We established longer term proof of concept of PK activation as a potential novel therapy in SCD.
More than 1-year treatment with mitapivat, a PK activator, improved hemolytic anemia and reduced vaso-occlusive events in patients with SCD.
Visual Abstract
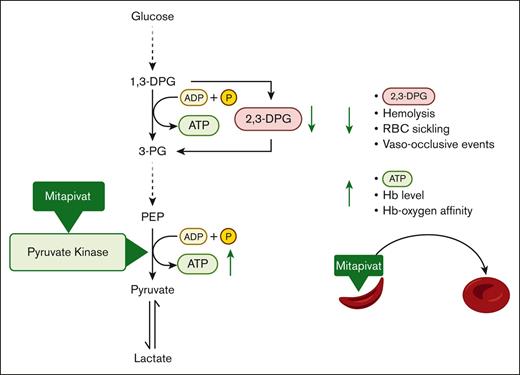
Effects of mitapivat-mediated pyruvate kinase activation in sickle cell disease. Mitapivat (AG-348) is an oral allosteric activator of pyruvate kinase (PK), a key enzyme in RBC glycolysis. PK activation decreases 2,3-DPG levels and increases ATP levels in RBCs. This study showed that mitapivat decreased parameters of hemolysis, RBC sickling and vaso-occlusive events and increased hemoglobin (Hb) level and Hb-oxygen affinity.1,3-DPG, 1,3-Disphosphoglycerate; ADP, adenosine diphosphate; P, phosphate; 2,3-DPG, 2,3-diphosphoglycerate; ATP, adenosine triphosphate; 3-PG, 3-Phosphoglycerate; PEP, phosphoenolpyruvate; RBC, red blood cell; Hb, hemoglobin.
Effects of mitapivat-mediated pyruvate kinase activation in sickle cell disease. Mitapivat (AG-348) is an oral allosteric activator of pyruvate kinase (PK), a key enzyme in RBC glycolysis. PK activation decreases 2,3-DPG levels and increases ATP levels in RBCs. This study showed that mitapivat decreased parameters of hemolysis, RBC sickling and vaso-occlusive events and increased hemoglobin (Hb) level and Hb-oxygen affinity.1,3-DPG, 1,3-Disphosphoglycerate; ADP, adenosine diphosphate; P, phosphate; 2,3-DPG, 2,3-diphosphoglycerate; ATP, adenosine triphosphate; 3-PG, 3-Phosphoglycerate; PEP, phosphoenolpyruvate; RBC, red blood cell; Hb, hemoglobin.
Targeting the primary pathogenic event of sickle cell disease (SCD), the polymerization of sickle hemoglobin (HbS), may prevent downstream clinical events. Mitapivat, an oral pyruvate kinase (PK) activator, has therapeutic potential by increasing adenosine triphosphate (ATP) and decreasing 2,3-diphosphoglycerate (2,3-DPG), a glycolytic red blood cell (RBC) intermediate. In the previously reported 8-week dose-finding period of this phase 2, investigator-initiated, open-label study, mitapivat was well tolerated and showed efficacy in SCD. Here, the 1-year fixed-dose extension period is reported in which 9 of 10 included patients (90%) aged ≥16 years with SCD (HbSS, HbS/β0, or HbS/β+) continued with mitapivat. Mostly mild treatment-emergent adverse events (AEs) (most commonly, transaminase increase and headache) were still reported. Apart from the reported nontreatment-related serious AE (SAE) of a urinary tract infection in the dose-finding period, 1 nontreatment-related SAE occurred in the fixed-dose extension period in a patient who died of massive pulmonary embolism due to COVID-19. Importantly, sustained improvement in Hb level (mean increase, 1.1 ± 0.7 g/dL; P = .0014) was seen, which was accompanied by decreases in markers of hemolysis. In addition, the annualized rate of vaso-occlusive events reduced significantly from a historic baseline of 1.33 ± 1.32 to 0.64 ± 0.87 (P = .0489) when combining the dose-finding period and fixed-dose extension period. Cellularly, the ATP:2,3-DPG ratio and Hb-oxygen affinity significantly increased and RBC sickling (point of sickling) nonsignificantly reduced. Overall, this study demonstrated 1-year safety and efficacy of treatment with mitapivat in SCD, supporting further evaluation in ongoing phase 2/3 study (RISE UP, NCT05031780). This trial was registered at https://www.clinicaltrialsregister.eu/ as NL8517 and EudraCT 2019-003438-18.
Introduction
Sickle cell disease (SCD) is a hereditary red blood cell (RBC) disorder that affects millions of people worldwide.1,2 SCD is caused by a single nucleotide mutation in the HBB gene (c.20A>T). Consequently, the produced sickle hemoglobin (HbS, βGlu6Val) polymerizes upon deoxygenation leading to rigid, poorly deformable, sickled RBCs. These damaged RBCs have a shortened lifespan and cause chronic hemolytic anemia, painful vaso-occlusive events (VOEs), and progressive end-organ damage resulting in strongly reduced quality of life and a decreased life expectancy.1,3,4
Therapeutic options for SCD have progressed slowly and are still limited.5 Curative therapies including hematopoietic stem cell transplantation and experimental gene therapy are only available to a small percentage of patients.6 To date, hydroxyurea, an inducer of fetal hemoglobin, is the only widely used agent that decreases the frequency of VOEs. However, hydroxyurea is limited by several side effects and not effective for all patients.7-10 In the last decade, the US Food and Drug Administration and/or European Medicines Agency approved other therapeutic agents (ie, L-glutamine, crizanlizumab, and voxelotor).11-13 Hereby, a new era for disease-modifying therapies targeting different processes in the pathophysiology of SCD has begun. However, there is still room for a novel therapeutic agent that improves both hemolytic anemia and the frequency of VOEs.14
A novel promising therapeutic target is to reduce HbS polymerization and RBC sickling by targeting RBC metabolism through activation of pyruvate kinase (PK). PK is a key enzyme in RBC metabolism, generating adenosine triphosphate (ATP) to maintain cellular homeostasis, membrane integrity, and deformability.15 It also modulates 2,3-diphosphoglycerate (2,3-DPG) levels in RBCs.16,17 2,3-DPG is an important glycolytic intermediate that decreases the oxygen affinity of hemoglobin (Hb) and, thus, contributes to the deoxygenation and polymerization of HbS in SCD.18,19 Mitapivat (AG-348) is an investigational, first-in-class, oral, small molecule allosteric activator of PK. It has recently been approved by the US Food and Drug Administration for adults with PK deficiency and shown to activate both wild-type and most mutant variants of the enzyme in RBCs.20-23 Reports of phase 1 studies in healthy individuals, phase 2 and 3 studies in patients with PK deficiency, and a phase 2 study in thalassemia showed that mitapivat had an acceptable safety profile. Effects consistent with PK activation and/or Hb responses were reported.17,21-24 Clinical studies in children, extension, and later phase studies are planned or ongoing (ClinicalTrials.gov identifiers: NCT04770753, NCT05144256, NCT03853798, NCT04770753, NCT04770779). In SCD, previously reported 6- to 8-week dose escalating studies, namely a phase 1 study (NCT04000165) and the dose-finding period of our phase 2 study (EudraCT 2019-003438-18), established proof of concept for the beneficial therapeutic effects of PK activation, such as increases in Hb level and decreases in RBC sickling in the short term.25,26
Here, we report the 1-year follow-up safety and efficacy results of treatment with mitapivat in patients with SCD.
