Noncoding Gata2 genetic variation reduces efficacy of single and multiagent clinical regimens to mobilize HSPCs.
Noncoding Gata2 genetic variation abrogates polyvinyl alcohol–induced hematopoietic stem/progenitor cell expansion.
Visual Abstract
Germline genetic variants alter the coding and enhancer sequences of GATA2, which encodes a master regulator of hematopoiesis. The conserved murine Gata2 enhancer (+9.5) promotes hematopoietic stem cell (HSC) genesis during embryogenesis. Heterozygosity for a single-nucleotide Ets motif variant in the human enhancer creates a bone marrow failure and acute myeloid leukemia predisposition termed GATA2 deficiency syndrome. The homozygous murine variant attenuates chemotherapy- and transplantation-induced hematopoietic regeneration, hematopoietic stem and progenitor cell (HSPC) response to inflammation, and HSPC mobilization with the therapeutic mobilizer granulocyte colony–stimulating factor (G-CSF). Because a Gata2 +9.5 variant attenuated G-CSF–induced HSPC expansion and mobilization, and HSC transplantation therapies require efficacious mobilization, we tested whether variation affects mechanistically distinct mobilizers or only those operating through select pathways. In addition to affecting G-CSF activity, Gata2 variation compromised IL-8/CXCR2- and VLA-4/VCAM1-induced mobilization. Although the variation did not disrupt HSPC mobilization mediated by plerixafor, which functions through CXCR4/CXCL12, homozygous and heterozygous variation attenuated mobilization efficacy of the clinically used plerixafor/G-CSF combination. The influence of noncoding variation on HSPC mobilization efficacy and function is important clinically because comprehensive noncoding variation is not commonly analyzed in patients. Furthermore, our mobilization-defective system offers unique utility for elucidating fundamental HSPC mechanisms.
Introduction
To obtain hematopoietic stem and progenitor cells (HSPCs) for transplantation, granulocyte colony–stimulating factor (G-CSF) is used to mobilize HSPCs into peripheral blood. Low levels of mobilization1-4 have been attributed to aging, history of ineffective mobilization, diabetes, prior chemotherapy, and low baseline bone marrow (BM) cellularity/CD34+ cell numbers.5 Poor mobilization in diabetes is associated with hyperglycemia6 and sympathetic denervation in the hematopoietic system.7 Sympathetic agonists increase mobilization.8 Genetic variation in patients with Fanconi anemia9 is associated with poor mobilization and decreased HSPCs in the marrow,10,11 although the mechanisms are unresolved.
Mechanisms mediating G-CSF–induced HSPC mobilization involve interplay among HSPCs, neutrophils, macrophages, and nonhematopoietic BM niche components.12 Disruption of the HSPC-expressed CXCR4 and niche-secreted SDF1 (CXCL12) HSC homing mechanism with plerixafor (AMD3100 and Mozobil)10,13 results in HSPC egress from the niche into peripheral blood. The plerixafor/G-CSF combination is leveraged clinically to increase CD34+ cell numbers. Disruption of the axis involving HSPC VLA-4 and niche VCAM1 with the VLA-4 antagonist BIO5192 or other inhibitors promotes mobilization.14-16 Inflammatory signals, including CXCL2 (Gro-beta; Mip2) and CXCL8 (IL-8), activate neutrophils and mobilize HSPCs.17-19 Although mice lack IL-8, CXCR2 activation with human (rh)IL-8, Gro-beta or truncated Gro-beta induces mobilization.18,20,21
We reasoned that variation in genes causing hematologic pathology predispositions may compromise mobilization. Germline variants alter coding and enhancer sequences of GATA2,22 which encodes a master regulator of hematopoiesis.23 The conserved murine Gata2 enhancer (+9.5) promotes hematopoietic stem cell (HSC) genesis during embryogenesis.24-26 Heterozygosity for a single-nucleotide Ets motif variant in the human enhancer creates a BM failure and acute myeloid leukemia predisposition termed GATA2 deficiency syndrome.27 The homozygous murine variant (+9.5(Ets)–/–) attenuates chemotherapy- and transplantation-induced hematopoietic regeneration and HSPC response to inflammation.28 Biallelic +9.5 enhancer variation25 (compound heterozygous; CH) blocks mobilization by multidose G-CSF administration.29 This system involves an allele harboring the +9.5 Ets motif variant and another with an inactivating enhancer deletion (Figure 1A). This “conditionally pathogenic” configuration creates a BM failure predisposition in mice29 and models humans in which epigenetic silencing of the normal allele exacerbates the impact of a germline predisposition variant.30 We asked if Gata2 variation interferes with all, or select, mobilization mechanisms and if heterozygosity, reflecting the human predisposition state, compromises mobilization.
