AMPK activation by GSK621 enhances preapoptotic surface exposure of CALR in AML.
AML cells primed by AMPK activation are potent dendritic cell activators and induce anticancer immunity in vivo.
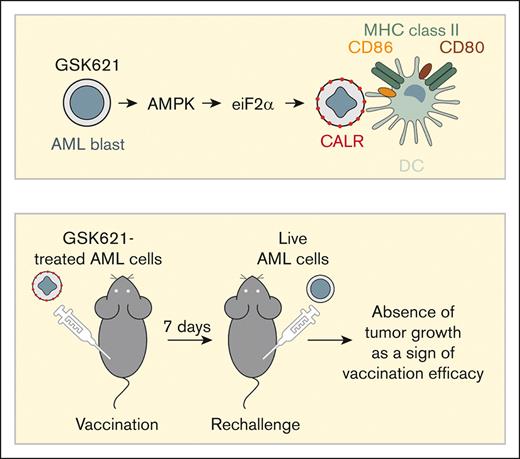
Visual Abstract
Survival of patients with acute myeloid leukemia (AML) can be improved by allogeneic hematopoietic stem cell transplantation (allo-HSCT) because of the antileukemic activity of T and natural killer cells from the donor. However, the use of allo-HSCT is limited by donor availability, recipient age, and potential severe side effects. Similarly, the efficacy of immunotherapies directing autologous T cells against tumor cells, including T-cell recruiting antibodies, chimeric antigen receptor T-cell therapy, and immune checkpoint inhibitors are limited in AML because of multiple mechanisms of leukemia immune escape. This has prompted a search for novel immunostimulatory approaches. Here, we show that activation of adenosine 5′-monophosphate–activated protein kinase (AMPK), a master regulator of cellular energy balance, by the small molecule GSK621 induces calreticulin (CALR) membrane exposure in murine and human AML cells. When CALR is exposed on the cell surface, it serves as a damage-associated molecular pattern that stimulates immune responses. We found that GSK621-treated murine leukemia cells promote the activation and maturation of bone marrow–derived dendritic cells. Moreover, vaccination with GSK621-treated leukemia cells had a protective effect in syngeneic immunocompetent recipients bearing transplanted AMLs. This effect was lost in recipients depleted of CD4/CD8 T cells. Together, these results demonstrate that AMPK activation by GSK621 elicits traits of immunogenic cell death and promotes a robust immune response against leukemia. Pharmacologic AMPK activation thus represents a new potential target for improving the activity of immunotherapy in AML.
Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy that arises from transformation of myeloid precursors. Although chemotherapy remains a cornerstone for the treatment of AML, new agents targeting molecular dependencies, such as fms-like tyrosine kinase 3 or B-cell lymphoma 2 (BCL2) inhibitors, are now available.1 However, most patients still succumb to relapsed/refractory disease, demonstrating a need for new therapeutic strategies.
Immunotherapy in the form of allogeneic hematopoietic stem cell transplantation (allo-HSCT) is widely used to consolidate remission induced by cytotoxic agents in patients with AML. Nonetheless, major side effects including infections and graft-versus-host disease considerably limit the use of allo-HSCT, especially in the elderly.2,3 As the antileukemic effects of allo-HSCT are mainly mediated by cellular immunity, new T-cell–activating immunotherapies are actively being investigated in AML, such as T-cell engaging antibodies, chimeric antigen receptors, and immune checkpoint inhibitors.4 However, antileukemic activity of these immunotherapies in AML appears to be modest, at least in studies to date.5
Among various modalities of cell death, immunogenic cell death (ICD) is relevant to cancer therapy because it promotes an antitumor immune response through tumor-specific antigenicity and adjuvanticity. This response is mediated by the release of damage-associated molecular patterns (DAMPs) that can activate dendritic cells (DCs) thus shaping the tumor immune microenvironment.6,7 Cellular changes in response to environmental or internal signals are critical in the adjuvanticity of cells undergoing ICD, and the integrated stress response (ISR) pathway recently emerged as a hallmark of ICD.8
ISR pathways converge on phosphorylation of eukaryotic translation initiation factor 2 subunit alpha (eIF2α), which is also a characteristic feature of ICD.9 Protein kinase RNA-like endoplasmic reticulum (ER) kinase (PERK) is an eIF2α kinase that participates in ER stress response, which activates the transcription factor activating transcription factor 4 (ATF4) involved in cell death and autophagy.10 eIF2α phosphorylation is required for exposure of the ER chaperone calreticulin (CALR) at the cell surface, which serves as an activating “eat-me” signal for DCs.11
Using the selective adenosine 5′-monophosphate–activated protein kinase (AMPK) activator GSK621, we recently showed that AMPK elicits a stress response through PERK and ATF4 activation, leading to enhanced mitochondrial susceptibility of AML to the BCL2 inhibitor venetoclax.12 Because AMPK activation induces eIF2α phosphorylation through PERK activation, we hypothesized that AMPK activation might also trigger ICD in AML.
Here, we tested this hypothesis and found that GSK621 induced multiple features of ICD in AML. These effects were AMPK dependent, involved the activation of PERK, and were seen in human and mouse AML cells, including in immunocompetent syngeneic in vivo models of vaccination and tumor challenge. Together, our results show a new function of AMPK in regulating stress-induced ICD, suggesting the potential of AMPK activation to favor antileukemic immune activity.
Methods
Patient samples
Patient AML cells were from fresh or viably cryopreserved bone marrow aspirates collected at the time of diagnosis. All patients consented to an institutional review board–approved banking protocol. Clinical details are in supplemental Table 1.
