Key Points
The median duration of response in next-generation sequencing MRD–negative responders was 53.4 months.
Pre-lymphodepletion maximum standardized uptake value and bulky disease, day +28 positron emission tomography–computed tomography and MRD negativity, and in vivo CAR T-cell kinetics affected PFS.
Abstract
High response rates have been reported after CD19-targeted chimeric antigen receptor–modified (CD19 CAR) T-cell therapy for relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL), yet the factors associated with duration of response in this setting are poorly characterized. We analyzed long-term outcomes in 47 patients with R/R CLL and/or Richter transformation treated on our phase 1/2 clinical trial of CD19 CAR T-cell therapy with an updated median follow-up of 79.6 months. Median progression-free survival (PFS) was 8.9 months, and the 6-year PFS was 17.8%. Maximum standardized uptake value (hazard ratio [HR], 1.15; 95% confidence interval [CI], 1.07-1.23; P < .001) and bulky disease (≥5 cm; HR, 2.12; 95% CI, 1.06-4.26; P = .034) before lymphodepletion were associated with shorter PFS. Day +28 complete response by positron emission tomography–computed tomography (HR, 0.13; 95% CI, 0.04-0.40; P < .001), day +28 measurable residual disease (MRD) negativity by multiparameter flow cytometry (HR, 0.08; 95% CI, 0.03-0.22; P < .001), day +28 MRD negativity by next-generation sequencing (HR, 0.21; 95% CI, 0.08-0.51; P < .001), higher peak CD8+ CAR T-cell expansion (HR, 0.49; 95% CI; 0.36-0.68; P < .001), higher peak CD4+ CAR T-cell expansion (HR, 0.47; 95% CI; 0.33-0.69; P < .001), and longer CAR T-cell persistence (HR, 0.56; 95% CI, 0.44-0.72; P < .001) were associated with longer PFS. The 6-year duration of response and overall survival were 26.4% and 31.2%, respectively. CD19 CAR T-cell therapy achieved durable responses with curative potential in a subset of patients with R/R CLL. This trial was registered at www.clinicaltrials.gov as #NCT01865617.
Introduction
CD19-targeted chimeric antigen receptor–modified (CD19 CAR) T-cell therapy has demonstrated high efficacy and curative potential in patients with relapsed and/or refractory (R/R) B-cell malignancies.1 We previously reported high antitumor efficacy for patients with R/R chronic lymphocytic leukemia (CLL) treated in a phase 1/2 clinical trial (NCT01865617) with an autologous CD19 CAR T-cell product (JCAR014) containing equal target doses of CD8+ and CD4+ CAR T-cells and a 4-1BB costimulatory domain but with limited duration of follow-up.2,3 Although long-term responses have been reported after CD19 CAR T-cell therapy for CLL, the factors associated with long-term outcomes have not been identified.4 Therefore, we now provide an update of our phase 1/2 clinical trial results with >6 years of median follow-up, including exploratory analyses of factors associated with long-term outcomes.
Methods
Study design
We included 49 patients with R/R CLL and/or Richter transformation treated on our phase 1/2 clinical trial of CD19 CAR T-cell therapy with JCAR014 (NCT01865617; supplemental Figure 1). Patients received JCAR014 without concurrent ibrutinib (n = 30; stopped before lymphodepletion [LD]) or with concurrent ibrutinib (n = 19; started at least 2 weeks before leukapheresis), as previously reported.3 Informed consent was obtained from each participant, and this study was approved by the Fred Hutchinson Cancer Center institutional review board. Details regarding study design and patient selection are available in the supplemental Methods.
CAR T-cell manufacturing
Autologous CD4+ and bulk CD8+ T cells were immunomagnetically selected and then modified with a lentivirus encoding a CAR containing a CD19-specific single-chain variable fragment, immunoglobulin G4 hinge, CD28 transmembrane domain, and 4-1BB and CD3z signaling domains. The CAR was separated by a ribosomal skip sequence from a truncated human epidermal growth factor receptor, which enabled CAR T-cell enumeration by flow cytometry and formulation of a CD4+:CD8+ CAR T-cell ratio of 1:1 for infusion, as previously described.2,3
LD regimens, CAR T-cell dose, and concurrent ibrutinib regimen
All patients underwent sequential or concurrent LD chemotherapy with cyclophosphamide (Cy) and fludarabine (Flu), Cy alone, or Flu alone. LD chemotherapy was followed by the infusion of 2 × 105, 2 × 106, or 2 × 107 CD19 CAR T cells per kilogram body mass. Patients in the concurrent ibrutinib cohort were scheduled to receive ibrutinib 420 mg per day beginning ≥2 weeks before leukapheresis and continuing until ≥3 months after CD19 CAR T-cell infusion. Dose reduction of ibrutinib for toxicity was permitted.
Procedures and end points
Measurable residual disease (MRD) in the bone marrow (BM) was evaluated by multiparameter flow cytometry (MFC; sensitivity, 10−4) and immunoglobulin heavy chain next-generation sequencing (NGS; clonoSEQ assay, Adaptive Biotechnologies, Seattle, WA; sensitivity, 10−6). CD4+ and CD8+ CAR T cells were defined as viable CD45+CD3+CD4+CD8−EGFRt+ or CD45+CD3+CD4−CD8+EGFRt+ events, respectively, using MFC. Absolute CAR T-cell counts were determined by multiplying the percentage of CAR T cells identified by flow cytometry in a CD45+ forward scatter–side scatter lymphocyte gate by the absolute lymphocyte count established by a complete blood count performed on the same day. CAR T-cell persistence was assessed using quantitative polymerase chain reaction to detect integrated transgene sequences. Serum cytokine concentrations (19 analytes) were measured by Luminex assay.
Response was defined as complete response (CR) or partial response (PR) per the 2018 International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria5 assessed approximately 28 days after CAR T-cell infusion. DOR was defined as the time from initial response assessment to relapse, progression, or death in patients in CR, CR with incomplete hematologic recovery (CRi), or PR by iwCLL criteria at day +28. The data cutoff date for this analysis was 1 March 2023. Cytokine release syndrome and neurotoxicity were graded using the Lee 2014 criteria6 and the Common Terminology Criteria for Adverse Events 4.037, respectively.
Statistical analyses
Median follow-up in the response-evaluable cohort was estimated by predicting the censoring distribution (inverse Kaplan-Meier method). DOR, progression-free survival (PFS), and overall survival (OS) were estimated using the Kaplan-Meier method. Univariate associations between patient, disease, CAR T-cell manufacturing, treatment-related variables, and MRD-negative CR by MFC or DOR were estimated using Cox regression (≥50 predictors). The proportional hazard assumption was tested by plotting the scaled Schoenfeld residuals against time. Because these were exploratory analyses, P values were not adjusted for multiplicity. CAR T-cell persistence (CAR transgene copies per μg genomic DNA) was modeled as a time-dependent covariate, with detectable CAR T-cell persistence defined as 10 CAR transgene copies per μg genomic DNA. All statistical analyses were performed using R software version 4.1.3 (R Core Team, Vienna, Austria).
Results
Patient, disease, and treatment characteristics
The median age at enrollment was 61 years (interquartile range [IQR], 55-67; Table 1). Patients were heavily pretreated (median prior lines of therapy, 5; IQR, 4-7), including prior purine analogs (n = 14, 29%), bendamustine (n = 25, 51%), and venetoclax (n = 19, 39%). Nearly all (n = 47; 96%) had intolerance to and/or progression on ibrutinib; all patients had stable disease (SD) or progressive disease (PD) on ibrutinib. Forty-six patients (94%) had high-risk cytogenetics (complex karyotype and/or 17p deletion). Nine patients (18%) had a prior history of (n = 7), or current (n = 2), Richter transformation.
