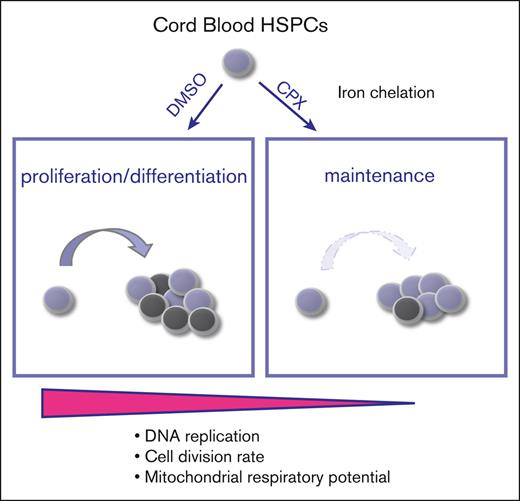
CPX is a regulator of HSCs and preserves the stem cell state in culture.
CPX-induced HSC maintenance is mediated by intracellular iron chelation, which restricts respiratory capacity and proliferation.
Visual Abstract
Culture conditions in which hematopoietic stem cells (HSCs) can be expanded for clinical benefit are highly sought after. To elucidate regulatory mechanisms governing the maintenance and propagation of human HSCs ex vivo, we screened libraries of annotated small molecules in human cord blood cells using an optimized assay for detection of functional HSCs during culture. We found that the antifungal agent ciclopirox ethanolamine (CPX) selectively supported immature CD34+CD90+ cells during culture and enhanced their long-term in vivo repopulation capacity. Purified HSCs treated with CPX showed a reduced cell division rate and an enrichment of HSC-specific gene expression patterns. Mechanistically, we found that the HSC stimulating effect of CPX was directly mediated by chelation of the intracellular iron pool, which in turn affected iron-dependent proteins and enzymes mediating cellular metabolism and respiration. Our findings unveil a significant impact of iron homeostasis in regulation of human HSCs, with important implications for both basic HSC biology and clinical hematology.
Introduction
Development of strategies to preserve and expand functional human hematopoietic stem cells (HSCs) ex vivo before transplantation has been a long-standing goal in the field of hematology to improve cell therapy applications. Umbilical cord blood (CB) is an abundant and readily available stem cell source for transplantation, however, the relatively low number of hematopoietic stem- and progenitor-cells (HSPCs) in a typical CB unit currently limits the use of CB to hematopoietic cell transplantation of pediatric patients.1 Successful ex vivo expansion of HSPCs would increase the utility of CB and allow transplantation also to adult patients. Moreover, gene-therapy applications and emerging gene-editing strategies for therapeutic targeting of HSCs would greatly benefit from robust culture conditions in which HSCs are preserved and ideally also expanded. Finally, it is likely that efforts to generate transplantable HSCs from pluripotent stem cells would be facilitated if conditions were established that allowed for the propagation of any HSCs generated.2
To date, several strategies for HSC expansion have shown promising potential, including the targeting of specific signaling pathways or epigenetic factors.3-6 Moreover, the negative impact of proinflammatory signaling and oxidative stress on HSC maintenance and function has been demonstrated in other studies, as well as the importance of intracellular calcium and copper homeostasis.7-11 Altogether, these efforts have led to a deeper understanding of the intricate complexity of gene regulation, signaling pathways, and metabolic networks in controlling the self-renewal and differentiation balance of HSCs.
To gain further insights into the processes governing the maintenance and propagation of human HSCs in culture, we screened several annotated small molecule libraries in primary human CB-derived HSPCs. We identified a top candidate, ciclopirox ethanolamine (CPX), which is an FDA-approved drug for the treatment of tropical fungal infections and a chelator of intracellular iron.12,13 In this report, we show that CPX by reducing the intracellular labile iron pool (LIP), triggers proliferative and metabolic alterations that preserves HSC integrity and protects the stem cell pool from exhaustion during culture.
Methods
CB collection and CD34+ cell isolation
Umbilical CB samples were collected at the end of full-term deliveries from Lund, Malmö, and Helsingborg hospitals in Sweden. Samples were collected with informed consent according to guidelines approved by the regional ethical committee. Mononuclear cells were separated from umbilical CB units through density-gradient centrifugation (LymphoprepTube, Axis-Shield Density Gradient Media #1019818) and CD34+ cells were isolated using magnetic beads (#130-046-703, Miltenyi Biotec). Small molecule libraries were purchased from Enzo Life Science (specified in supplemental Table 1). Compounds tested in this study were dissolved in dimethyl sulfoxide (DMSO) and the following were added: Ciclopirox (Enzo Life Science), SR1 (STEMCELL Technologies), Ly2228820 (Selleck Chem) and UM171 (STEMCELL Technologies).