Cell lines
Murine C1498 cells were purchased from the American Type Culture Collection (ATCC TIB-49) in 2018. Human AML cell lines were obtained from the ATCC between 2009 and 2015. Short tandem repeat profiling was performed periodically and mycoplasma negativity was verified yearly. Cells were cultured at 37°C in 5% CO2 with minimum essential medium α (Gibco, 12561-072), supplemented with 10% fetal bovine serum (Sigma Aldrich, F2442), 1 mM L-alanyl-L-glutamine (Corning, 25-015-CI), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Generation of CRISPR knockout (KO) cells
Murine cells
To generate controls for surface CALR flow cytometry, C1498 cell lines were retrovirally transduced with a pRetro_CALR-DAF_DsRed,13 or Calr was deleted by CRISPR/CRISPR-associated protein 9 (Cas9). Cells were transfected with lentivirus containing single guide RNAs (sgRNAs) targeting Calr, or a nontargeting guide. For murine AML cells on a transgenic Cas9 background (see further sections), sgRNAs were cloned in the lentiviral vector pLKO5.sgRNA.EFS.tRFP (Addgene no. 57823), cells were infected, and red fluorescent protein–positive cells were sorted. sgRNA sequences: Calr (sgRNA no. 1: TATGTTTGGATTCGACCCAG; sgRNA no. 2: ATAGATGGCAGGGTCTGCGG; sgRNA no. 3: CGTAAAATTTGCCAGAACTG); Prkaa1 (sgRNA no. 1: CCTGTGACAATAATCCACAC; sgRNA no. 2: TGAAGGGTTTAAGTACTGAG; sgRNA no. 3: GAAGATTCGGAGCCTTGACG); Rosa26 (sgRNA no. 1: TGCAAGTTGAGTCCATCCGC; and sgRNA no. 2: GGGTAAAACGACTCCCCCAG); and nontargeting control (GGAGCGCACCATCTTCTTCA).
Human cells
Lentiviral and retroviral infection
Virus was produced following standard methods in 293 T cells using Lipofectamine 2000 (Thermo Fisher no. 11668019) and either psPAX2 (second-generation lentiviral packaging plasmid; Addgene no. 12260) and pMD2.G (vesicular stomatitis virus glycoprotein envelope–expressing plasmid; Addgene no. 12259) for lentivirus, or pECO-PAC2 for retrovirus.
MLL-AF9 mouse primary cell line generation
All animal experiments were performed with approval from the Dana-Farber Cancer Institute Institutional Animal Care and Use Committee. Murine ckit+ cells were sorted from whole bone marrow of Rosa26-Cas9 knockin C57BL/6J mice, transduced with mixed lineage leukemia-ALL1-fused gene from chromosome 9 (MLL-AF9) retrovirus and reinjected to sublethally irradiated C57BL/6J mice to generate a murine model of AML.17 Mice were monitored by peripheral blood sampling and euthanized when overt AML was diagnosed (>20% circulating green fluorescent protein–positive blasts or poor body condition). Briefly, single-cell suspensions from femurs and tibias of a leukemic mouse were harvested by spin isolation18 and seeded in methylcellulose media (Methocult, Stem Cell Technologies, M3234), supplemented with interleukin-6 (IL-6; 10 ng/mL), stem cell factor SCF (10 ng/mL), and IL-3 (6 ng/mL) (GoldBio). After serial replating, at a point when >90% of the colonies were granulocyte colony-forming units, cells were switched to liquid culture in media containing IL-3 (10 ng/mL, then 1 ng/mL). Cells were replated repeatedly, and the percentage green fluorescent protein–positive cells were regularly monitored until the AML cells were able to grow in cytokine-free media (Figure 1D). Dead cells were periodically removed by magnetic separation following the manufacturer’s instructions (Dead Cell Removal kit, Miltenyi Biotec, 130-090-101).
GSK621 induces preapoptotic surface CALR exposure in murine and human cells. (A) C1498 cells were treated at the indicated concentrations with GSK621 for 48 hours, and the percentage apoptotic cells was measured by flow cytometry using annexin V/propidium iodide (PI) staining (early apoptotic cells: annexin V–positive/PI−; late apoptotic: annexin V–positive/PI+). Ordinary two-way analysis of variance (ANOVA) with Dunnett multiple comparisons test. (B) Flow cytometry of C1498 cells used as controls for surface CALR staining. CALR KO or constitutive cell surface CALR were stained with a CALR–AF647 antibody or isotype control. Graphs represent mean fluorescence intensity (MFI) ± standard deviation (SD). (C) Fold change in MFI relative to vehicle of surface CALR staining on live, unpermeabilized cells by flow cytometry (MFI ± standard error of the mean) after 6 hours of treatment with the indicated drugs (GSK621, MTO, or AraC); n = 3 biological replicates. Ordinary two-way ANOVA with Dunnett multiple comparisons test. (D) Schematic of the procedure to generate MLL-AF9 transformed AML cells from leukemia-bearing mice. After multiple replatings in methylcellulose supplemented with IL-6 (10 ng/mL), stem cell factor (SCF, 10 ng/mL), and IL-3 (6 ng/mL), colonies were enriched in granulocyte colony-forming units (CFU-G; picture). Colonies were then transferred in liquid media and grown in suspension. (E) MLL-AF9 cells were treated at various concentrations with GSK621 for 48 hours and percentages of apoptotic cells were measured as described earlier. Ordinary two-way ANOVA with Šidák multiple comparisons test. (F) Representative dot plots of surface CALR staining in unpermeabilized MLL-AF9 cells treated for 24 hours with GSK621 30 μM or dimethyl sulfoxide (DMSO). (G) MFI of surface CALR staining measured on live, unpermeabilized MLL-AF9 cells; n = 3 replicates. Paired t test. (H) Percent of CALR+ PI− blasts of 3 samples from patients with AML after treatment with GSK621 (20 μM), MTO (0.5 nM), or DMSO. Human AML samples 1 and 2 were cryopreserved, thawed, and treated with GSK621. Sample 3 was received fresh and treated immediately. Ordinary one-way ANOVA with Dunnett multiple comparisons test. (I) Representative dot plots for sample 3.
