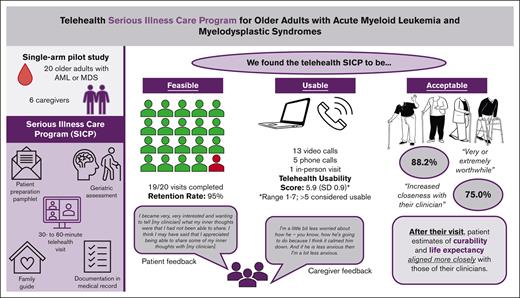
We found a telehealth-delivered SICP to be feasible and usable for older adults with AML and MDS.
The majority of patients found the telehealth SICP to be worthwhile and would recommend it to others.
Visual Abstract
Older patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) feel shocked and bewildered when diagnosed. Serious illness conversations (SICs) may increase disease understanding and preparations for the future. However, SICs often happen late, in part because of clinician-perceived patient discomfort. Telehealth may promote patient comfort by allowing SICs to take place at home. This study assesses the feasibility and usability of a telehealth-delivered Serious Illness Care Program (SICP) for older adults with AML and MDS. We conducted a single-arm pilot study including 20 older adults with AML and MDS. Feasibility was measured using retention rate, with >80% considered feasible. Usability was measured using telehealth usability questionnaire (TUQ; range, 1-7): >5 considered usable. We collected other outcomes including acceptability and disease understanding and conducted post-visit qualitative interviews to elicit feedback. Hypothesis testing was performed at α = 0.10 owing to the pilot nature and small sample size. Retention rate was 95% (19/20); mean TUQ scores were 5.9 (standard deviation [SD], 0.9) and 5.9 (SD, 1.1) for patients and caregivers, respectively. We found the SICP to be acceptable. The majority of patients found the SICP to be very or extremely worthwhile (88.2%; 15/17), and reported it increased closeness with their clinician (75.0%; 12/16). After their visit, patient estimates of curability, and overall life expectancy aligned more closely with those of their clinicians. In qualitative interviews, most patients said that they would recommend this program to others (89.5%, 17/19). This study demonstrated that delivery of the telehealth SICP to older patients with AML and MDS is feasible, usable, and acceptable. This trial is registered at www.clinicaltrials.gov as #NCT04745676.
Introduction
Patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) often experience intense emotions, such as shock, anger, and anxiety when receiving and navigating their diagnosis.1-4 The emotions may make processing and understanding their diagnosis and treatment options difficult.1,5 In addition, there is very little time between diagnosis and treatment decision-making.6 Older adults, compared with younger individuals, face additional challenges. This includes a higher prevalence of aging-related vulnerabilities, such as physical and cognitive impairments that increase the risks of adverse events and mortality.7-9 Therefore, older patients with AML and MDS often make treatment decisions with limited understanding of their diagnosis and lack of time for emotional coping.
Serious illness conversations (SICs) may increase patients’ understanding of their disease, promote hope and illness acceptance, and better prepare them for the future.10 The geriatric assessment (GA) uses validated tools to identify aging-related vulnerabilities (eg, functional impairment and cognitive impairment) that are associated with poor outcomes. GA may help clinicians better identify aging-related vulnerabilities and inform management discussions. Integration of a GA into SICs may help clinicians better tailor SICs based on age-related vulnerabilities and enhance the quality of conversations.11 Furthermore, many older adults with cancer prefer to maintain some sense of control at the end-of-life (EOL), and early SICs may allow patients to discuss their EOL wishes before they are unable to do so.12 In a cross-sectional study of 200 patients with cancer, 82.5% of patients wished that they had an SIC with their physician, and 94% preferred to have these discussions early.13 Therefore, routine SICs have the potential to both mitigate emotional distress and address aging-related concerns that older adults with AML and MDS often have to navigate.
We and others have shown that SICs often happen late in the disease course and are often limited by clinic time and clinician-perceived patient discomfort.14-16 One strategy to address perceived patient discomfort is the use of telehealth, which can promote patient comfort by allowing SICs to take place when patients are at home.17,18 We previously conducted a qualitative study to adapt the SIC program (SICP) for delivery via telehealth to promote early SICs among older adults with AML and MDS.19 The primary aim of this pilot study was to assess the feasibility and usability of the adapted SICP via telehealth for older adults with AML and MDS.
Methods
Study design, population, and setting
We conducted a single-arm pilot study at the University of Rochester Medical Center/Wilmot Cancer Institute in Rochester, New York and recruited patients and their caregivers from June 2022 to March 2023. Patients enrolled in this study were aged ≥60 years, had a diagnosis of AML or MDS, were being managed in the outpatient setting, were able to speak English (because the adapted SICP is written in English only), and were able to provide informed consent. Caregivers aged ≥18 years old were enrolled if identified by the patient when asked if there was a “family member, partner, friend, or caregiver with whom you discuss or who can be helpful in health-related matters” and able to provide consent. Patients could enroll in this study with or without caregivers (up to a maximum of 2 caregivers were allowed to enroll), but caregivers could only enroll if their respective patient consented to participate. Caregivers who did not formally enroll in the study could join the SICP visits. Oncologists and advance practice providers who had provided care for at least 1 patient aged ≥60 years with AML/MDS in the past year were also enrolled. This study was approved by the University of Rochester Research Subjects Review Board.