Methods
Study oversight and patient selection
This phase 2, investigator-initiated, open-label, single center study of mitapivat in patients with SCD (the ESTIMATE study) was approved by the Medical Research Ethics Committee Utrecht, the Netherlands (no. 20-220; https://www.clinicaltrialsregister.eu/ NL8517; EudraCT 2019-003438-18) and was conducted in accordance with the Declaration of Helsinki. The study was sponsored by Julius Clinical, Zeist, the Netherlands. The study drug was provided by Agios Pharmaceuticals, Inc, Cambridge, MA. All patients provided written informed consent before enrollment. Inclusion criteria included: patients aged ≥16 years with SCD (homozygous HbS [HbSS], HbS/β0-thalassemia, or HbS/β+-thalassemia), prior VOEs (1-10 VOEs in the 12 months prior to initiation of study treatment) or SCD-related complications, and a Hb level >4.0 g/dL and ≤11.1 g/dL (see supplemental Methods for the full eligibility criteria). VOEs were defined as acute pain events that resulted in a health care provider contact and treatment with oral or parenteral narcotics or parenteral nonsteroidal anti-inflammatory drugs; acute chest syndrome, priapism, hepatic sequestration, or splenic sequestration. For patients receiving hydroxyurea, the dose must have been stable for ≥3 months. Major exclusion criteria were treatment with regular RBC transfusions and significant comorbidities (supplemental Methods).
Study design and end points
The ESTIMATE study comprised different phases, with the main results of the 8-week dose-finding period previously reported, and the 1-year fixed-dose extension period is reported here (Figure 1).26 After a screening period, initial dosing of mitapivat was 20 mg twice daily with a maximum of 2 sequential dose escalations (to 50 or 100 mg twice daily) in the dose-finding period, unless dosage-limiting side effects or an excessive Hb response, per protocol definition a Hb level >12.9 g/dL, occurred. Patients continued in the fixed-dose extension period if they safely tolerated mitapivat and showed evidence of clinical activity (ie, a significant change in the point of sickling [PoS] or increase in Hb level of ≥1 g/dL compared with baseline), followed by the maximum 24-month prolonged fixed-dose extension period. Dose reduction was allowed at any time. Patients who received ≥1 dose and withdrew, discontinued, or interrupted dosing after a 1-to 2-week dose taper to prevent hemolysis would return for a safety follow-up visit. Mitapivat was administered orally as 20- or 50-mg tablets.
Study schema of the ESTIMATE study. Safety and efficacy analysis were planned after completion of the 1-year fixed-dose extension period. BID, twice daily; D, study visit day of the dose-finding period with D0 at the start; M, study visit month of the prolonged fixed-dose extension period with M0 at the start corresponding to W52 of the fixed-dose extension period; W, study visit week of the fixed-dose extension period with W0 at the start corresponding to D56 of the dose-finding period.
Study schema of the ESTIMATE study. Safety and efficacy analysis were planned after completion of the 1-year fixed-dose extension period. BID, twice daily; D, study visit day of the dose-finding period with D0 at the start; M, study visit month of the prolonged fixed-dose extension period with M0 at the start corresponding to W52 of the fixed-dose extension period; W, study visit week of the fixed-dose extension period with W0 at the start corresponding to D56 of the dose-finding period.
The dose-finding period and (prolonged) fixed-dose extension period shared the same primary, secondary, and exploratory end points. The primary end points were safety, evaluated by the frequency and severity of adverse events (AEs) and graded according to Common Terminology Criteria for Adverse Events version 5.0 (National Cancer Institute, Bethesda, MD),27 and efficacy of treatment with mitapivat, evaluated by Hb response and antisickling response. AEs were classified as pretreatment AEs, treatment-emergent AEs (TEAEs), or posttreatment AEs. All relationships of AEs to the study drug were adjudicated by the principal investigator, coinvestigator, and sponsor. According to the study protocol, VOEs were not reported as AEs unless a VOE was atypical for the patient either in severity or presentation. However, SCD low-grade pain events, for example, those that occurred at home and were treated with oral opioids but did not result in a health care provider contact, were reported as AEs. Hb response was defined as the percentage of patients who had an improvement in mean Hb level of ≥1 g/dL compared with baseline during the fixed-dose extension period. Antisickling response was defined as the percentage of patients who had ≥10% improvement in mean PoS using oxygen gradient ektacytometry compared with baseline during the fixed-dose extension period. Secondary end points included changes in hematological parameters including Hb, absolute reticulocyte count, total bilirubin, lactate dehydrogenase (LDH), levels of ATP and 2,3-DPG, and Hb-oxygen affinity, as reflected by the oxygen tension at which Hb is 50% saturated (P50). In addition, surrogate markers of organ damage or biomarkers associated with mortality were evaluated (N-terminal prohormone of brain natriuretic peptide, C-reactive protein, LDH/carboxy hemoglobin ratio, D-dimer, von Willebrand Factor antigen, erythropoietin, and urinary albumin to creatinine ratio).28,29 Exploratory end points included changes in annualized rates of VOEs and annualized SCD-related hospital admission days.