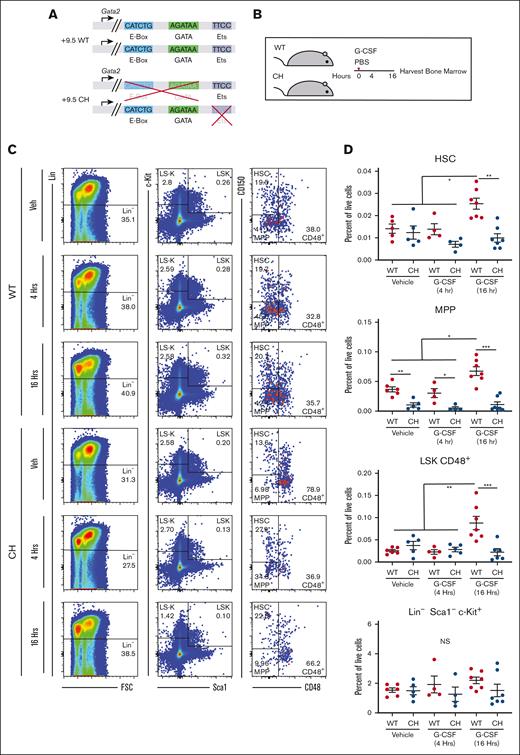
Gata2 germline variation blocks HSPC expansion after acute G-CSF treatment. (A) Configuration of Gata2 variant alleles in WT and CH. (B) Mice were treated with 1 dose of 125 mg/kg rhG-CSF or vehicle and harvested after 4 or 16 hours. (C) Representative flow cytometric plots for BM HSPC quantification. The numbers represent the percentage of the parent cell population. N = 6 to 9 per condition, 4 experiments (D) BM HSPC quantification. HSC: Lin− Sca1+ Kit+ (LSK) CD48– CD150+; MPP: LSK CD48– CD150–; LSK CD48+; Lin– Sca1– c-Kit+. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. FSC, forward scatter; PBS, phosphate-buffered saline.
Gata2 germline variation blocks HSPC expansion after acute G-CSF treatment. (A) Configuration of Gata2 variant alleles in WT and CH. (B) Mice were treated with 1 dose of 125 mg/kg rhG-CSF or vehicle and harvested after 4 or 16 hours. (C) Representative flow cytometric plots for BM HSPC quantification. The numbers represent the percentage of the parent cell population. N = 6 to 9 per condition, 4 experiments (D) BM HSPC quantification. HSC: Lin− Sca1+ Kit+ (LSK) CD48– CD150+; MPP: LSK CD48– CD150–; LSK CD48+; Lin– Sca1– c-Kit+. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. FSC, forward scatter; PBS, phosphate-buffered saline.
Methods
Age- and sex-matched mice were used for all experiments. Protocols were approved by the UW-Madison Institutional Animal Care and Use Committee in accordance with Association for Assessment and Accreditation of Laboratory Animal Care International regulations. rhG-CSF (Zarxio, 125 μg/kg) was administered subcutaneously as a single dose or twice a day (8 doses) beginning in the evening of the first day. Mice were harvested 3 hours after the final dose. For alternative mobilizers, plerixafor 5 mg/kg was administered intraperitoneally (IP), BIO5192 (R&D systems, 5051/10) 1 mg/kg was administered IV, and rhIL-8 (Peprotech, 200-08M) 1 mg/kg was injected IP. Mice were harvested after 15 minutes, 1 hour, or 2 hours. Colony forming unit (CFU) assays, transcript analysis, and flow cytometry were performed as described.29 5-ethynyl-2’-deoxyuridine (EdU); (Sigma, 900584-50 mg) 500 μg was injected IP 4 hours before harvest. EdU incorporation was detected using Click-iT EdU Alexa Fluor 647 Flow Cytometry Assay Kit (ThermoFisher, C10419) with modifications.31 Annexin V binding was detected using Alexa Fluor 647 Annexin V (BioLegend, 640943) as described.32 Complete blood counts were performed using an Oxford Science Inc GENESIS.