Study procedure and data collection
Patients were identified by the study team, and eligibility was confirmed with both the primary oncologist and principal investigator (K.P.L.). Eligible participants were approached via telephone. Participants who consented to participate completed baseline measures (demographics and cancer health literacy).20 Cancer health literacy was measured using the 6-item Cancer Health Literacy Test (CHLT-6). CHLT-6 is a validated measure with a Cronbach α of 0.96 to 0.99.20 Correct responses to the questions were scored as 1 and summed. Participants were classified as having adequate cancer health literacy (total score, 4-6) or limited cancer literacy (total score <4).20 Clinical information was collected by the study team from the electronic health record (EHR). A 30- to 60-minute SICP visit with the oncology clinician was scheduled within 2 months of consent. Telehealth was defined as the visit taking place via video call or telephone in this study. Participants then completed postintervention measures and participated in an audio-recorded, semistructured interview via telephone to discuss their experience and provide feedback. Audio-recorded postintervention interviews were transcribed verbatim and uploaded to MAXQDA software (VERBI Software GmBH) for analysis.
Intervention description
The adapted SICP includes19,21 (1) GA to evaluate aging-related vulnerabilities obtained from the patient and summarized for clinicians, (2) patient preparation pamphlet, (3) clinician preparation email, (4) SIC conversation guide (SICG), (5) EHR documentation template for clinicians (supplemental Figure 1), and (6) family guide. The patient preparation letter, SICG, and family guide have previously been published.19,22 Before the SICP visit, the study team completed a GA with the patient that was provided to clinicians. The GA included activities of daily living (ADL), instrumental ADLs, fall history, nutritional status, and cognition assessed using the Short Orientation–Memory-Concentration test of cognitive impairment.23-26 Patients were provided with the patient preparation pamphlet via email or mailed to their home, and clinicians were provided with a preparation email. The preparation email included access to a University of Rochester Medical Center compliant Zoom link with details of the day and time of the visit, summary of the patient’s GA, a copy of the SICG, and information regarding the EHR documentation template. Clinicians documented their visit in the EHR using the documentation template. After the visit, patients were provided with the family guide via email or mailed to their home.
Measures
Feasibility and usability
The primary outcome measures for this study were feasibility and usability. Feasibility was measured using retention rate, defined as completion of the SICP visit, with >80% retention considered feasible. Usability was measured using the Telehealth Usability Questionnaire (TUQ [range, 1-7; higher score is better]) with an average score of >5 considered usable.27
Other outcome measures
To inform future trials, we also collected other patient and caregiver measures. Patient measures included advanced care planning engagement, psychological health, quality of life (QOL), disease understanding, acceptability of the SICP, and satisfaction with communication. Caregiver measures included psychological health, QOL, disease understanding, and satisfaction with communication.
Patient measures: baseline and after intervention
Advance care planning (ACP) engagement was assessed using an adapted 15-item questionnaire on patient readiness and self-efficacy (range, 1-5; higher score is better).28
Psychological health was assessed using the validated 7-item Generalized Anxiety Disorder-7 ([GAD-7] range, 0-21; lower score is better), the validated 9-item Patient Health Questionnaire-9 ([PHQ-9] range, 0-27; lower score is better), and the 1-item distress thermometer (range, 0-10; lower score is better).29-31 QOL was assessed using the validated 44-item functional assessment of cancer therapy-leukemia ([FACT-Leu] range, 0-176; higher score is better).32 The FACT-Leu assessment includes 5 domains: physical well-being (7-item), social/family well-being (7-item), emotional well-being (6-item), and functional well-being (7-item), and additional concerns (17-item).32 Disease understanding was assessed using a 5-item questionnaire on prognosis.33 Patients were asked to estimate the curability of their cancer with treatment and their life expectancy, and these results were compared with responses of clinicians.33 For example, in order to measure the alignment of life expectancy between patients and clinicians, they were asked the following question: “Considering your (the patient’s) health, and your (the patient’s) underlying medical conditions, what would you estimate your (the patient’s) overall life expectancy to be?” Response options were ≤6 months, 7 to 12 months, 1 to 2 years, 2 to 5 years, and >5 years. We described the distribution of responses to this question for patients at baseline and after intervention, as well as for clinicians, and compared the responses at both time points. Responses were considered more aligned if they were similar to each other.
Patient measures: baseline (only)
Social support was assessed using the validated 13-item Older Americans Resources and Services Medical Social Support survey.34 This survey assesses the frequency and availability of social interaction and emotional support for older adults. It also evaluates the patient’s perception of their support persons.