Methodology and assessments
After the 8-week dose-finding period, each patient was scheduled for 7 planned study visits in the 1-year fixed-dose extension period (week 4, 8, 16, 24, 40, and 52 of the fixed-dose extension period).26 Specific attention was paid to AEs, compliance (based on pill counts and diaries), concomitant medication, vital signs (blood pressure, heart rate, and pulse oximetry) and physical examination. Safety assessments also included viral and pregnancy tests (during screening), 12-lead electrocardiogram (at baseline, the end of the dose-finding period, and week 52 of the fixed-dose extension period) and bone density scans (at baseline and week 52 of the fixed-dose extension period). Blood samples were collected at all visits. On indication for quality analysis in research assays, healthy control blood from our institutional donor service with ethical approval (no. 18/774) was used. In brief, routine laboratory tests and PoS measurements were performed at all visits within 24 hours.19,30,31 Oxygen gradient ektacytometry using a Laser Optical Rotational Red Cell Analyzer (Lorrca; RR Mechatronics, Zwaag, the Netherlands) measures RBC deformability at 37°C under constant shear stress (30 Pa) from the maximum elongation index (EImax) upon gradual deoxygenation with nitrogen to a minimum elongation index (EImin), representing minimal deformability resulting from RBC sickling upon deoxygenation.19 The PoS depicts the PO2 at which sickling is initiated and is defined as the PO2 at which the EImax is reduced by 5% during deoxygenation.19,31 At baseline, the end of the dose-finding period, and week 24 and week 52 of the fixed-dose extension period, additional laboratory tests including the P50 using a Hemox Analyzer (TCS Scientific Corp, New Hope, PA) and blood and urinary markers of SCD pathophysiology, organ damage and mortality were measured.32 Quantitative analysis of ATP and 2,3-DPG, corrected for Hb, was based on reported methods on snap frozen whole blood using liquid chromatography tandem mass spectrometry (UltiMate 3000, Thermo Fisher Scientific, San Jose, CA).33
Statistical analysis
The study was powered to evaluate changes in RBC sickling. The number needed to test a change of 10% in PoS, with intrasubject coefficients of variation <5%, would be well powered with 5 patients.19 To account for noncompliance, which is described to be high in this patient population, we planned to enroll 10 patients.34 Categorical data for safety and efficacy were summarized by counts or percentages, and continuous data by descriptive statistics (mean ± standard deviation). For continuous laboratory parameters, the mean of screening and day 0 were defined as baseline. Historical VOE frequency was based on chart review and patient recall (with source document verification) for VOEs in the 2 years before screening. The safety analysis set included all patients who received ≥1 dose of study treatment. Efficacy analysis based on the intention-to-treat principle included all patients who were dosed during the fixed-dose extension period. Intention-to-treat analysis was also used to evaluate the annualized VOE rate and annualized SCD-related hospital admission days on study drug treatment, combining the dose-finding period and the fixed-dose extension period. The per protocol set included all patients who were dosed and had Hb and PoS values at the start of the fixed-dose extension period and week 52. The strict per protocol set was used to analyze effectivity during study drug compliant periods, defined as ≥80% of pills taken between the prior and current visit as determined with pill counts. The strict per protocol set was also used to evaluate the annualized VOE rate and annualized SCD-related hospital admission days after exclusion of events with documented noncompliance the week before. Data were analyzed using IBM SPSS Statistics (version 27.0.0.0) and Graphpad Prism (version 9.3.0). The paired sample t test or Wilcoxon signed-rank test were used when appropriate, depending on whether the differences between pairs (the mean values of scheduled visits of the fixed-dose extension period vs baseline) were normally distributed based on the Shapiro-Wilk test. A P value ≤.05 was defined as statistically significant, while keeping in mind that multiple statistical tests have been performed.
Data management
A data management plan was generated. All study data from source documents were entered into Castor Electronic Data Capture that allows for audit trail. Data management included the designing of manual checks to ensure that data were entered logically and in adherence to the study protocol. The logic checks included some general checks (eg, dates are not entered in future and dates are entered in chronological order). Furthermore, some project-specific checks were designed such as completion of dosing diary and compliance. The logic behind the checks was tested on the data exports obtained from Castor Electronic Data Capture, and records for which inconsistent data entry was observed were flagged. Manual queries were raised for these data points and followed up until resolution. Data monitoring was performed by a certified clinical research associate of the sponsor with details provided in a monitoring plan.
Results
Patients
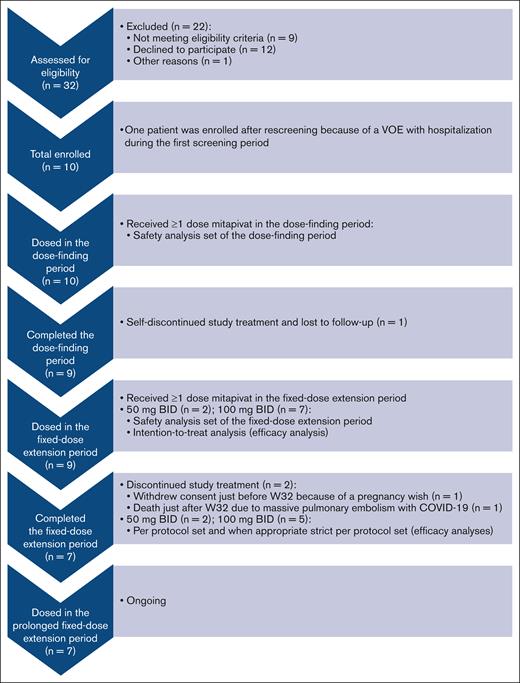
In total 10 patients were enrolled (September 2020-December 2021), dosed in the dose-finding period, and included in the safety analysis set (median total treatment duration, 59 weeks [range, 1 day-61 weeks]) (Figure 2; Table 1).26 Because 1 patient self-discontinued treatment and was lost to follow-up shortly after first dosing in the dose-finding period, 9 of 10 patients (90%) were dosed during the fixed-dose extension period and included in the intention-to-treat analysis (median total treatment duration, 60 weeks [range, 39-61 weeks]). Baseline characteristics for patients dosed in the fixed-dose extension period were: median age of 30 years (range, 16-59 years), 5 of 9 (56%) were female, and 6 of 9 (67%) were on stable-dose hydroxyurea (Table 1). Of the 9 patients, 7 (78%) had homozygous HbSS; 1 (11%) had HbS/β0 thalassemia; and 1 (11%) had HbS/β+ thalassemia. Median VOEs experienced was 1 (range, 0-7), with a median of 8 days (range, 0-42 days) of hospitalization for SCD-related complications in the 24 months before screening. Of the 9 patients, 7 (78%) completed the fixed-dose extension period and could be included in the per protocol set analysis and, when applicable, in the strict per protocol set analysis; 2 (22%) discontinued study treatment in the fixed-dose extension period (just before and after week 32). One patient withdrew consent because of a pregnancy wish; and another patient died due to massive pulmonary embolism with COVID-19 unrelated to study drug (see “Safety”). Most patients received mitapivat 100 mg twice daily (n = 7; 78%). Two (22%) received 50 mg twice daily after a single dose reduction per investigator decision, because transaminase increase >2.5× baseline was an AE of special interest at that time according to the study protocol and investigator’s brochure. The median compliance rate for mitapivat in the fixed-dose extension period was 97% (range, 62%-100%).
Study flowchart for the reported fixed-dose extension period analysis. The study flowchart of this nonrandomized phase 2 trial is adapted from the format of the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for randomized trials. Examples why patients did or could not participate included personal reasons such as a busy schedule, not willing to participate in experimental studies, or the COVID-19 pandemic and medical reasons such as Hb level >11.1 g/dL at baseline, no adequate organ function (ie, elevated transaminase levels), or a wish for children. n, number of patients.
Study flowchart for the reported fixed-dose extension period analysis. The study flowchart of this nonrandomized phase 2 trial is adapted from the format of the Consolidated Standards of Reporting Trials (CONSORT) flow diagram for randomized trials. Examples why patients did or could not participate included personal reasons such as a busy schedule, not willing to participate in experimental studies, or the COVID-19 pandemic and medical reasons such as Hb level >11.1 g/dL at baseline, no adequate organ function (ie, elevated transaminase levels), or a wish for children. n, number of patients.