Adult C57BL/6 recipient mice (CD45.1+, 6- to 8-week-old; Stock # 002014, Jackson Labs) were lethally irradiated using an XRAD 320 irradiator for a single dose of 8 Gy. Competitor BM cells were harvested from individual 8-week-old mice (CD45.1+). A total of 106 BM cells were mixed with 50 mL of peripheral blood (6 total per group; 2 experiments). Transplant-recipient mice were maintained on Irradiated Uniprim Diet (Envigo; Cat# TD.06596) for 2 weeks. Blood obtained from the retro-orbital venous sinus was isolated after transplantation and analyzed using flow cytometry for donor-derived hematopoiesis.29
For HSC culture, BM from 8–12-week-old age- and sex- matched mice was isolated in phosphate-buffered saline. Fifty Lin− Sca1+ Kit+ (LSK) CD34– CD48– CD150+ cells were isolated using fluorescence-activated cell sorter and plated in the central wells of fibronectin-coated 96-well plates (Corning #354409) containing 0.2 mL polyvinyl alcohol (PVA) medium (Ham F-12 Nutrient Mix liquid medium (Gibco #11765-054) with 10 mM HEPES (Gibco #15630-080), 1× penicillin-streptomycin–Glutamine (Gibco #10378-016), insulin-transferrin-selenium–ethanolamine (Gibco #51500-056), 100 ng/mL recombinant animal-free murine thrombopoietin (Peprotech #AF-315-14), and 10 ng/mL recombinant animal-free murine stem cell factor (Peprotech #AF-250-03).33,34 PVA (1 mg/mL; 87%-90%–hydrolyzed; Sigma, #P8136) was included as indicated. Medium changes were initiated 5 days after plating and repeated every 2 to 3 days.
Group sizes required for each experiment followed the National Academies recommended calculation to detect a P value. The results are presented as box andwhisker plots, with bounds from the 25th to the 75th percentiles, the median line, and whiskers ranging from minimum to maximum values or as individual values summarized by median and standard error. Alternatively, individual data points were plotted with median and standard error values. Multiple independent cohorts were used in each experiment. Statistical comparisons were performed using 2-tailed Student t tests (significance cutoff of P < .05), with correction of statistical overrepresentation of functions calculated using the Benjamini-Hochberg multiple tests correction procedure or 1-way analysis of variance (ANOVA) followed by Tukey multiple comparisons test. GraphPad Prism (GraphPad Software) was used to perform statistical analyses.
Results
Short-term G-CSF treatment induces HSPC metabolic gene signatures,35 cell cycle entry, and expansion.36,37 Because CH have decreased multipotent progenitors (MPPs; LSK CD150– CD48–) and common myeloid progenitors (Lin− Sca1− Kit+ FcγR− CD34+),29 we asked if mobilization defects reflected decreased HSPCs in BM and if Gata2 variation affects HSPC proliferation before circulation. Wild-type (WT) BM immunophenotypic HSCs expanded 1.8- (P = .01) and 1.4-fold (P = .03) at 8 and 16 hours, respectively (supplemental Figure 1). No population frequency changes were detected at 4 hours. (Figures 1B-D; supplemental Figure 1). We assessed whether CH were competent to expand under these conditions. At 16 hours, WT HSCs (LSK CD48– CD150+; P = .001) and LSK CD48– CD150– MPPs (P = .01) expanded 1.8-fold relative to vehicle treatment; CH HSCs and MPPs were unchanged in response to G-CSF. As seen previously, CH MPPs were 3.5-fold lower than WT in vehicle-treated samples and 5.8-fold lower than WT after 4 hours of G-CSF. G-CSF expanded the multipotent LSK CD48+ population 3.5-fold in WT without affecting the heterogeneous Lin–Kit+Sca1– population. CH variation abrogated expansion of immunophenotypic HSPC expansion after G-CSF treatment.