Patient measures: postintervention (only)
Acceptability of the SICP was assessed using a 23-item acceptability survey.21 Satisfaction with communication was assessed using the adapted 6-item Health Care Communication Questionnaire ([HCCQ] range, 0-20; higher score is better) and the 1-item Heard and Understood question (range, 0-4; higher score is better).35,36 Satisfaction with communication about other medical issues and aging concerns was assessed using the adapted 7-item HCCQ-Age (range, 0-28; higher score is better).11 Therapeutic alliance was assessed using a modified Human Connection Scale (range, 16-64; higher score is better).37 Acceptance of illness was assessed using the validated 5-item Peace, Equanimity, and Acceptance in Cancer Experience ([PEACE] range, 5-20; higher score is better) questionnaire.38
Caregiver measures: baseline and postintervention
Psychological health was assessed using the validated GAD-7, the validated PHQ-9, and the distress thermometer.29-31 QOL was assessed using the validated 35-item Caregiver QOL Index for caregivers (range, 0-140; higher score is better).39 Disease understanding was assessed using a similar questionnaire as was used for the patients.33 Caregivers were asked to estimate the curability of the patient’s cancer with treatment and life expectancy for the patient.33
Caregiver: postintervention (only)
Similar measures were used to assess satisfaction with communication for caregivers. Caregiver HCCQ also included 2 additional sections: HCCQ-Age about patients (range, 0-28) and HCCQ-Age about caregivers (range, 0-20). One section assessed the caregiver’s perception of the patient’s communication with the clinician (7-item). The second section assessed the caregiver’s own communication with the clinician (5-item).11,35,36
Data analysis
We used descriptive statistics to summarize demographics, feasibility, usability, and patient and caregiver measures. For measures collected at both baseline and postintervention, we used paired t tests or Wilcoxon signed-rank tests to examine change from baseline to postintervention depending on the distribution of data. Hypothesis testing was performed at α = 0.10 (2-tailed) given the pilot nature of the study and small sample size. We anticipated that our sample size would be sufficient based on prior research and also published guidance on usability studies.40-42 We anticipated that ∼20% of the participants would withdraw before postintervention assessment owing to death. With 20 patients enrolled, we anticipated at least 16 patients to be evaluable. When we estimated retention rate and usability, a 95% confidence interval would span ≥25%. We conducted all quantitative analyses using SAS statistical software, version 9.4 (SAS Institute Inc, Cary, NC). For qualitative analyses, 2 coders used open coding and focused content analysis to independently code transcripts for themes. Discrepancies were resolved through consensus between coders. Thematic saturation was achieved, and data were reported using Consolidated Criteria for Reporting Qualitative Research guidelines (supplemental Tables 1 and 2).
Results
Demographics and GA
Participant demographics and patient clinical characteristics are shown in Table 1. Mean ages of patients and caregivers were 75 years (standard deviation [SD], 5.9; range, 63-87) and 64 years (SD, 13.7; range, 44-77), respectively. The majority of participants were White (patients: 85%, 17/20; caregivers 100%, 6/6) and non-Hispanic (patients: 80.0%, 16/20; caregivers: 83.3%, 5/6). Results of the GA for patients are shown in supplemental Table 4.
Participant demographics
| Variable . | Patients (N = 20) . | Caregivers (N = 6) . | Clinicians (N = 9) . |
|---|---|---|---|
| Age, mean (SD, range) | 75 (5.9, 63-87) | 64 (13.7, 44-77) | 44 (11.7, 31-66) |
| Number of years in practice after completion of training, mean (SD, range) | _ | _ | 11 (10.0, 2-30) |
| Discipline, n (%) | |||
| Oncology physician | _ | _ | 5 (55.6%) |
| Advanced practice provider | _ | _ | 4 (44.4%) |
| Sex, n (%) | |||
| Male | 11 (55.0%) | 2 (33.3%) | 3 (33.3%) |
| Female | 9 (45.0%) | 4 (66.7%) | 6 (66.7%) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 16 (80.0%) | 5 (83.3%) | 9 (100.0%) |
| Unknown/not reported | 4 (20.0%) | 1 (16.7%) | 0 (0.0%) |
| Race, n (%) | |||
| White | 17 (85.0%) | 6 (100.0%) | 8 (88.9%) |
| Black or African American | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) |
| Asian | (0.0%) | 0 (0.0%) | 1 (11.1%) |
| Unknown/not reported | 2 (10.0%) | 0 (0.0%) | 0 (0.0%) |
| Education, n (%) | |||
| High school or below | 6 (30.0%) | 0 (0.0%) | _ |
| Training after high school or at least some college/university | 1 (5.0%) | 3 (50.0%) | _ |
| College/university graduate | 6 (30.0%) | 2 (33.3%) | _ |
| Postgraduate level | 7 (35.0%) | 1 (16.7%) | _ |
| Marital status, n (%) | |||
| Married | 12 (60.0%) | 5 (83.3%) | _ |
| Single | 3 (15.0%) | 1 (16.7%) | _ |
| Widowed | 5 (25.0%) | 0 (0.0%) | _ |
| Employment status, n (%) | |||
| Employed | 1 (5.0%) | 3 (50.0%) | _ |