Baseline characteristics of patients with SCD treated with mitapivat in the 8-week dose-finding period and the 1-year fixed-dose extension period
| . | Dose-finding period (n = 10) . | Fixed-dose extension period (n = 9) . |
|---|---|---|
| Age in y, median (range) | 26 (16-59) | 30 (16-59) |
| Female, n (%) | 6 (60) | 5 (56) |
| SCD genotype, n (%) | ||
| HbSS | 8 (80) | 7 (78) |
| HbS/β0-thalassemia | 1 (10) | 1 (11) |
| HbS/β+-thalassemia | 1 (10) | 1 (11) |
| Concomitant hydroxyurea therapy, n (%) | 6 (60) | 6 (67) |
| VOE∗ rate in previous 24 mo, median (range) | 2 (0-7) | 1 (0-7) |
| SCD-related hospital admission days in previous 24 mo, median (range) | 9 (0-42) | 8 (0-42) |
| RBC units in previous 12 mo, median (range) | 1 (0-3) | 1 (0-3) |
| . | Dose-finding period (n = 10) . | Fixed-dose extension period (n = 9) . |
|---|---|---|
| Age in y, median (range) | 26 (16-59) | 30 (16-59) |
| Female, n (%) | 6 (60) | 5 (56) |
| SCD genotype, n (%) | ||
| HbSS | 8 (80) | 7 (78) |
| HbS/β0-thalassemia | 1 (10) | 1 (11) |
| HbS/β+-thalassemia | 1 (10) | 1 (11) |
| Concomitant hydroxyurea therapy, n (%) | 6 (60) | 6 (67) |
| VOE∗ rate in previous 24 mo, median (range) | 2 (0-7) | 1 (0-7) |
| SCD-related hospital admission days in previous 24 mo, median (range) | 9 (0-42) | 8 (0-42) |
| RBC units in previous 12 mo, median (range) | 1 (0-3) | 1 (0-3) |
Includes VOEs as defined per study protocol based on chart review and patient recall for up to 24 months prior to study enrollment.
Safety
TEAEs were reported in all 10 patients receiving mitapivat in the dose-finding period and/or fixed-dose extension period, regardless of causality (Table 2). The majority of TEAEs were grade 1 and transient. The most commonly reported TEAEs (occurring in >2 patients) were transaminase increase (alanine aminotransferase increased in 7 of 10 [70%]; aspartate aminotransferase increased in 6 [60%]; all grade 1) and headache (n = 4 [40%]; grade 1-2). Two patients (20%) had a TEAE of grade ≥3, both assessed as nontreatment related. One was the previously reported nontreatment-related grade 4 TEAE in the dose-finding period of a serious AE (SAE) of a urinary tract infection.26 This patient was lost to follow-up (Figure 2). The 1 nontreatment-related grade 5 TEAE in the fixed-dose extension period was an SAE of massive pulmonary embolism due to COVID-19 (severe acute respiratory syndrome coronavirus 2 infection), which resulted in death of the patient. No other patients discontinued study treatment because of TEAEs. Next to the VOEs that are separately reported in the (additional) efficacy results, 2 patients experienced TEAEs of SCD low-grade pain events (Table 2). One patient had only mild SCD low-grade pain events (grade 1) and took paracetamol and oral nonsteroidal anti-inflammatory drugs at home. Another patient, who also had 1 VOE, experienced mild to moderate SCD low-grade pain events (grade 1-2), for which he occasionally took oral narcotics at home. No other SCD low-grade pain events were treated with oral narcotics at home.
TEAEs of patients with SCD treated with mitapivat (n = 10): safety analysis set of the 8-week dose-finding period and 1-year fixed-dose extension period
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|---|
| Total | 34 (77%) | 8 (18%) | 0 (0%) | 1 (2%)∗ | 1 (2%)† |
| Alanine aminotransferase increased | 7 (70%) | ||||
| Aspartate aminotransferase increased | 6 (60%)‡ | ||||
| Headache | 3 (30%) | 1 (10%) | |||
| Dyspepsia | 2 (20%) | ||||
| Abdominal pain | 2 (20%) | ||||
| Insomnia | 2 (20%) | ||||
| Diarrhea | 2 (20%) | ||||
| SCD low-grade pain events§ | 1 (10%) | 1 (10%) | |||
| Lymphocyte count increased | 2 (20%) | ||||
| Influenza-like symptoms | 1 (10%) | ||||
| Vaccination site lymphadenopathy | 1 (10%) | ||||
| Rhinorrhea | 1 (10%) | ||||
| Testosterone (total/free) increased | 1 (10%) | ||||
| Palpitations | 1 (10%) | ||||
| Hearing impaired | 1 (10%) | ||||
| Dysesthesia | 1 (10%) | ||||
| Iron deficiency | 1 (10%) | ||||
| Bloating | 1 (10%) | ||||
| Lung infection | 1 (10%) | ||||
| Pruritus | 1 (10%) | ||||
| Hemolysis | 1 (10%) | ||||
| Gastritis | 1 (10%) | ||||
| Urinary tract infection | 1 (10%)∗ | ||||
| Pulmonary embolism due to COVID-19 | 1 (10%)† |
| . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|---|
| Total | 34 (77%) | 8 (18%) | 0 (0%) | 1 (2%)∗ | 1 (2%)† |
| Alanine aminotransferase increased | 7 (70%) | ||||
| Aspartate aminotransferase increased | 6 (60%)‡ | ||||
| Headache | 3 (30%) | 1 (10%) | |||
| Dyspepsia | 2 (20%) | ||||
| Abdominal pain | 2 (20%) | ||||
| Insomnia | 2 (20%) | ||||
| Diarrhea | 2 (20%) | ||||
| SCD low-grade pain events§ | 1 (10%) | 1 (10%) | |||
| Lymphocyte count increased | 2 (20%) | ||||
| Influenza-like symptoms | 1 (10%) | ||||
| Vaccination site lymphadenopathy | 1 (10%) | ||||
| Rhinorrhea | 1 (10%) | ||||
| Testosterone (total/free) increased | 1 (10%) | ||||
| Palpitations | 1 (10%) | ||||
| Hearing impaired | 1 (10%) | ||||
| Dysesthesia | 1 (10%) | ||||
| Iron deficiency | 1 (10%) | ||||
| Bloating | 1 (10%) | ||||
| Lung infection | 1 (10%) | ||||
| Pruritus | 1 (10%) | ||||
| Hemolysis | 1 (10%) | ||||
| Gastritis | 1 (10%) | ||||
| Urinary tract infection | 1 (10%)∗ | ||||
| Pulmonary embolism due to COVID-19 | 1 (10%)† |
Data are n (%). Patients with multiple AEs within a preferred term were counted only once in that preferred term. For participants with multiple occurrences of an AE, the AE with the worst Common Terminology Criteria for Adverse Events grade is included in the summary.
Grade 4 TEAE was a SAE, assessed as nontreatment-related, in a noncompliant patient with an urinary tract infection, requiring intensive care unit admission for 1 night.
Grade 5 TEAE was a SAE, assessed as nontreatment-related, in a patient with a massive pulmonary embolism due to COVID-19.
One patient had an aspartate aminotransferase increase grade 1 after an elective cholecystectomy. Mitapivat dosage was reduced from 100 mg twice daily to 50 mg twice daily, and the level normalized at the following visit.