Flow cytometry and cell sorting
Before analysis or sorting, cells were labeled with different combinations of the following fluorochrome–conjugated antihuman antibodies: CD14 (HCD14), CD19 (HIB19), CD3 (UCHT1), CD33 (P67.6), CD34 (8G12), CD38 (HIT2), CD45 (HI30), CD45RA (HI100), CD49f (GoH3), CD90 (5E10), CD133 (13A4), and CD71 (L01.1). Dead cells were excluded with 7-aminoactinomycin D (7AAD, Sigma Aldrich). Apoptotic cells were stained with the Annexin V apoptosis detection kit, according to manufacturer’s protocol (BD Bioscience). All data were collected on fluorescence-activated cell sorter (FACS) Canto II or LSRII analyzer (Becton Dickinson) and analyzed with FlowJo software. Cells were sorted on a FACS Aria II or III (Becton Dickinson).
Primary small molecule screening and dose response validation
A total of 584 small molecules at 2 concentrations (0.5 and 10 Μm; supplemental Table 1) were screened on CB CD34+ (10 000 cells), plated in U-shape 96 well plates and grown in 100 μL of SFEM (STEMCELL Technologies) supplemented with 100 ng/mL of stem cell factor, TPO, and FLt3L (Peprotech). Cultures were kept at 37°C and 5% CO2. After 6 days, cells were washed and stained with CD34 (581) and CD90 (5E10) (BioLegend) antibodies in the same plate and analyzed by FACS Canto II. Titration experiments followed the same method. Throughout this study, culture condition and duration has been similar to the above-mentioned screen procedure.
Human engraftment assay
All experiments with mice were reviewed and conducted under approved protocol from Lund/Malmö Local Ethical committee. Nonobese diabetic.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (NSG; Jackson Laboratory) were sublethally irradiated (300 cGy) before transplantation. Cultured equivalent of 30 000 input CD34+ cells were injected intravenously into NSG mice aged 10 to 12 weeks. Human cell contribution in Perioheral Blood (PB) and Bone Marrow (BM) of NSG was assessed at 6, 12, and 16 (for PB) and 16 weeks (for BM) after transplantation. For secondary transplantion, a half femur equivalent of BM from primary NSG recipients was injected into secondary sublethally irradiated NSG mice. Human cell contribution was assessed from 16 to 18 weeks after transplant in PB and BM. For the limit dilution analysis, BM samples were assessed at week 18 after transplant. Mice with myeloid chimerism >1% were considered engrafted for the scid repopulating cell (SRC) quantification, which was performed using Extreme Limiting Dilution Analysis online tool.14
CFC assay
Colony-forming cell (CFC) potential was established by plating magnetically-enriched CD34+ cells after 6 days of culture in presence of DMSO or CPX in methylcellulose (Methocult H4230, STEMCELL Technologies), supplemented with stem cell factor (25 ng/mL), granulocyte-macrophage colony-stimulating factor (50 ng/mL), interleukin-3 (25 ng/mL), and erythropoietin (5 U/mL, Janssen). Hematopoietic colonies were scored after 14 days.
Cell division history assay
To assess cell division history, 1000 CD34+CD38-CD45RA-CD90+ were sorted at day 0 and resuspended in 37°C warm phosphate-buffered saline (PBS). While vortexing, an equal volume of 1μM carboxyfluorescein succinimidyl ester (CFSE) (Sigma Aldrich #21888) solution was added to the cell suspension. After 15 minutes incubation at 37°C, the reaction was stopped by a 2-minute incubation with fetal calf serum at room temperature, and unbound CFSE was washed away twice with PBS. Cells were stained for CD34 and CD90 expression and analyzed by FACS either at day 0 or at day 6 of culture.
Microarray analysis
Sorted, CB-derived CD34+CD38–CD90+CD45RA– cells were cultured with either CPX or DMSO. CD90+ cells were resorted into RLT buffer after 24 hours and 6 days of culture. Subsequent sample processing and analysis on Affymetrix human genome U133+ array were performed at KFB (Regensburg, Germany). Genes that showed a minimum of 1.5-fold modulation were considered for further analysis. Microarray data are available under the accession GSE159100.
Intracellular iron binding measurement and iron preloading experiment
CB-CD34+ cells (3 × 104) were plated and resuspended in RPMI medium containing 50 nM calcein-AM (Fisher Scientific #C3100MP) and incubated at 37°C for 5 minutes. After calcein loading, cells were washed with prewarmed PBS and plated in the presence of CPX or DMSO condition for 1 hour. Fluctuation in intracellular calcein fluorescence was measured by flow cytometry. Geometric mean fluorescence intensity was calculated by FlowJo analysis software. Iron preloading experiment was done using FAC (Sigma Aldrich) at 200 μg/mL for 1 hour. After this time, cells were spun down and new media containing CPX or DMSO were added to the culture. The experimental end point was FACS analysis of CD34 and CD90 expression at day 6.