| Retired | 18 (90.0%) | 3 (50.0%) | _ |
| Homemaker | 1 (5.0%) | 0 (0.0%) | _ |
| Caregiver living with the patient, n (%) | |||
| Partner (spouse/significant other) | 11 (55.0%) | _ | _ |
| Grandchild/grandchildren | 1 (5.0%) | _ | _ |
| None | 8 (40.0%) | _ | _ |
| Caregiver who is not living with the patient, n (%) | |||
| Partner (spouse/significant other) | 2 (10.0%) | _ | _ |
| Child/children | 4 (20.0%) | _ | _ |
| None | 14 (70.0%) | _ | _ |
| Caregiver’s relationship to the patient, n (%) | |||
| Partner (spouse/significant other) | _ | 4 (66.7%) | _ |
| Child/children | _ | 2 (33.3%) | _ |
| Patient’s diagnosis, n (%) | |||
| AML | 11 (55.0%) | _ | _ |
| MDS | 9 (45.0%) | _ | _ |
| Initial treatment, n (%) | |||
| Intensive treatment | 5 (25.0%) | _ | _ |
| Lower intensity treatment | 13 (65.0%) | _ | _ |
| Best supportive care | 2 (10.0%) | _ | _ |
| AML risk group (2017 ELN), n (%) | |||
| Low or intermediate | 7 (35.0%) | _ | _ |
| High | 3 (15.0%) | _ | _ |
| Unknown | 1 (5.0%) | _ | _ |
| MDS IPSS-R score, n (%) | |||
| Low or intermediate | 5 (25.0%) | _ | _ |
| High | 4 (20.0%) | _ | _ |
| ECOG (KPS) performance status at time of visit, n (%) | |||
| 0 (90-100) | 3 (15.0%) | _ | _ |
| 1 (70-80) | 8 (40.0%) | _ | _ |
| 2 (50-60) | 7 (35.0%) | _ | _ |
| Not recorded | 2 (10.0%) | _ | _ |
| Cancer health literacy, n (%) | |||
| Adequate | 20 (100.0%) | 6 (100.0%) | _ |
| Variable . | Patients (N = 20) . | Caregivers (N = 6) . | Clinicians (N = 9) . |
|---|---|---|---|
| Age, mean (SD, range) | 75 (5.9, 63-87) | 64 (13.7, 44-77) | 44 (11.7, 31-66) |
| Number of years in practice after completion of training, mean (SD, range) | _ | _ | 11 (10.0, 2-30) |
| Discipline, n (%) | |||
| Oncology physician | _ | _ | 5 (55.6%) |
| Advanced practice provider | _ | _ | 4 (44.4%) |
| Sex, n (%) | |||
| Male | 11 (55.0%) | 2 (33.3%) | 3 (33.3%) |
| Female | 9 (45.0%) | 4 (66.7%) | 6 (66.7%) |
| Ethnicity, n (%) | |||
| Not Hispanic or Latino | 16 (80.0%) | 5 (83.3%) | 9 (100.0%) |
| Unknown/not reported | 4 (20.0%) | 1 (16.7%) | 0 (0.0%) |
| Race, n (%) | |||
| White | 17 (85.0%) | 6 (100.0%) | 8 (88.9%) |
| Black or African American | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) |
| Asian | (0.0%) | 0 (0.0%) | 1 (11.1%) |
| Unknown/not reported | 2 (10.0%) | 0 (0.0%) | 0 (0.0%) |
| Education, n (%) | |||
| High school or below | 6 (30.0%) | 0 (0.0%) | _ |
| Training after high school or at least some college/university | 1 (5.0%) | 3 (50.0%) | _ |
| College/university graduate | 6 (30.0%) | 2 (33.3%) | _ |
| Postgraduate level | 7 (35.0%) | 1 (16.7%) | _ |
| Marital status, n (%) | |||
| Married | 12 (60.0%) | 5 (83.3%) | _ |
| Single | 3 (15.0%) | 1 (16.7%) | _ |
| Widowed | 5 (25.0%) | 0 (0.0%) | _ |
| Employment status, n (%) | |||
| Employed | 1 (5.0%) | 3 (50.0%) | _ |
| Retired | 18 (90.0%) | 3 (50.0%) | _ |
| Homemaker | 1 (5.0%) | 0 (0.0%) | _ |
| Caregiver living with the patient, n (%) | |||
| Partner (spouse/significant other) | 11 (55.0%) | _ | _ |
| Grandchild/grandchildren | 1 (5.0%) | _ | _ |
| None | 8 (40.0%) | _ | _ |
| Caregiver who is not living with the patient, n (%) | |||
| Partner (spouse/significant other) | 2 (10.0%) | _ | _ |
| Child/children | 4 (20.0%) | _ | _ |
| None | 14 (70.0%) | _ | _ |
| Caregiver’s relationship to the patient, n (%) | |||
| Partner (spouse/significant other) | _ | 4 (66.7%) | _ |
| Child/children | _ | 2 (33.3%) | _ |
| Patient’s diagnosis, n (%) | |||
| AML | 11 (55.0%) | _ | _ |
| MDS | 9 (45.0%) | _ | _ |
| Initial treatment, n (%) | |||
| Intensive treatment | 5 (25.0%) | _ | _ |
| Lower intensity treatment | 13 (65.0%) | _ | _ |
| Best supportive care | 2 (10.0%) | _ | _ |
| AML risk group (2017 ELN), n (%) | |||
| Low or intermediate | 7 (35.0%) | _ | _ |
| High | 3 (15.0%) | _ | _ |
| Unknown | 1 (5.0%) | _ | _ |
| MDS IPSS-R score, n (%) | |||
| Low or intermediate | 5 (25.0%) | _ | _ |
| High | 4 (20.0%) | _ | _ |
| ECOG (KPS) performance status at time of visit, n (%) | |||
| 0 (90-100) | 3 (15.0%) | _ | _ |
| 1 (70-80) | 8 (40.0%) | _ | _ |
| 2 (50-60) | 7 (35.0%) | _ | _ |
| Not recorded | 2 (10.0%) | _ | _ |
| Cancer health literacy, n (%) | |||
| Adequate | 20 (100.0%) | 6 (100.0%) | _ |
Feasibility
We approached 29 patients, and 21 consented (consent rate, 72%). One patient died between consent and baseline, resulting in a total sample of 20 patients enrolled. Of the 20 enrolled patients, 1 died before their scheduled SICP visit, resulting in a total of 19 SICP visits (retention rate, 95%; primary feasibility metric). Five patients did not consent because they did not feel that the visit would be helpful (eg, family members or clinicians already knew their wishes); 2 patients did not want to complete surveys, and 1 patient was concerned about knowing too much about their diagnosis. Thirteen SICP visits were completed using video calls via Zoom; 5 using telephone calls (4 patients preferred phone over video call; 1 patient had technical difficulty with Zoom), and 1 visit was in person (patient was not comfortable with phone or video).