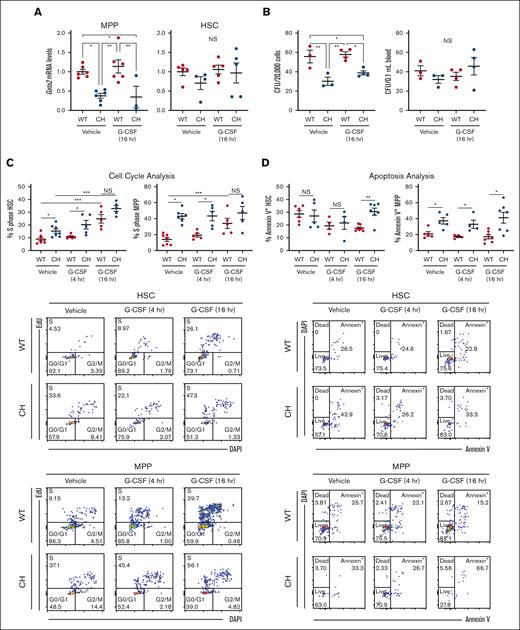
We asked if increased Gata2 expression in HSPC populations is associated with HSPC expansion in response to G-CSF. Gata2 expression in WT and CH long-term (LT)-HSCs was comparable in the steady state29 or after G-CSF (Figure 2A). CH variation impaired Gata2 expression in MPPs in a G-CSF–independent manner. Gata2 expression was 2.5-fold lower (P = .003) in vehicle-treated CH MPP vs WT and 3.3-fold lower (P = .037) in CH MPP vs WT 16 hours after G-CSF treatment (Figure 2A). Because the LSK CD48- CD150- MPP pool contains additional MPP subtypes, including MPP5 (LSK CD48− CD150− CD34+ Flt3−) and MPP6 (LSK CD48− CD150− CD34− Flt3−), we asked whether the decreased LSK CD48− CD150− population corresponded to MPP5, MPP6, or both. MPP5 and MPP6 were 1.7-fold (P = .0001 and .003) lower in untreated animals (supplemental Figure 2).
G-CSF–independent and -dependent CH HSPC defects. (A) Messenger RNA quantification from FACS-isolated HSC (LSK CD150+ CD48−) or MPP (LSK CD150– CD48–). N = 3 to 6 per condition from 3 experiments. (B) CFU from 2 × 105 total BM cells or 0.1 mL of peripheral blood. N = 3 to 4 per condition from 3 experiments. ∗P < .05, ∗∗P < .01; 1-way ANOVA followed by Tukey multiple comparisons test. (C) Top: EdU incorporation to quantify cell entry into S phase within HSCs and MPPs. Bottom: representative flow cytometric plots. N = 4 to 7 per condition, 5 experiments. (C) Top: annexin V binding to quantify HSC and MPP apoptosis. Bottom: representative flow cytometric plots. N = 4 to 7 per condition, 5 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 2-tailed unpaired Student t test with Benjamini-Hochberg correction. DAPI, 4′,6-diamidino-2-phenylindole; FACS, fluorescence-activated cell sorter.
G-CSF–independent and -dependent CH HSPC defects. (A) Messenger RNA quantification from FACS-isolated HSC (LSK CD150+ CD48−) or MPP (LSK CD150– CD48–). N = 3 to 6 per condition from 3 experiments. (B) CFU from 2 × 105 total BM cells or 0.1 mL of peripheral blood. N = 3 to 4 per condition from 3 experiments. ∗P < .05, ∗∗P < .01; 1-way ANOVA followed by Tukey multiple comparisons test. (C) Top: EdU incorporation to quantify cell entry into S phase within HSCs and MPPs. Bottom: representative flow cytometric plots. N = 4 to 7 per condition, 5 experiments. (C) Top: annexin V binding to quantify HSC and MPP apoptosis. Bottom: representative flow cytometric plots. N = 4 to 7 per condition, 5 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 2-tailed unpaired Student t test with Benjamini-Hochberg correction. DAPI, 4′,6-diamidino-2-phenylindole; FACS, fluorescence-activated cell sorter.
We asked if decreased immunophenotypic populations resulted in reduced functional activity in BM and peripheral blood. CH had 1.8-fold fewer CFU than WT BM in the steady state (P = .007) and 1.5-fold fewer 16 hours after G-CSF (P = .017), reflecting a paucity of functional HSPCs. No differences in colony counts from peripheral blood were detected, confirming that HSPCs have not egressed from the BM38 (Figure 2B). Thus, CH mice exhibit decreased HSPCs and failed to rapidly elevate HSPCs after G-CSF administration.
We asked if defective expansion resulted from inability of CH HSPCs to enter the cell cycle. No increases were detected at 4 hours. By contrast, 16 hours after G-CSF administration, WT BM EdU+ (S phase) HSCs increased 2.8-fold (Figure 2C). CH HSCs had a 1.7-fold (P = .04) higher percentage of cells in S phase and 1.9-fold (P = .04) higher percentage 4 hours after G-CSF. At 16 hours, no differences were detected. WT and CH LSK CD48+ cell numbers in S phase did not differ at any time, although both genotypes exhibited greater than twofold increases at 16 hours (supplemental Figure 3A). No differences were detected with Lin– Kit+ Sca1– progenitors. Cycling CH MPPs were 2.7-fold higher than WT in the steady state, 2.2-fold higher at 4 hours, and comparable by 16 hours (Figure 2C).