Sample preparation for metabolomic profiling and analysis
CB-CD34+ cells (1 × 106) were treated with either CPX (1.5 μM) or control (0.1% DMSO) for 12 hours. Cells were harvested and washed with 150 mM ice-cold ammonium acetate on ice and then resuspended in 2.5 mL of fresh ice-cold ammonium acetate. Next, cells were transferred to a 15 m polypropylene tube (BD-Falcon Cat# 352097 or Fisher Cat#14-959-70C) and spun down at 200 g for 2 minutes at 4°C. The supernatant was carefully removed, and cell pellet was resuspended in 0.75 mL of ice-cold 150 mM ammonium acetate and transferred into ice-cold cryovials. Supernatant was carefully removed, and cell pellet was snap-frozen in liquid nitrogen until metabolite profiling. Metabolite extraction and profiling was done as previously described,15 and metabolites with fold changes >1.2 (CPX/DMSO) were submitted to MetaboAnalyst software v4.0.
Extracellular flux analysis
Oxygen consumption rate (OCR) was measured on CB-CD34+ cells using the XFe-96 Extracellular Flux Analyzer (Seahorse Bioscience, Agilent). Cells (5 × 104; in triplicates) were placed in 180 μL XF assay media (nonbuffered Dulbecco Modified Eagle Medium with 10 mM glucose, 2 mM Glutamax, and 1 mM sodium pyruvate) in Cell-Tak-coated plates and monitored in basal conditions and after injection of oligomycin (4 μM), FCCP (2 μM), rotenone (1 μM), and antimycin A (40 μM; Sigma). The calculations for basal respiration (OCRbasal – OCRRotenone/AntimycinA), adenosine triphosphate production (OCRbasal - OCROligomycin), proton leak (OCROligomycin – OCRRotenone/AntimycinA), maximal respiration (OCRFCCP – OCRRotenone/AntimycinA), and spare respiratory capacity (OCRFCCP – OCRbasal) were performed for triplicates in each condition.
Statistical analysis
Statistical significance was calculated using student t test (2-tailed) with GraphPad Prism software unless otherwise stated. For in vivo data, statistical significance was tested using Mann-Whitney nonparametric t test. Statistical significance in the figures are indicated by ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001. Error bars indicate standard error of the mean unless otherwise stated and n represents number of independent experiments.
Results
A small molecule screen identifies CPX as a candidate modifier of human HSCs ex vivo
To identify modifiers of human HSCs ex vivo, we first set out to define relevant phenotypic markers of CB-derived HSCs during culture as there is a dissociation between stem cell phenotype and function upon culture.16,17 We monitored the expression of several stemness-related markers18 in cultures of bulk CD34+ cells (supplemental Figure 1A), as well as HSC-enriched CD34+CD38loCD90+CD45RA– cells (supplemental Figure 1B) and highly purified CD34+CD38–CD90+CD45RA–CD49f+ cells (supplemental Figure 1C). Cells were grown in the presence or absence of the p38α inhibitor Ly2228820 (Ly) that was previously reported to enhance HSC propagation in vitro.7 Although lack of expression of both CD38 and CD45RA defines functional HSCs in freshly isolated cells, these markers are globally downregulated upon culture and not useful to further enrich HSCs from cultured cells (supplemental Figure 1A-C). Similarly, CD49f expression, which positively marks uncultured functional HSCs,18 was only detected in nontransplantable CD34lo/– cells upon culture. However, both CD13319 and, most notably, CD90 distinctively separated the CD34+ population in all culture conditions (supplemental Figure 1A-C). Although CD133 marked a large portion (>1 out of 3) of the CD34+ cells, CD90 was expressed on a discrete subset, indicating that it might define a highly enriched HSC population. Indeed, we detected robust lymph-myeloid long-term repopulating activity from the CD34+CD90+ population but not from up to 10-fold higher doses of CD34+CD90- cells, regardless of starting cell population, culture time, or presence of the p38 inhibitor (supplemental Figure 1D-H). By contrast, >95% of progenitor cells with CFC potential were contained in the CD34+CD90– population (supplemental Figure 1I-J). Thus, in cultured CB cells, CD90 expression selectively marks the functional HSC pool and dissociates it from the vast majority of progenitor cells, which is in agreement with other studies.17,20-22