Patient and disease characteristics
| Characteristic . | N = 49 . |
|---|---|
| Age (year) | |
| Median (IQR) | 61.0 (55.0-67.0) |
| Range | 40.0-73.0 |
| Sex | |
| Female | 16 (33%) |
| Male | 33 (67%) |
| ECOG score | |
| 0 | 24 (49%) |
| 1 | 25 (51%) |
| Prior history of or current Richter transformation | 9 (18%) |
| Prior history of Richter transformation | 7 (14%) |
| Current Richter transformation | 2 (4%) |
| No. of prior therapies | |
| Median (IQR) | 5.0 (4.0-7.0) |
| Range | 1.0-10.0 |
| Prior fludarabine | 14 (29%) |
| Prior bendamustine | 25 (51%) |
| Prior venetoclax | 19 (39%) |
| High-risk cytogenetics∗ | 46 (94%) |
| Complex karyotype† (n = 48) | 36 (75%) |
| 17p deletion | 33 (67%) |
| Intolerance to and/or progression on ibrutinib | 47 (96%) |
| Intolerance to ibrutinib | 5 (10%) |
| Progression on ibrutinib | 43 (88%) |
| Prior allogeneic HCT | 7 (14%) |
| Bridging chemotherapy | 8 (16%) |
| Absolute lymphocyte count (109/L) | |
| Median (IQR) | 1.8 (1.0-7.1) |
| Range | 0.2-58.9 |
| Absolute CD4+T-cell count (cells per μL) | |
| Median (IQR) | 547.3 (238.2-937.0) |
| Range | 59.8-4408.7 |
| Absolute CD8+T-cell count (cells per μL) | |
| Median (IQR) | 350.4 (197.8-735.1) |
| Range | 12.4-9403.2 |
| Percentage of CLL cell count in the blood by MFC (% of WBCs; n = 47) | |
| Median (IQR) | 22.3 (4.0-60.0) |
| Range | 0.0-92.0 |
| Marrow CLL burden | |
| Percentage of CLL cells in the BM by IHC (%) (n = 43) | |
| Median (IQR) | 60.0 (12.5-70.0) |
| Range | 0.0-90.0 |
| Percentage of CLL cells in BM by MFC (%) | |
| Median (IQR) | 49.4 (14.1-73.5) |
| Range | 0.0-96.0 |
| Serum LDH concentration (U/L) | |
| Median (IQR) | 200.0 (153.0-308.0) |
| Range | 94.0-1872.0 |
| Tumor cross-sectional area‡(mm2) | |
| Median (IQR) | 3092.9 (1489.7-4436.4) |
| Range | 0.1-20 406.2 |
| Maximum SUV (n = 37) | |
| Median (IQR) | 5.1 (3.7-7.5) |
| Range | 0.0-27.5 |
| Bulky disease§ | 13 (27%) |
| LD regimen | |
| Concurrent Cy/Flu‖ | 27 (55%) |
| Sequential Cy/Flu¶ | 19 (39%) |
| Cy only (2 g/m2 × 1 day) | 1 (2%) |
| Flu only (25 mg/m2 × 3 days) | 2 (4%) |
| CAR T-cell dose level | |
| 2 × 105 cells per kg | 5 (10%) |
| 2 × 106 cells per kg (recommended phase 2 dose)# | 43 (88%) |
| 2 × 107 cells per kg | 1 (2%) |
| Characteristic . | N = 49 . |
|---|---|
| Age (year) | |
| Median (IQR) | 61.0 (55.0-67.0) |
| Range | 40.0-73.0 |
| Sex | |
| Female | 16 (33%) |
| Male | 33 (67%) |
| ECOG score | |
| 0 | 24 (49%) |
| 1 | 25 (51%) |
| Prior history of or current Richter transformation | 9 (18%) |
| Prior history of Richter transformation | 7 (14%) |
| Current Richter transformation | 2 (4%) |
| No. of prior therapies | |
| Median (IQR) | 5.0 (4.0-7.0) |
| Range | 1.0-10.0 |
| Prior fludarabine | 14 (29%) |
| Prior bendamustine | 25 (51%) |
| Prior venetoclax | 19 (39%) |
| High-risk cytogenetics∗ | 46 (94%) |
| Complex karyotype† (n = 48) | 36 (75%) |
| 17p deletion | 33 (67%) |
| Intolerance to and/or progression on ibrutinib | 47 (96%) |
| Intolerance to ibrutinib | 5 (10%) |
| Progression on ibrutinib | 43 (88%) |
| Prior allogeneic HCT | 7 (14%) |
| Bridging chemotherapy | 8 (16%) |
| Absolute lymphocyte count (109/L) | |
| Median (IQR) | 1.8 (1.0-7.1) |
| Range | 0.2-58.9 |
| Absolute CD4+T-cell count (cells per μL) | |
| Median (IQR) | 547.3 (238.2-937.0) |
| Range | 59.8-4408.7 |
| Absolute CD8+T-cell count (cells per μL) | |
| Median (IQR) | 350.4 (197.8-735.1) |
| Range | 12.4-9403.2 |
| Percentage of CLL cell count in the blood by MFC (% of WBCs; n = 47) | |
| Median (IQR) | 22.3 (4.0-60.0) |
| Range | 0.0-92.0 |
| Marrow CLL burden | |
| Percentage of CLL cells in the BM by IHC (%) (n = 43) | |
| Median (IQR) | 60.0 (12.5-70.0) |
| Range | 0.0-90.0 |
| Percentage of CLL cells in BM by MFC (%) | |
| Median (IQR) | 49.4 (14.1-73.5) |
| Range | 0.0-96.0 |
| Serum LDH concentration (U/L) | |
| Median (IQR) | 200.0 (153.0-308.0) |
| Range | 94.0-1872.0 |
| Tumor cross-sectional area‡(mm2) | |
| Median (IQR) | 3092.9 (1489.7-4436.4) |
| Range | 0.1-20 406.2 |
| Maximum SUV (n = 37) | |
| Median (IQR) | 5.1 (3.7-7.5) |
| Range | 0.0-27.5 |
| Bulky disease§ | 13 (27%) |
| LD regimen | |
| Concurrent Cy/Flu‖ | 27 (55%) |
| Sequential Cy/Flu¶ | 19 (39%) |
| Cy only (2 g/m2 × 1 day) | 1 (2%) |
| Flu only (25 mg/m2 × 3 days) | 2 (4%) |
| CAR T-cell dose level | |
| 2 × 105 cells per kg | 5 (10%) |
| 2 × 106 cells per kg (recommended phase 2 dose)# | 43 (88%) |
| 2 × 107 cells per kg | 1 (2%) |
Unless otherwise noted, data are n (%).
All variables were assessed before LD chemotherapy, unless specified.
ECOG, Eastern Cooperative Oncology Group; IHC, immunohistochemistry; LDH, lactate dehydrogenase; WBC, white blood cell.
Defined as 17p deletion and/or complex karyotype.
Defined as ≥3 chromosomal abnormalities.
For patients with evaluable nodal disease (n = 45); the sum of the product of the diameters of up to 6 of the largest lymph nodes or masses evaluated on the pre-LD CT.
Defined as largest lymph node ≥5 cm.
Cy 300 to 500 mg/m2 × 3 days + Flu 20 to 30 mg/m2 × 3 days.
Cy 30 to 60 mg/kg or 1 g/m2 × 1 day, then Flu 25 mg/m2 × 3 or 5 days.
One patient with Richter transformation was treated on a dose-dense protocol, in which a second infusion of CAR T cells was administered on day +14 without interval LD.
Early outcomes
Cytokine release syndrome and neurotoxicity occurred in 40 (82%; grade ≥3, 7 [14%]) and 16 (33%; grade ≥3, 13 [27%]) patients, respectively. Two patients died before response assessment (grade 5 cytokine release syndrome and neurotoxicity, n = 1 and presumed ibrutinib-related cardiac arrhythmia; n = 1), as previously reported.3 Of the 3 patients who received Cy or Flu alone, all had CAR T-cell expansion; day +28 restaging outcomes were SD (n = 1) and PD (n = 2).
For the remaining 47 evaluable patients, the overall response rate at day 28 after CAR T-cell infusion (day +28) was 70% (CR, 6% [n = 3]; CRi, 11% [n = 5]; and PR, 53% [n = 25]). Among the patients with BM disease before LD, 70% (32 of 46) had BM MRD negativity by MFC on day +28. Among patients with pre-LD BM disease, day +28 MRD negativity by MFC and available data, 62% (18 of 29) had BM MRD negativity by NGS.