GSK621 induces preapoptotic surface CALR exposure in murine and human cells. (A) C1498 cells were treated at the indicated concentrations with GSK621 for 48 hours, and the percentage apoptotic cells was measured by flow cytometry using annexin V/propidium iodide (PI) staining (early apoptotic cells: annexin V–positive/PI−; late apoptotic: annexin V–positive/PI+). Ordinary two-way analysis of variance (ANOVA) with Dunnett multiple comparisons test. (B) Flow cytometry of C1498 cells used as controls for surface CALR staining. CALR KO or constitutive cell surface CALR were stained with a CALR–AF647 antibody or isotype control. Graphs represent mean fluorescence intensity (MFI) ± standard deviation (SD). (C) Fold change in MFI relative to vehicle of surface CALR staining on live, unpermeabilized cells by flow cytometry (MFI ± standard error of the mean) after 6 hours of treatment with the indicated drugs (GSK621, MTO, or AraC); n = 3 biological replicates. Ordinary two-way ANOVA with Dunnett multiple comparisons test. (D) Schematic of the procedure to generate MLL-AF9 transformed AML cells from leukemia-bearing mice. After multiple replatings in methylcellulose supplemented with IL-6 (10 ng/mL), stem cell factor (SCF, 10 ng/mL), and IL-3 (6 ng/mL), colonies were enriched in granulocyte colony-forming units (CFU-G; picture). Colonies were then transferred in liquid media and grown in suspension. (E) MLL-AF9 cells were treated at various concentrations with GSK621 for 48 hours and percentages of apoptotic cells were measured as described earlier. Ordinary two-way ANOVA with Šidák multiple comparisons test. (F) Representative dot plots of surface CALR staining in unpermeabilized MLL-AF9 cells treated for 24 hours with GSK621 30 μM or dimethyl sulfoxide (DMSO). (G) MFI of surface CALR staining measured on live, unpermeabilized MLL-AF9 cells; n = 3 replicates. Paired t test. (H) Percent of CALR+ PI− blasts of 3 samples from patients with AML after treatment with GSK621 (20 μM), MTO (0.5 nM), or DMSO. Human AML samples 1 and 2 were cryopreserved, thawed, and treated with GSK621. Sample 3 was received fresh and treated immediately. Ordinary one-way ANOVA with Dunnett multiple comparisons test. (I) Representative dot plots for sample 3.
Murine bone marrow–derived dendritic cells (BMDCs)
BMDCs were generated from bone marrow isolated from 8- to 10-week-old C57BL/6J mice.19 Cells were isolated from leg bones by centrifugation,18 then cultured in the presence of recombinant murine granulocyte-macrophage colony stimulating factor (20 ng/mL) for 7 days. Fresh culture medium supplemented with recombinant murine granulocyte-macrophage colony stimulating factor was added on day 3. On day 7, the percent CD11c+ cells (BMDCs) was determined by flow cytometry and used for further experiments.
Coculture experiments
On day 6 of BMDC differentiation, murine AML cells (C1498 or MLL-AF9 transformed cells) were treated with drugs or vehicle in a separate flask. On day 7 of differentiation, BMDCs and tumor cells were mixed at a ratio ranging from 1:2 to 1:20. After overnight coculture, BMDC activation was measured by flow cytometry using CD11c (marker of DCs), with CD80, CD86, and major histocompatibility complex II (MHCII; activation markers). As a positive control to induce activation markers without AML coculture, BMDCs were incubated with 0.1 μg/mL lipopolysaccharide (LPS) overnight.
Vaccination assay
Female C57BL/6J mice, 6- to 8-week old were obtained from The Jackson Laboratory. AML cells were treated in vitro with GSK621 30 μM or vehicle for 24 hours. After 24 hours, the cells were fixed in formalin 1% (in phosphate-buffered saline [PBS]) and then washed 5 times in PBS, as previously described.20 After the last wash, cells were resuspended in PBS at 20 × 106 cells per mL. Mice received a first subcutaneous injection of 100 μL (2 × 106 fixed cells) in the left flank, followed by an identical boost 1 week later in the same flank. One week after the vaccination boost, the animals were challenged with 850 000 live cells resuspended in PBS-Matrigel (50:50), in the right flank. The group designated as unvaccinated received 100 μL of PBS only for the 2 vaccination steps. All subcutaneous injections were done under isoflurane anesthesia. For T-cell depletion, animals received intraperitoneal injections of 200 μg each of mouse anti-CD4 and anti-CD8 antibodies (InVivoMAb anti-mouse CD4, Clone: GK1.5, BioXcell no. BE0003-1; InVivoMAb anti-mouse CD8α, Clone 2.43, BioXcell no. BE0061), or immunoglobulin G (IgG) control antibody (InVivoMAb rat IgG2b isotype control, BioXcell no. BE0090). Injections of antibodies started 2 days before challenge and were continued until the end of the experiment. Tumor growth was monitored with digital calipers every 2 to 3 days. Tumor volumes were approximated as follows: volume = (width2 × length) / 2, in which length is the longer tumor diameter, and width is the perpendicular tumor diameter. All experiments followed Institutional Animal Care and Use Committee–approved protocols. The study end points were defined per animal facility policies and required euthanasia for any mouse bearing a subcutaneous tumor reaching 2 cm in any dimension, an ulcerated tumor, or overall poor body condition or distress.
Western blotting
Cells were lysed in Laemmli buffer supplemented with dithiothreitol (50 mM) and sodium orthovanadate (2 mM). Blots were imaged using an Image Quant LAS-4000. Antibodies used were actin (MilliporeSigma, no. A5441); AMPKα (Cell Signaling Technology, no. 2532S); CALR (Cell Signaling Technology, no. 12238S); Cas9 (Cell Signaling Technology, no. 14697S); eIF2α (Cell Signaling Technology, no. 9722S); phospho-AMPKα (Thr172); Cell Signaling Technology no. 2535S); and phospho-eIF2α (Ser51; Cell Signaling Technology, no. 3398S).
Flow cytometry
Antibody staining
Cells were washed in PBS (PBS 1×, pH 7.4; Gibco, 10010049) supplemented with 1% bovine serum albumin (Fisher BioReagents, BP1600100) and incubated with Fc block, following the manufacturer’s protocols (mouse: anti-mouse CD16-CD32, ThermoFisher Scientific, no. BDB553141; and Human BD Fc Block, no. 564219). Cells were stained with indicated antibodies for 30 minutes at 4°C in the dark.