Although immunophenotypic CH HSCs and MPPs exhibited increased cycling, their numbers decreased relative to WT. This did not reflect ectopic mobilization as peripheral blood CFU in the steady state or 16 hours after treatment was unaltered (Figure 2B). We asked if CH variation rendered these populations apoptotic.32,39 Although Annexin V+ CH HSCs increased 1.8-fold relative to WT (P = .002) 16 hours after G-CSF administration, there was no increase with vehicle alone. CH MPP apoptosis increased (1.6- to 3.1-fold vs WT MPP) (P = .02, .04, and .02 with vehicle, 4 hours, and 16 hours G-CSF, respectively) regardless of treatment (Figure 2D; supplemental Figure 3B). GATA2 was therefore required for MPP population homeostasis.
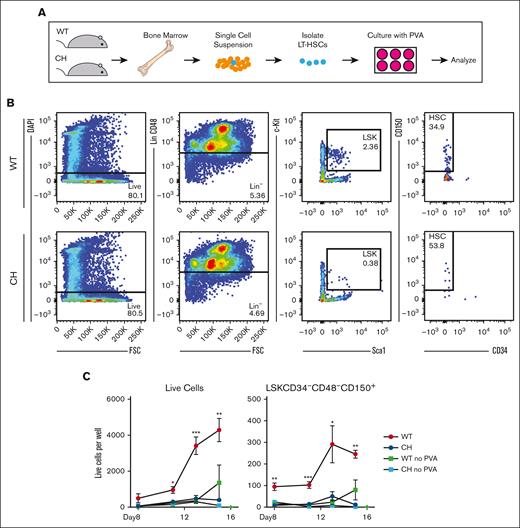
We tested whether CH HSC expansion defects could be overcome by PVA, which supports expansion of HSPCs ex vivo.33 Defined serum-free medium containing PVA expands purified HSPCs (LSK CD150+ CD34–) more than 100-fold over a month culture.33 We asked if WT and CH LT-HSCs (LSK CD150+ CD34– CD48–) had similar growth potential over 15 days (Figure 3A-B). Fifty cells were seeded per well. By day 15, WT cultures had a mean of 4286 cells per well, an 86-fold expansion (Figure 3C). In contrast, CH cultures had an average of 396 cells/well, a less than eightfold (P = .0072 vs WT) expansion. Under these conditions, the number of WT immunophenotypic LT-HSCs (LSK CD150+ CD34– CD48–) increased fivefold, CH LT-HSCs decreased to an average of 10 cells per well (P = .0007 vs WT) (Figure 3C). Neither culture expanded in medium lacking PVA. Thus, GATA2 is required for HSPC expansion ex vivo and in vivo.
Gata2 germline variation prevents polyvinyl alcohol-supported HSPC expansion ex vivo. (A) Schematic for isolation and culture of LT-HSCs in defined medium.34 (B) Representative flow cytometric plots for FACS-isolation of LT-HSCs (LSK CD150+ CD48− CD34–). (C) Flow cytometric quantification of total live cells and LT-HSCs (LSK CD150+ CD48− CD34–) per well. N = 3 replicates per condition, average of 3 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. DAPI, 4′,6-diamidino-2-phenylindole.
Gata2 germline variation prevents polyvinyl alcohol-supported HSPC expansion ex vivo. (A) Schematic for isolation and culture of LT-HSCs in defined medium.34 (B) Representative flow cytometric plots for FACS-isolation of LT-HSCs (LSK CD150+ CD48− CD34–). (C) Flow cytometric quantification of total live cells and LT-HSCs (LSK CD150+ CD48− CD34–) per well. N = 3 replicates per condition, average of 3 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. DAPI, 4′,6-diamidino-2-phenylindole.
To analyze relationships between mobilization and expansion defects, we used mobilization strategies involving distinct mechanisms. We asked if Gata2 variation attenuated mobilization via disrupting the CXCR4/CXCL12 chemokine axis with the CXCR4 antagonist plerixafor, stromal/neutrophil CXCR2 activation with rhIL-8, or VLA-4/VCAM1 binding with VLA-4 inhibitor BIO5192. Although the mobilization kinetics differed slightly, maximal mobilization by these mechanisms occurs within 1 hour, before complex BM remodeling.10,14,18 To ensure that the differences in mobilization did not reflect differences in mobilization kinetics between genotypes, we analyzed mobilization at 3 times.