SCD low-grade pain events are not meeting the protocol definition of a VOEs. According to the study protocol, VOEs were not reported as (serious) AE unless a VOE was atypical for the patient either in severity or presentation.
Efficacy on laboratory end points
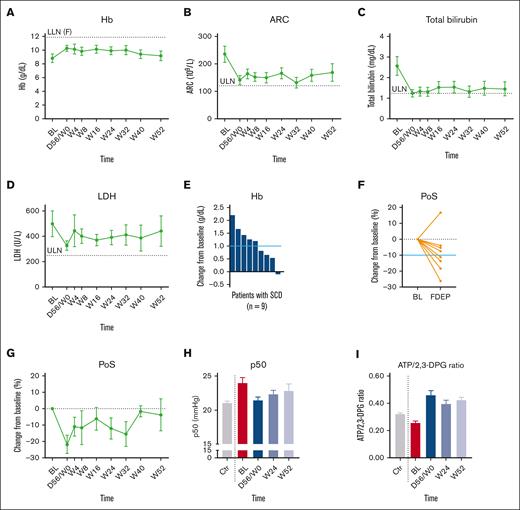
Intention-to-treat analysis showed significant improvements in multiple primary and secondary end points (Table 3; Figure 3A-I). In the fixed-dose extension period, a Hb response was found in 5 of 9 patients (56%) (Figure 3E). Hb level significantly increased (mean increase, 1.1 ± 0.7 g/dL; P = .0014; Figure 3A). The largest increase was 2.2 g/dL, and no excessive Hb response (>12.9 g/dL), occurred. Markers of hemolysis (mean absolute reticulocyte count, total bilirubin, and LDH level) decreased by 32%, 43%, and 18%, respectively, which was observed in all patients (Figure 3B-D). Notably, 1 patient with preexisting iron overload was able to have 2 phlebotomies before his last scheduled visit in the fixed-dose extension period. This was also the only patient who received (exchange) transfusion during the SAE in the fixed-dose extension period with no influence on study laboratory results. Most oxygen gradient ektacytometry-derived parameters improved: mean decrease in PoS was 4.1 ± 5.6 mmHg (P = .0802), EImax increase was 0.028 ± 0.016 (P = .0017), and EImin increase was 0.034 ± 0.024 (P = .0054), which indicate reduced RBC sickling and improved RBC deformability (Table 3). Four of 8 patients (50%) showed an antisickling response (Figure 3F-G). Notably, due to technical issues, the oxygen gradient ektacytometry fixed-dose extension period data are missing for 1 patient, a week 52 visit (n = 1 patient), and 4 visits from week 24 to week 52. An increase in Hb-oxygen affinity was found as reflected by a left shift of the oxygen equilibrium curves toward normal (mean P50 reduction of 1.5 ± 1.1 mmHg; P = .0032; Figure 3H). This was accompanied by a decrease in 2,3-DPG level (mean decrease of 2.4 ± 1.2 mg/gHb; P = .0004) and increase in ATP:2,3-DPG ratio (mean increase of 0.14 ± 0.08; P = .0009; Figure 3I), reflecting restoration of compromised RBC metabolism in SCD. Surrogate markers of organ damage and biomarkers associated with mortality did not significantly change in the fixed-dose extension period (supplemental Table 1).
Changes in Hb level, markers of hemolysis, biochemical parameters, RBC parameters of functional assays, and markers of SCD-related complications from baseline to the end of the dose-finding period and mean of the fixed-dose extension period (intention-to-treat analysis)
| . | Baseline (n = 9) . | End of the dose-finding period (n = 9) . | Mean of the fixed-dose extension period (n = 9) . | P (baseline vs mean of the fixed-dose extension period) . |
|---|---|---|---|---|
| Hb level and markers of hemolysis | ||||
| Hb, g/dL | 8.8 (1.8) | 10.3 (1.3) | 9.9 (1.8) | .0014 |
| Reticulocytes | ||||
| ARC, 109 per L | 235 (88) | 141 (50) | 156 (50) | .0038 |
| % of RBCs | 8.2 (2.3) | 4.2 (1.4) | 5.0 (1.4) | .0003 |
| Total bilirubin, mg/dL | 2.6 (1.3) | 1.2 (0.5) | 1.4 (0.7) | .0025 |
| LDH, U/L | 500 (307) | 328 (113) | 401 (224) | .0217 |
| Biochemical parameters | ||||
| ATP, mg/gHb | 2.9 (0.7) | 3.6 (0.5) | 3.6 (0.5) | .1386 |
| 2,3-DPG, mg/gHb | 11.4 (1.0) | 7.9 (1.1) | 9.0 (1.1) | .0004 |
| ATP:2,3-DPG ratio | 0.25 (0.05) | 0.46 (0.09) | 0.40 (0.06) | .0009 |
| RBC parameters of functional assays | ||||
| PoS, mmHg | 40.2 (8.8)∗ | 33.1 (9.7)∗ | 36.2 (6.3)∗ | .0802 |
| EImax, EI | 0.450 (0.074)∗ | 0.477 (0.059)∗ | 0.478 (0.061)∗ | .0017 |
| EImin, EI | 0.067 (0.048)∗ | 0.116 (0.049)∗ | 0.101 (0.066)∗ | .0054 |
| P50, mmHg | 24.0 (2.4) | 21.5 (1.4) | 22.5 (1.8) | .0032 |
| Markers of SCD-related complications | ||||
| Annualized VOE rate Dose-finding period + fixed-dose extension period† Fixed-dose extension period | 1.33 (1.32) | 0.64 (0.87) | .0489† | |
| 0.72 (2.17) | 0.60 (0.78) | .0625 | ||
| Annualized SCD-related hospital admission days | 5.3 (7.0) | 0.0 (0.0) | 4.1 (5.6) | .4452 |
| . | Baseline (n = 9) . | End of the dose-finding period (n = 9) . | Mean of the fixed-dose extension period (n = 9) . | P (baseline vs mean of the fixed-dose extension period) . |
|---|---|---|---|---|
| Hb level and markers of hemolysis | ||||
| Hb, g/dL | 8.8 (1.8) | 10.3 (1.3) | 9.9 (1.8) | .0014 |
| Reticulocytes | ||||
| ARC, 109 per L | 235 (88) | 141 (50) | 156 (50) | .0038 |
| % of RBCs | 8.2 (2.3) | 4.2 (1.4) | 5.0 (1.4) | .0003 |
| Total bilirubin, mg/dL | 2.6 (1.3) | 1.2 (0.5) | 1.4 (0.7) | .0025 |
| LDH, U/L | 500 (307) | 328 (113) | 401 (224) | .0217 |
| Biochemical parameters | ||||
| ATP, mg/gHb | 2.9 (0.7) | 3.6 (0.5) | 3.6 (0.5) | .1386 |
| 2,3-DPG, mg/gHb | 11.4 (1.0) | 7.9 (1.1) | 9.0 (1.1) | .0004 |
| ATP:2,3-DPG ratio | 0.25 (0.05) | 0.46 (0.09) | 0.40 (0.06) | .0009 |
| RBC parameters of functional assays | ||||
| PoS, mmHg | 40.2 (8.8)∗ | 33.1 (9.7)∗ | 36.2 (6.3)∗ | .0802 |
| EImax, EI | 0.450 (0.074)∗ | 0.477 (0.059)∗ | 0.478 (0.061)∗ | .0017 |
| EImin, EI | 0.067 (0.048)∗ | 0.116 (0.049)∗ | 0.101 (0.066)∗ | .0054 |
| P50, mmHg | 24.0 (2.4) | 21.5 (1.4) | 22.5 (1.8) | .0032 |
| Markers of SCD-related complications | ||||
| Annualized VOE rate Dose-finding period + fixed-dose extension period† Fixed-dose extension period | 1.33 (1.32) | 0.64 (0.87) | .0489† | |
| 0.72 (2.17) | 0.60 (0.78) | .0625 | ||
| Annualized SCD-related hospital admission days | 5.3 (7.0) | 0.0 (0.0) | 4.1 (5.6) | .4452 |
Data are presented as mean (standard deviation). P values derived from paired sample t test or Wilcoxon signed-rank test when appropriate to compare baseline values with the mean values of the fixed-dose extension period (unless otherwise stated), but not with the mean values of the end of the dose-finding period (in italics).