Apoptosis
Cells were washed in binding buffer 1× (annexin V–binding buffer, Thermo Fisher Scientific, BDB556454) and incubated for 15 minutes at room temperature in the dark with fluorochrome-conjugated annexin V and live/dead cell dye. Cells were analyzed using a CytoFLEX S (Beckman Coulter) or a BD LSRFortessa X-20. At least 10 000 events in the population of interest were recorded. Data were analyzed using FlowJo version 10.8 (BD Life Sciences). Reagents used were propidium iodide staining solution (ThermoFisher Scientific, no. BDB556463); 4′,6-diamidino-2-phenylindole (Cell Signaling Technology, no. 4083S); Zombie Green (BioLegend, no. 423111); Zombie Aqua (BioLegend, no. 423101); anti-CALR antibody, ER marker (Abcam, no. ab 2907); goat anti-rabbit IgG (H+L), secondary antibody Alexa Fluor 488 (Invitrogen, no. A-11034); Alexa Fluor 647 anti-CALR antibody (EPR3924), ER Marker (Abcam, no. ab196159); allophycocyanin anti-human CD45 (BioLegend, no. 368512); annexin V–Pacific Blue (BioLegend, no. 640918); allophycocyanin anti-mouse CD11c (BioLegend, no. 117310); Brilliant Violet 510 anti-mouse CD86 (BioLegend, no. 105039); PE anti-mouse I-A/I-E (MHCII) (BioLegend, no. 107607); and PerCP/cyanine5.5 anti-mouse CD80 (BioLegend, no. 104721).
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 9, GraphPad Software, La Jolla, CA. Statistical tests used for each data presentation are specified in the legends.
Results
GSK621 induces preapoptotic CALR membrane exposure in murine and human AML cells
We recently showed that AMPK activation by GSK621 induced a stress response through PERK activation and eIF2α phosphorylation.12 Given that eIF2α phosphorylation is a hallmark of ICD, we hypothesized that GSK621 might elicit CALR membrane exposure and enhanced adjuvanticity in AML. First, we tested the effects of GSK621 on the C1498 murine AML cell line,21 derived from a spontaneous leukemia in a C57BL/6 mouse (supplemental Figure 1A). GSK621 induced dose-dependent apoptosis after 48 hours of exposure, similar to the effects seen in human AML cells14 (Figure 1A). Next, to establish controls for the specificity of surface CALR quantification by flow cytometry, we generated positive control C1498 cells with constitutive CALR membrane expression, or negative control cells with Calr KO using CRISPR/Cas9 (supplemental Figure 1B). We observed that CALR staining was similar to the isotype control in Calr KO cells, whereas signal intensity was markedly increased in cells with enforced CALR surface expression (Figures 1B; supplemental Figure 1C). Next, we treated parental C1498 cells with GSK621 and observed that CALR membrane expression increased within 6 hours, in a dose-dependent manner (Figures 1C; supplemental Figure 1D), supporting the hypothesis that AMPK activation induces ICD. CALR expression was also induced by mitoxantrone (MTO), an anthracycline known to induce ICD.22 In contrast, the antileukemic drug cytarabine (AraC) did not induce CALR membrane expression to the same level (Figure 1C), despite inducing a comparable degree of cell death (supplemental Figure 1E).
To test this hypothesis in a distinct and genetically defined AML model, we transduced bone marrow cells from C57BL/6 mice to express the human MLL-AF9 (also known as KMT2A::MLLT3) fusion oncogene. Subsequent transplantation into syngeneic recipient mice led to leukemia development, as performed previously.23 We cultured the resulting mouse AML cells ex vivo in methylcellulose and performed serial replating to generate a murine AML cell line (designated, MLL-AF9 cells; Figure 1D). In these AML cells, GSK621 treatment for 48 hours induced dose-dependent apoptosis (Figure 1E), which was preceded by CALR surface exposure in viable cells at 24 hours of drug exposure (Figure 1F-G).
Finally, we exposed bone marrow samples from 3 patients with AML (supplemental Table 1) to GSK621. We observed increased CALR membrane expression in live bone marrow blasts at levels comparable with those induced by MTO (Figure 1H-I). Interestingly, induction of apoptosis and CALR exposure were detected on leukemia blasts but not on granulocytes and lymphocytes from the same bone marrow sample, which supports a potential therapeutic window (supplemental Figure 1F). Collectively, these results show that GSK621 induces an immunostimulatory DAMP in the form of CALR membrane exposure in murine and human leukemia cells.
CALR surface exposure is dependent on AMPK
To determine whether GSK621-induced CALR membrane exposure was AMPK dependent, we used MOLM-14 and OCI-AML2 human AML cell lines depleted of the AMPKα1 subunit by CRISPR/Cas9 editing (AMPK KO), which fully abrogates AMPK activity14 (Figure 2A). We observed that incubation with GSK621 had no impact on whole cell CALR protein abundance in AMPK KO or in control (nontargeting sgRNA; NTG) cells (supplemental Figure 2A). However, CALR cell surface expression was strongly induced by GSK621 in control but not in AMPK KO cells, arguing that GSK621-induced CALR membrane upregulation was on target (Figure 2B-C). GSK621 did not possess intrinsic fluorescence or promote isotype control staining, further supporting the specificity of cell surface CALR detection (supplemental Figure 2C). In contrast, CALR membrane exposure was strongly increased by idarubicin, an anti-AML chemotherapy known to induce ICD,22 in cells with or without AMPK (Figure 2D). Other anti-AML chemotherapies, such as cytarabine and venetoclax, had less impact on CALR membrane exposure (Figure 2D). Notably, all these agents caused comparable cell death in MOLM-14 cells at the same concentrations, supporting that specific cytotoxic drugs induce CALR exposure (supplemental Figure 2B).