Using univariate logistic regression, higher peak CD8+ (odds ratio [OR], 9.10; 95% confidence interval [CI], 2.92-50.6; P = .002) and CD4+ CAR T-cell expansion (OR, 5.57; 95% CI, 2.17-19.6; P = .002) were associated with higher odds of day +28 MRD negativity by MFC. Male sex was associated with lower odds of day +28 MRD negativity by MFC (OR, 0.11; 95% CI, 0.01-0.67; P = .047). There was no difference in the median number of prior therapies between male and female patients (male, 5 [IQR, 4.25-6] vs female, 5 [IQR, 4-7]; P = .77). We estimated lower odds of day +28 MRD negativity by MFC for patients who received a higher number of prior therapies (OR, 0.70; 95% CI, 0.46-1.00; P = .067; supplemental Table 1), although we could not exclude a null effect at the .05 level.
In the concurrent ibrutinib cohort, 5 patients (26%) continued on ibrutinib for 90 days after CAR T-cell infusion, per study protocol. One patient (5%) was lost to follow-up before day +90. Six (32%) patients stopped ibrutinib before day +90. Seven (37%) patients continued ibrutinib beyond day +90.
Long-term outcomes
In the response-evaluable cohort (n = 47), median follow-up was 79.6 months (IQR, 60.5-87.5), median DOR was 18.9 months (95% CI, 9.66-55.6), and the 6-year DOR estimate was 26.4% (95% CI, 14.8-47.2; Figure 1A). For all patients who received CAR T-cell infusion (n = 49), median PFS and OS rates were 8.9 months (95% CI, 3.0-19.9) and 25.0 months (95% CI, 11.5-62.1), respectively; the 6-year PFS and OS rates were 17.8% (95% CI, 9.7-32.8) and 31.2% (95% CI, 20.3-48.1), respectively (Figure 1B-C). The 2 patients with current Richter transformation experienced CR (n = 1) and CRi (n = 1) but relapsed 9.8 and 3.0 months after CAR T-cell infusion, respectively.
Long-term outcomes of patients treated with CD19 CAR T-cell therapy with JCAR014. (A) DOR of patients who were in CR or PR by iwCLL criteria on day +28. (B) PFS and (C) OS of all patients who received CAR T-cell infusion.
Long-term outcomes of patients treated with CD19 CAR T-cell therapy with JCAR014. (A) DOR of patients who were in CR or PR by iwCLL criteria on day +28. (B) PFS and (C) OS of all patients who received CAR T-cell infusion.
For patients with CR/CRi or PR on day +28 by iwCLL criteria (n = 33), we observed prolonged CAR persistence up to, or beyond, 1 year after CAR T-cell infusion in 7 of 9 patients with ongoing responses at last follow-up, with longest measured persistence of 86.0 months. For patients with subsequent relapse or progression with available data (n = 19; death before confirmed loss of CAR persistence in 1 patient), we observed a loss of CAR persistence by 1 year in all but 1 patient (95%; Figure 2). CD19 expression data was available in 11 patients at the time of relapse or progression, of which 9 (82%) had CD19+ disease.
Duration of CAR transgene persistence and B-cell aplasia in the peripheral blood relative to clinical events. Data are from 33 patients with CR, CRi, and PR by 2018 iwCLL criteria on day +28.
Duration of CAR transgene persistence and B-cell aplasia in the peripheral blood relative to clinical events. Data are from 33 patients with CR, CRi, and PR by 2018 iwCLL criteria on day +28.
Four patients (9%) died in CR/PR after CAR T-cell therapy (n = 1, diffuse alveolar hemorrhage and pulmonary aspergillosis; n = 1, progressive multifocal leukoencephalopathy; n = 1, myocardial infarction; and n = 1, therapy-related myelodysplastic syndrome transformed to acute myeloid leukemia). These 4 deaths were not attributed to CAR T-cell therapy. For the 20 patients who were in persistent remission ≥1 year after infusion, adverse events occurring ≥1 year after infusion were as follows: hypogammaglobulinemia (n = 5 [25%]), secondary malignancy (n = 4 [20%]), infection (n = 4 [20%]), neurologic disorders (n = 2 [10%]), and other (n = 1 [5%]).
Subsequent therapies after JCAR014 treatment in the response-evaluable cohort
Of 34 patients with relapse or progression after JCAR014, 16 patients (47%) underwent a second CD19-targeted CAR T-cell infusion at a median of 10.1 months (range, 1.0-87.1 months) after the initial infusion. The first 2 infusions for the patient treated on the dose-dense protocol were considered to be the patient’s first infusion. Indications for the second CAR T-cell infusion were as follows: SD or PD after the first CAR T-cell infusion (n = 6) and relapse or PD after initial response to the first CAR T-cell infusion (n = 10). Two patients received US Food and Drug Administration–approved commercial CAR T-cell products for Richter transformation (lisocabtagene maraleucel, n = 1 [mixed response] and axicabtagene ciloleucel, n = 1 [CR]). Thirteen patients (38%) went on to receive a repeat infusion of JCAR014, as previously reported,8 whereas 1 patient (3%) received an investigational CAR T-cell product.
Nine patients (26%) received allogeneic hematopoietic cell transplantation (HCT) for PD at a median of 14.9 months (range, 2.7-58.2 months) after the initial infusion, including 4 patients who had already received a second CAR T-cell infusion. The outcomes of these patients were as follows: transplant-related mortality (n = 1), in remission since HCT (n = 2), relapse after HCT (n = 6).
Predictors of PFS
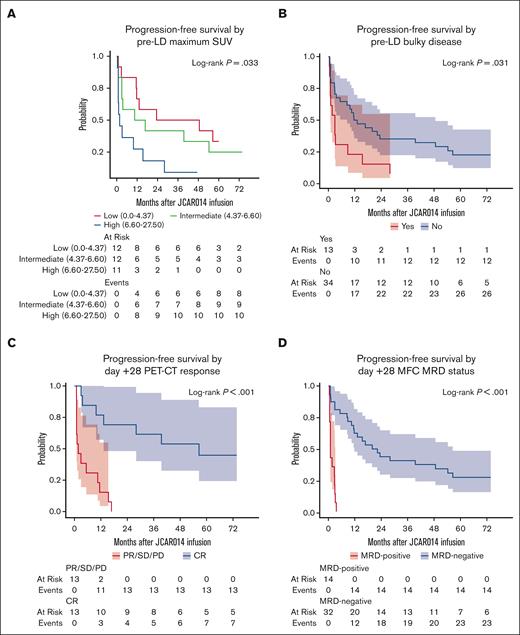
In univariate Cox regression, we identified both pretreatment and posttreatment factors associated with PFS (Table 2). Pretreatment factors associated with worse PFS included younger age (hazard ratio [HR], 1.04; 95% CI, 1.00-1.09; P = .048), pre-LD maximum standardized uptake value (SUV; HR, 1.15; 95% CI, 1.07-1.23; P < .001), pre-LD bulky disease (≥5 cm; HR, 2.12; 95% CI, 1.06-4.26; P = .034), and non-Cy/Flu LD (HR, 4.17; 95% CI, 1.18-14.7; P = .027). The association between pre-LD maximum SUV and PFS was near linear without a threshold effect (supplemental Figure 2). In a Cox regression model including both pre-LD maximum SUV and bulky disease, pre-LD maximum SUV remained independently associated with PFS (supplemental Table 2).