ARC, absolute reticulocyte count.
Due to technical issues of the oxygen gradient ektacytometer, data are missing for 1 patient a week 52 visit (n = 1 patient), and 4 visits from week 24 to week 52 in the fixed-dose extension period (n = 2 patients).
Intention-to-treat analysis of baseline vs the total period on study drug treatment (the dose-finding period and the fixed-dose extension period combined) instead of only the fixed-dose extension period.
Improvements of efficacy parameters in the dose-finding period and the fixed-dose extension period of treatment with mitapivat treatment in patients with SCD (intention-to-treat analysis). (A) Hb level increases were sustained over time in patients with SCD compared with baseline (n = 9 BL-W24; n = 8 W32; n = 7 at W40 and W52). (B-D) The increase in Hb level is accompanied by decreases of laboratory markers of hemolysis: (B) ARC, (C) Total bilirubin, and (D) LDH toward the upper limit of normal. (E) Five of 9 patients (56%) with SCD showed a mean increase in Hb level ≥1 g/dL in the fixed-dose extension period from baseline (solid, light blue, horizontal reference line). (F-G) Oxygen gradient ektacytometry showed in 4 of 8 patients (50%) with SCD a mean decrease in PoS of ≥10% in the fixed-dose extension period from baseline (solid, light blue, horizontal reference line), which indicates that initiation of RBC sickling occurs at lower PO2 levels. Notably, fixed-dose extension period data are missing for 1 patient, a week 52 visit (n = 1 patient), and 4 visits from week 24 to week 52 (n = 2 patients). (H) Treatment with mitapivat increased Hb-oxygen affinity in patients with SCD, reflected by a decreases in P50 from baseline (red) to the end of the dose-finding period (darkest blue), week 24 of the fixed-dose extension period (middle blue), and week 52 of the fixed-dose extension period (lightest blue) toward P50 values of untreated healthy controls (Ctr; gray; n = 45). (I) The ATP:2,3-DPG ratio increased upon treatment with mitapivat, also compared with untreated healthy controls (Ctr; gray; n = 40). Means are presented. Error bars represent standard errors of the mean. Dashed, gray, horizontal reference lines represent LLN, ULN or 0% change from baseline as indicated. Dashed, gray, vertical reference lines separate untreated healthy controls (on the left) with different timepoints of the treated patients (on the right). ARC, absolute reticulocyte count; BL, baseline; D56/W0, day 56 of the dose-finding period corresponding with W0 of the fixed-dose extension period; F, female; LLN, lower limit of normal; ULN, upper limit of normal; W, study visit week of the fixed-dose extension period.
Improvements of efficacy parameters in the dose-finding period and the fixed-dose extension period of treatment with mitapivat treatment in patients with SCD (intention-to-treat analysis). (A) Hb level increases were sustained over time in patients with SCD compared with baseline (n = 9 BL-W24; n = 8 W32; n = 7 at W40 and W52). (B-D) The increase in Hb level is accompanied by decreases of laboratory markers of hemolysis: (B) ARC, (C) Total bilirubin, and (D) LDH toward the upper limit of normal. (E) Five of 9 patients (56%) with SCD showed a mean increase in Hb level ≥1 g/dL in the fixed-dose extension period from baseline (solid, light blue, horizontal reference line). (F-G) Oxygen gradient ektacytometry showed in 4 of 8 patients (50%) with SCD a mean decrease in PoS of ≥10% in the fixed-dose extension period from baseline (solid, light blue, horizontal reference line), which indicates that initiation of RBC sickling occurs at lower PO2 levels. Notably, fixed-dose extension period data are missing for 1 patient, a week 52 visit (n = 1 patient), and 4 visits from week 24 to week 52 (n = 2 patients). (H) Treatment with mitapivat increased Hb-oxygen affinity in patients with SCD, reflected by a decreases in P50 from baseline (red) to the end of the dose-finding period (darkest blue), week 24 of the fixed-dose extension period (middle blue), and week 52 of the fixed-dose extension period (lightest blue) toward P50 values of untreated healthy controls (Ctr; gray; n = 45). (I) The ATP:2,3-DPG ratio increased upon treatment with mitapivat, also compared with untreated healthy controls (Ctr; gray; n = 40). Means are presented. Error bars represent standard errors of the mean. Dashed, gray, horizontal reference lines represent LLN, ULN or 0% change from baseline as indicated. Dashed, gray, vertical reference lines separate untreated healthy controls (on the left) with different timepoints of the treated patients (on the right). ARC, absolute reticulocyte count; BL, baseline; D56/W0, day 56 of the dose-finding period corresponding with W0 of the fixed-dose extension period; F, female; LLN, lower limit of normal; ULN, upper limit of normal; W, study visit week of the fixed-dose extension period.
Efficacy on clinical end points
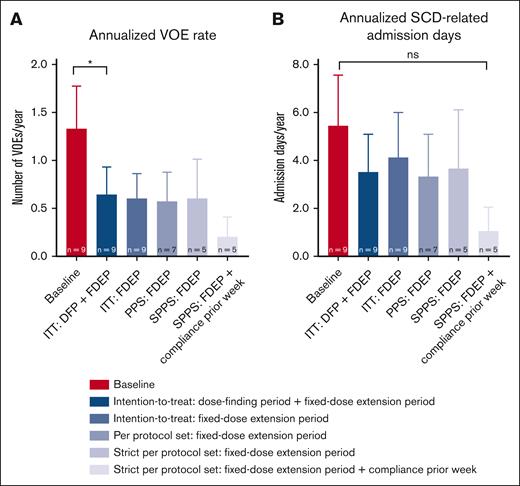
In the intention-to-treat analysis of the fixed-dose extension period, mean annualized VOE rate dropped from 1.33 ± 1.32 during the 2-year prior history to 0.60 ± 0.78 during treatment with mitapivat (P = .0625; Table 3; Figure 4A). When taking the total treatment period with mitapivat of the dose-finding period and the fixed-dose extension period together in the intention-to-treat analysis, mean annualized VOE rate decreased significantly to 0.64 ± 0.87 (P = .0489) (Table 3; Figure 4A). In 3 of 5 VOEs (60%), patients were documented noncompliant in the week before the event. In addition, clear VOE triggers (ie, a lung infection requiring antibiotic treatment [n = 2] and gastro-intestinal complaints after alcohol intake [n = 1]) were reported. One of the VOEs without documented noncompliance was triggered by COVID-19 during the reported SAE; the last VOE had no known trigger. The annualized SCD-related hospital admission days did not significantly change (Table 3; Figure 4B).