Five patients identified a total of 6 caregivers for enrollment (1 patient had 2 caregivers enrolled), all of whom consented (consent rate: 100%). All consented caregivers participated in SICP visits (retention rate: 100%). Nine patients could not identify a primary caregiver, and 6 patients did not want to burden their caregivers with surveys. Nonenrolled caregivers were present in 9 of the 19 SICP visits. Supplemental Table 3 describes caregiver presence at visits.
Usability
The adapted SICP was usable, with mean TUQ scores of 5.9 (SD, 0.9) and 5.9 (SD, 1.1) for patients and caregivers, respectively.
Other outcome measures
SICP acceptability (patients)
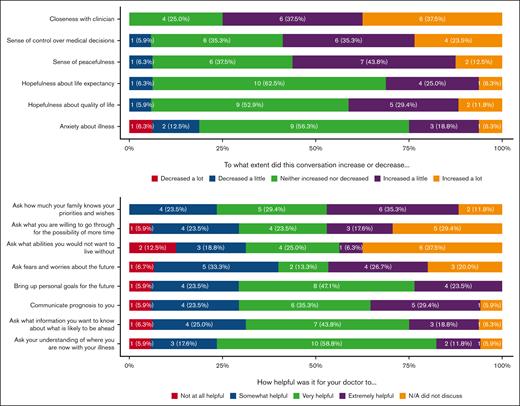
Results of the patient acceptability survey are shown in Figure 1. Fifteen patients (88.2%; 15/17) found this conversation to be very or extremely worthwhile. The majority of patients felt that the SICP increased their sense of peacefulness (56.3%; 9/16), sense of control over medical decisions (58.8%; 10/17), and closeness with their clinician (75.0%; 12/16). Most patients found it very or extremely helpful for their clinician to ask about their understanding of where they are with their illness (70.6%; 12/17), communicate their prognosis to them (64.7%; 11/17), bring up their personal goals for the future (70.6%; 12/17), and ask about how much their family knows about their priorities and wishes (64.7%; 11/17).
The majority of patients (56.3%; 9/16) felt that this conversation took place at the right time, but some patients (18.8%, 3/16) wished their doctor had raised these topics earlier.
Patient measures: baseline and after intervention
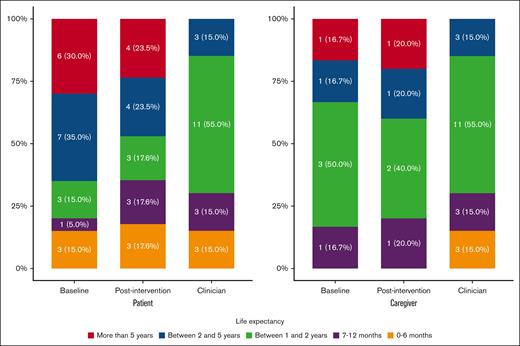
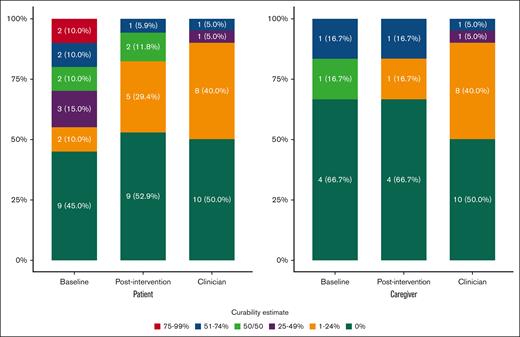
Other patient measures completed at baseline and after intervention are shown in Table 2. After intervention, ACP engagement scores numerically increased (+0.4; P = .12). No significant changes in psychological health (ie, GAD-7, PHQ-9, distress) or QOL (FACT-Leu) occurred after SICP visits. After the SICP visit, patients’ life expectancy and curability estimates aligned more closely with their clinicians’ estimates (Figures 2 and 3).