In contrast to CH mobilization with G-CSF, plerixafor increased peripheral blood CFU in WT 3.3- to 4.1-fold at all times analyzed. CH CFU increased 3.4- and 4.1-fold at 1- and 2-hours after G-CSF administration, respectively (Figure 4A-B). CH mobilization was not decreased relative to WT at any time, demonstrating that the CXCR4/CXCL12 mobilization axis is intact in CH. Maximal rhIL-8–mediated mobilization in WT was achieved by 1 hour (3.9-fold over vehicle for WT). CH mobilization was not increased at any time analyzed and was significantly lower (P = .0003) than WT at the 1-hour time. Although VLA-4 inhibition with BIO5192 increased WT CFU maximally at 15 minutes (5.5-fold relative to vehicle), CH CFUs were significantly lower at 15 minutes (P = .016) and did not increase relative to vehicle treatment. Individual agents increased neutrophil numbers in peripheral blood in WT.18,40,41 Consistent with CFU analyses, CH responded to plerixafor, but not IL-8 or BIO5192 (Figure 4C). Although the CXCR4/CXCL12 pathway was insensitive to Gata2 variation, mobilization by means of CXCR2 activation or VLA-4 inhibition was impaired.
Gata2 germline variation selectively attenuates acute HSPC mobilization. (A) Mice were treated with 5 mg/kg plerixafor (CXCR4 antagonist), 1 mg/kg rhIL-8 (CXCR2 agonist), or 1 mg/kg BIO5192 (VLA-4 antagonist) and harvested after 0.25, 1, or 2 hours. (B) CFU from 0.1 mL of peripheral blood. (C) Peripheral blood neutrophil quantification. N = 3 to 12 per condition, 9 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. PBS, phosphate-buffered saline.
Gata2 germline variation selectively attenuates acute HSPC mobilization. (A) Mice were treated with 5 mg/kg plerixafor (CXCR4 antagonist), 1 mg/kg rhIL-8 (CXCR2 agonist), or 1 mg/kg BIO5192 (VLA-4 antagonist) and harvested after 0.25, 1, or 2 hours. (B) CFU from 0.1 mL of peripheral blood. (C) Peripheral blood neutrophil quantification. N = 3 to 12 per condition, 9 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. PBS, phosphate-buffered saline.
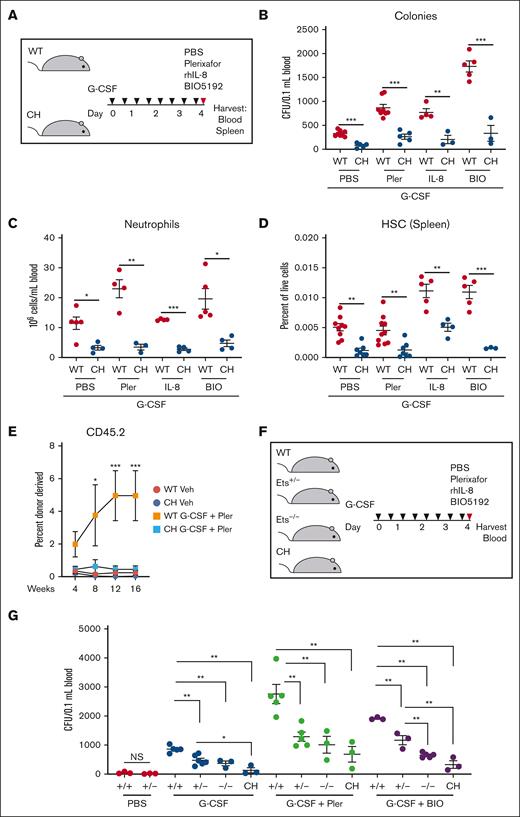
G-CSF treatment of WT mice decreases CXCL12 levels in the BM niche, increases neutrophil activation, and decreases VCAM1/VLA-4 interaction.42-44 We tested whether targeting these pathways collectively with G-CSF restores mobilization in CH. Synergistic mobilization was detected in WT with dual treatments of G-CSF and plerixafor, IL-8, or BIO5192 (2.6-, 2.3-, and 5.8-fold increases in CFU; P = .0002, .0007, and .0001, respectively) relative to G-CSF alone (Figure 5A-B). The CH variant reduced CFU, circulating neutrophils, and splenic HSC frequencies (Figure 5A-D). We tested whether these deficits resulted in decreased repopulating activity. Competitive transplantations with peripheral blood revealed that G-CSF + plerixafor increased WT donor–derived hematopoiesis 24-, 20-, and 18-fold at 8, 12, and 16 weeks, respectively, relative to the vehicle-treated control (Figure 5E). Treatment of CH did not increase donor-derived contributions. CH mice lacked the rapid HSPC expansion after G-CSF administration, yielding fewer cells to mobilize. Normal mobilization was not achieved even with plerixafor, which was insensitive to CH variation in the single-dose paradigm. The G-CSF + plerixafor mobilizing regimen did not elevate CH repopulating activity.