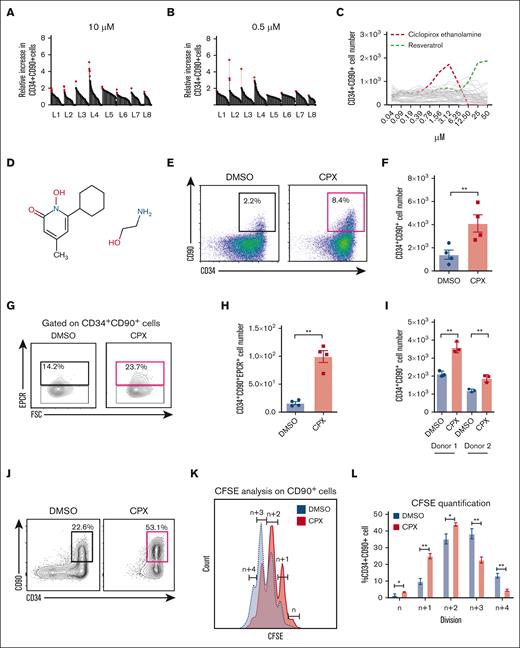
Having validated CD90 as a reliable marker to track human HSC activity in culture, we next performed a high-throughput screen to identify small molecules supporting the propagation of CD34+CD90+ cells (supplemental Figure 2A). We screened >500 annotated compounds at 2 concentrations of 10μM and 0.5μM in CB-derived CD34+ cells (supplemental Tables 1 and 2) and identified 48 primary candidates (Figure 1A-B) that increased the number of CD34+CD90+ cells compared with DMSO-treated controls. Next, from titration experiments, we identified 2 compounds, the antioxidant resveratrol, as well as the antifungal and iron chelating agent CPX,23 showing a particularly large increase of CD34+CD90+ cells (Figure 1C). Several studies have discussed the impact of alterations in iron level and metabolism in regulation of HSPCs in normal and malignant conditions.15,24 However, knowledge about the exact mechanism of iron-mediated regulation of HSPC function remains limited and therefore we selected CPX for further investigation (Figure 1D). We found that CPX was beneficial within a narrow concentration range (0.78-3.12 uM) and toxic at higher concentrations. Of note, when the screen was analyzed for an increase of total CD34+ cells, CPX was not among the high-scoring compounds (supplemental Table 3). This explains why CPX had not been picked up in previous large-scale small molecule screens targeted at CD34+ cell expansion,3,4 and it further underscores the potential of our CD34+CD90+ assay to detect HSC-specific outcomes independent of progenitor proliferation.
Small molecule screening identifies CPX as a candidate modifier of human HSPCs ex vivo. (A-B) Results from primary screening of 584 small molecules at 2 different concentrations on CB-CD34+ cells. Y-axis represents the fold increase in CD34+CD90+ cell number after 6 days for each compound tested, relative to DMSO control, and red dots represent selected candidates from each library (L1-L8). (C) Dose titration of 48 top candidates from the primary screen. (D) Chemical structure of CPX. (E) Representative FACS plots and (F) numbers of CD34+CD90+ cells after 6 days culture of bulk CB CD34+ cells (n = 4). (G) Representative FACS plot and (H) number of CD34+CD90+EPCR+ cells after culture of bulk CD34+ cells in presence and absence of CPX. (I) Numbers of CD34+CD90+ cells after 6 days culture of human BM-CD34+ cells from 2 healthy donors. (J) Representative FACS plots from 1000 CB-derived CD34+CD38–CD45RA–CD90+ cells cultured for 6 days. (K) Cell division history measured by CFSE labeling on CD90+ cells and (L) quantification of cell frequency in each division. Number of divisions are quantified as relative number of divisions between the 2 conditions by referring to the highest CFSE peak as n number of divisions and the subsequent peaks as n + 1, n + 2, etc.
Small molecule screening identifies CPX as a candidate modifier of human HSPCs ex vivo. (A-B) Results from primary screening of 584 small molecules at 2 different concentrations on CB-CD34+ cells. Y-axis represents the fold increase in CD34+CD90+ cell number after 6 days for each compound tested, relative to DMSO control, and red dots represent selected candidates from each library (L1-L8). (C) Dose titration of 48 top candidates from the primary screen. (D) Chemical structure of CPX. (E) Representative FACS plots and (F) numbers of CD34+CD90+ cells after 6 days culture of bulk CB CD34+ cells (n = 4). (G) Representative FACS plot and (H) number of CD34+CD90+EPCR+ cells after culture of bulk CD34+ cells in presence and absence of CPX. (I) Numbers of CD34+CD90+ cells after 6 days culture of human BM-CD34+ cells from 2 healthy donors. (J) Representative FACS plots from 1000 CB-derived CD34+CD38–CD45RA–CD90+ cells cultured for 6 days. (K) Cell division history measured by CFSE labeling on CD90+ cells and (L) quantification of cell frequency in each division. Number of divisions are quantified as relative number of divisions between the 2 conditions by referring to the highest CFSE peak as n number of divisions and the subsequent peaks as n + 1, n + 2, etc.