Calreticulin surface exposure is AMPK dependent in AML cells. (A) Western blot showing knockdown efficiency of AMPKα in MOLM14 and OCI-AML2 cells transduced with a nontargeting control sgRNA guide (NTG) or PRKAA1 sgRNA. (B) Representative dot plots of MOLM14 and OCI-AML2 surface CALR. Percentages represent the fraction of CALR+ among 4′,6-diamidino-2-phenylindole (DAPI)-negative cells. (C) Control (NTG) or AMPK KO MOLM14 and OCI-AML2 cells were treated with GSK621 (30 μM) or vehicle for 24 hours, and surface exposure of CALR was measured by flow cytometry. Results are MFI of CALR on live (DAPI negative) cells. Baseline fluorescence intensity of the isotype control stained or unstained DMSO treated cells are shown on the same graphs. Ordinary one-way ANOVA with Šidák multiple comparisons test. (D) MOLM14 AMPK KO or control cells (MOLM14 NTG) were treated for 24 hours with DMSO, GSK621 (30 μM), idarubicin (IDA; 25 nM), venetoclax (VEN; 500 nM), or AraC (2 μM), and surface CALR was measured by flow cytometry. The MFI of 3 independent replicates is shown. Ordinary two-way ANOVA with Dunnett multiple comparisons test for comparison to DMSO within each cell line group (NTG and AMPK KO), and ordinary two-way ANOVA with Tukey multiple comparisons test to compare GSK621 treatment in the NTG vs AMPK KO cell line. (E) Western blot showing the expression of a V5-tagged truncated form of AMPKα1 lacking its regulatory region (AMPK 1-312; selected in blasticidin; predicted size: 37.2 kDa). (F) MFI of surface CALR on AMPK KO MOLM14 cells with or without AMPK (1-312) rescue after 24 hours treatment with DMSO, GSK621, VEN, or AraC. Ordinary two-way ANOVA with Šidák multiple comparisons test. (G) MOLM14 cells lacking endogenous PERK expression were generated by CRISPR/Cas9 editing of EIF2AK3 at an intron-exon junction, compared with NTG cells. Cells were rescued with doxycycline-inducible expression of wild-type (WT) PERK bearing a Myc 9E10 tag. Western blot showing the indicated proteins in PERK knockdown and rescue cells, with and without treatment with GSK621 (30 μM). (H) Surface exposure of CALR was measured by flow cytometry before and after induction of PERK rescue, with or without GSK621 treatment. Ordinary two-way ANOVA with Šidák multiple comparisons test.
Calreticulin surface exposure is AMPK dependent in AML cells. (A) Western blot showing knockdown efficiency of AMPKα in MOLM14 and OCI-AML2 cells transduced with a nontargeting control sgRNA guide (NTG) or PRKAA1 sgRNA. (B) Representative dot plots of MOLM14 and OCI-AML2 surface CALR. Percentages represent the fraction of CALR+ among 4′,6-diamidino-2-phenylindole (DAPI)-negative cells. (C) Control (NTG) or AMPK KO MOLM14 and OCI-AML2 cells were treated with GSK621 (30 μM) or vehicle for 24 hours, and surface exposure of CALR was measured by flow cytometry. Results are MFI of CALR on live (DAPI negative) cells. Baseline fluorescence intensity of the isotype control stained or unstained DMSO treated cells are shown on the same graphs. Ordinary one-way ANOVA with Šidák multiple comparisons test. (D) MOLM14 AMPK KO or control cells (MOLM14 NTG) were treated for 24 hours with DMSO, GSK621 (30 μM), idarubicin (IDA; 25 nM), venetoclax (VEN; 500 nM), or AraC (2 μM), and surface CALR was measured by flow cytometry. The MFI of 3 independent replicates is shown. Ordinary two-way ANOVA with Dunnett multiple comparisons test for comparison to DMSO within each cell line group (NTG and AMPK KO), and ordinary two-way ANOVA with Tukey multiple comparisons test to compare GSK621 treatment in the NTG vs AMPK KO cell line. (E) Western blot showing the expression of a V5-tagged truncated form of AMPKα1 lacking its regulatory region (AMPK 1-312; selected in blasticidin; predicted size: 37.2 kDa). (F) MFI of surface CALR on AMPK KO MOLM14 cells with or without AMPK (1-312) rescue after 24 hours treatment with DMSO, GSK621, VEN, or AraC. Ordinary two-way ANOVA with Šidák multiple comparisons test. (G) MOLM14 cells lacking endogenous PERK expression were generated by CRISPR/Cas9 editing of EIF2AK3 at an intron-exon junction, compared with NTG cells. Cells were rescued with doxycycline-inducible expression of wild-type (WT) PERK bearing a Myc 9E10 tag. Western blot showing the indicated proteins in PERK knockdown and rescue cells, with and without treatment with GSK621 (30 μM). (H) Surface exposure of CALR was measured by flow cytometry before and after induction of PERK rescue, with or without GSK621 treatment. Ordinary two-way ANOVA with Šidák multiple comparisons test.
To confirm target specificity, using AMPK KO cells, we re-expressed an active truncated form of AMPK (AMPK 1-312, missing an inhibitory domain16) that is not targeted by the AMPK sgRNA (Figure 2E-F; supplemental Figure 2D). Expression of AMPK 1-312 allowed membrane CALR induction in AMPK KO cells treated with GSK621 (Figure 2F). This was in contrast to treatment with venetoclax or cytarabine, which did not induce surface CALR regardless of AMPK expression status (Figure 2F). These data support a model in which CALR exposure can be induced in AML by specific agents and via AMPK-dependent or AMPK-independent pathways.
We recently showed that AMPK activation by GSK621 induces an ER stress response dependent on PERK activation.12 Because the ISR is known to induce CALR membrane exposure and ICD,9,24 we investigated the role of PERK in AMPK-induced CALR membrane expression. We used PERK KO MOLM-14 cells in which we could conditionally re-express PERK by a doxycycline-inducible construct.12 We observed that PERK expression was important for eIF2α phosphorylation induced by GSK621 (Figure 2G). Moreover, CALR membrane expression was enhanced by GSK621 treatment in PERK-expressing MOLM-14 cells but was unaffected by PERK expression in the absence of GSK621 (Figure 2H). Collectively, these results show that AMPK activation by GSK621 induced ICD markers, including eIF2α phosphorylation and CALR membrane exposure, which were at least partially dependent on PERK.