Baseline and/or postintervention measures for patients and caregivers
| . | Baseline . | Postintervention . | Change from baseline to postintervention . | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | n . | P-value . | |
| Patient | |||||||
| ACP engagement survey (range, 1-5) | 4.1 (1.1) | 18 | 4.6 (0.4) | 15 | 0.4 (1.0) | 14 | .12 |
| GAD-7 (range, 0-21) | 1.9 (1.9) | 19 | 2.2 (2.9) | 17 | 0.1 (2.2) | 17 | .83 |
| PHQ-9 (range, 0-27) | 4.1 (3.1) | 19 | 4.3 (4.6) | 17 | 0.4 (4.2) | 17 | .73 |
| Distress (range, 0-10) | 2.2 (2.2) | 20 | 2.2 (1.7) | 17 | 0.3 (2.4) | 17 | .62 |
| FACT-Leu (range, 0-176) | 129.5 (16.3) | 20 | 127.4 (24.0) | 18 | −3.3 (17.6) | 18 | .44 |
| HCCQ (range, 0-20) | _ | _ | 18.3 (2.1) | 18 | _ | _ | _ |
| HCCQ-Age (range, 0-28) | _ | _ | 22.5 (5.9) | 18 | _ | _ | _ |
| Heard and understood (range, 0-4) | _ | _ | 3.5 (0.5) | 18 | _ | _ | _ |
| Human Connection Scale (range, 16-64) | _ | _ | 57.9 (4.7) | 17 | _ | _ | _ |
| PEACE questionnaire (range, 5-20) | _ | _ | 17.5 (2.0) | 18 | _ | _ | _ |
| Caregiver | |||||||
| GAD-7 (range, 0-21) | 6.5 (6.6) | 6 | 3.7 (2.4) | 6 | –2.8 (5.0) | 6 | .23 |
| PHQ-9 (range, 0-27) | 5.3 (5.0) | 6 | 4.3 (3.1) | 6 | –1.0 (3.0) | 6 | .46 |
| Distress (range, 0-10) | 4.2 (2.3) | 6 | 2.8 (2.3) | 6 | –1.3 (1.4) | 6 | .06 |
| Caregiver QOL index (range, 0-140) | 99.2 (20.1) | 6 | 105.2 (16.5) | 6 | 6.0 (8.3) | 6 | .14 |
| HCCQ (HCCQ; range, 0-20) | _ | _ | 18.2 (2.9) | 6 | _ | _ | _ |
| Heard and understood (range, 0-4) | _ | _ | 3.7 (0.5) | 6 | _ | _ | _ |
| HCCQ-Age about patients (range, 0-28) | _ | _ | 24.8 (3.1) | 6 | _ | _ | _ |
| HCCQ-Age about caregivers (range, 0-20) | _ | _ | 16.7 (3.1) | 6 | _ | _ | _ |
| . | Baseline . | Postintervention . | Change from baseline to postintervention . | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) . | n . | Mean (SD) . | n . | Mean (SD) . | n . | P-value . | |
| Patient | |||||||
| ACP engagement survey (range, 1-5) | 4.1 (1.1) | 18 | 4.6 (0.4) | 15 | 0.4 (1.0) | 14 | .12 |
| GAD-7 (range, 0-21) | 1.9 (1.9) | 19 | 2.2 (2.9) | 17 | 0.1 (2.2) | 17 | .83 |
| PHQ-9 (range, 0-27) | 4.1 (3.1) | 19 | 4.3 (4.6) | 17 | 0.4 (4.2) | 17 | .73 |
| Distress (range, 0-10) | 2.2 (2.2) | 20 | 2.2 (1.7) | 17 | 0.3 (2.4) | 17 | .62 |
| FACT-Leu (range, 0-176) | 129.5 (16.3) | 20 | 127.4 (24.0) | 18 | −3.3 (17.6) | 18 | .44 |
| HCCQ (range, 0-20) | _ | _ | 18.3 (2.1) | 18 | _ | _ | _ |
| HCCQ-Age (range, 0-28) | _ | _ | 22.5 (5.9) | 18 | _ | _ | _ |
| Heard and understood (range, 0-4) | _ | _ | 3.5 (0.5) | 18 | _ | _ | _ |
| Human Connection Scale (range, 16-64) | _ | _ | 57.9 (4.7) | 17 | _ | _ | _ |
| PEACE questionnaire (range, 5-20) | _ | _ | 17.5 (2.0) | 18 | _ | _ | _ |
| Caregiver | |||||||
| GAD-7 (range, 0-21) | 6.5 (6.6) | 6 | 3.7 (2.4) | 6 | –2.8 (5.0) | 6 | .23 |
| PHQ-9 (range, 0-27) | 5.3 (5.0) | 6 | 4.3 (3.1) | 6 | –1.0 (3.0) | 6 | .46 |
| Distress (range, 0-10) | 4.2 (2.3) | 6 | 2.8 (2.3) | 6 | –1.3 (1.4) | 6 | .06 |
| Caregiver QOL index (range, 0-140) | 99.2 (20.1) | 6 | 105.2 (16.5) | 6 | 6.0 (8.3) | 6 | .14 |
| HCCQ (HCCQ; range, 0-20) | _ | _ | 18.2 (2.9) | 6 | _ | _ | _ |
| Heard and understood (range, 0-4) | _ | _ | 3.7 (0.5) | 6 | _ | _ | _ |
| HCCQ-Age about patients (range, 0-28) | _ | _ | 24.8 (3.1) | 6 | _ | _ | _ |
| HCCQ-Age about caregivers (range, 0-20) | _ | _ | 16.7 (3.1) | 6 | _ | _ | _ |
Patient and caregiver life expectancy estimates at baseline and after intervention compared with clinician estimates.