Changes in annualized rates of VOEs and annualized SCD-related hospital admission days (intention-to-treat, per protocol set, and strict per protocol set analyses). (A) The annualized rate of VOEs reduced significantly from 1.33 ± 1.32 to 0.64 ± 0.87 (P = .0489) in the patients with SCD treated with mitapivat when combining the study drug treatment periods of the dose-finding period and the fixed-dose extension period in the intention-to-treat analysis. In the other efficacy analyses of the fixed-dose extension period including the (strict) per protocol set analyses, also with documented compliance ≥80% in the week before the event (on the right), this trend was also seen, although nonsignificantly. (B) The annualized SCD-related hospital admission days also reduced, although nonsignificantly, in all efficacy analyses. Means are presented. Error bars represent standard errors of the mean; ∗P < .05. DFP, 8-week dose-finding period; FDEP, 1-year fixed-dose extension period; ITT, intention-to-treat; PPS, per protocol set (all patients who were dosed and had Hb and oxygen gradient ektacytometry assessments at the start of the FDEP and week 52); SPPS, strict per protocol set (all patients of the PPS who were study drug compliant).
Changes in annualized rates of VOEs and annualized SCD-related hospital admission days (intention-to-treat, per protocol set, and strict per protocol set analyses). (A) The annualized rate of VOEs reduced significantly from 1.33 ± 1.32 to 0.64 ± 0.87 (P = .0489) in the patients with SCD treated with mitapivat when combining the study drug treatment periods of the dose-finding period and the fixed-dose extension period in the intention-to-treat analysis. In the other efficacy analyses of the fixed-dose extension period including the (strict) per protocol set analyses, also with documented compliance ≥80% in the week before the event (on the right), this trend was also seen, although nonsignificantly. (B) The annualized SCD-related hospital admission days also reduced, although nonsignificantly, in all efficacy analyses. Means are presented. Error bars represent standard errors of the mean; ∗P < .05. DFP, 8-week dose-finding period; FDEP, 1-year fixed-dose extension period; ITT, intention-to-treat; PPS, per protocol set (all patients who were dosed and had Hb and oxygen gradient ektacytometry assessments at the start of the FDEP and week 52); SPPS, strict per protocol set (all patients of the PPS who were study drug compliant).
Additional efficacy analyses
Generally, per protocol set analysis with 7 of 9 patients (78%), excluding 2 patients who discontinued study treatment before the end of the fixed-dose extension period, and strict per protocol set analysis with 5 of 9 (56%) patients, excluding 2 other patients with noncompliance at all visits of the fixed-dose extension period, showed the same trends although less statistical significance was reached with the exception of the PoS and ATP level (supplemental Tables 2 and 3). In the strict per protocol set analysis, the annualized VOE rate and annualized SCD-related hospital admission days were the lowest after exclusion of events with documented noncompliance in the week before the event (Figure 4A-B). Except for 1 patient with HbS/β0 thalassemia, concomitant hydroxyurea, and iron deficiency at the end of the fixed-dose extension period, improvements in Hb levels were observed in all individuals (supplemental Figure 1A). Improvements in markers of hemolysis were observed in all patients regardless of SCD genotype and concomitant hydroxyurea use (supplemental Figure 1B-D).
Discussion
In the 1-year fixed-dose extension period of this phase 2, open-label study, we show that treatment with mitapivat, a PK activator, continued to improve hemolytic anemia in patients with SCD. Moreover, consistent with PK activation, the level of 2,3-DPG decreased, the ATP/2,3-DPG ratio increased, and Hb-oxygen affinity improved. Although exploratory, our data might suggest that mitapivat has the potential to improve clinical outcomes based on the significant decrease in annualized VOE rate when combining the 8-week dose-finding period and the 1-year fixed-dose extension period. Most TEAEs were grade 1, and no treatment-related TEAEs of grade ≥3 were reported. Overall, to the best of our knowledge, this is the first study that reports proof of concept of more than 1-year treatment with mitapivat in patients with SCD.
The provided evidence for an acceptable safety profile of mitapivat in SCD is consistent with those of studies in patients with PK deficiency and thalassemia.21,23,24 In the phase 1 study of treatment with mitapivat in SCD, insomnia, diarrhea, increased aspartate aminotransferase, and headache were also among the most commonly reported TEAEs although, for example, hypertension was reported more often in that study.25 No new safety concerns were reported in our study. Two SAEs, assessed as nontreatment-related, were reported; 1 grade 4 TEAE of a urinary tract infection in the dose-finding period and 1 grade 5 TEAE of massive pulmonary embolism due to COVID-19 in the fixed-dose extension period.26 Most VOEs (1 of 1 [100%] in the dose-finding period and 4 of 5 [80%] in the fixed-dose extension period) had known possible triggers.26 Notably, in 3 of 6 VOEs (50%), an additional trigger could have been noncompliance with mitapivat the week before the start of the VOE. In another study, 6- to 8-week treatment with mitapivat showed that most VOEs occurred during tapering or when not on study drug.25 This could suggest that without mitapivat’s beneficial effects, patients may return to their baseline risk of VOEs. In our study, some patients who missed several doses, including the patient with the least overall compliance in the fixed-dose extension period, did not experience any VOEs. VOEs are not unexpected in patients with SCD. The question remains whether the VOEs that occurred would have been the same in frequency and severity without study drug treatment. More longer term and placebo-controlled phase 2/3 studies could help to elucidate whether mitapivat protects patients from VOEs.