Gata2 germline variation attenuates combinatorial HSPC mobilization. (A) Mice were given 8 doses of 125 mg/kg rhG-CSF over 5 days followed by vehicle, 5 mg/kg plerixafor, 1 mg/kg rhIL-8, or 1 mg/kg BIO5192 and harvested after 1 hour. (B) CFU from 0.1 mL of peripheral blood. (C) Peripheral blood neutrophil quantification. (D) Splenic LT-HSC (LSK CD150+ CD48–) quantification. N = 3 to 10 per condition, 7 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 2-tailed unpaired Student t test with Benjamini-Hochberg correction. (E) Repopulating activity in peripheral blood 16 weeks after competitive transplant of 50 μL peripheral blood with 106 BM competitor cells. N = 5 to 6 per condition from 2 experiments. ∗P < .05, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. (F-G) Monoallelic Ets motif variation attenuates mobilization. WT, monoallelic (Ets+/–), homozygous biallelic (Ets–/–), and CH variants were tested. CFU from 0.1 mL of peripheral blood. N = 3 to 6 per condition, 6 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. PBS, phosphate-buffered saline.
Gata2 germline variation attenuates combinatorial HSPC mobilization. (A) Mice were given 8 doses of 125 mg/kg rhG-CSF over 5 days followed by vehicle, 5 mg/kg plerixafor, 1 mg/kg rhIL-8, or 1 mg/kg BIO5192 and harvested after 1 hour. (B) CFU from 0.1 mL of peripheral blood. (C) Peripheral blood neutrophil quantification. (D) Splenic LT-HSC (LSK CD150+ CD48–) quantification. N = 3 to 10 per condition, 7 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 2-tailed unpaired Student t test with Benjamini-Hochberg correction. (E) Repopulating activity in peripheral blood 16 weeks after competitive transplant of 50 μL peripheral blood with 106 BM competitor cells. N = 5 to 6 per condition from 2 experiments. ∗P < .05, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. (F-G) Monoallelic Ets motif variation attenuates mobilization. WT, monoallelic (Ets+/–), homozygous biallelic (Ets–/–), and CH variants were tested. CFU from 0.1 mL of peripheral blood. N = 3 to 6 per condition, 6 experiments. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001; 1-way ANOVA followed by Tukey multiple comparisons test. PBS, phosphate-buffered saline.
Because GATA2 deficiency syndrome and somatic GATA2 variation involve heterozygous alleles, we tested whether monoallelic Ets motif variation28,29 disrupts mobilization. Monoallelic variation did not decrease circulating, steady-state HSPCs. G-CSF increased CFU 28.5-fold (P < .0001) relative to vehicle-treated WT mice (Figure 5F-G). Mobilization of Gata2 Ets+/– HSPCs were reduced significantly (P = .003). CH variation severely disrupted mobilization, with 7.3-fold fewer CFU than Gata2 Ets+/– (P = .02). Synergistic G-CSF/plerixafor treatment increased CFU 89-fold in WT vs untreated (P < .0001), and Gata2 Ets+/– mobilization was reduced (P = .006) and not improved relative to Gata2 Ets–/– or CH. Ets+/– mobilization with G-CSF/BIO5192 was also impaired. Thus, Gata2 heterozygosity, modeling a human pathological state, impaired G-CSF-induced HSPC mobilization with all regimens tested.
Discussion
Considering the complex cell-intrinsic and -extrinsic mechanisms governing HSPC mobilization, genetic variation in genes encoding a plethora of components is almost certainly an important determinant of mobilization efficiency. The many unanswered questions include: is variation in genes encoding cell-intrinsic regulators of HSPC development and function an important determinant of mobilization efficiency? Although mobilization differs among inbred mouse strains10 and genetic background influences mobilization efficiency,45 the mechanisms are not established. In patients, failure to mobilize has been attributed to age, prior failure to mobilize, disease (eg, diabetes), prior chemotherapy, and low baseline BM cellularity and/or CD34+ cell numbers.5 Heritability estimations suggested that genetic variation plays a role in determining circulating CD34+ levels,46 and a genome-wide association study analysis demonstrated that PPM1H, CXCR4, ENO1-RERE, ITGA9, ARHGAP45, CEBPA, TERT, and MYC are associated with steady-state levels of circulating CD34+.47 Although CXCR4 variation may dictate CD34+ levels in the steady state, the relationship between CXCR4 polymorphism and mobilization efficiency is unclear.48,49
Prior research has focused on the role of genetic variation within genes encoding the receptors involved in the mobilization mechanisms analyzed herein. Not all genetic variation alters mobilization efficiency, even when occurring in genes encoding established regulators of mobilization. In most cases, CXCL12 single-nucleotide polymorphisms are not associated with CD34+ yield,48,50,51 although a 3′ variant is associated with high yield.52 As with CXCR4, VCAM1 polymorphisms can contribute or have no impact on mobilization efficiency.48,53 Given the millions of single-nucleotide polymorphisms in the human genome, there are many unknowns regarding the broader impact of genetic variation on mobilization efficiency.