Patient and caregiver life expectancy estimates at baseline and after intervention compared with clinician estimates.
Patient and caregiver curability estimates at baseline and after intervention compared with clinician estimates.
Patient and caregiver curability estimates at baseline and after intervention compared with clinician estimates.
Patient measures: after intervention (only)
Mean scores for HCCQ, Heard and Understood, Human Connection Scale were 18.3 (SD 2.1), 3.5 (SD 0.5), and 57.9 (SD 4.7), respectively.
Caregiver measures: baseline and after intervention
Caregiver measures completed at baseline and after intervention are shown in Table 2. After intervention, caregiver depression (−1.0 [SD, 3.0]; P = .46) and anxiety (−2.8 [SD, 5.0]; P = .23) numerically decreased, and a statistically significant decrease in distress (−1.3 [SD, 1.4]; P = .06) was noted. Self-reported QOL also increased for caregivers (+6.0 [SD, 8.3]; P = .14). After the SICP visit, caregivers’ life expectancy and curability estimates were generally unchanged (Figures 2 and 3).
Caregiver measures: after intervention (only)
Mean scores for HCCQ and Heard and Understood were 18.2 (SD, 2.9) and 3.7 (SD, 0.5), respectively.
Qualitative feedback
Three major qualitative themes emerged from postintervention interviews: (1) Participants appreciated the comfort of telehealth during their SICP visit; (2) participants felt that the SICP visit provided them with the opportunity to share their wishes, and (3) participants felt that the SICP visit eased their worries. Almost all patients (94.7%; 18/19) and caregivers (83.3%; 5/6) felt that the patient was prioritized at the SICP visit. Similarly, almost all (89.5%; 17/19) patients and (100%; 6/6) caregivers would recommend a SICP visit to other individuals with the same diagnosis. One patient would recommend the visit later in the illness, and 1 patient would recommend the visit if it included a written summary in addition to the conversation.
Participants appreciated the comfort of telehealth during their SICP visit
Patients felt that having their SICP visit from home via telehealth, video, or phone was comforting. Patients also emphasized the convenience of telehealth, because it reduced the need to travel.
Patient 8: “It seems like it's easier to talk to the doctor if you're sitting in the office talking to him, (but) I felt more comfortable sitting in my own chair talking to my doctor, seeing her face (and) seeing her reactions.”
Participants felt that the SICP visit provided them with the opportunity to share their wishes.
Patients appreciated sharing their wishes regarding EOL care with their clinician and found it helpful to have their family members participate in the discussion.
Patient 7: “There were some questions about end of life and my wishes and things like that. I was very glad that these things were all in the open because my husband was on the phone. Though we have talked about them, my husband and I, I just wanted to confirm to him how I feel, and (my clinician) made it so simple.”
Patients felt that it was important for their clinician to understand what was important to them, and for many patients in this study, their family is their priority and their major source of strength.
Patient 3: “You mean what’s important to us and that kind of stuff? That’s an easy answer. That’s my wife, my family, and friends. I want to hang around and enjoy (them). As long as I can do that, it keeps me going. I think it is (helpful) for (my clinician) and us (to share that). I think anytime you get a chance with someone you respect to talk about that, the questions and answers mean more.”
Caregivers agreed that being part of this conversation helped open up discussions between patients and their families.
Caregiver 2: “I think it was helpful because it definitely opened up areas of conversation between us that maybe we would have never explored.”
Participants felt that the SICP visit eased their worries
Although some patients were initially hesitant about sharing their fears with their clinician, patients felt comfortable discussing their concerns during the SICP visit.
Patient 6: “In the beginning I was a little bit leery about talking about what was bothering me. But after we got into a discussion I became very, very interested and wanting to tell (my clinician) what my inner thoughts were that I had not been able to share. I think I may have said that I appreciated being able to share some of my inner thoughts with (my clinician).”
In addition, sharing their worries allowed patients to feel better prepared and less fearful about the future.
Patient 4: “My one fear would be that…when they get down towards the end (my) doctors kind of bail…just go into hospice or go home and die. And I heard him say very clearly ‘I will not abandon you,’ which meant a lot.”
Caregivers similarly felt that the SICP visit eased the patient’s worries, thereby also decreasing their anxiety.
Caregiver 5: “Yeah. Actually, I’m a little bit less worried about how he, you know, how he’s going to do because I think it calmed him down. And if he is less anxious then I’m a lot less anxious.”
Discussion
In this pilot study, we found that telehealth delivery of the adapted SICP for older adults with AML and MDS was feasible (retention rate: 95%) and usable. In qualitative interviews, the majority of patients (89.5%) would recommend a SICP visit to others. Participants appreciated the comfort of telehealth and felt that the SICP provided them with the opportunity to share their wishes. After their SICP visit, patients’ estimates of their curability and life expectancy aligned more closely with those of their clinicians, and postintervention satisfaction with communication was relatively high.