CPX enhances the propagation of CD34+CD90+ cells in cultured CB- and BM-derived HSPCs
To further validate the potential HSC-promoting activity of CPX, we first cultured bulk CB-CD34+ cells for 6 days and confirmed a marked increase in both frequency and number of CD34+CD90+ cells (3-fold; P < .01) in the presence of CPX (Figure 1E-F). In addition, we also evaluated the output of CD34+CD90+EPCR+ cells because EPCR expression has been associated with functional HSC activity in ex vivo cultured HSPCs.25,26 Similar to CD34+CD90+ cells, we could see a significant increase in the number of CD34+CD90+EPCR+ cells upon CPX treatment as compared with DMSO control (Figure 1G-H). Because HSCs from different stages of ontogeny are functionally different,27 we treated adult BM-derived CD34+ cells with CPX and found a similar increase in CD34+CD90+ cell numbers (Figure 1I). CPX did not appear to affect total cell survival as both the number of viable cells and the frequency of apoptotic cells in the CD34+CD90+ population were unaltered (supplemental Figure 2B-C). Next, we tested the effect of CPX on HSC-enriched CD34+CD38-CD45RA-CD90+ cells from CB and observed that it strongly preserved their immature phenotype with >50% of the cultured cells remaining CD34+CD90+ after 6 days (Figure 1J). As the HSC state is typically associated with a low cellular turnover, we next asked whether CPX affected the rate of cell division of cultured CD34+CD90+ cells. By tracking cell division history using CFSE labeling, we found that CPX treatment led to a slower cell division rate of CD34+CD90+ cells (Figure 1K-L). By contrast, cell cycle analysis performed at day 3 did not reveal any significant changes in cell cycle distribution, indicating that the slower division rate, at least in part, may be because of prolonged cell cycle transit rather than a shift between quiescent and actively cycling states (supplemental Figure 2D).
Altogether, these data suggest that CPX selectively impedes the cell division rate of HSCs during culture and preserves their immature state.
CPX enhances the long-term engraftment capacity of ex vivo cultured HSPCs
To evaluate the functional capability of CPX-treated cells, we cultured CD34+ cells in presence of CPX or DMSO for 6 days and then assayed for colony-forming ability using methylcellulose medium. CPX-treated cells generated similar numbers of myeloid colonies (CFU-GM) and showed a trend toward higher numbers of erythroid colonies (BFU-E) than control condition, whereas immature multilineage colonies (CFU-GEMM) were detected exclusively in the CPX-treated cultures (supplemental Figure 3A). This indicates that CPX treatment may preferentially support the more immature progenitor populations.
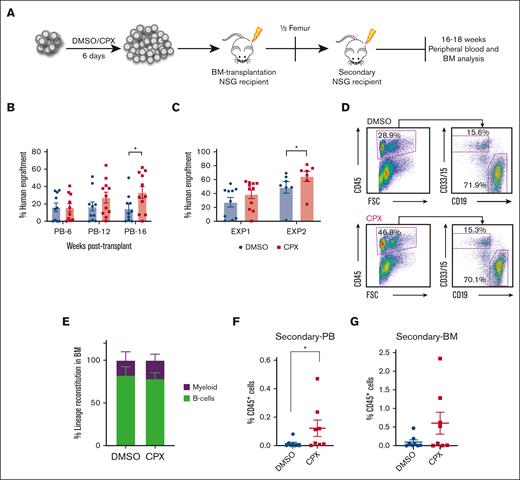
Next, we transplanted the cultured output of 30 000 initially seeded CB-CD34+ cells into sublethally irradiated NSG mice after 6 days culture in the presence or absence of CPX in 2 separate experiments with independent batches of pooled Umbilical Cord Blood (UCB) from multiple units (Figure 2A). Mice that underwent transplantation with CPX-treated cells showed similar short-term (6 weeks) human engraftment compared with controls but displayed significantly higher engraftment 16 weeks after transplantation in peripheral blood (Figure 2B). When bone marrow was analyzed after 16 weeks, many mice displayed saturating engraftment levels (>40%), but there was still a clear trend toward higher engraftment from the CPX-treated cells in 1 experiment (Exp 1, Figure 2C) and significantly higher chimerism levels in a subsequent experiment (Exp 2, Figure 2C). The engrafted cells contributed to both myeloid and lymphoid lineages (Figure 2D-E). In order to further evaluate the impact of CPX treatment on long-term HSC repopulation ability, we transplanted BM from engrafted primary recipients into secondary NSG recipients. Eighteen weeks after transplant, we observed significantly better engraftment in the PB of the CPX group and a similar trend in BM engraftment of the secondary recipients (Figure 2F-G). Altogether, our data suggest that CPX enhances the ex vivo maintenance of functional long-term repopulating HSCs.
CPX treatment improves long-term reconstitution capability of cultured CB-CD34+ cells. (A) Schematic representation of transplantation experiment. (B) Percentage of human CD45+ cells in the PB (6, 12, 16 weeks) and (C) BM (16 weeks) of NSG recipients. (D-E) Representative FACS plots and quantification of lineage distribution for B cells (CD19) and myeloid cells (CD33/CD15) in BM. (F-G) Secondary transplantation: human chimerism level in PB (F) and BM (G) of secondary NSG recipients from 16 to 18 weeks after transplantations.