Univariate Cox regression model evaluations of predictors of PFS
| Characteristic . | N . | Event, n . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Age | 47 | 38 | 0.96 | 0.92-1.00 | .048 |
| Sex | 47 | 38 | |||
| Female | — | — | |||
| Male | 1.03 | 0.52-2.05 | .93 | ||
| ECOG PS | 47 | 38 | |||
| 0 | — | — | |||
| 1 | 1.60 | 0.84-3.06 | .15 | ||
| Prior history of or current Richter transformation | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.68 | 0.76-3.68 | .20 | ||
| Prior history of Richter transformation | 47 | 38 | |||
| 0 | — | — | |||
| 1 | 1.57 | 0.65-3.78 | .31 | ||
| Current Richter transformation | 47 | 38 | |||
| 0 | — | — | |||
| 1 | 1.76 | 0.41-7.50 | .44 | ||
| No. of prior therapies | 47 | 38 | 1.04 | 0.88-1.22 | .68 |
| Prior fludarabine | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.95 | 0.47-1.91 | .88 | ||
| Prior bendamustine | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.98 | 0.52-1.86 | .96 | ||
| Prior venetoclax | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.87 | 0.97-3.61 | .061 | ||
| Complex karyotype∗ | 46 | 37 | |||
| No | — | — | |||
| Yes | 1.82 | 0.79-4.17 | .16 | ||
| 17p deletion | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.30 | 0.63-2.69 | .47 | ||
| Intolerance to and/or progression on ibrutinib | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.38 | 0.09-1.63 | .19 | ||
| Intolerance to ibrutinib | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.79 | 0.28-2.23 | .66 | ||
| Progression on ibrutinib | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.13 | 0.44-2.89 | .80 | ||
| Prior allogeneic HCT | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.53 | 0.19-1.50 | .23 | ||
| Concurrent ibrutinib with CD19 CAR T-cell therapy | 47 | 38 | |||
| Ibrutinib | — | — | |||
| No ibrutinib | 1.16 | 0.60-2.23 | .66 | ||
| Bridging chemotherapy after leukapheresis | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.42 | 0.62-3.23 | .40 | ||
| Absolute lymphocyte count (109/L) | 47 | 38 | 0.98 | 0.95-1.01 | .15 |
| Percentage of CLL cell count in the blood by MFC (% of WBCs) | 45 | 37 | 0.99 | 0.98-1.00 | .13 |
| Percentage of CLL cells in the BM by IHC (%) | 41 | 32 | 0.99 | 0.98-1.00 | .084 |
| Percentage of CLL cells in the BM by MFC (%) | 47 | 38 | 0.99 | 0.98-1.00 | .13 |
| Serum LDH concentration (log10U/L) | 47 | 38 | 1.28 | 0.43-3.81 | .66 |
| Tumorcross-sectionalarea (log10mm2) | 46 | 37 | 1.17 | 0.90-1.52 | .24 |
| Maximum SUV | 35 | 27 | 1.15 | 1.07-1.23 | <.001 |
| Bulky disease† | 47 | 38 | |||
| No | — | — | |||
| Yes | 2.12 | 1.06-4.26 | .034 | ||
| Concurrent or sequential LD | 47 | 38 | |||
| Concurrent Cy/Flu | — | — | |||
| Sequential Cy/Flu | 0.74 | 0.37-1.47 | .39 | ||
| Other | 4.17 | 1.18-14.7 | .027 | ||
| CAR T-cell dose level (cells per kg) | 47 | 38 | |||
| 2 × 105 | — | — | |||
| 2 × 106 | 1.14 | 0.40-3.23 | .81 | ||
| 2 × 107 | 2.62 | 0.28-24.1 | .40 | ||
| CRS grade‡ | 47 | 38 | 0.98 | 0.71-1.36 | .90 |
| Neurotoxicity grade§ | 47 | 38 | 0.97 | 0.77-1.23 | .82 |
| Day +28 nodal response by CT | 42 | 33 | |||
| PR/SD/PD | — | — | |||
| CR | 0.55 | 0.17-1.79 | .32 | ||
| Day +28 nodal response by PET-CT | 26 | 20 | |||
| PR/SD/PD | — | — | |||
| CR | 0.13 | 0.04-0.40 | <.001 | ||
| Day +28 response by 2018 iwCLL criteria | 47 | 38 | |||
| PR/SD/PD | — | — | |||
| CR/CRi | 0.59 | 0.25-1.42 | .24 | ||
| Day +28 BM MRD by MFC | 46 | 37 | |||
| Positive | — | — | |||
| Negative | 0.08 | 0.03-0.22 | <.001 | ||
| Day +28 BM MRD by IGH NGS | 29 | 21 | |||
| Positive | — | — | |||
| Negative | 0.21 | 0.08-0.51 | <.001 | ||
| Peak CD4+CAR T-cell expansion (log10cells per μL) | 47 | 38 | 0.47 | 0.33-0.69 | <.001 |
| Peak CD8+CAR T-cell expansion (log10cells per μL) | 47 | 38 | 0.49 | 0.36-0.68 | <.001 |
| CAR T-cell persistence (CAR transgene copies per μg genomic DNA)‖ | — | — | 0.56 | 0.44-0.72 | <.001 |
| Characteristic . | N . | Event, n . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Age | 47 | 38 | 0.96 | 0.92-1.00 | .048 |
| Sex | 47 | 38 | |||
| Female | — | — | |||
| Male | 1.03 | 0.52-2.05 | .93 | ||
| ECOG PS | 47 | 38 | |||
| 0 | — | — | |||
| 1 | 1.60 | 0.84-3.06 | .15 | ||
| Prior history of or current Richter transformation | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.68 | 0.76-3.68 | .20 | ||
| Prior history of Richter transformation | 47 | 38 | |||
| 0 | — | — | |||
| 1 | 1.57 | 0.65-3.78 | .31 | ||
| Current Richter transformation | 47 | 38 | |||
| 0 | — | — | |||
| 1 | 1.76 | 0.41-7.50 | .44 | ||
| No. of prior therapies | 47 | 38 | 1.04 | 0.88-1.22 | .68 |
| Prior fludarabine | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.95 | 0.47-1.91 | .88 | ||
| Prior bendamustine | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.98 | 0.52-1.86 | .96 | ||
| Prior venetoclax | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.87 | 0.97-3.61 | .061 | ||
| Complex karyotype∗ | 46 | 37 | |||
| No | — | — | |||
| Yes | 1.82 | 0.79-4.17 | .16 | ||
| 17p deletion | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.30 | 0.63-2.69 | .47 | ||
| Intolerance to and/or progression on ibrutinib | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.38 | 0.09-1.63 | .19 | ||
| Intolerance to ibrutinib | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.79 | 0.28-2.23 | .66 | ||
| Progression on ibrutinib | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.13 | 0.44-2.89 | .80 | ||
| Prior allogeneic HCT | 47 | 38 | |||
| No | — | — | |||
| Yes | 0.53 | 0.19-1.50 | .23 | ||
| Concurrent ibrutinib with CD19 CAR T-cell therapy | 47 | 38 | |||
| Ibrutinib | — | — | |||
| No ibrutinib | 1.16 | 0.60-2.23 | .66 | ||
| Bridging chemotherapy after leukapheresis | 47 | 38 | |||
| No | — | — | |||
| Yes | 1.42 | 0.62-3.23 | .40 | ||
| Absolute lymphocyte count (109/L) | 47 | 38 | 0.98 | 0.95-1.01 | .15 |
| Percentage of CLL cell count in the blood by MFC (% of WBCs) | 45 | 37 | 0.99 | 0.98-1.00 | .13 |
| Percentage of CLL cells in the BM by IHC (%) | 41 | 32 | 0.99 | 0.98-1.00 | .084 |
| Percentage of CLL cells in the BM by MFC (%) | 47 | 38 | 0.99 | 0.98-1.00 | .13 |
| Serum LDH concentration (log10U/L) | 47 | 38 | 1.28 | 0.43-3.81 | .66 |
| Tumorcross-sectionalarea (log10mm2) | 46 | 37 | 1.17 | 0.90-1.52 | .24 |
| Maximum SUV | 35 | 27 | 1.15 | 1.07-1.23 | <.001 |
| Bulky disease† | 47 | 38 | |||
| No | — | — | |||
| Yes | 2.12 | 1.06-4.26 | .034 | ||
| Concurrent or sequential LD | 47 | 38 | |||
| Concurrent Cy/Flu | — | — | |||
| Sequential Cy/Flu | 0.74 | 0.37-1.47 | .39 | ||
| Other | 4.17 | 1.18-14.7 | .027 | ||
| CAR T-cell dose level (cells per kg) | 47 | 38 | |||
| 2 × 105 | — | — | |||
| 2 × 106 | 1.14 | 0.40-3.23 | .81 | ||
| 2 × 107 | 2.62 | 0.28-24.1 | .40 | ||
| CRS grade‡ | 47 | 38 | 0.98 | 0.71-1.36 | .90 |
| Neurotoxicity grade§ | 47 | 38 | 0.97 | 0.77-1.23 | .82 |
| Day +28 nodal response by CT | 42 | 33 | |||
| PR/SD/PD | — | — | |||
| CR | 0.55 | 0.17-1.79 | .32 | ||
| Day +28 nodal response by PET-CT | 26 | 20 | |||
| PR/SD/PD | — | — | |||
| CR | 0.13 | 0.04-0.40 | <.001 | ||
| Day +28 response by 2018 iwCLL criteria | 47 | 38 | |||
| PR/SD/PD | — | — | |||
| CR/CRi | 0.59 | 0.25-1.42 | .24 | ||
| Day +28 BM MRD by MFC | 46 | 37 | |||
| Positive | — | — | |||
| Negative | 0.08 | 0.03-0.22 | <.001 | ||
| Day +28 BM MRD by IGH NGS | 29 | 21 | |||
| Positive | — | — | |||
| Negative | 0.21 | 0.08-0.51 | <.001 | ||
| Peak CD4+CAR T-cell expansion (log10cells per μL) | 47 | 38 | 0.47 | 0.33-0.69 | <.001 |
| Peak CD8+CAR T-cell expansion (log10cells per μL) | 47 | 38 | 0.49 | 0.36-0.68 | <.001 |
| CAR T-cell persistence (CAR transgene copies per μg genomic DNA)‖ | — | — | 0.56 | 0.44-0.72 | <.001 |
PFS of response-evaluable patients (n = 47).