The majority of patients found their SICP visit to be worthwhile, although some patients wished their doctor had brought this conversation up earlier. Patients in this study felt that the SICP provided them with the opportunity to share wishes that they might not have been able to discuss during a routine clinic visit and assisted in creating dialogue with their family members regarding current and ongoing care. Understanding patient care preferences is especially important for patients who are at high risk for rapid clinical decline.43,44 Older adults with AML and MDS often receive life-sustaining treatment at the EOL and die in the hospital, which may not be concordant with their wishes.45,46 Bereaved families of patients with cancer have regretted not talking about death sufficiently, and some of these regrets may be attributed to the family’s uncertainty regarding the patient’s terminal prognosis.47 SICs encourage ongoing care communication and may increase illness understanding, allowing patients and their families to make value-aligned medical decisions in the present as well as at the EOL.
We found that patients’ disease understanding improved after their SICP visit, and curability and life expectancy estimates aligned more closely with those of their clinicians, but disease understanding did not change at postintervention for caregivers. This may be because at baseline, caregiver knowledge aligned closely with clinicians. Specifically, at baseline, 50% (3/6) of caregiver’s curability and life expectancy estimates aligned with clinician estimates. For patients, improved prognostic awareness may not improve depression, anxiety, or QOL.48 On the other hand, certain coping strategies improve QOL and reduce depression in patients who experience psychological distress from accurate prognostic awareness.49 Therefore, considering the variable impact of accurate prognostic awareness on psychological health, it is important that SICs offer coping strategies for both patients and caregivers in discussions of prognosis.
Communication scores for participants in this study were high with mean HCCQ scores of 18.3 (SD, 2.1) and 18.2 (SD, 2.9) for patients and caregivers, respectively. Participants felt heard by their clinicians, and therapeutic alliance as assessed by the Human Connection Scale was relatively high (57.9 [SD, 4.7]). These scores for communication are higher than those reported in previous work, including 1 study that assessed communication with older adults with cancer using the GA, which had a mean HCCQ Score of 16.8 (SD, 3.2) and another study that assessed therapeutic alliance between oncologists and patients with advanced cancer, which had a mean Human Connection Scale score of 56.4 (SD, 7.4).11,50 Poor patient-physician communication may lead to confusion and loss of confidence in the care team, whereas strong therapeutic alliance is associated with decreased symptom burden and lower psychological distress.50,51 In addition, high Human Connection Scale scores have been associated with a lower likelihood of intensive care unit use at EOL for patients with cancer.50 Therefore, the SICP has the potential to improve patient-clinician communication, facilitating a stronger alliance between patients and their care team that may lead to better patient-reported outcomes and lower intensity EOL care.
This study has several strengths. First, we focused on a vulnerable population, ie, older adults with AML and MDS. Second, we used telehealth in order to increase accessibility to SICs for patients. Third, we used both quantitative and qualitative methods to comprehensively assess the impact of the SICP on care for participants in this study. This study also has limitations. First, it is a single center, single-arm study with a small sample size. Second, our participants were mostly White and non-Hispanic; therefore our results may not be generalizable to individuals of other races and ethnicities.
In this single-arm pilot study, we found the adapted telehealth SICP to be both feasible and usable. Participants in this study found participating in the SICP to be worthwhile and would recommend this program to others. The adapted SICP has the potential to improve patient disease understanding, strengthen the patient-clinician relationship, and help clinicians align care with what matters most to each patient and their family.
Acknowledgments
This work was supported by University of Rochester Clinical and Translational Science Award number TL1 TR002000 from the National Center for Advancing Translational Sciences of the National Institutes of Health (M.L.). This work was also supported by National Cancer Institute at the National Institutes of Health grants UG1CA189961, K99CA237744l, and R00CA237744 (K.P.L.), National Institute on Aging at the National Institutes of Health grants R03AG073985 (K.P.L.), R33AG059206 (H.D.K.), and K02AG062745 (B.M.K.), Conquer Cancer American Society of Clinical Oncology and Walther Cancer Foundation Career Development Award (K.P.L.), and the Wilmot Research Fellowship Award (K.P.L.). The authors thank the Cancer and Aging Research Group and the Stakeholders for Care in Oncology and Research for our Elders Board who provided feedback. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Susan Rosenthal for her editorial feedback on this manuscript.
Authorship
Contribution: M.L. contributed in conceptualization and design, data collection, data analysis, and interpretation, and manuscript writing; S.M.-H. contributed in data analysis and interpretation; Y.W. contributed in data analysis and interpretation, and manuscript writing; J.H.M., S.N., R.B., T.C., H.K., and B.K. contributed in conception and design; K.P.L. contributed in conceptualization and design, data collection, data analysis, and interpretation, and manuscript writing; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: K.P.L. has served as a consultant to Pfizer and Seagen and has received honoraria from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Kah Poh Loh, Division of Hematology/Oncology, Department of Medicine, James P. Wilmot Cancer Center, 601 Elmwood Ave, Box 704, Rochester, NY 14642; email: kahpoh_loh@urmc.rochester.edu.
References
Author notes
Data from this study are available for sharing upon reasonable request from the corresponding author, Kah Poh Loh (kahpoh_loh@urmc.rochester.edu).
The full-text version of this article contains a data supplement.