CPX treatment improves long-term reconstitution capability of cultured CB-CD34+ cells. (A) Schematic representation of transplantation experiment. (B) Percentage of human CD45+ cells in the PB (6, 12, 16 weeks) and (C) BM (16 weeks) of NSG recipients. (D-E) Representative FACS plots and quantification of lineage distribution for B cells (CD19) and myeloid cells (CD33/CD15) in BM. (F-G) Secondary transplantation: human chimerism level in PB (F) and BM (G) of secondary NSG recipients from 16 to 18 weeks after transplantations.
We further performed limit dilution analysis to be able to more accurately assess the SRC number upon treatment with CPX. Therefore, the cultured equivalents of 500, 2000, and 8000 CB-CD34+ cells as well as their uncultured counterparts were transplanted into NSG mice. Human chimerism was evaluated 18 weeks after transplant and showed a trend toward an increase in number of SRCs in the CPX-treated group compared with both uncultured cells and DMSO control, adding further support to our previous transplantation experiments (supplemental Figure 3B; supplemental Table 4). Altogether, these data suggest that CPX treatment supports the propagation of functional long-term HSCs by preserving their undifferentiated state. However, it remains unresolved whether CPX treatment is sufficient to support robust expansion of bona fide HSCs in this context.
Next, we, therefore, asked whether CPX may cooperate with other molecules that recently have been reported to also enhance the short-term stem cell activity of cultured human HSPCs. Combining CPX with either the aryl hydrocarbon receptor antagonist, Stemregenin 1 (SR1),3 the p38 inhibitor Ly2228820,7 or the pyrimidoindole derivative UM1714 led to an increase in both frequency and number of CD34+CD90+ cells compared with each individual compound alone (supplemental Figure 4A), suggesting independent mechanisms of action and that the HSC-preserving activity of CPX may be beneficial together with other small molecules to promote optimal HSPC expansion. We further assessed the number of immature cells with CFU-GEMM potential that were produced from the different combinations and observed a particularly profound increase in the number of CFU-GEMM colonies when combining CPX and SR1, compared with either agent alone (supplemental Figure 4B).
The HSC supportive effect of CPX is mediated by iron chelation
To better understand the mechanism of action of CPX on HSPCs, we performed global transcriptomic profiling on CB-CD34+CD90+ cells after 24 hours or 6 days of treatment with CPX or DMSO, which identified 382 and 522 differentially expressed genes, respectively (supplemental Figure 5A). Gene set enrichment analysis on differentially expressed genes after 24 hours of CPX treatment revealed a distinct signature of reduced RNA polymerase I transcription, a rate-limiting factor for ribosomal RNA synthesis and cell cycle progression28,29 (supplemental Figure 5B; supplemental Table 5). Moreover, we observed a strong enrichment of HSC-associated gene signature in the CPX-treated CD34+CD90+ cells at day 6, compared with their DMSO-treated counterparts, further indicating that the HSC state is preserved by CPX30 (supplemental Figure 5C; supplemental Table 6). In addition, we could see a trend toward an enrichment of genes that are downregulated in HSCs upon ex vivo activation, compared with their noncultured quiescent counterparts, as reported by García-Prat et al,31 suggesting that CPX-treated cells preserve some molecular features of quiescent HSCs after 6 days in culture (supplemental Figure 5D).
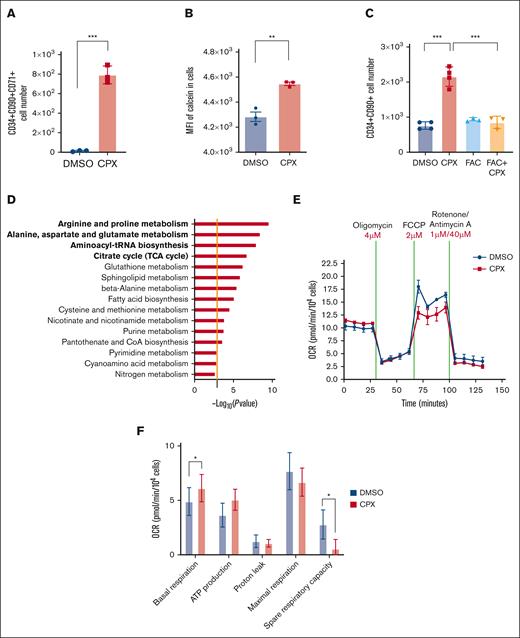
Further analysis of the global gene expression data from CPX-treated cells showed an enrichment of genes responsible for iron transport and uptake (supplemental Figure 5E; supplemental Table 7), which is in agreement with the well-known iron chelation activity of CPX and an indication of a compensatory responses to restore the intracellular iron concentration.15,32 In line with this observation, we detected an upregulation of transferrin receptor (TFRC/CD71) in CD34+CD90+ cells after 6 days of treatment with CPX (Figure 3A). To assess whether CPX directly alters iron homeostasis of CB-HSPCs, we quantified free intracellular iron levels by staining CD34+ cells with the cell permeable dye, Calcein-AM, whose fluorescent activity is quenched upon binding intracellular iron.33 We detected a significant increase in calcein fluorescence as early as 1 hour after CPX treatment, demonstrating that CPX reduces the cytoplasmic LIP (Figure 3B). To assess whether CPX-dependent modulation of iron homeostasis was responsible for the enhanced stem cell output, we pretreated CB-CD34+ cells with ferric ammonium citrate (FAC) for 1 hour before CPX treatment. We found that iron preloading, although not affecting control cells, completely reverted the CPX-induced increase of CD34+CD90+ cell number (Figure 3C). In addition, iron preloading led to a substantial reduction in the number of CD90+CD71+ cells after 6 days (supplemental Figure 5F). Taken together, these findings strongly suggest that the main biological effects of CPX in HSCs are mediated by its iron chelation function.
