phase 2 studies of tisagenlecleucel for both B-ALL and adult diffuse large B-cell lymphoma were used to examine how dose is related to efficacy, safety, and exposure.13 In patients with B-ALL, logistic regression analyses showed that a higher CAR T-cell dose was associated with a higher probability of response.13 There was no increase in CRS incidence or severity across dose ranges, and patients achieved comparable early response rates independently of dose. In this study, increased doses were not associated with increased toxicity.
In a recent study by Pasquini et al,7 real-world outcomes of patients with ALL and non-Hodgkin lymphoma treated with tisagenlecleucel were evaluated using the Center for International Bone Marrow Transplant Registry database. In this evaluation, logistic regression was used to assess the association between best overall response and CAR T-cell dose infused. In patients with ALL with a weight of ≤50 or >50 kg, dose did not affect best overall response. Our study additionally analyzed survival outcomes (ie, OS, EFS, and RFS), with all 3 outcomes affected by cell dose, where higher cell dose was associated with significant improvement in OS, EFS, and RFS.
The dose groups compared in our current study were based on dose quartiles generated to interrogate patterns of association between dose and outcome, not to identify an optimal dose threshold or dose range. In the higher dose quartiles, improved cytogenetics were seen compared with lower dose quartiles, which potentially could have affected the ability to collect appropriate T cells for CAR T-cell generation and could also have served as a confounder in our data set. Therefore, the impact of cytogenetics on posttisagenlecleucel outcomes needs to be examined in additional studies.
Further research is warranted to elucidate the relationship between threshold dosing and post-CAR outcomes. Our results, however, indicate that higher tisagenlecleucel doses are associated with superior survival outcomes in the real-world setting and suggest further exploration of higher dosing strategies, within the safe range of tisagenlecleucel, in the clinical setting.
Acknowledgments
The authors acknowledge the following individuals for their major roles in supporting successful execution of this multi-institutional study: Sharon Mavroukakis and Emily Egeler for regulatory support; Anika Dove and Daisy Torres for administrative support; Neil Morimoto for legal counsel and contracting; and Anne Marcy, Michelle Fujimoto, Jennifer Sheppard, Jean Sosna, Victoria Koch, Katie Doherty, Emily Bakinowski, Elizabeth Klein, Daritzya Baraja, Courtney Newbold, Glenn McWillians, Maggie Dyer, Kasey Abrahamnson, Angie Peltz, Ahmed Tahoun, Mary Suarez, Megan Hanby, Stacy Cooper, and Brad Muller for data management.
This work was supported by a St. Baldrick’s/Stand Up 2 Cancer Pediatric Dream Team Translational Cancer Research Grant (C.L.M.). Stand Up 2 Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. C.L.M. is a member of the Parker Institute for Cancer Immunotherapy, which supports the Stanford University Cancer Immunotherapy Program. The work was also supported by the Virginia and D.K. Ludwig Fund for Cancer Research.
Authorship
Contribution: L.M.S. performed administrative duties; C.B. and L.M.S. designed the data collection tool; H.E.S. and L.M.S. designed this study; A.E. performed statistical analysis; and all authors were involved in conception and design, collection and assembly of patient data, data analysis and interpretation, manuscript writing, and final manuscript approval and are accountable for all aspects of this work.
Conflict-of-interest disclosure: H.E.S. has served on an advisory committee and speaker’s bureau for Novartis. M.R.V. has served on an advisory committee for Novartis, has been a consultant for and is a current equity holder in Fate Therapeutics and Bmogen, and has been a consultant for UpToDate. C.L.P. has served on an advisory committee for Novartis. S.P.M. has served on an advisory committee for Novartis and Jazz Pharmaceuticals. G.D.M. served on the ELIANA trial steering committee and speaker’s bureau and has served as a consultant and received honoraria from Novartis. P.A.B. has served on an advisory committee for Novartis, Kite, Takeda, Janssen, Kura, Servier, and Jazz Pharmaceuticals. M.Q. has served as a consultant for Novartis and Mesoblast. M.H. has served on an advisory board for Sobi and Novartis. P.S. has served as a consultant for Takeda and Mesoblast. K.J.C. has served as a consultant for and received research funding from Novartis, has served as a consultant for Mesoblast, and has received research funding from Celgene. C.L.M. has served as a consultant for and is a current equity holder in Lyell Immunopharma and Apricity Health, has served as a consultant for NeoImmune Tech, Nektar Therapeutics, and Bristol Myers Squibb, and is a current equity holder in Allogene. T.W.L. reports consultancy relationships with Novartis, Cellectis, Bayer, Deciphera, Jumo Health, and Y396 mAbs Therapeutics and research funding from Pfizer, Novartis, and Bayer. The remaining authors declare no competing financial interests.
Correspondence: Liora M. Schultz, Division of Hematology/Oncology, Department of Pediatrics, Stanford University School of Medicine, 1000 Welch Rd, Palo Alto, CA 94304; e-mail: liora.schultz@gmail.com.
References
Author notes
Data are available on request from the corresponding author, Liora M. Schultz (liora.schultz@gmail.com).
The full-text version of this article contains a data supplement.