AML cells pretreated with GSK621 induce bone marrow DC maturation
Maturation of antigen-presenting cells is a surrogate readout of tumor cell immunogenicity in coculture experiments.25 We examined the effect of GSK621-treated murine AML cells on syngeneic BMDCs in coculture assays ex vivo. We used LPS as a positive control to induce direct BMDC activation and maturation.26 First, we incubated C1498 cells with vehicle (dimethyl sulfoxide), GSK621, MTO, or AraC. These cells were then cocultured with syngeneic BMDCs, and we measured DC activation by the co-expression of CD80/MHCII or CD86/MHCII among CD11c-positive BMDCs, using 2 ratios of DCs to tumor cells to mimic the heterogeneity of bone marrow infiltration in patients with AML (Figure 3A; supplemental Figure 3A). We observed that C1498 cells previously exposed to GSK621 or MTO significantly induced CD80 and CD86 expression on BMDCs to a level similar to that of LPS-treated BMDCs, whereas AraC-treated cells induced more modest BMDC maturation (Figure 3B-C). As an additional control, we used 3 cycles of freeze/thaw to induce nonspecific death in AML cells. We observed that a 1:1 or 1:10 ratio of DCs to freeze/thaw–killed tumor cells was insufficient to trigger BMDC activation (supplemental Figure 3B-C). We repeated this assay using GSK621-treated MLL-AF9 AML cells and similarly observed that coculture with BMDCs significantly induced CD80+ and CD86+ expression (Figure 3D). Together, these results show that AMPK activation in AML cells enables them to induce DC maturation.
AML cells pretreated with GSK621 induce murine BMDC maturation in vitro. (A) Experimental schema for coculture experiments. BMDCs were generated over 7 days from fresh bone marrow cells harvested from female C57BL/6 mice. BMDC maturation was measured by flow cytometry after overnight coculture with tumor cells pretreated for 24 hours before mixing. (B) Representative dot plots of the percent activated DCs after overnight coculture with cells pretreated with DMSO, AraC (10 μM), GSK621 (30 μM), or MTO (100 nM), with a DC–to–tumor cell ratio of 1:2. Controls are BMDCs treated overnight with LPS (0.1 μg/mL) or vehicle (PBS). (C) Graphs representing the level of maturation of BMDCs as measured by percentage of MHCII+CD80+ or MHCII+CD86+ cells after overnight coculture with C1498 cells pretreated as indicated, with 2 ratios of BMDC to tumor cells; n = 3 replicates (mean ± SD). BMDCs alone were treated with LPS as a positive control to induce activation markers. Ordinary one-way ANOVA with Dunnett multiple comparisons test for multiple comparison to DMSO within each ratio of cocultures, and a t test to compare BMDC cultured without tumor cells, with or without LPS. (D) Coculture experiments using murine AML cells driven by MLL-AF9. Cells were pretreated for 24 hours with GSK621 or vehicle, then mixed overnight with BMDCs in a 1:8 ratio (1 BMDC for 8 AML cells). Percent of live coculture-matured DCs as defined by DAPI-negative, CD11+ cells expressing MHCII and CD80, or MHCII and CD86, measured by flow cytometry. Ordinary one-way ANOVA with Šidák multiple comparisons test.
AML cells pretreated with GSK621 induce murine BMDC maturation in vitro. (A) Experimental schema for coculture experiments. BMDCs were generated over 7 days from fresh bone marrow cells harvested from female C57BL/6 mice. BMDC maturation was measured by flow cytometry after overnight coculture with tumor cells pretreated for 24 hours before mixing. (B) Representative dot plots of the percent activated DCs after overnight coculture with cells pretreated with DMSO, AraC (10 μM), GSK621 (30 μM), or MTO (100 nM), with a DC–to–tumor cell ratio of 1:2. Controls are BMDCs treated overnight with LPS (0.1 μg/mL) or vehicle (PBS). (C) Graphs representing the level of maturation of BMDCs as measured by percentage of MHCII+CD80+ or MHCII+CD86+ cells after overnight coculture with C1498 cells pretreated as indicated, with 2 ratios of BMDC to tumor cells; n = 3 replicates (mean ± SD). BMDCs alone were treated with LPS as a positive control to induce activation markers. Ordinary one-way ANOVA with Dunnett multiple comparisons test for multiple comparison to DMSO within each ratio of cocultures, and a t test to compare BMDC cultured without tumor cells, with or without LPS. (D) Coculture experiments using murine AML cells driven by MLL-AF9. Cells were pretreated for 24 hours with GSK621 or vehicle, then mixed overnight with BMDCs in a 1:8 ratio (1 BMDC for 8 AML cells). Percent of live coculture-matured DCs as defined by DAPI-negative, CD11+ cells expressing MHCII and CD80, or MHCII and CD86, measured by flow cytometry. Ordinary one-way ANOVA with Šidák multiple comparisons test.
GSK621-treated AMLs induce a vaccination effect in vivo in immunocompetent mice
To establish that AMPK activation results in clinically relevant ICD, we investigated the potential of GSK621-treated cells to facilitate an antitumor immune response in vivo using vaccination assays.25 In these experiments, syngeneic murine AML cells were incubated in vitro with GSK621, and then injected subcutaneously into the left flank of immunocompetent C57BL/6 mice (vaccine site). After 7 days, we injected live AML cells into the right flank of vaccinated mice (challenge site), and followed tumor growth on the challenge site (Figure 4A). In the first experiment using C1498 AML cells, we observed that mice vaccinated with GSK621- or AraC-treated cells had significantly reduced tumor growth and improved tumor-free survival compared with unvaccinated mice (supplemental Figure 4A-B). However, there was no statistical difference between the GSK621 and AraC groups, possibly because of the rapid growth of C1498 cells in all assay arms using this model. Indeed, all animals vaccinated with dimethyl sulfoxide-treated cells had to be censored because they developed tumors at the vaccination site before the challenge site could be assessed. Nonetheless, using an enzyme-linked immunospot assay we confirmed that splenocytes from mice vaccinated with GSK621-treated AML produced interferon gamma when cultured specifically in the presence of parental leukemia cells (Figure 4B), which suggested the induction of tumor-specific cellular immunity.