CPX induces intracellular iron deprivation that mediates the HSC supportive effect. (A) Numbers of CD34+CD90+CD71+ cells after 6 days culture (n = 3). (B) Calcein-AM staining in CD34+ cells treated with CPX or DMSO for 1 hour (n = 3). (C) Quantification of CD34+CD90+ cell number after 6 days culture with DMSO, CPX, or ferric amonium citrate (FAC) (n = 3). (D) Pathway analysis of altered metabolites from CB-CD34+ cells treated with CPX or DMSO, the orange line represents the cutoff of significance (P = .05). (E-F) OCR measured by Seahorse assay on CB-CD34+ cells.
CPX induces intracellular iron deprivation that mediates the HSC supportive effect. (A) Numbers of CD34+CD90+CD71+ cells after 6 days culture (n = 3). (B) Calcein-AM staining in CD34+ cells treated with CPX or DMSO for 1 hour (n = 3). (C) Quantification of CD34+CD90+ cell number after 6 days culture with DMSO, CPX, or ferric amonium citrate (FAC) (n = 3). (D) Pathway analysis of altered metabolites from CB-CD34+ cells treated with CPX or DMSO, the orange line represents the cutoff of significance (P = .05). (E-F) OCR measured by Seahorse assay on CB-CD34+ cells.
To further explore the mechanism by which iron chelation preserves the function of HSC during culture, we analyzed the metabolome of CPX-treated CB CD34+ cells. We observed a reduction in biosynthesis of some amino acids and altered aminoacyl-tRNA charging, which is consistent with reduced protein synthesis and slower cell proliferation (Figure 3D; supplemental Tables 8 and 9). Furthermore, accumulation of citric acid and reduction of several intermediate metabolites such as succinic acid, malate, and fumaric acid suggested reduced activity of the tricarboxylic acid (TCA) cycle34 upon CPX treatment, indicative of an altered energy-consuming state. To investigate whether CPX treatment directly affects mitochondrial respiration, we performed a mitochondrial stress test using the Seahorse extracellular flux analyzer. We observed that CPX treatment altered mitochondrial oxidative phosphorylation (OXPHOS), specifically decreasing the spare respiratory capacity compared with control but also a significant increase in the basal respiratory level (Figure 3E-F). Overall, these findings suggest that CPX-mediated iron deprivation, limits the mitochondrial respiratory mechanism and possibly restricts transition of primitive cells toward more energy-consuming state of proliferation.
Discussion
In this study, we developed and optimized a high content small molecule screen applied to primary human HSPCs. We show that CD90 expression directly correlates with the long-term repopulating activity of cultured CB-derived HSPCs and thus constitutes a valuable indicator of HSC content in cultured cells. From the screen, we identified CPX as a novel candidate regulator of human HSCs that promotes the ex vivo maintenance of cultured HSCs.
CPX is a hydroxypyridone derivative and an FDA-approved drug for the topical treatment of a wide range of fungal infections.12,35 CPX has been shown to chelate intracellular iron and, thereby, affect iron-dependent proteins and cellular processes. Iron chelators have profound antileukemic activity as demonstrated in both preclinical and clinical investigations.23,36,37 Oral administration of CPX was shown to prevent primary acute myeloid leukemia cell engraftment in nonobese diabetic/severe combined immunodeficiency mice, possibly by targeting leukemic stem cells.23 However, a role of CPX in regulation of normal HSCs has not been described previously.
Here we show that CPX treatment of primary human HSPCs in culture enhanced the output of both phenotypic and functional long-term repopulating HSCs, which was associated with a slower cell division rate of the HSCs. Moreover, enrichment of HSC-associated gene signatures of ex vivo cultured HSCs treated with CPX point toward a direct and specific impact of CPX on the most primitive HSC population.