CRS, cytokine release syndrome; ECOG PS, Eastern Cooperative Oncology Group performance status; IGH, immunoglobulin heavy chain; IHC, immunohistochemistry; LDH, lactate dehydrogenase; WBC, white blood cell.
Defined as ≥3 chromosomal abnormalities.
Defined as largest lymph node ≥ 5 cm.
By Lee 2014 CRS criteria.6
By Common Terminology Criteria for Adverse Events version 4.03 criteria.7
Modeled as a time-dependent continuous covariate (limit of quantitation: 10 copies per μg genomic DNA).
Posttreatment factors associated with longer PFS were day +28 CR by positron emission tomography–computed tomography (PET-CT) (HR, 0.13; 95% CI, 0.04-0.40; P < .001), day +28 MRD negativity by MFC (HR, 0.08; 95% CI, 0.03-0.22; P < .001), day +28 MRD negativity by NGS (HR, 0.21; 95% CI, 0.08-0.51; P < .001), higher peak CD8+ CAR T-cell expansion (HR, 0.49; 95% CI, 0.36-0.68; P < .001), higher peak CD4+ CAR T-cell expansion (HR, 0.47; 95% CI, 0.33-0.69; P < .001), and longer CAR T-cell persistence (HR, 0.56; 95% CI, 0.44-0.72; P < .001). The median PFS estimates for patients who had MFC MRD–negative and NGS MRD–negative results on day +28 were 21.1 months (95% CI, 11.7-56.5) and 48.6 months (95% CI, 23.4-not reached), respectively. Among the 7 patients who were progression free at 5 years, 7 (100%) had day +28 MRD negativity by MFC, and 5 (83%; 1 missing) had day +28 MRD negativity by NGS. Univariate Cox regression models of PFS by pre-LD maximum SUV, pre-LD bulky disease, day +28 PET-CT response, and day +28 MFC MRD status are shown in Figure 3.
Pre- and post-treatment factors associated with PFS. (A) PFS by pre-LD maximum SUV; (B) PFS categorized by pre-LD bulky disease; (C) PFS by day +28 PET-CT response; (D) PFS by day+28 MRD status by MFC.
Pre- and post-treatment factors associated with PFS. (A) PFS by pre-LD maximum SUV; (B) PFS categorized by pre-LD bulky disease; (C) PFS by day +28 PET-CT response; (D) PFS by day+28 MRD status by MFC.
We could not confirm associations between PFS and pre-LD CLL BM burden by MFC (P = .13), tumor cross-sectional area (P = .24), prior intolerance to (P = .66) or progression on ibrutinib (P = .80), concurrent ibrutinib treatment (P = .66), CAR T-cell dose (2 × 106/kg vs 2 × 105/kg [P = .81]; 2 × 107/kg vs 2 × 105/kg [P = .40]), and day +28 iwCLL response (P = .24).
In our exploratory analyses, we also observed an association between higher pre-LD, day +0, and peak serum levels of several cytokines with shorter PFS (supplemental Table 3). Because these cytokines have been associated with more aggressive disease biology and impaired T-cell function, we studied correlations between select cytokines and variables associated with aggressive disease in addition to in vivo CAR T-cell kinetics. We found that pre-LD interleukin-10 (IL-10), tumor necrosis factor receptor 55 (TNFRp55), and TNFRp75 were associated with pre-LD disease burden as measured by serum lactate dehydrogenase, maximum SUV, and cross-sectional tumor area; pre-LD TNFRp55 and TNFRp75 were additionally associated with lower peak CAR T-cell expansion (supplemental Figure 3).
Predictors of DOR
In univariate Cox regression, day +28 CR by PET-CT (HR, 0.17; 95% CI, 0.05-0.64; P = .008), day +28 MRD negativity by MFC (HR, 0.03; 95% CI, 0.01-0.15; P < .001), day +28 MRD negativity by NGS (HR, 0.25; 95% CI, 0.09-0.68; P = .006), higher peak CD8+ CAR T-cell expansion (HR, 0.53; 95% CI, 0.33-0.85; P = .009), higher peak CD4+ CAR T-cell expansion (HR, 0.49; 95% CI, 0.28-0.85; P = .011), and longer CAR T-cell persistence (HR, 0.56; 95% CI, 0.39-0.81; P = .002) were associated with longer DOR (Table 3). The median DORs in responders with MFC MRD–negative results (n = 27) and responders with NGS MRD–negative results (n = 17) were 27.1 months (95% CI, 14.4 to not reached) and 53.4 months (95% CI, 27.1 to not reached), respectively (Figure 4A-B). Presence of current Richter transformation (HR, 5.14; 95% CI, 1.10-23.9; P = .037) and SD by day +28 CT (vs PR: HR, 8.33; 95% CI, 1.54-50; P = .014 vs CR: HR; 9.09; 95% CI, 1.20-50; P = .033) were associated with shorter DOR. Among patients who had MRD-negative results by MFC on day +28, those in CR by PET-CT had significantly longer DOR than those in PR (not reached vs 6.95 months; P < .001; supplemental Figure 4). We could not confirm associations between DOR and pre-LD CLL BM burden by immunohistochemistry (P = .40), tumor cross-sectional area (P = .65), prior intolerance to (P = .48) or progression on (P = .78) ibrutinib, concurrent ibrutinib treatment (P = .67), CAR T-cell dose (2 × 106/kg vs 2 × 105/kg, P = .44; 2 × 107/kg vs 2 × 105/kg, P = .16), and day +28 iwCLL response (P = .85).