AML cells pretreated with GSK621 induce a vaccination effect in vivo. (A) Schema of the vaccination assay performed with C1498 cells or MLL-AF9 cells in immunocompetent C57BL/6 mice. (B) Representative interferon gamma enzyme-linked immunospot (ELISpot) assay using single-cell suspensions from the spleens of 1 animal of each arm of the experiment activated by culture with the parental AML cells. Splenocytes were cocultured with a syngeneic C57BL/6 melanoma cell line (B16F10) or the ovalbumin antigen alone (OVA) as negative controls, or anti-CD3 as a nonspecific positive control. Each column represents ELISpots from splenocytes of 1 spleen seeded in triplicates. (C) Kaplan-Meier curves showing the overall survival in each group (5 mice per group) vaccinated with MLL-AF9 cells pretreated as indicated and fixed before injection, then challenged with live MLL-AF9 cells. Log-rank test. (D) Tumor growth on the challenge site of individual animals in panel C. Of note, none of the mice vaccinated with GSK621-treated cells developed tumors (red line on x-axis). (E) Kaplan-Meier curves showing the overall survival in each group (5 mice per group) vaccinated with MLL-AF9 cells pretreated with GSK621 before injection, then challenged with live MLL-AF9 cells. One group was depleted of CD4/CD8 T cells with intraperitoneal injections of neutralizing antibodies starting 2 days before the challenge (dashed line). The mice of the other group received a control IgG (solid line). Log-rank test. (F) Tumor growth on the challenge site of individual animals in panel E.
AML cells pretreated with GSK621 induce a vaccination effect in vivo. (A) Schema of the vaccination assay performed with C1498 cells or MLL-AF9 cells in immunocompetent C57BL/6 mice. (B) Representative interferon gamma enzyme-linked immunospot (ELISpot) assay using single-cell suspensions from the spleens of 1 animal of each arm of the experiment activated by culture with the parental AML cells. Splenocytes were cocultured with a syngeneic C57BL/6 melanoma cell line (B16F10) or the ovalbumin antigen alone (OVA) as negative controls, or anti-CD3 as a nonspecific positive control. Each column represents ELISpots from splenocytes of 1 spleen seeded in triplicates. (C) Kaplan-Meier curves showing the overall survival in each group (5 mice per group) vaccinated with MLL-AF9 cells pretreated as indicated and fixed before injection, then challenged with live MLL-AF9 cells. Log-rank test. (D) Tumor growth on the challenge site of individual animals in panel C. Of note, none of the mice vaccinated with GSK621-treated cells developed tumors (red line on x-axis). (E) Kaplan-Meier curves showing the overall survival in each group (5 mice per group) vaccinated with MLL-AF9 cells pretreated with GSK621 before injection, then challenged with live MLL-AF9 cells. One group was depleted of CD4/CD8 T cells with intraperitoneal injections of neutralizing antibodies starting 2 days before the challenge (dashed line). The mice of the other group received a control IgG (solid line). Log-rank test. (F) Tumor growth on the challenge site of individual animals in panel E.
To overcome the problem of aggressive growth of the untreated vaccine cells, we performed similar experiments using MLL-AF9–driven AMLs and modified our protocol by fixing treated cells before vaccination using 1% formalin, which is known to preserve the expression of cell surface molecules for tumor immunization.20 We observed that the difference in CALR staining between GSK621 and vehicle-treated cells after fixation (supplemental Figure 4C) was similar to that seen in unfixed cells. Using this model, we observed an absence of tumor growth at the challenge site and 100% survival in the cohort of mice vaccinated with GSK621-treated AML cells, which was statistically improved compared with the other groups (Figure 4C-D). To explore the role of cellular immunity in the vaccination effect, we performed the same experiment in recipient mice treated with antibodies to deplete T cells before challenge. We reproduced the vaccination effect induced by GSK621-treated cells in immunocompetent mice receiving control IgG antibodies. In contrast, all CD4/CD8-depleted mice developed tumors that led to 100% mortality of this cohort, showing that the protective antitumor immune response induced by AMPK-activated leukemic cells was dependent on T cells in the vaccine recipients (Figure 4E-F). Collectively, these results indicate that AMPK activation by GSK621 elicits ICD in AML models.
Discussion
AML has been treated by allo-HSCT for decades, a modality of immunotherapy in which graft-versus-leukemia activity is achieved by T cells and natural killer cells from the donor.27 However, the antileukemic activity of allo-HSCT is, for the most part, inseparable from graft-versus-host disease, which causes considerable morbidity and mortality.28 Therefore, improving cellular immunity to eradicate leukemic cells represents an important therapeutic goal. Directing T cells toward leukemia cells may be achieved by T-cell recruiting antibody constructs such as bispecific T-cell engagers against leukemia antigens, or by immune checkpoint inhibitors such as anti–programmed cell death protein 1 (PD-1)/programmed death ligand 1 antibodies.4 Moreover, autologous T cells can be modified to express chimeric antigen receptors targeting AML antigens such as CD33 and CD123.29 However, although bone marrow–infiltrating T-cell number is preserved or even increased in patients with AML compared with in healthy individuals, these cells are functionally impaired.30 This impairment may drive the disappointing results of T-cell immunotherapies to date. Immune evasion may involve defective antigen presentation, upregulation of checkpoint molecules, and other mechanisms of direct suppression of immune cells.31 Therefore, new strategies enhancing leukemia adjuvanticity could represent an improvement for AML immunotherapy.32
Here, we showed that AMPK activation using the small molecule GSK621 resulted in the expression of an immunostimulatory DAMP in the form of CALR membrane exposure by murine and human AML cells. We previously demonstrated that AMPK activation induced a stress response involving the PERK/eIF2α/ATF4 signaling pathway, resulting in mitochondrial priming.12 Interestingly, here we found that CALR membrane exposure induced by GSK621 in preapoptotic leukemic cells was dependent on AMPK and involved eIF2α phosphorylation. However, AMPK-induced CALR membrane expression was only partly dependent on PERK, suggesting that other effectors downstream of AMPK contributed to this immunostimulatory signal in AML. In melanoma, the stress kinases protein kinase R and general control nonderepressible 2 were shown to be key mediators of eIF2α phosphorylation in vitro33 and might be pathways to explore in future work.
CALR surface exposure is associated with increased circulating natural killer, CD4+, and CD8+ cell populations including T cells directed against leukemia-associated antigens, and correlates with improved survival in patients with AML.34 This suggests that pharmacological activation of AMPK to increase CALR could represent a new strategy to increase the anticancer immune response. We observed that idarubicin, a widely used anti-AML chemotherapy, also strongly induced CALR membrane exposure in AML, as reported,35 and its effect was independent of AMPK. However, previous work demonstrated that PERK was necessary for anthracycline-induced surface CALR exposure,24 which suggests a possible cross talk between the DNA damage response and ER stress, independent of AMPK.36 The precise mechanism of ICD induction by other antileukemic therapies should be studied in future experiments.