The significant increase in Hb level and decrease in markers of hemolysis indicate that mitapivat enhanced survival of RBCs. A prior risk reduction meta-analysis in patients with SCD demonstrated that an increase in Hb level of ≥1 g/dL resulted in a decreased risk of SCD-related complications including cerebrovascular disease, albuminuria, elevated pulmonary artery systolic pressure, and mortality of 41%, 53%, 57%, and 64%, respectively.35 This suggests that mitapivat has the potential to improve SCD-related complications, although, in our small study, we could not demonstrate changes in markers associated with organ damage or mortality. The risk/benefit ratio of therapies aimed at increasing Hb-oxygen affinity in SCD, which may be offset by adverse effects on oxygen release in tissues, has been discussed.36-38 So far, studies with an inhibitor of HbS polymerization, voxelotor, which needs higher therapeutic concentrations to allosterically bind to Hb than mitapivat to PK,17,39,40 showed no concerns regarding impaired oxygen delivery to tissues.41,42 In our study, no increase in erythropoietin levels and no issues due to tissue hypoxia were reported, partly postulated by the fact that 2,3-DPG binds and dissociates rapidly, thereby locally modulating Hb-oxygen affinity.36,43 In addition, concerns have been raised about increasing the Hb level >10 g/dL because of case reports of viscosity-related complications in patients with SCD who received RBC transfusions.44 A phase 3 study of senicapoc (a Gardos channel inhibitor) and a phase 3 study of voxelotor in SCD showed an increase in Hb level and reductions in some markers of hemolysis but no change in VOE rate.13,45,46 In the senicapoc study, a higher VOE rate was reported in the subgroup of patients who did not concomitantly use hydroxyurea. However, a recent reanalysis of this senicapoc study showed that the VOE rate in patients with a Hb response was not significantly different when compared with the placebo group.47 As for voxelotor, the annualized rate of VOEs was the lowest among patients who reached the highest Hb levels (ie, ≥12 g/dL). In the meantime, real-world data of voxelotor showed a significantly reduced annualized rate of VOEs in patients who had ≥1 VOE in the year before initiation of voxelotor.48 Together with our clinical end points, the absence of an increased incidence of VOEs suggests that increasing Hb level and thereby possibly blood viscosity does not necessarily negatively affect patients with SCD. For mitapivat, clinical efficacy may be related to the upstream mechanism of action (improvement of the ATP/2,3-DPG ratio) that results in reduced HbS polymerization, improvement of RBC sickling and RBC deformability (EImax and EImin) by supporting RBC membrane integrity, and improving cellular hydration and anti-sickling effects.16,49-54 This is also consistent with findings of mouse and ex vivo studies with another PK activator, etavopivat (FT-4202), which showed decreases in PoS and P50.55,56 Notably, no excessive Hb response or issues were reported in our study.
Even though the annualized VOE rate and markers associated with organ damage or mortality were reported, our study was not enriched or powered to evaluate this as efficacy end points. This is a limitation of our study. In addition, the number of SCD-related hospital admission days was obtained from the date of admission up to and including the date of discharge without correction for the exact time of admission or discharge. Furthermore, health-related quality of life assessments were, although important, not yet evaluated in this small, unblinded study without placebo controls. Due to technical issues of the Lorrca device, the PoS could not be evaluated at every visit. These missing data could well have led to the finding of the nonstatistically significant decrease in PoS in the intention-to-treat analysis; however, the decrease was statistically significant in the (strict) per protocol set analyses. In addition, efficacy end points differ among the intention-to-treat, per protocol set, and strict per protocol set analyses, which is partly due to less adherence to mitapivat in the 1-year fixed-dose extension period, especially when compared with the 8-week dose-finding period.26 For example, the ATP level significantly increased in the (strict) per protocol set, whereas this was not the case in the intention-to-treat analysis. It is known that across chronic diseases including SCD, various intentional and unintentional nonadherence factors compromise therapeutic benefits.57 Finally, although our study showed that all patients with HbSS, HbS/β0 thalassemia, and HbS/β+ thalassemia with or without concomitant hydroxyurea had decreases in markers of hemolysis, larger studies with a generally balanced distribution of SCD genotypes and concomitant hydroxyurea are needed to confirm this.
In conclusion, we established longer term proof of concept for PK activation as a potential therapeutic option in SCD. Treatment with mitapivat demonstrated durable beneficial mechanistic effects and a favorable safety profile at doses up to 100 mg twice daily for >1 year in SCD. Clinically meaningful improvements in hemolytic anemia, various RBC parameters, and annualized VOE rate were shown. Collectively, these results support further evaluation of safety and efficacy of mitapivat in the ongoing randomized placebo-controlled phase 2/3 RISE UP study (NCT05031780).
Acknowledgments
The authors sincerely thank the patients who participated in this study. The authors also thank all members of the Sickle Cell Outcome Research consortium, the Netherlands, for recruitment and helpful comments on the study design, conduct, protocol and manuscript. Data management was in part provided by Kanaka Soman of Julius Clinical, Zeist, the Netherlands. The visual abstract was created with BioRender.com.
This study was supported in part by research funding from Agios Pharmaceuticals Inc, Cambridge, MA.
Authorship
Contribution: R.v.W. and E.J.v.B. directed the research; M.J.v.D., M.A.E.R., B.A.v.O., R.v.W., and E.J.v.B. designed the research and developed methodology; J.J.M.J. supervised the ATP level and 2,3-DPG level measurements; M.J.v.D., C.D., A.W.R, M.H.C., E.N., B.J.B., M.B. and E.J.v.B. recruited the patients and provided patient data; M.J.v.D., B.A.v.O., J.B., C.D., and E.J.v.B. performed the research and acquired data; M.J.v.D., R.v.W., and E.J.v.B. analyzed and interpreted data; M.J.v.D. wrote the manuscript; W.W.v.S., R.E.G.S., R.v.W. and E.J.v.B. reviewed the manuscript; and all authors revised the manuscript critically and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.A.E.R. and R.v.W. receive research funding from Axcella Therapeutics and Pfizer. M.A.E.R., R.v.W., and E.J.v.B. receive research funding from and are consultants for Agios Pharmaceuticals Inc. R.v.W. and E.J.v.B. are consultants for Pfizer. M.H.C. (institution) has received investigator-initiated research and travel grants as well as speaker fees over the years from the Netherlands Organisation for Scientific Research and Netherlands National Research Agenda, the Netherlands Organization for Health Research and Development (ZonMw), the Dutch Innovatiefonds Zorgverzekeraars, Stichting Haemophilia, Baxter/Baxalta/Shire/Takeda, Pfizer, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, Roche, and Nordic Pharma,; and has served as a steering board member for Roche, Bayer, and Novartis. E.N. receives research funding from Novartis and Emmaus and participates in advisory board of Novartis. B.J.B. receives research funding from Sanquin, Pfizer, and Novartis; and has participated in the advisory boards of Novartis, Global Blood Therapeutics /Pfizer, Novo Nordisk, Celgene, Chiesi, CSL Behring, and bluebird bio. R.E.G.S. has received research funding and/or speaker fees from Bayer, CSL Behring, Hemab, NovoNordisk, Octapharma, Sanofi, and Sobi (all paid to institution). The remaining authors declare no competing financial interests.
Correspondence: Eduard J. van Beers, Center for Benign Hematology, Thrombosis and Hemostasis - Van Creveldkliniek, University Medical Center Utrecht, Utrecht University, Room C.01.412, PO Box 85500, Heidelberglaan 100, 3508 GA Utrecht, The Netherlands; email: e.j.vanbeers-3@umcutrecht.nl.
References
Author notes
∗R.v.W. and E.J.v.B. contributed equally to this study.
The full-text version of this article contains a data supplement.
Original data and protocol are available on request from the corresponding author, Eduard J. van Beers (e.j.vanbeers-3@umcutrecht.nl).
Data will be shared as allowed by General Data Protection Regulation and European Union privacy laws, depending on location of the new data controller/processor and applicable national law.