Univariate Cox regression models of predictors of DOR
| Characteristic . | N . | Event, n . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Age | 33 | 24 | 0.96 | 0.91-1.02 | .21 |
| Sex | 33 | 24 | |||
| Female | — | — | |||
| Male | 0.89 | 0.39-2.04 | .79 | ||
| ECOG score | 33 | 24 | |||
| 0 | — | — | |||
| 1 | 1.82 | 0.81-4.08 | .15 | ||
| Prior history of or current Richter transformation | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.50 | 0.51-4.40 | .46 | ||
| Prior history of Richter transformation | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.83 | 0.19-3.54 | .80 | ||
| Current Richter transformation | 33 | 24 | |||
| No | — | — | |||
| Yes | 5.14 | 1.10-23.9 | .037 | ||
| No. of prior therapies | 33 | 24 | 1.03 | 0.84-1.26 | .79 |
| Prior fludarabine | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.68 | 0.25-1.82 | .44 | ||
| Prior bendamustine | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.02 | 0.46-2.28 | .96 | ||
| Prior venetoclax | 33 | 24 | |||
| No | — | — | |||
| Yes | 2.86 | 1.26-6.52 | .012 | ||
| Complex karyotype∗ | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.85 | 0.69-4.99 | .22 | ||
| 17p deletion | 33 | 24 | |||
| No | — | — | |||
| Yes | 2.14 | 0.73-6.31 | .17 | ||
| Intolerance to and/or progression on ibrutinib | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.29 | 0.04-2.30 | .24 | ||
| Intolerance to ibrutinib | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.60 | 0.14-2.54 | .48 | ||
| Progression on ibrutinib | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.19 | 0.35-4.00 | .78 | ||
| Prior allogeneic HCT | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.39 | 0.09-1.67 | .20 | ||
| Concurrent ibrutinib with CD19 CAR T-cell therapy | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.19 | 0.53-2.70 | .67 | ||
| Bridging chemotherapy after leukapheresis | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.87 | 0.26-2.92 | .82 | ||
| Absolute lymphocyte count (109/L) | 33 | 24 | 0.97 | 0.93-1.01 | .15 |
| Percentage of CLL cell count in the blood by MFC (% of WBCs) | 32 | 24 | 0.98 | 0.97-1.00 | .057 |
| Percentage of CLL cells in the BM by IHC (%) | 30 | 21 | 0.99 | 0.98-1.01 | .40 |
| Percentage of CLL cells in the BM by MFC (%) | 33 | 24 | 0.99 | 0.98-1.01 | .32 |
| Serum LDH concentration (log10U/L) | 33 | 24 | 0.89 | 0.21-3.80 | .87 |
| Tumorcross-sectionalarea (log10mm2) | 32 | 23 | 1.06 | 0.82-1.38 | .65 |
| Maximum SUV | 25 | 17 | 1.14 | 0.94-1.37 | .18 |
| Bulky disease† | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.91 | 0.75-4.85 | .17 | ||
| Concurrent or sequential LD | 33 | 24 | |||
| Concurrent Cy/Flu | — | — | |||
| Sequential Cy/Flu | 0.69 | 0.30-1.58 | .38 | ||
| CAR T-cell dose level (cells per kg) | 33 | 24 | |||
| 2 × 105 | — | — | |||
| 2 × 106 | 0.65 | 0.22-1.94 | .44 | ||
| 2 × 107 | 5.32 | 0.51-55.5 | .16 | ||
| CRS grade‡ | 33 | 24 | 0.89 | 0.59-1.35 | .59 |
| Neurotoxicity grade§ | 33 | 24 | 1.01 | 0.76-1.35 | .94 |
| Day +28 nodal response by CT | 29 | 20 | |||
| SD | — | — | |||
| PR | 0.12 | 0.02-0.65 | .014 | ||
| CR | 0.11 | 0.02-0.83 | .033 | ||
| Day +28 nodal response by PET-CT | 19 | 13 | |||
| PR | — | — | |||
| CR | 0.17 | 0.05-0.64 | .008 | ||
| Day +28 response by 2018 iwCLL criteria | 33 | 24 | |||
| PR | — | — | |||
| CR/CRi | 0.91 | 0.36-2.30 | .85 | ||
| Day +28 BM MRD by MFC | 33 | 24 | |||
| Positive | — | — | |||
| Negative | 0.03 | 0.01-0.15 | <.001 | ||
| Day +28 BM MRD by IGH NGS | 25 | 17 | |||
| Positive | — | — | |||
| Negative | 0.25 | 0.09-0.68 | .006 | ||
| Peak CD4+CAR T-cell expansion (log10cells per μL) | 33 | 24 | 0.49 | 0.28-0.85 | .011 |
| Peak CD8+CAR T-cell expansion (log10cells per μL) | 33 | 24 | 0.53 | 0.33-0.85 | .009 |
| CAR T-cell persistence (CAR transgene copies per μg genomic DNA)‖ | — | — | 0.56 | 0.39-0.81 | .002 |
| Characteristic . | N . | Event, n . | HR . | 95% CI . | P value . |
|---|---|---|---|---|---|
| Age | 33 | 24 | 0.96 | 0.91-1.02 | .21 |
| Sex | 33 | 24 | |||
| Female | — | — | |||
| Male | 0.89 | 0.39-2.04 | .79 | ||
| ECOG score | 33 | 24 | |||
| 0 | — | — | |||
| 1 | 1.82 | 0.81-4.08 | .15 | ||
| Prior history of or current Richter transformation | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.50 | 0.51-4.40 | .46 | ||
| Prior history of Richter transformation | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.83 | 0.19-3.54 | .80 | ||
| Current Richter transformation | 33 | 24 | |||
| No | — | — | |||
| Yes | 5.14 | 1.10-23.9 | .037 | ||
| No. of prior therapies | 33 | 24 | 1.03 | 0.84-1.26 | .79 |
| Prior fludarabine | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.68 | 0.25-1.82 | .44 | ||
| Prior bendamustine | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.02 | 0.46-2.28 | .96 | ||
| Prior venetoclax | 33 | 24 | |||
| No | — | — | |||
| Yes | 2.86 | 1.26-6.52 | .012 | ||
| Complex karyotype∗ | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.85 | 0.69-4.99 | .22 | ||
| 17p deletion | 33 | 24 | |||
| No | — | — | |||
| Yes | 2.14 | 0.73-6.31 | .17 | ||
| Intolerance to and/or progression on ibrutinib | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.29 | 0.04-2.30 | .24 | ||
| Intolerance to ibrutinib | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.60 | 0.14-2.54 | .48 | ||
| Progression on ibrutinib | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.19 | 0.35-4.00 | .78 | ||
| Prior allogeneic HCT | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.39 | 0.09-1.67 | .20 | ||
| Concurrent ibrutinib with CD19 CAR T-cell therapy | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.19 | 0.53-2.70 | .67 | ||
| Bridging chemotherapy after leukapheresis | 33 | 24 | |||
| No | — | — | |||
| Yes | 0.87 | 0.26-2.92 | .82 | ||
| Absolute lymphocyte count (109/L) | 33 | 24 | 0.97 | 0.93-1.01 | .15 |
| Percentage of CLL cell count in the blood by MFC (% of WBCs) | 32 | 24 | 0.98 | 0.97-1.00 | .057 |
| Percentage of CLL cells in the BM by IHC (%) | 30 | 21 | 0.99 | 0.98-1.01 | .40 |
| Percentage of CLL cells in the BM by MFC (%) | 33 | 24 | 0.99 | 0.98-1.01 | .32 |
| Serum LDH concentration (log10U/L) | 33 | 24 | 0.89 | 0.21-3.80 | .87 |
| Tumorcross-sectionalarea (log10mm2) | 32 | 23 | 1.06 | 0.82-1.38 | .65 |
| Maximum SUV | 25 | 17 | 1.14 | 0.94-1.37 | .18 |
| Bulky disease† | 33 | 24 | |||
| No | — | — | |||
| Yes | 1.91 | 0.75-4.85 | .17 | ||
| Concurrent or sequential LD | 33 | 24 | |||
| Concurrent Cy/Flu | — | — | |||
| Sequential Cy/Flu | 0.69 | 0.30-1.58 | .38 | ||
| CAR T-cell dose level (cells per kg) | 33 | 24 | |||
| 2 × 105 | — | — | |||
| 2 × 106 | 0.65 | 0.22-1.94 | .44 | ||
| 2 × 107 | 5.32 | 0.51-55.5 | .16 | ||
| CRS grade‡ | 33 | 24 | 0.89 | 0.59-1.35 | .59 |
| Neurotoxicity grade§ | 33 | 24 | 1.01 | 0.76-1.35 | .94 |
| Day +28 nodal response by CT | 29 | 20 | |||
| SD | — | — | |||
| PR | 0.12 | 0.02-0.65 | .014 | ||
| CR | 0.11 | 0.02-0.83 | .033 | ||
| Day +28 nodal response by PET-CT | 19 | 13 | |||
| PR | — | — | |||
| CR | 0.17 | 0.05-0.64 | .008 | ||
| Day +28 response by 2018 iwCLL criteria | 33 | 24 | |||
| PR | — | — | |||
| CR/CRi | 0.91 | 0.36-2.30 | .85 | ||
| Day +28 BM MRD by MFC | 33 | 24 | |||
| Positive | — | — | |||
| Negative | 0.03 | 0.01-0.15 | <.001 | ||
| Day +28 BM MRD by IGH NGS | 25 | 17 | |||
| Positive | — | — | |||
| Negative | 0.25 | 0.09-0.68 | .006 | ||
| Peak CD4+CAR T-cell expansion (log10cells per μL) | 33 | 24 | 0.49 | 0.28-0.85 | .011 |
| Peak CD8+CAR T-cell expansion (log10cells per μL) | 33 | 24 | 0.53 | 0.33-0.85 | .009 |
| CAR T-cell persistence (CAR transgene copies per μg genomic DNA)‖ | — | — | 0.56 | 0.39-0.81 | .002 |
CRS, cytokine release syndrome; ECOG, Eastern Cooperative Oncology Group; IGH, immunoglobulin heavy chain; IHC, immunohistochemistry; LDH, lactate dehydrogenase; WBC, white blood cell.