This prompts the question of why we cannot simply rely on conventional chemotherapy to induce ICD in AML? In addition to causing myelosuppression and organ toxicity, anthracyclines have immunosuppressive activity by increasing regulatory T cells (Tregs) and tolerogenic DCs, which may limit antileukemia immunity.37 Cytarabine reportedly modulates checkpoint molecules and possibly promotes AML immunogenicity.38 However, we observed that cytarabine did not induce CALR membrane exposure to the same degree as GSK621, and it only modestly primed leukemic cells to trigger BMDC maturation ex vivo. Thus, the “3 + 7” induction regimen of anthracycline plus cytarabine that is the backbone of AML therapy has complex effects on immunity, which might still be enhanced by adding AMPK agonism. In further support of using AMPK activation as immune stimulation, AMPK loss in Tregs promotes tumor growth by increasing the expression of PD-1, whereas treatment of Tregs with the AMPK activator AICAR decreases PD-1 expression.39 This suggests that AMPK activation could exert anticancer immunostimulatory effects on the tumor microenvironment as well as on AML cells. In fact, trials have reported activity by combining immune checkpoint blockade with metformin, an inhibitor of complex I mitochondria respiratory chain that stimulates anticancer immunity, in part, through AMPK activation.40 Together, our results suggest that AMPK activation could represent an attractive strategy to kill AML blasts and simultaneously increase their adjuvanticity. Alternatively, addition of AMPK activation to conventional AML chemotherapies might enhance immunogenicity without relying solely on the AMPK activator for tumor debulking.
To establish that AMPK activation resulted in ICD, we used vaccination assays in AML models in syngeneic immunocompetent mice. Even though AML mainly spreads in the bone marrow and blood, we challenged vaccinated mice by the subcutaneous injection of leukemic cells, leading to the development of in situ tumors using established tumor immunology assays.14 This provided straightforward and reproducible measurements of tumor growth. In future studies, it may be important to test systemic leukemia growth, because AML cells may interact differently with the immune system in the bone marrow, blood, and in lymphoid organs such as the spleen. Despite these limitations, GSK621 pretreatment of either C1498- or MLL-AF9–driven AML cells induced a robust vaccination effect in vivo that was dependent on a T-cell systemic adaptive immune response specific to AML blasts.
In summary, we show that DAMP production by GSK621-treated AML cells primes BMDCs in vitro and elicits an adaptive T-cell immune response in vivo in immunocompetent hosts. This strategy could be exploited to increase AML adjuvanticity to improve endogenous immune responses and/or the efficacy of T-cell–based immunotherapies. The best timing of such treatments should be determined in clinical trials, possibly as an “immune priming” before, or during, initiation of induction chemotherapy, or later as part of consolidation. For instance, AMPK activation could be combined with other immunotherapies for patients who have achieved chemotherapy-induced remission, such as with DC-AML fusion vaccines41 or immune checkpoint blockade.
Acknowledgments
The authors thank the Translational Immunogenomics Laboratory at the Dana-Farber Cancer Institute; in particular, Derin Keskin, for scientific advice and technical support for immune monitoring ex vivo. The authors thank Sabrina Spaggiari from Institut Gustave Roussy for technical support and advice on preliminary in vitro experiments.
This work was supported by grants from the French Institut National du Cancer and the National Institutes of Health.
Authorship
Contribution: J.M. was responsible for study conceptualization, resource provision, data curation, data analysis, supervision, funding acquisition, validation, investigation, methodology, and writing the original draft of the manuscript; M.G. and L.P. were responsible for providing resources, data analysis, investigation, and methodology; R.A.B. was responsible for investigation; O.K. and G.K. were responsible for methodology; J.-E.S. was responsible for providing resources and methodology; and J.T. and A.A.L. were responsible for conceptualization, resources, supervision, funding acquisition, and reviewing and editing the manuscript.
Conflict-of-interest disclosure: J.M. was funded by the French National Institute for Health and Medical Research (INSERM, Plan Cancer InCa, formation à la recherche translationnelle en cancérologie), the Ligue Contre le Cancer, the Monahan Foundation (partner of the Fullbright Foundation), L’Oréal UNESCO for Women in Science French Young Talents program, and the Philippe Foundation. M.G. was funded by the Cancéropôle Grand Sud-Ouest. M.G., L.P., and J.-E.S. report funding from the Laboratoire d'Excellence Toulouse Cancer (TOUCAN and TOUCAN2.0; contract ANR11-LABEX), the French Institut National du Cancer (PLBIO 2020-010), the Fondation ARC, and the Ligue Contre le Cancer. O.K. reports grants from the French Institut National du Cancer and the DIM Elicit initiative of the Ile de France and is a cofounder of Samsara Therapeutics. A.A.L. was funded by the National Institutes of Health/National Cancer Institute (CA225191), the Ludwig Center at Harvard, Alex’s Lemonade Stand Foundation, and is a scholar of the Leukemia & Lymphoma Society. G.K. declares having held research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sotio, Tollys, Vascage, and Vasculox/Tioma; has received consulting/advisory honoraria from Reithera; serves on the board of directors of the Bristol Myers Squibb Foundation France; is a scientific cofounder of everImmune, Osasuna Therapeutics, Samsara Therapeutics, and Therafast Bio; is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis, and metabolic disorders; has received research funding from AbbVie and Stemline Therapeutics; and received consulting fees from Adaptimmune, Cimeio, IDRx, N-of-one, Qiagen, and Stemline Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Andrew A. Lane, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Mayer 413, Boston, MA 02215; email: andrew_lane@dfci.harvard.edu.
References
Author notes
∗M.G. and L.P. contributed equally to this study.
†J.T. and A.A.L. contributed equally to this study.
Data are available on request from the corresponding author, Andrew A. Lane (andrew_lane@dfci.harvard.edu).
The full-text version of this article contains a data supplement.