It is instructive to consider variation in genes encoding cell-intrinsic regulators of HSPC development and function. Our results established Gata2 variation as a vital determinant of mobilization efficiency. Although patients with pathogenic GATA2 variants display altered chemotaxis in response to CXCL12,54 we demonstrated that the CXCR4/CXCL12 axis is intact in CH, unlike other mobilization regimens tested. Lymphoma, multiple myeloma, and Fanconi anemia are associated with inadequate mobilization.1,9,55,56,Fancc–/– and Fanca–/– HSPCs retain the capacity to mobilize in response to plerixafor and Rac inhibition,10,57,58 although whether the mechanism that impairs mobilization is identical to that used in CH is unclear. As with CH, these patients frequently have low HSPC levels and/or dysfunctional HSPCs. Because proinflammatory cytokine levels correlate with mobilization,59 and GATA2 alterations can dysregulate cytokine production,60,61 it seems reasonable that GATA2 alterations generate HSPC intrinsic and extrinsic defects that compromise GATA2-dependent mobilization.
Our mobilization-defective system (Figure 6) offers unique utility for elucidating fundamental HSPC mechanisms. It will be instructive to evaluate noncoding variation genome-wide with patients exhibiting suboptimal mobilization and evaluate a spectrum of variants linked to BM failure to establish rules, which may unveil innovations to surmount mobilization defects and pinpoint contexts in which specific variants preclude mobilization.
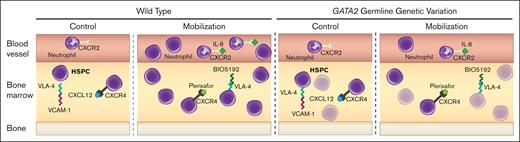
GATA2 genetic variation reduces HSPC activity and mobilization efficiency. Before treatment, WT quiescent HSPCs are maintained in BM through retention mechanisms including CXCR4/CXCL12, VLA-4/VCAM1, and low inflammation (IL-8). During mobilization, these mechanisms are disrupted, leading to HSPC expansion and circulation. Gata2 variation decreases HSPC levels, reducing the available HSPCs to mobilize and compromises VLA-4/VCAM1- and IL-8–induced mobilization mechanisms.
GATA2 genetic variation reduces HSPC activity and mobilization efficiency. Before treatment, WT quiescent HSPCs are maintained in BM through retention mechanisms including CXCR4/CXCL12, VLA-4/VCAM1, and low inflammation (IL-8). During mobilization, these mechanisms are disrupted, leading to HSPC expansion and circulation. Gata2 variation decreases HSPC levels, reducing the available HSPCs to mobilize and compromises VLA-4/VCAM1- and IL-8–induced mobilization mechanisms.
Acknowledgments
The work was supported by National Institutes of Health (NIH) grant DK68634 (E.H.B.), Edward P. Evans Foundation (E.H.B.), 1U01CA257666-01A1 (E.H.B.), ASH Scholar Award (A.A.S.), Leukemia and Lymphoma Society Career Development Program (A.A.S.), Carbone Cancer Center P30CA014520, and NIH shared instrumentation grant 1S10RR025483-01.
Authorship
Contribution: A.A.S. and E.H.B. conceived and designed the research, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emery H. Bresnick, Wisconsin Blood Cancer Research Institute, Department of Cell and Regenerative Biology, Carbone Cancer Center, University of Wisconsin School of Medicine and Public Health, 4009 WIMR, 1111 Highland Ave, Madison, WI 53705; email: ehbresni@wisc.edu.
References
Author notes
All data generated and analyzed during this study are included in this article and its supplemental information files. Original data and further information on resources and reagents are available upon reasonable request from the corresponding author, Emery Bresnick (ehbresni@wisc.edu).
The full-text version of this article contains a data supplement.