Defined as ≥3 chromosomal abnormalities.
Defined as largest lymph node ≥ 5 cm.
By Lee 2014 CRS criteria.6
By Common Terminology Criteria for Adverse Events version 4.03 criteria.7
Modeled as a time-dependent continuous covariate (limit of quantitation: 10 copies per μg genomic DNA).
DOR and OS by day +28 MRD status. DOR by day +28 MRD status by (A) MFC and (B) immunoglobulin heavy chain (IGH) NGS. OS by day +28 MRD status by (C) MFC and (D) NGS.
DOR and OS by day +28 MRD status. DOR by day +28 MRD status by (A) MFC and (B) immunoglobulin heavy chain (IGH) NGS. OS by day +28 MRD status by (C) MFC and (D) NGS.
We also observed an association between higher day +0 soluble interleukin-6 receptor (sIL-6R; HR, 0.08; 95% CI, 0.01-0.90; P = .041) and higher frequency of CD4+ T central memory cells in the CD3+CD4+ gate (HR, 0.96; 95% CI, 0.93-1.0; P = .023) and longer DOR (supplemental Table 4). There was a trend toward association between the frequencies of CD8+ T central memory cells (P = .08) and CD8+ naïve cells (P = .058) in the peripheral blood mononuclear cell gate and shorter DOR. We could not confirm an association between pre-LD or peak sIL-6R and DOR, nor an association between day +0 sIL-6R and PFS or OS. We did not observe an association between day +0 sIL-6R and peak CAR T-cell expansion, disease burden (pre-LD CLL BM burden, serum lactate dehydrogenase, maximum SUV, or cross-sectional tumor area), or day +28 MFC MRD–negative CR/CRi by iwCLL criteria. Furthermore, we did not observe an association between frequency of CD4+ T central memory cells in the CD3+CD4+ gate and peak CAR T-cell expansion or day +28 MFC MRD–negative CR/CRi by iwCLL criteria.
In a multivariable Cox regression model including day +28 PET-CT response, day +28 MRD negativity by MFC, and peak CD8+ CAR T-cell expansion, the first 2 predictors remained independently associated with DOR (supplemental Table 5).
Predictors of OS in the response-evaluable cohort
In univariate Cox regression, day +28 CR by PET-CT (HR, 0.25; 95% CI, 0.09-0.74; P = .012), day +28 MRD negativity by MFC (HR, 0.23; 9% CI, 0.11-0.49; P < .001), day +28 MRD negativity by NGS (HR, 0.35; 9% CI, 0.12-0.96; P = .042), peak CD4+ CAR T-cell expansion (HR, 0.66; 95% CI, 0.46-0.96; P = .028), and peak CD8+ CAR T-cell expansion (HR, 0.56; 95% CI, 0.39-0.81; P = .002) were associated with a longer OS (supplemental Table 6). The median OS estimates for patients who had MFC MRD–negative and NGS MRD–negative results were 53.3 months (95% CI, 43.0 to not reached) and not reached (95% CI, 50.3 to not reached), respectively (Figure 4C-D). We could not confirm an impact of CAR T-cell persistence on OS (supplemental Table 6). Factors associated with shorter OS included Eastern Cooperative Oncology Group performance status of 1, maximum SUV, and bulky disease (supplemental Table 6).
Impact of concurrent ibrutinib treatment and prior therapies on clinical outcomes and in vivo CAR T-cell kinetics
In the response-evaluable cohort (n = 47), concurrent ibrutinib was associated with higher median peak CD4+ CAR T-cell expansion (1.36 [IQR, 1.04-1.87] vs 0.64 [IQR, −0.02 to 1.08] log10 cells per μL; P = .009). We could not confirm an impact of concurrent ibrutinib on median peak CD8+ CAR T-cell expansion, overall response, OS, PFS, or DOR. Among patients with a day +28 response by iwCLL criteria, the number of patients receiving concurrent ibrutinib per responder category was as follows: CR by day +28 PET-CT response, 5 of 13 (38%); day +28 MRD negativity by MFC, 12 of 27 (44%); and day +28 MRD negativity by NGS, 11 of 17 (65%). We could not confirm an impact of prior fludarabine or bendamustine exposure on peak CAR T-cell expansion, overall response, OS, PFS, or DOR. We also could not confirm an association between the time between the last bendamustine-containing regimen to leukapheresis and peak CAR T-cell expansion (CD8+: P = .36 and CD4+: P = .83), OS (P = .15), PFS (P = .84), or DOR (P = .88). Prior venetoclax exposure was associated with shorter OS (HR, 2.10; 95% CI, 1.03-4.29; P = .04) and DOR (HR, 2.86; 95% CI, 1.26-6.52; P = .012) but not peak CAR T-cell expansion, overall response, or PFS.
Discussion
CD19 CAR T-cell therapy has revolutionized the treatment of patients with high-risk B-cell malignancies. Although high response rates have been reported by our group and others in patients with R/R CLL,2,3,9-13 long-term outcomes after CD19 CAR T-cell therapy are not well characterized. With a median follow-up of >6 years in 47 response-evaluable patients, our study, to our knowledge, offers the longest median follow-up to date in a large cohort of patients with R/R CLL treated with CD19 CAR T-cell therapy10,11,13-15 and provides a comprehensive analysis of >50 clinically relevant predictors of PFS and DOR, including CAR T-cell kinetics, MRD status, cytokine production, and CAR T-cell manufacturing data. We demonstrate that in a heavily pretreated, ibrutinib-refractory/intolerant population with high-risk cytogenetics, CD19 CAR T-cell therapy induced durable responses, with ∼25% of responders still in remission at 6 years. Our findings suggest that CD19 CAR T-cell therapy may be curative in a subset of responders with MRD-negative results. We identified key pretreatment predictors of PFS, namely, maximum SUV and bulky disease (largest lesion diameter of ≥5cm), which can be routinely assessed in clinical practice and potentially guide patient selection. Furthermore, we found that depth of response (ie, day +28 MRD negativity) and in vivo CAR T-cell kinetics were strongly associated with long-term outcomes.
When assessing day +28 responses after CAR T-cell therapy, we observed that in a subset of patients (n = 6), there was discrepancy between BM and nodal responses. Among patients who had MFC MRD–negative results on day +28, a portion still had nodal disease on PET-CT, indicating that CAR T-cell therapy was able to induce deep marrow responses but not a metabolic nodal response. The median DOR in this subset was very short (7 months). These results suggest that it may be more difficult for CAR T-cells to overcome the nodal tumor microenvironment compared to the BM tumor microenvironment. Supporting our observations, Mittal et al have demonstrated that the nodal tumor microenvironment is enriched with a more aggressive molecular signature consisting of proliferation and immune suppression.16
We also found that higher serum levels of IL-10, TNFRp55, and macrophage inflammatory protein 1β correlated with disease bulk and had a detrimental impact on PFS. This is consistent with published data highlighting that IL-10, TNFRp55, and macrophage inflammatory protein 1β promote CLL cell growth by inhibiting antitumor responses and activating prosurvival signals.17-19 In addition, TNFRp55 was associated with lower peak CAR T-cell expansion in vivo, suggesting a deleterious effect on CAR T-cell function. We speculate that the effect of these cytokines is twofold: (1) maintenance of an immunosuppressive CLL tumor microenvironment and (2) direct inhibition of CAR T-cell antitumor activity. Further research is warranted to characterize the mechanisms underlying CAR T-cell therapy failure for patients with CLL.