Upregulation of genes responsible for iron uptake and transport upon CPX treatment was quite expected given the ability of CPX on chelating intracellular LIP. This effect is considered as a compensatory response mechanism in order to restore the intracellular iron concentration. The observation that iron preloading abolished the positive impact of CPX on cultured HSPCs led us to strongly conclude that CPX-mediated stem cell stimulating effect is attributed to its iron chelating mechanism. Because iron is a vital component of many physiological systems, including iron-dependent enzymes that play a role in cell proliferation and cell cycle division, it is possible that the slower division rate of primitive CB-derived cells could be because of a mild block or lower activity of such enzymes. A similar mechanism was recently described for the thrombopoietin receptor agonist eltrombopag, known for its clinical utility in treatment of patients with bone marrow failure by stimulating multilineage hematopoiesis.15 Kao et al demonstrated HSC supportive effects of eltrombopag in both mice and humans and showed that these were mediated through the iron chelation activity of eltrombopag, rather than its functions as a thrombopoietin receptor agonist.15 This is consistent with our findings for CPX and consolidates the importance of intracellular iron homeostasis for HSC regulation and maintenance.
Iron is present as a cofactor in a plethora of proteins, such as iron-sulfur (Fe-S) cluster proteins and heme-containing proteins, because of its facile ability to undergo redox cycling between ferric (Fe3+) and ferrous ion (Fe2+),38 and it, therefore, plays a vital role in mitochondrial respiratory complexes. In agreement with this, we found that CPX treatment affect the mitochondrial respiratory potential of HSCs mainly reducing their spare respiratory capacity. Previously, Fryknäs et al also found that iron chelation in quiescent tumor cells reduced the mitochondrial spare respiratory capacity leaving limited metabolic plasticity for cancer cells.39
Therefore, a possible scenario is that iron chelation induced by CPX delays the DNA replication as a result of the impact of iron deprivation on iron-dependent enzymes such as ribonucleotide reductase.23 This is in line with altered pyrimidine and purine metabolites in our metabolome analysis, which may result in a delayed transition through the cell cycle. In addition, CPX treatment limited the mitochondrial respiratory potential of HSCs and may, thereby, introduce a road-block for the transition of quiescent HSCs toward the proliferation state that relies mainly on high oxidative energy consumption.40 Therefore, CPX treatment preserves the HSC state and protects the stem cell pool from exhaustion during culture. This finding was strongly supported by CPX-mediated metabolomic changes, as evidenced by reduced level of several amino acids, alerted protein synthesis, and reduced TCA cycle activity; increased level of polyamines including spermidine and spermine is in line with a previously reported connection between polyamine levels and stemness.41 In addition, we observe a reduction in S1P and other sphingolipid metabolites, which were previously reported to regulate HSCs function. Sphingolipid composition was recently reported to differ along the human hematopoietic hierarchy, and sphingolipid modulation through inhibition of sphingolipid synthesis led to an increase in functional LT-HSCs.22 Collectively, we report here a previously unknown ability of CPX on preserving the immature state of human HSCs during ex vivo culture. The crucial role of iron homeostasis for human HSCs was reported recently; however, to our knowledge, here we show for the first time the impact of LIP deprivation on CB-HSC maintenance during ex vivo culture. To achieve the optimal expansion of CB-HSPCs for successful transplantation, it is of great importance to identify conditions that can robustly amplify short-term progenitor cells while maintaining and/or expanding long-term HSCs to promote hematopoietic recovery. Although our finding indicates that CPX preferentially supports the maintenance of long-term regenerative activity of cultured HSCs, it may not be sufficient in the context of robust expansion of CB-HSPCs for transplantation purposes. However, CPX in combination with previously identified HSC expansion molecules such as SR1, P38 inhibitors, and UM171 resulted in an additional increase in the number of phenotypic HSPCs, and it will be of interest to further investigate and rigorously test combinations of CPX with these molecules for expansion of CB-HSPCs.
Acknowledgments
This work was funded by grants from the Swedish Research Council, the Swedish Cancer Foundation, the Swedish Pediatric Cancer Foundation, Knut och Alice Wallenbergs Stiftelse, and the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program (grant agreement number 648894) to J.L. The work was further supported by the HematoLinné and StemTherapy programs at Lund University.
Authorship
Contribution: M.S.T and J.L. planned the study and designed the experiments; M.S.T performed the majority of experiments with assistance from A.B, A.S., N.M., C.K., L.O., A.R., K.Z., A.G.A., J.R., and K.P.; M.S.T analyzed the data; F.E. and R.O planned and assisted with chemical screening; Y-R.K. and B.W. performed metabolomic profiling and analyzed the data; and M.S.T and J.L. wrote the manuscript with input from the other authors.
Conflict-of-interest disclosures: The authors declare no competing financial interests.
Correspondence: Jonas Larsson, Molecular Medicine and Gene Therapy, Lund Stem Cell Center, Lund University, BMC A12, 221 84, Lund, Sweden; email: jonas.larsson@med.lu.se.
References
Author notes
∗A.B. and F.E. contributed equally to this work.
Microarray data are available under the accession GSE159100. Data are available on request from the corresponding author, Jonas Larsson (jonas.larsson@med.lu.se).
The full-text version of this article contains a data supplement.