Consistent with prior studies of CD19 CAR T-cell therapy in CLL10,11,14 and other high-risk B-cell malignancies,1,20,21 we found that CAR T-cell peak expansion and persistence were associated with improved outcomes. These associations highlight CAR T-cell fitness as a key target to improve the antitumor effects of CAR T-cell therapy.22-24 Strategies to bolster CAR T-cell function and persistence include improving the CAR construct, for example by targeting the CD19 CAR to the T-cell receptor α constant locus25 or using a fully human CD19 binder,26,27 or combinatorial strategies such as concurrent PD-L1 blockade.28 Other strategies to improve patient outcomes include moving CAR T-cell therapy to an earlier therapy line29,30 and avoiding lymphotoxic therapies such as fludarabine and bendamustine, which have been associated with poorer CAR T-cell expansion, response rates, and PFS.31
Our findings are relevant to contextualize the updated results from the TRANSCEND CLL 004 trial (JCAR017; lisocabtagene maraleucel). Although we observed a higher overall response rate by 2018 iwCLL criteria after JCAR014 compared with after JCAR017 treatment (70% vs 43%, respectively), the rates of MRD negativity in BM by NGS were comparable (62% vs 59%, respectively), and median PFS and DOR were shorter (PFS: 8.9 vs 11.9 months; DOR: 18.9 vs 35.3 months, respectively).13 Our rates of high-risk cytogenetics, median lines of prior therapy, and rate of prior ibrutinib failure were similar to the TRANSCEND CLL 004 trial.13 Although JCAR014 and JCAR017 comprise the same CAR construct and are both administered in a defined composition of equal target doses of CD4+ and CD8+ CAR T cells, there are significant differences between these 2 products: a different CAR T-cell dose (per kilogram vs total dose, respectively), ex vivo manufacturing (antigen-presenting cell vs cytokine based, respectively), and end-product formulation (fresh vs cryopreserved, respectively).32,33 Additional follow-up in the TRANSCEND trial will reveal whether lisocabtagene maraleucel has curative potential, and may elucidate additional factors associated with PFS and DOR.
Limitations of this study include the single-arm design, the relatively small sample size, and variations in the LD regimen used, which might have affected outcomes. Because of our cohort size, limited multivariable analyses were performed. Because of missing data, we did not have CAR T-cell persistence and B-cell aplasia data available for the full follow-up period for each patient. Despite these limitations, we were still able to demonstrate a strong association between longitudinal CAR T-cell persistence and PFS.
This 6-year follow-up update of our phase 1/2 clinical trial demonstrates durable responses in patients with high-risk R/R CLL with MRD-negative response after CD19 CAR T-cell therapy. We identified both pretreatment (maximum SUV and bulky disease) and posttreatment variables (day +28 PET-CT response, day +28 MRD status, peak CAR T-cell expansion, and CAR T-cell persistence) that were strongly associated with PFS. We expect CD19 CAR T-cell therapy to become a critical addition to the therapeutic armamentarium for high-risk CLL. Pending additional results, the CD19 CAR T-cell product JCAR017 is anticipated to be approved by the US Food and Drug Administration in the near future based on the TRANSCEND CLL 004 clinical trial.12,13
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH) National Cancer Institute (R01 CA136551, P30 CA15704, R35 CA197734, and 2P30 CA015704-45), NIH National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK56465), NIH National Heart, Lung, and Blood Institute (T32 HL007093), the Life Sciences Discovery Fund, the Bezos Family Foundation, the National Gene Vector Biorepository at Indiana University (funded by NIH National Heart, Lung, and Blood Institute grant 75N92019D00018), and Juno Therapeutics (a Bristol Myers Squibb company).
Authorship
Contribution: E.C.L. and J.G. conceptualized and designed the study, and analyzed and interpreted the data; E.C.L., C.J.T., and J.G. collected and assembled the data; all authors were responsible for writing the manuscript and approval of the final version; and all authors are accountable for all aspects of the work.
Conflict-of-interest disclosure: A.V.H. reports honoraria from Bristol Myers Squibb and Novartis and research funding from Juno Therapeutics (a Bristol Myers Squibb company) and Nektar Therapeutics. E.L.K. reports research funding from Juno Therapeutics (a Bristol Myers Squibb company). A.C. serves on advisory boards of Affini-T, Metagenomi, SignalOne Bio, TScan Therapeutics; reports consultancy with BioNTech and Ridgeline Discovery GmbH; reports equity interest in Adaptive Biotechnologies Corporation, Affini-T, Metagenomi, SignalOne Bio, and TScan Therapeutics; and reports patents with Adaptive Biotechnologies Corporation, Affini-T, Amazon.com, Cullinan, ElevateBio, Lonza Walkersville, and Celgene. M.S. reports consulting for, and serving on advisory boards, steering committees, or data safety monitoring committees of, AbbVie, Genentech, AstraZeneca, Pharmacyclics, BeiGene, Bristol Myers Squibb, MorphoSys/Incyte, TG Therapeutics, Kite Pharma, Eli Lilly, Adaptimmune, Mustang Bio, Merck, Fate Therapeutics, MEI Pharma, and Atara Biotherapeutics; reports research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, Atara Biotherapeutics, Genmab, MorphoSys/Incyte, and Vincerx; and declares spouse employment by Bristol Myers Squibb. B.G.T. reports patents with/royalties from Mustang Bio; reports consulting for Mustang Bio and Proteios; and declares research funding from Bristol Myers Squibb and Mustang Bio. R.D.C. reports honoraria from Amgen, Jazz, Servier, Kite/Gilead, and Pfizer; consultancy or advisory role at Amgen and Kite/Gilead; received research funding from Pfizer, Amgen, Servier, Incyte, Kite, and Vanda; serves on the data safety monitoring committee of Pepromene Bio; serves on the independent response review committee of Autolus; and declares that his spouse has been employed by and owned stock in Seagen. S.R.R. is a cofounder of Lyell Immunopharma and Juno Therapeutics (a Bristol Myers Squibb company); received research funding from Lyell Immunopharma and Bristol Myers Squibb; declares intellectual property license agreement with Lyell Immunopharma and Bristol Myers Squibb; serves on advisory boards of Juno Therapeutics and Adaptive Biotechnologies; and is a member of board of directors of Ozette Technologies. C.J.T. declares research funding from Juno Therapeutics (a Bristol Myers Squibb company) and Nektar Therapeutics; serves on current scientific and clinical advisory boards of Caribou Biosciences, T-CURX, Myeloid Therapeutics, Cargo Therapeutics, and ArsenalBio; reports past scientific and clinical advisory boards membership for Precision Biosciences, Eureka Therapeutics, and Century Therapeutics; declares ad hoc advisory board membership/consulting for (last 12 months) Nektar Therapeutics, Allogene, Sobi, Legend Bio, Syncopation Life Sciences, Century Therapeutics, and Bristol Myers Squibb; reports stock options in Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, and ArsenalBio; serves on the data safety monitoring committee of Kyverna; and declares patents and the right to receive payment from Fred Hutchinson Cancer Center as an inventor on patents related to CAR T-cell therapy. D.G.M. declares serving as ad hoc consultant for, and having received honoraria from, Bristol Myers Squibb, Caribou Biosciences, Inc, Celgene, Genentech, Incyte, Juno Therapeutics, Kite, and Lilly; received research funding from Fred Hutchinson Cancer Research Center; has received research funding, including salary support, from the following companies for clinical trials as a principal investigator or subinvestigator: Kite Pharma, Juno Therapeutics, Celgene, and Legend Biotech; owns patents and the rights to royalties from Fred Hutchinson Cancer Research Center for patents licensed to Juno Therapeutics (a Bristol Myers Squibb company); has stock options in A2 Biotherapeutics, and Navan Technologies; reports memberships with compensation in A2 Biotherapeutics; is a member of the scientific advisory board of Navan Technologies, Chimeric Therapeutics, and Genentech; and is a member and chair of the Lymphoma Steering Committee, Bristol Myers Squibb member of the JCAR017 EAP-001 safety review committee; Bristol Myers Squibb member of the CLL strategic council; ImmPACT Bio, member of the clinical advisory board, CD19/CD20 bispecific CAR-T Cell Therapy Program; Gilead Sciences, member of the scientific review committee, Research Scholars Program in Hematologic Malignancies; Interius Biotherapeutics, Inc, clinical advisory board member; and reports memberships without compensation with Bristol Myers Squibb, member of the JCAR017-BCM-0 scientific steering committee. J.G. reports ad hoc consultancy for, and having received honoraria from, Sobi, Legend Biotech, Janssen, Kite Pharma, and MorphoSys; received research funding from Sobi, Juno Therapeutics (a Bristol Myers Squibb company), Celgene (a Bristol Myers Squibb company), and Angiocrine Bioscience; and served on an independent data review committee for Century Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jordan Gauthier, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North D3-100, Seattle, WA; e-mail: jgauthier@fredhutch.org.
References
Author notes
Data are available on request from the corresponding author, Jordan Gauthier (jgauthier@fredhutch.org).
The full-text version of this article contains a data supplement.